Abstract
Aging and challenging signal-in-noise conditions are known to engage use of cortical resources to help maintain speech understanding. Extensive corticothalamic projections are thought to provide attentional, mnemonic and cognitive-related inputs in support of sensory inferior colliculus (IC) inputs to the medial geniculate body (MGB). Here we show that a decrease in modulation depth, a temporally less distinct periodic acoustic signal, leads to a jittered ascending temporal code, changing MGB unit responses from adapting responses to responses showing repetition-enhancement, posited to aid identification of important communication and environmental sounds. Young-adult male Fischer Brown Norway rats, injected with the inhibitory opsin archaerhodopsin T (ArchT) into the primary auditory cortex (A1), were subsequently studied using optetrodes to record single-units in MGB. Decreasing the modulation depth of acoustic stimuli significantly increased repetition-enhancement. Repetition-enhancement was blocked by optical inactivation of corticothalamic terminals in MGB. These data support a role for corticothalamic projections in repetition-enhancement, implying that predictive anticipation could be used to improve neural representation of weakly modulated sounds.
Keywords: Auditory thalamus, less distinct modulated stimuli, sensory adaptation, repetition-enhancement
Introduction
Speech intelligibility can be maintained in noisy backgrounds and in the aged auditory system by increased use of linguistic/contextual redundancies engaged to substitute for sensory deficits (Warren, 1970; Wingfield, 1975; Peelle & Wingfield, 2016; Pichora-Fuller et al., 2016; Anderson et al., 2020). For young-adults in cluttered acoustic environments and older individuals affected by age-related hearing loss (presbycusis), higher-order/cortical resources are brought into play to help disambiguate acoustic signals (Shinn-Cunningham & Wang, 2008; Davis et al., 2011; Obleser, 2014; Başkent et al., 2016; Vaden et al., 2016; Pichora-Fuller et al., 2017). Peripheral deficits only partially account for the age-related loss of speech understanding (Humes et al., 2012; Roque et al., 2019). Sensory declines in aging may be simulated in young participants by decreasing the temporal distinctiveness of presented acoustic stimuli either by adding noise or decreasing modulation depth, resulting in a temporally jittered ascending acoustic code showing decreases in envelope-locked responses (Dubno et al., 1984; Fitzgibbons & Gordon-Salant, 1994; Pichora-Fuller et al., 2007; Dimitrijevic et al., 2016; Mamo et al., 2016). Studies in non-human primates and rabbits using amplitude modulated stimuli have reported an increased neural jitter by decreasing the modulation depth of amplitude-modulated stimuli (Nelson & Carney, 2007; Malone et al., 2010). Recent studies support use of increased top-down predictive resources to help decode challenging sensory stimuli such as in speech-in-noise or less temporally distinct speech (Pichora-Fuller et al., 2017; Anderson & Karawani, 2020).
Sensory adaptation has been observed in thalamus and cortex, for all sensory modalities, with declining responses for repeated stimuli (Ulanovsky et al., 2003; Bartlett & Wang, 2005; Pérez-González & Malmierca, 2014). In contrast to sensory adaptation, repetition-enhancement, perhaps prediction, to a repeating stimulus has been reported when acoustic signals were less temporally distinct, attended to, expected for statistical regularities, and/or with stimuli presented at higher rates in challenging conditions (Luce & Pisoni, 1998; Heinemann et al., 2011; de Gardelle et al., 2013; Müller et al., 2013; Kommajosyula et al., 2019). The current study was designed to examine the role of corticothalamic/top-down projections to medial geniculate body (MGB) in mediating repetition adaptation/enhancement responses to repeating stimuli of different modulation depths.
The auditory thalamus is a key subcortical structure suggested to play a critical role in auditory processing. Sensory systems show attention/task/context-dependent changes in thalamic activity, likely reflecting increasingly engaged corticofugal circuits (von Kriegstein et al., 2008; Saalmann & Kastner, 2011; Diaz et al., 2012; Mihai et al., 2019; Tabas & von Kriegstein, 2021). The MGB receives top-down/corticofugal information from extensive descending corticothalamic (CT) projections (Rouiller & Welker, 1991; Winer et al., 2001; He, 2003; Bartlett, 2013; Guo et al., 2017; Parras et al., 2017). These excitatory CT projections originate from cortical layer 5&6 neurons and terminate on the distal dendrites of MGB neurons in all subdivisions, including the lemniscal ventral division and the non-lemniscal dorsal and medial divisions (Bartlett et al., 2000; Winer et al., 2005; Smith et al., 2007). Additionally, MGB receives state and salience-related information from serotonergic/noradrenergic and cholinergic projections (McCormick & Pape, 1990; Sottile et al., 2017; Schofield & Hurley, 2018). MGB neurons show stimulus specific adaptation (SSA) to repeated identical stimuli, which upon presentation of an oddball signal show a significant mismatch signal, thought to code for deviance detection and prediction error (Anderson & Malmierca, 2013; Malmierca et al., 2015; Parras et al., 2017). MGB unit responses show altered tuning and gain changes with manipulation of the auditory cortex/corticofugal influences (Orman & Humphrey, 1981; He, 2003; Tang et al., 2012; Malmierca et al., 2015). A recent study by Guo et al. (2017) showed increased detection of acoustic signals involving CT projections, and CT projections have been shown to be involved in the processing of complex auditory stimuli (Ono et al., 2006; Rybalko et al., 2006; Homma et al., 2017). However, little is known about how CT inputs can alter MGB response properties to repeating signals. The aim of the current study is to examine the impact corticothalamic inputs have on the coding of random vs. repeating sinusoidal amplitude-modulated (SAM) stimuli of differing modulation depths.
Previous MGB single unit studies found that age- and decreased temporal precision (decreased modulation depth or adding noise to the envelope) of the temporal cue significantly increased MGB unit preference (discharge-rate) for repeating SAM stimuli (Cai et al., 2016b; Kommajosyula et al., 2019). Repetition-enhancement was absent in single-units recorded from MGB in anesthetized rats, suggesting that anesthesia affected thalamic and cortical responses to abolish repetition enhancement (Cai et al., 2016b). Collectively, these findings suggest that temporally less distinct acoustic cues and variability due to aging engage top-down/corticofugal influences to enhance responses evoked by a repeating, weakened ascending temporal code. The present study examined MGB single unit responses to determine if increased preference for a repeating less temporally distinct SAM stimulus could be reversed by CT blockade in young, awake rats.
Materials and Methods
Male Fischer 344 x Brown Norway (FBN) rats (n = 7), aged 4–6 months old, obtained from the NIA Aging Rodent Resource Colony supplied by Charles River, were individually housed on a reverse 12:12-h light-dark cycle with ad libitum access to food and water. FBN rats have a long life-span and lower tumor load than other commonly used rat aging models. They have been characterized as a rat model of aging (Cai et al., 2018), and age-related changes in central auditory structures have been extensively studied (Caspary et al., 2008; Caspary & Llano, 2018; Mafi et al., 2020). Procedures were performed in accordance with guidelines and protocols approved (Ref. No. 41-018-004) by the Southern Illinois University School of Medicine Lab Animal Care and Use Committee.
Microinjection
Adenoviral vectors (AAV-CAG-ArchT-GFP, AAV serotype 1) with light-activated proton pump and eYFP expressed under the control of a CAG (CMV enhancer, chicken beta-Actin promoter and rabbit beta-Globin splice acceptor site) were obtained from the University of North Carolina Vector Core (Chapel Hill, NC). Young-adult FBN rats were anesthetized initially with ketamine (105 mg/kg)/xylazine (7 mg/kg) and maintained with isoflurane (0.5–1%) throughout the duration of the surgery. A small hole was drilled into the skull and dura mater removed. Viral vectors were injected intracranially into left auditory cortex using the Neurostar stereotaxic drill and injection system (stereodrive 015.838, injectomate IM28350, stereodrill DR352; Neurostar, Germany). Coordinates of the injection sites were primary auditory cortex (A1) layers 5 and 6 (L5 and L6), entry at 22° angle laterally (−8.93, −1.8, 4.37 mm relative to bregma). Animals were allowed to recover for 21 days to allow viral expression to transport to the level of CT terminals in the MGB (Fig. 1A).
Figure 1: Targeting corticothalamic projections and acoustic stimuli.
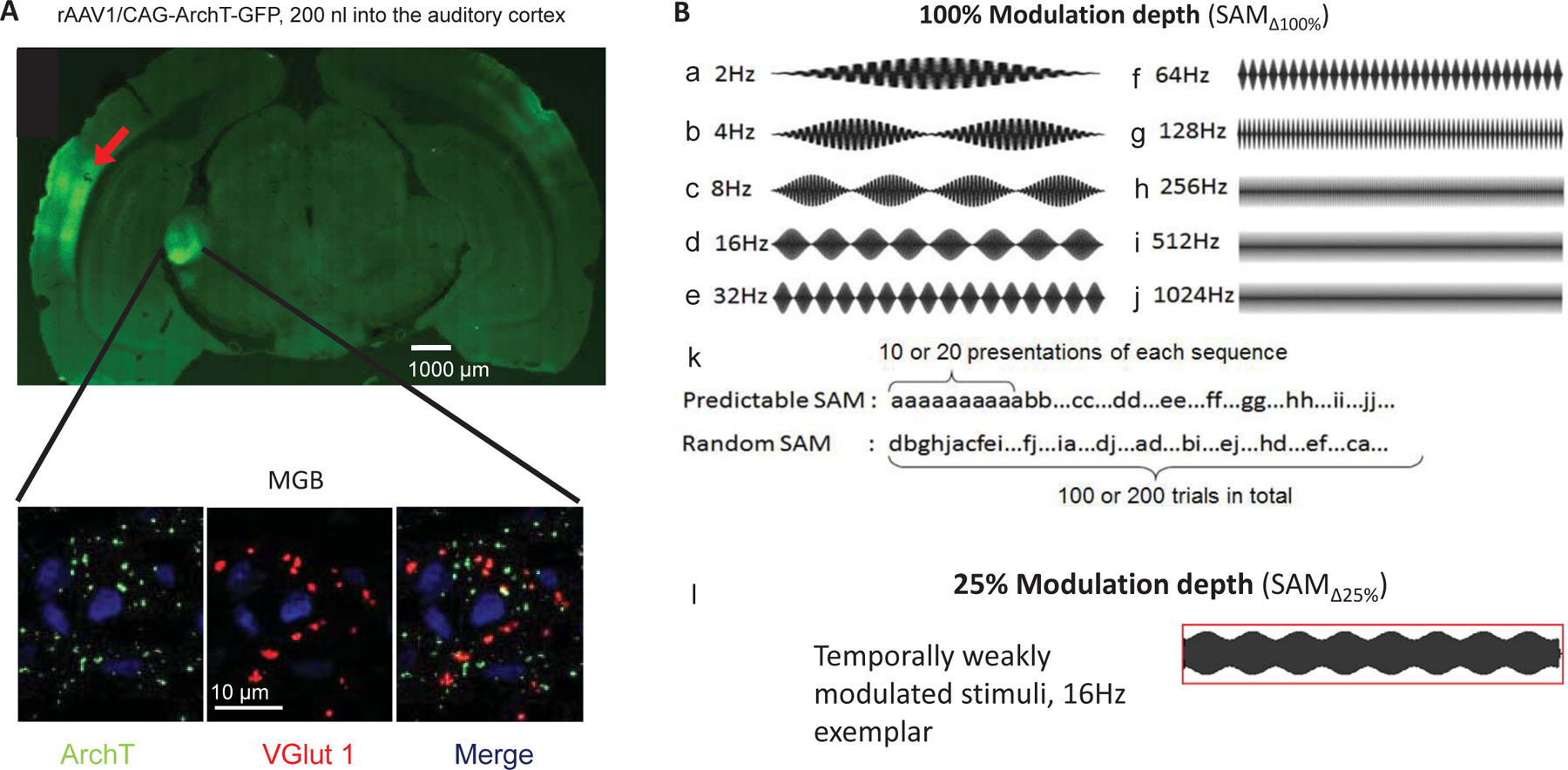
A: Confocal image showing a wide-field and inset of AI GFP-labeled (green) viral injection site and excitatory corticothalamic (CT) projection expressing the ArchT pump. Insets show MGB neurons (63x) receiving labeled projection terminals (ArchT, green), and labeled with glutamatergic marker (VGlut1, red) as well as the nuclear marker (DAPI, blue). Merged image depicts colocalization of ArchT with VGlut1. B: Sets of sinusoidally amplitude modulated (SAM) stimuli used in the present study. Standard (100% modulation depth ) SAM stimuli with either a tone or broadband noise carrier in 500 ms epochs from 2 Hz to 1024 Hz modulation frequencies [] (B, a-j). Stimuli were presented at between 2 Hz to 1024 Hz as either predictable/repeating or random sets (B, k). Exemplar waveforms of temporally weakly modulated/less distinct SAM (25% modulation depth []) at 16 Hz (B, l).
Acoustic brainstem response (ABR) recording
To ensure normal hearing thresholds, prior to optetrode implantation and 14–21 days after microinjection, auditory brainstem responses (ABR) were collected from all rats as previously described (Wang et al., 2009; Cai et al., 2016b).
Awake recordings
Three days following ABR testing, rats began 6–10 day acclimation training in a modified Experimental Conditioning Unit (ECU; Braintree Scientific, Braintree, MA) with free access to water and food reward (1/4 to 1/2 Froot™ Loop) until they could remain quiet/still for up to 3 hours. Prior to surgical implantation, VersaDrive8 optical tetrode drives (Neuralynx, Bozeman, MT) with an additional drive shaft for optical probe were assembled and loaded similarly to VersaDrive4 previously described (Richardson et al., 2013; Kalappa et al., 2014; Cai et al., 2016b). In a dark sound proof booth, there were no other known distractors to divide the rat’s attention during this passive listening task, with SAM stimuli presented from a speaker located above the rat’s head. We recorded 20–25, 45 minute-sessions from each rat. After isolation of a single-unit, spontaneous activity, rate-level functions, and response maps were collected before collecting unit responses to SAM stimulus set. Of the 80 units studied, 95% were clearly isolated single-units (high signal-in-noise ratio, similar amplitude and shape as single units or sorted using principal component analysis) the remaining 5% of units were from small inseparable unit clusters (2–3) are included since no differences in response properties were observed.
All recordings were completed within a 4 week period following implantation recovery. When recordings were complete, rats were anesthetized with ketamine and xylazine as described above and current pulses (5–10 μA for 5 s, nano Z, Neuralynx, Bozeman, MT) were passed through the tips of each tetrode wire, producing a small electrolytic lesions. Rats were cardiac perfused with phosphate-buffered saline (0.1 M, pH 7.4) followed by 4% paraformaldehyde (Sigma, St. Louis, MO), brains were removed, post-fixed for 24 h in 4% paraformaldehyde at 4°C, transferred to 20% sucrose and stored at 4°C until sectioned. To assess the position of recordings, frozen coronal sections (30–35 μm thick) were slide mounted with electrode tracks and lesion sites visible using phase-contrast microscopy. Based on each recording site relative to the final location of the tetrode tip, dimensions of the optetrode placement and MGB anatomy, an approximate location of each recorded unit was derived (Paxinos & Watson, 1998).
Electrophysiological recordings and optical stimulation
Stimulus paradigms and single unit sorting/recording procedures were the same as for awake rats as in previous studies (Kommajosyula et al., 2019). Briefly, extracellularly recoded single spikes, signal to noise ratio of at least 10:1, and with similar waveform were isolated/threholded with small spike unit clusters sorted using of principal component analysis. Stimulus presentation real-time data display and analysis used ANECS software (Dr. K. Hancock, Blue Hills Scientific, Boston, MA). Acoustic signals were generated using a 16-bit D/A converter (TDT RX6, TDT System III, Tucker Davis Technologies, Alachua, FL), and transduced by a Fostex tweeter (model FT17H, Fostex, Middleton, WI) placed 30 cm above animal’s head. The Fostex tweeter was calibrated off-line using a ¼ inch microphone (model: 4938; Brüel & Kjær, Naerum, Denmark) placed at the approximate location of the raťs head. ANECS generated calibration tables in dB sound pressure level (SPL) were used to set programmable attenuators (TDT PA5) to achieve pure-tone levels accurate to within 2 dB SPL for frequencies up to 45 kHz. The TDT generated “sync-pulse” was connected to an LED optical system (200 µm, 0.39 NA, Thorlabs Inc., NJ) with LED driver (M565F3, LEDD1B, Thorlabs Inc.). Optical stimuli from LED driver were calibrated prior to experiments using optical power meter (S121C and PM121D, Thorlabs Inc., NJ). Optical stimuli were 565 nm wavelength as determined to be the best wavelength for photo-inhibition mediated by ArchT (Han et al., 2011). Optogenetic stimulus parameters were chosen to allow for simultaneous stimulation of sound and optical stimuli based on previous and our own preliminary studies: 2.56 mw (~20.38 mW/mm2) intensity presented for 20–40 ms and at 10 Hz regardless of modulation frequencies () (Kato et al., 2017; Natan et al., 2017; Bigelow et al., 2019).
Experimental design: SAM stimulus paradigms and data acquisition
The present study compared the single unit responses in response to three paradigms presented in either a random or repeating paradigm:1) Fully modulated SAM (), considered the standard clear temporal signal; 2) SAM at 25% modulation depth () considered a less temporally distinct signal; 3) with during corticothalamic blockade (+ CT blockade) (Fig.1B & 2). There were only small differences (< 2 dB) in total energy levels between the standard () and lower modulation depth stimuli. We will interchangeably use standard () and less temporally distinct SAM () across the manuscript. The less temporally distinct SAM stimulus was chosen, in part, as a surrogate for aging to reproduce prior results (Cai et al., 2016a; Kommajosyula et al., 2019). Kommajosyula et al. (2019) found that with1.0kHz noise jittering the envelope gave similar results to . The SAM carrier was generally BBN, but the uniťs (characteristic frequency) CF was used as carrier if the unit was more strongly driven by CF-tones. Rate modulation transfer functions (rMTFs) and temporal modulation transfer functions (tMTFs) were collected at 30–35 dB above CF or BBN threshold. SAM stimuli were of 450 ms duration, presented at 2/sec with a 4 ms raise-fall; were stepped between 2 and 1024 Hz (Fig. 1B). SAM stimuli were presented as two separate sets: pseudorandomly, from now on referred to as random across trial (interleaved) or identical repeating/blocks of SAM, with each repeated (10 times) before being stepped to the next in a stepped increasing order (Fig. 1B). To control for order of presentation during repeating trials, we tested stepped in descending steps/reverse order, from 1024 to 2 Hz and found that presentation order (descending or ascending) made no difference on spike count. All reported data for repeating SAM trials were stepped from 2 to 1024 Hz. Spikes were collected over a 500 ms period following stimulus onset, with 10 stimulus repetitions at each envelope frequency. Responses to CT blockade examined the role of CT MGB projection during stimuli. The effect of CT blockade on coding was collected from a subset of MGB units neurons. Data were collected every day for 3–4 weeks after implantation. Data were recorded only if single-unit responses were repeatable and consistent across multiple trials.
Figure 2: Effects of stimulus modulation depth on temporal locking properties of MGB units:
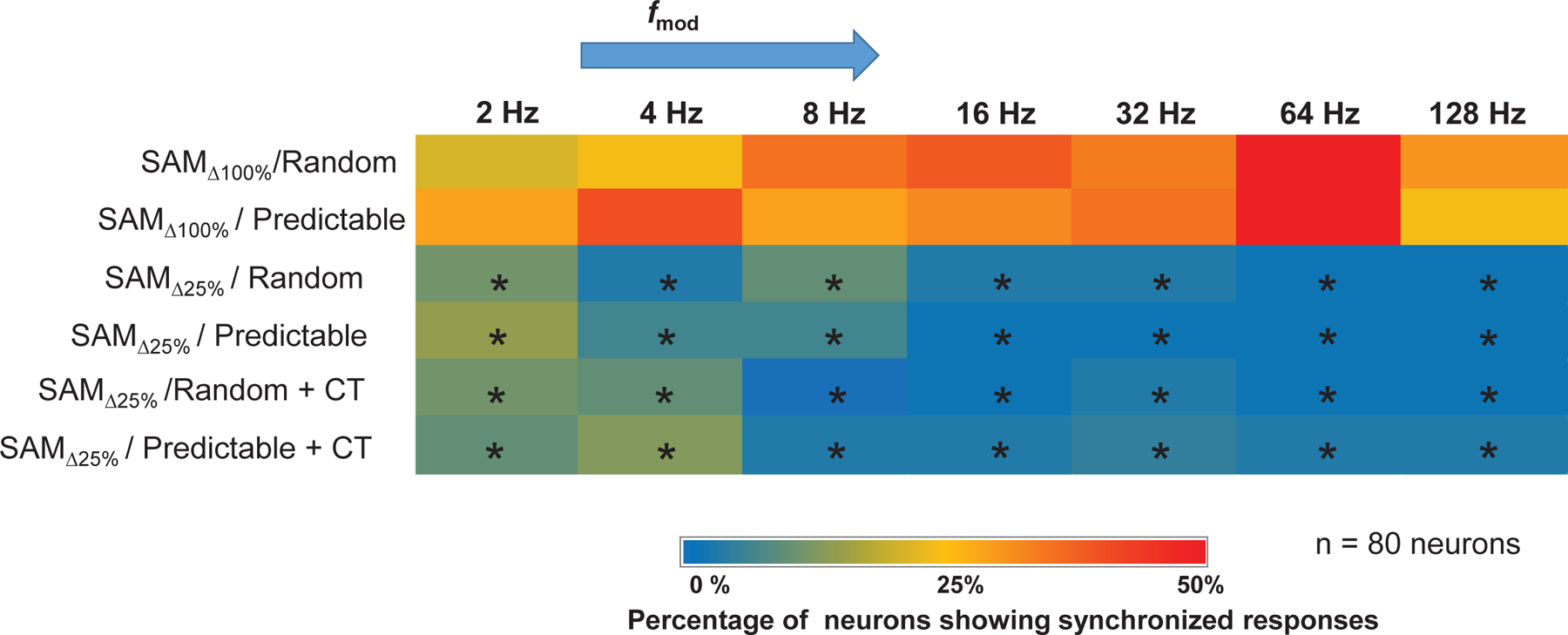
To assess the ability of units to temporally follow the SAM stimulus, the Rayleigh score for each (2–128) was used to generate a heat map based on the temporal responses of all 80 MGB units studied. MGB units might lock to a single or multi based on the Rayleigh score. Warmth of color indicates the percentage of neurons (out of 80) showing temporal-locking (Rayleigh statistic ≥ 13.8) to the SAM stimuli. Hot colors (red) indicate a higher percentage of units showing temporal-locking (e.g at 64 Hz ), whereas cool colors (blue) indicate a lower percentage of units showing temporal locking (e.g. at 16 Hz ). Significant differences were observed between and regardless of order of presentation, with and without CT blockade (Wilcoxon test followed by Bonferroni correction, p < 0.05).
Rate-level functions and spontaneous activity (250 epochs of 250 ms each) were recorded in presence and absence of optical blockade. Broadband noise (BBN) (200 ms, 4 ms rise-fall, 2/sec) stimuli were stepped in rate-level functions (0 dB to 80 dB) and responses were collected over a 500 ms period. Response maps were used to determine the CF of sorted single units (Cai & Caspary, 2015). Real-time single unit activity was sampled at 100 kHz and archived for off-line analysis.
Immunohistochemistry
Free-floating slices were processed in parallel and treated with 0.2% Triton-X for 1 h and incubated for 2 h in blocking solution containing PBS with 0.1% Triton-X, 1.5% normal donkey serum and 3% bovine serum albumin. Sections were transferred to primary antibody solution containing monoclonal mouse anti-vesicular glutamate transporter 1 (VGlut1) antibody (1:750; Millipore, Burlington, MA) in blocking buffer and incubated overnight at room temperature. After washing in PBS, sections were incubated with secondary antibody as follows: donkey anti-mouse IgG (Alexa Fluor 647, 1:150, Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. As a negative control, the primary antibody was omitted. Sections were mounted onto slides, cover slipped with VectaShield (Vector Laboratories) and imaged with a Zeiss LSM 800 confocal microscope. Injection of Arch T virus into deep layers of auditory cortex led to expression of GFP tagged ArchT within 4 weeks in the CT terminals at the level of medial geniculate body, as shown by colocalization (yellow) (Fig. 1A).
Statistical data analysis
Data were collected for MGB single units with or and CT-blockade as between subject variables. Normality assumptions were met and ANOVA was run to determine significance at the p < 0.05 level. Bonferroni corrections were utilized for pairwise comparisons to maintain a type I error level of 5% or less.
Responses were analyzed offline. Phase locking ability was evaluated by the standard vector strength equation: , where total number of spikes and the phase of observed spike relative to modulation frequency (Goldberg & Brown, 1969; Yin et al., 2011). Statistical significance was assessed using the Rayleigh statistic to account for differences in the number of driven spikes, with Rayleigh statistic values greater than 13.8 considered to be statistically significant (Mardia & Jupp, 2000) (Fig. 2). To compare number of units showing phase locking, a Wilcoxon test was used followed by a Bonferroni correction for multiple comparisons.
Rate-level functions determined using spike rate in response to BBN were quantified across intensities and compared between control and CT blockade paradigms using repeated measures ANOVA with Bonferroni correction. Spontaneous activity measured using spike rate across 250 ms epochs in 10 ms bins were compared between control and CT blockade paradigms using repeated measures ANOVA with Bonferroni correction. Preliminary analysis involved differences between order of presentation and across stimulus conditions using total spike counts from 10 trials at 10 different . Differences between orders of presentation were compared across random or repeating presentation of stimuli between , , and blockade condition using repeated measures ANOVA followed by post-hoc Bonferroni corrections.
Differences between stimulus conditions were compared using a preference ratio (PR) calculated across all (PR = total spikes in repeating trials/total spikes in random trials). A ratio smaller than 0.95 suggests the unit is a random preferring unit; a ratio larger than 1.05 suggest the unit is repetition preferring unit; while a ratio between the range of 0.95 and 1.05 were considered non-selective units (Fig. 3). The rationale for use of 10 % change in firing as a criteria was based on previous studies (Ghitza et al., 2006; Cai & Caspary, 2015; Cai et al., 2016b). Chi-Square test was used to compare the PR across conditions.
Figure 3: Random vs. predictable/repetition preference with and without CT blockade.
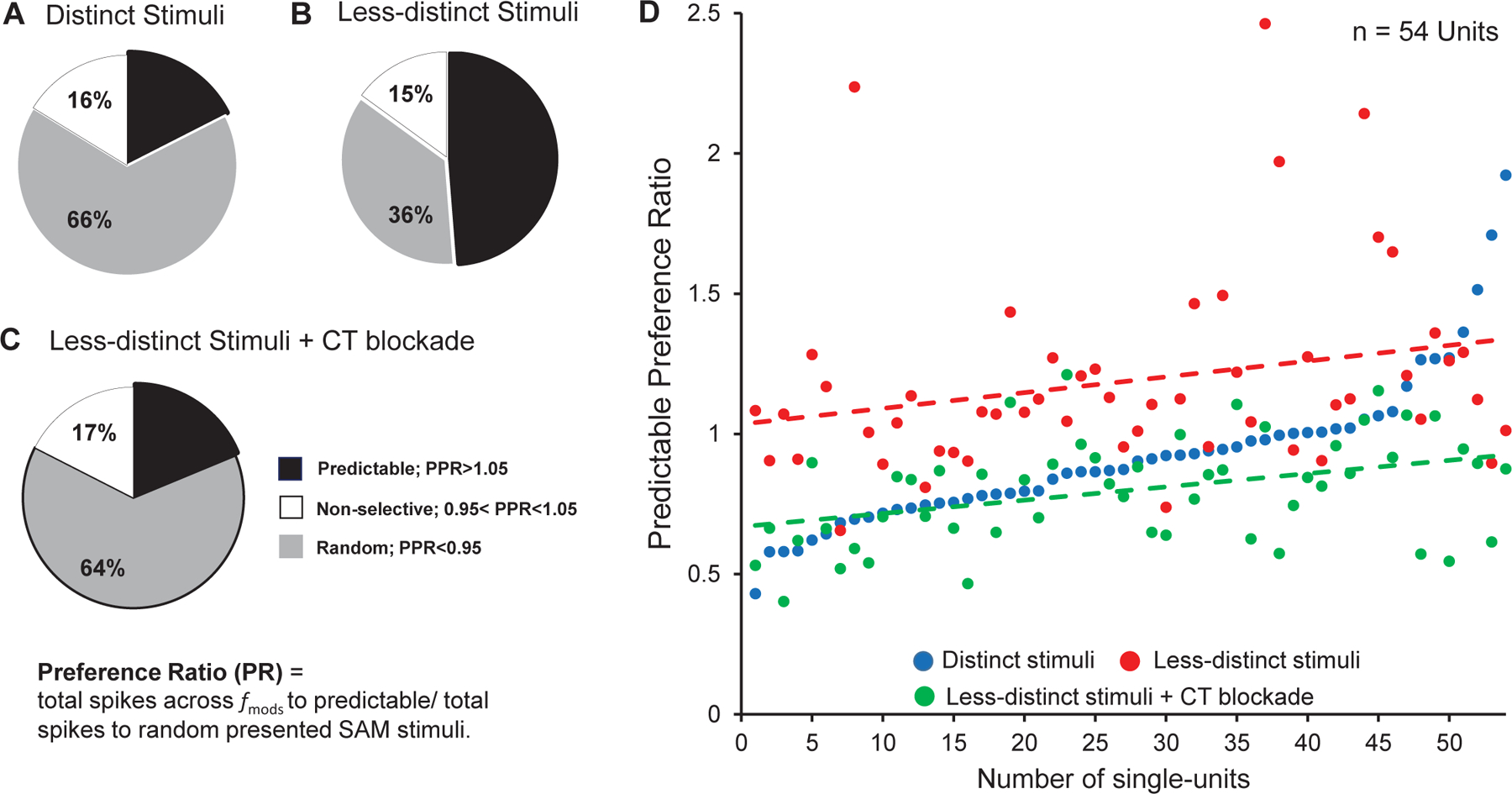
Preference ratios (PR) (total spikes to predictable trials/total spikes to random trials) across all in response to distinct, less distinct SAM stimuli, less-distinct stimuli with corticothalamic blockade (CT blockade). A: Unit recording from awake rat MGB showed a clear preference for random distinct stimuli. B: Responses to predictable (repeating) SAM stimuli increased from 18% (14/80), to 49% (39/80), in response to across . C: Optical CT blockade reversed the predictable preference of MGB neurons to 19% (14/80, in response to less SAM. Significant differences were seen between vs. , vs. blockade and blockade vs. + recovery (Chi-Square test, p < 0.05). D: PR values plotted on a continuum of increasing PPI values for each of 54 MGB units showing differential responses to distinct, (blue dots) vs. less-distinct, stimuli (red dots) and with CT blockade (green dots). The green trend line shows that CT blockade dramatically decreased the PR in response to (red trend-line) approaching the response to stimuli (blue dots).
Modulation transfer functions (MTFs) were determined using spike rate (rMTF) measurements at each tested. The rMTF data were used for further quantitative analyses. A predictable preference index (PPI) was calculated using the area under the curve (AUC) and the equation: PPI = [(AUCREP-AUCRAN)/(AUCREP+AUCRAN)], modified from the novelty response index (Lumani & Zhang, 2010; Cai et al., 2016b). The area under successive frequency segments of the rMTF curve (AUC) values were based on rMTF curve calculated using GraphPad Prism. The range of PPI values varied between −1 to +1: +1 represented a repetition preferring unit response, and −1 represented a random preferring unit response (Figs 5 and 6). By calculating the AUC for specific ranges, changes between sets of could be compared. Repeated-measures ANOVA followed by post-hoc Tukey correction for multiple comparisons was used to compare PPI values.
Figure 5: Predictable preference index (PPI) for MGB unit’s sensitive to stimulus depth of modulation:
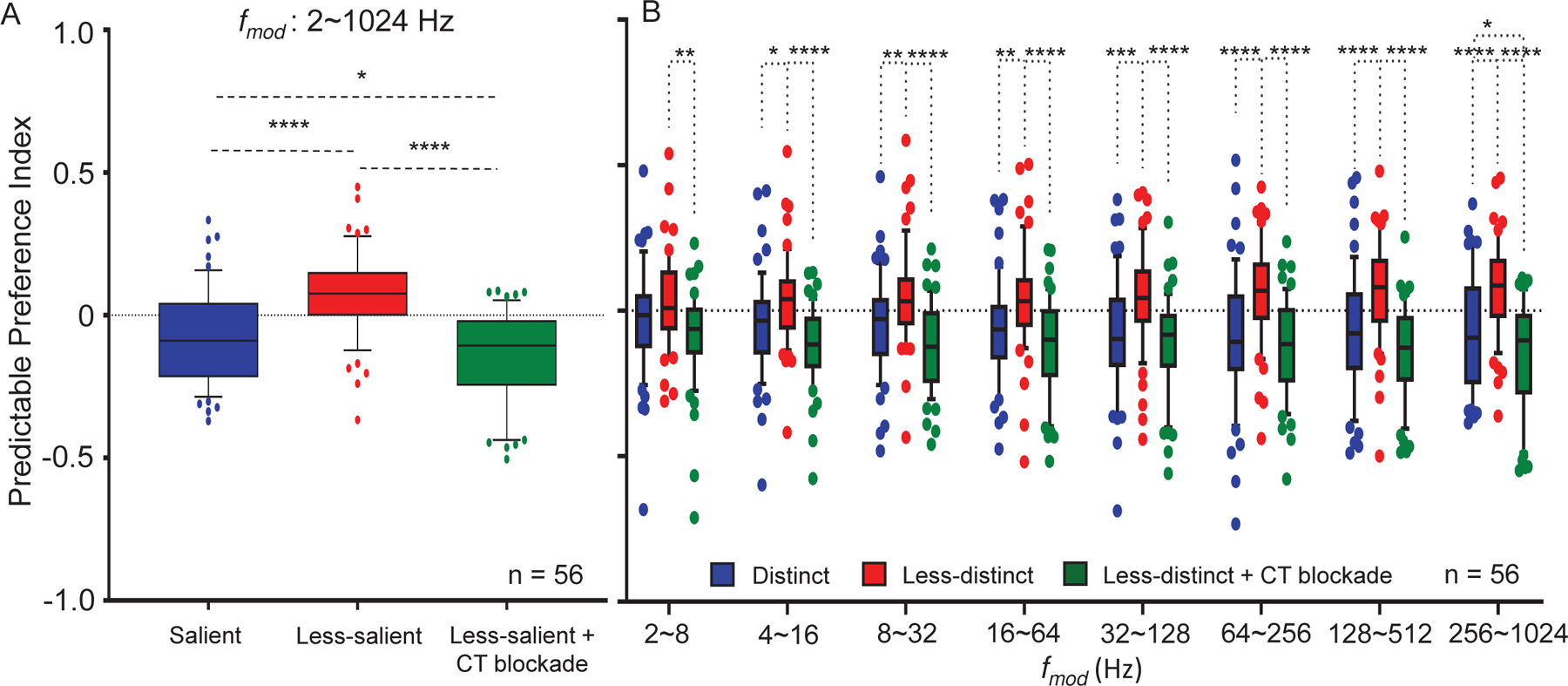
PPI’s were calculated (see text) for MGB responses to random and predictable trials across all combined and for specific subsets of A. For all combined, MGB units (n = 56) showed significant increases in PPI values (red bar) when switching from to less distinct stimuli (blue bar). The observed increase in PPI was reversed (green bar) with corticothalamic (CT) blockade. B. PPI values for MGB neurons showed significantly increased PPIs to especially at higher with CT blockade reversing these increases. (Data are presented as the mean ± SEM; repeated-measures ANOVA followed by post hoc Tukey’s correction were used for analyses (Graphpad). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Figure 6: MGB region specific changes in predictable preference index (PPI) for unit’s sensitive to stimulus depth of modulation:
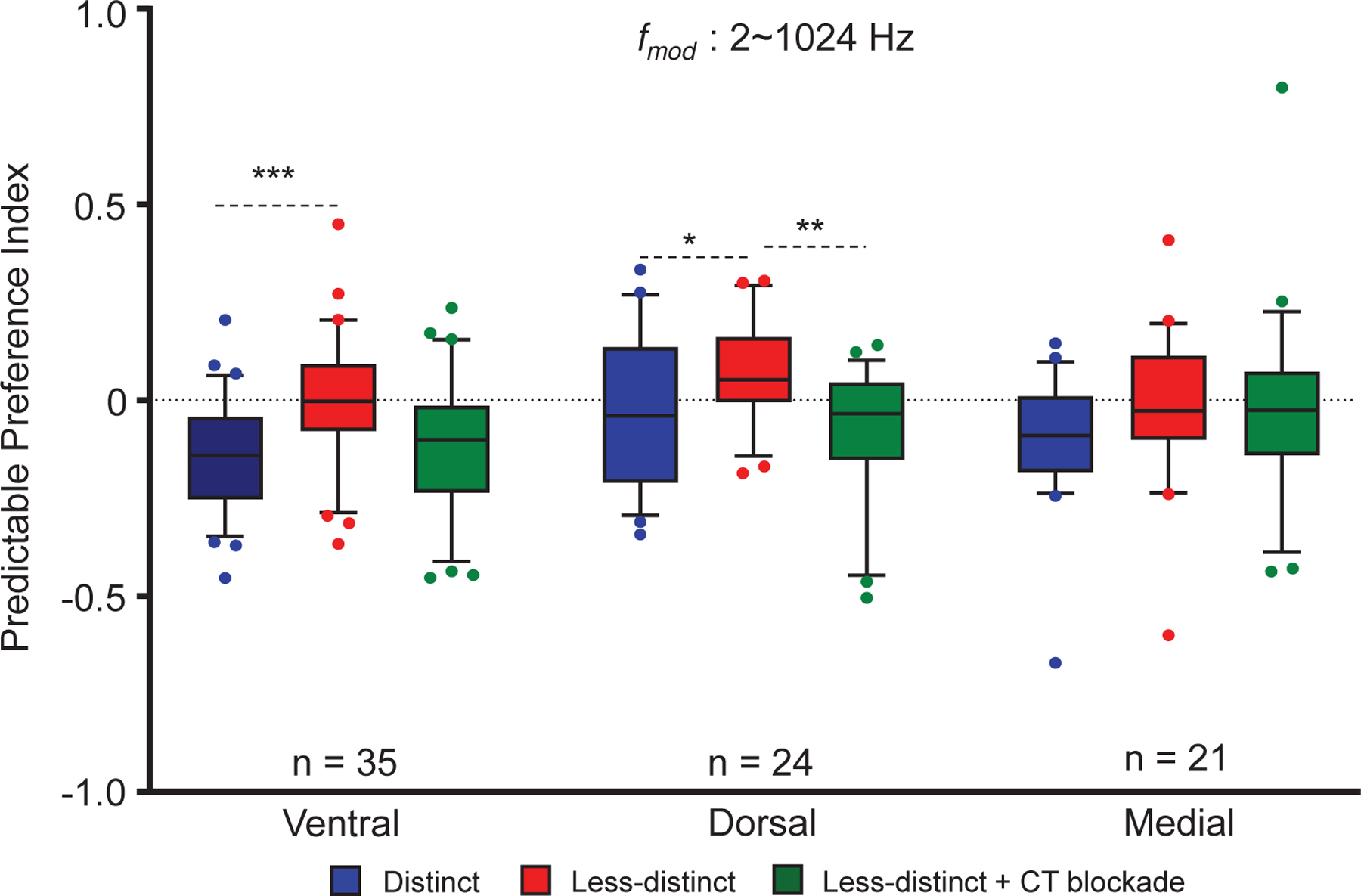
PPI’s were calculated (see text) for MGB units located in the three major divisions of the MGB. Responses to random vs. predictable SAM across all combined with and without CT blockade. Across , dorsal (24) and ventral (39), MGB units showed significant increases in PPI values (red bar) when switching from to . Corticothalamic (CT) blockade reversed this significant increase for dorsal and ventral MGB units. These changes were not observed in the medial division. Data are presented as the mean ± SEM; repeated-measures ANOVA followed by post hoc Tukey’s correction were used for analyses (Graphpad). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Trial-to-trial responses to repeating/predictable SAM presentation showed repetition-enhancement at temporally challenging (higher frequency) ( 128 Hz-1024 Hz) (Cai et al., 2016b; Kommajosyula et al., 2019). Differences in firing rate trend-line slopes between the three groups (standard SAM were compared using two-tailed ANCOVA, followed by Friedman test with a post-hoc Wilcoxon test to analyze spike rate differences at each trial (Fig. 7).
Figure 7:
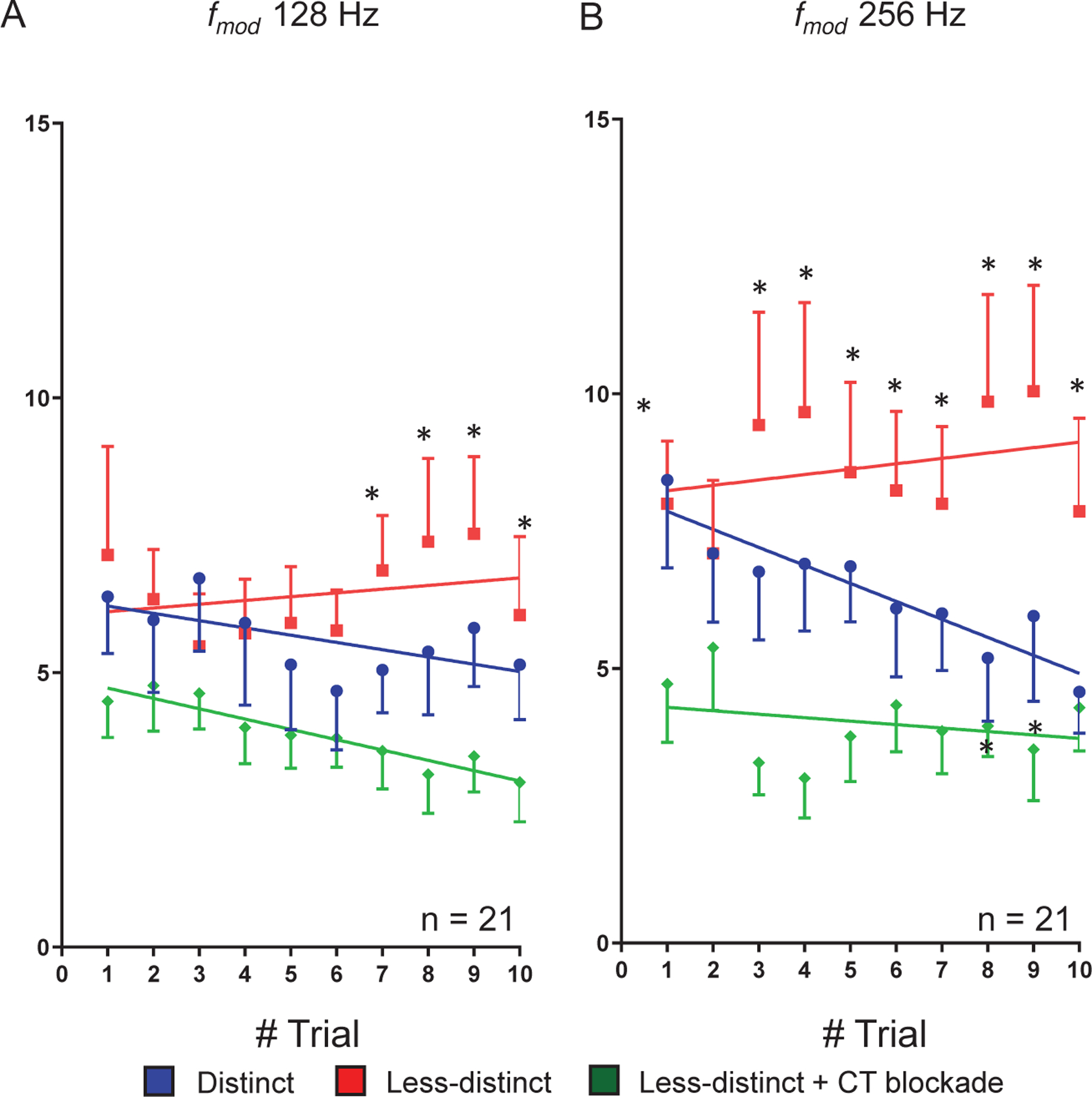
Trial-by-trial response analysis to to with and without corticothalamic (CT) blockade. Single-units showing PPI changes larger than 0.3 at high when switch from to are included in the trail by trial analysis. Group (n = 21) trial-by-trial responses to predictable SAM at 128Hz (A) and 256Hz (B). These units show adapting responses to 10 presentations of repeating salient stimuli (blue dot). Decreasing SAM modulation depth switched the trial-by-trial responses from adapting to predictable with spikes increasing with each successive presentation of the stimulus (red dot). Optical CT blockade reversed the predictive response (green dot). Trend line slopes were significantly different for the three conditions for average spikes to predictable presentation of at 128 Hz (A, ANCOVA, two-tailed, p < 0.05). Differences were significant at individual trial 7, 8, 9 and 10 in between and stimulus conditions (p < 0.05, Friedman test followed Wilcoxon test) (A). Similarly, Trend line slopes were significantly different for the three conditions for average spikes to predictable presentation at 256 Hz (B) (ANCOVA, two-tailed, p < 0.05). Differences were significantly different at trial 1, 3, 4, 5, 6, 7, 8, 9, and 10 between vs. with CT blockade. There were significant differences between and stimuli at trial 8 and 9 in their firing rates (B) (p < 0.05, Friedman test followed Wilcoxon test).
Repeated measures ANOVA followed by post-hoc Bonferroni corrections were used to test statistical significance. Statistical analysis was performed using GraphPad Prism 6 and IBM SPSS version 24. All values are expressed as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001, were treated as statistical significance level.
Results
Eighty MGB units, responding to sinusoidal amplitude modulation stimuli (SAM) were recorded from the MGB in awake, passively listening, young-adult FBN rats. Consistent with previous studies, MGB single-unit responses to SAM stimuli showed band-pass, low-pass, high-pass, mixed or atypical rMTFs, showing synchronized and asynchronized or mixed responses (Bartlett & Wang, 2007).
Basic response properties with CT blockade
There were no significant changes in spontaneous activity with CT blockade compared to control condition (13.85 ± 1.27 vs 13.26 ± 1.34, n = 45; p = 0.282). Rate-level functions showed significant decreases in responses across intensities with CT blockade compared to control (Multivariate ANOVA, p = 0.040) with significant differences for comparisons at a couple of intensities (Table 1).
Table 1:
Rate-level functions under control and CT blockade conditions for 60 units
| Intensity (dB) | Control (Mean±SEM) | CT blockade (Mean±SEM) | p-value* |
|---|---|---|---|
| 0 | 15.927±1.5 | 15.493±1.5 | 0.564 |
| 10 | 15.75±1.7 | 14.306±1.5 | 0.152 |
| 20 | 15.195±1.5 | 13.941±1.5 | 0.07 |
| 30 | 14.797±1.5 | 13.997±1.5 | 0.346 |
| 40 | 16.0396±1.7 | 13.472±1.4 | 0.004 |
| 50 | 14.854±1.5 | 13.920±1.5 | 0.17 |
| 60 | 15.429±1.5 | 13.791±1.4 | 0.031 |
| 70 | 15.462±1.4 | 15.008±1.5 | 0.437 |
| 80 | 18.062±1.8 | 18.182±1.7 | 0.908 |
Comparisons are made between control (column 2) vs. CT blockade (column 3) in 60 neurons
Column 4 represents the Bonferroni-corrected p-values for comparisons between MGB single-unit responses to control and CT blockade.
Decrease in modulation depth decreases envelope-locking of MGB neurons
Decreasing modulation depth to decreased envelope locking of MGB units studied relative to stimuli, as measured using the Rayleigh score across (2–128 Hz) (Fig. 2). A higher percentage of MGB units showed temporal locking (Rayleigh statistic ≥13.8) to the standard stimuli () than to the stimuli across tested (table 2). CT blockade did not alter percentages of envelope-locking responses to less-distinct/ stimuli across tested. These data show decreased temporal locking in response to stimuli and that temporal locking was relatively independent of top-down modulation. These results are similar to findings showing decreases in temporal locking when adding noise to the SAM periodic envelope (Kommajosyula et al., 2019). Here we focus on rate responses of MGB single-units and the effect of CT projections on MGB single-unit response properties.
Table 2:
Bonferroni-corrected p-values for percentage of envelope-locking units with changing sound stimuli modulation depth
| range (Hz) | vs. * |
vs. * +CT blockade* |
||
|---|---|---|---|---|
| Random | Repeating | Random | Repeating | |
| 2 | 0.037 | 0.0079 | 0.037 | 0.0014 |
| 4 | 0.00082 | 0.000012 | 0.0071 | 0.000098 |
| 8 | 0.00016 | 0.00048 | 0.00000072 | 0.00016 |
| 16 | 0.0000072 | 0.000034 | 0.0000042 | 0.000058 |
| 32 | 0.000034 | 0.000012 | 0.000034 | 0.000034 |
| 64 | 0.0000001 | 0.0000001 | 0.0000001 | 0.00000019 |
| 128 | 0.000058 | 0.00048 | 0.000058 | 0.00082 |
Each row represents the Bonferroni-corrected p-values following the Wilcoxon tests for corresponding (in Hz) in the first column of the row. Comparisons are made between salient vs. less-salient (column 2) and salient vs. less-salient+ CT blockade (column 3) in all the of neurons (n=80).
Decreased modulation depth and CT blockade significantly alter MGB unit rate response to random vs. repeating SAM
Total spike counts in response to SAM stimuli presented in random or repeating trials were compared across stimulus sets with and without CT blockade (standard SAM at 100% depth of modulation []), less distinct (SAM at 25% depth of modulation ), less distinct blockade) (Fig. 1B and methods for details). Consistent with Kommajosyula et al. (2019), 66% (56 of 80) MGB units preferred randomly presented stimuli (Fig. 3A). When modulation depth was reduced to , there was a significant increase in the percentage of MGB units showing a rate preference for repeating stimuli (18% vs. 49%, X2(4, N = 80) = 88.789, p = 2.3812E-18) (Fig. 3A&B). This switch in preference toward repeating less distinct was reversed by CT blockade in MGB (49% vs. 19%, X2(4, N = 80) = 84.884, p = 1.6054E-17) (Fig. 3B&C). Following termination of CT optical blockade, MGB unit responses returned to showing increased response preference for repeating less distinct/ (19% vs. 39%, X2(6,N = 80) =106.386, p = 1.1628E-20, data not shown).
Ninety percent (72/80) of MGB units changed their PRs toward repeated stimuli in response to the switch in modulation depth/CT blockade (change in PR > 0.1). Seventy-five percent (54/72) of those units shifted their preference from repeated back to random stimuli with CT blockade at . The PR scores for each of the 54 MGB units were plotted on a continuum of increasing PR score for , with PR for (with or without CT blockade) also plotted for each unit (Fig. 3D). PR trend lines show an increase in PR to repeating stimuli when switching from to for most units (Fig. 3D-red line). CT blockade during stimuli (green trend line) returns the PR or preference for random stimuli, to levels which approximate but are below responses for . Reducing SAM modulation depth increased repetition-enhancement in 54/72 neurons, while CT blockade reversed the switch from repetition-enhancement to adapting responses (Fig. 3D).
The 18 remaining MGB units of the 72 units did not show a change in PR with a decrease in SAM temporal distinctiveness ( to ) but showed increase in PR, or a preference for repeated stimuli when switched to with optical CT blockade. Eight MGB neurons unresponsive to optical blockade were not included in the analysis.
Changes in response to modulation depth and CT blockade are shown for an exemplar MGB unit (Fig.4). Switching to less-distinct showed a two-fold increase in responses to repeating trials across a range of modulation frequencies, which was reversed by CT blockade (Fig. 4B&C).
Figure 4: Exemplar MGB unit showing differential responses to SAM presentation order, modulation depth and CT blockade:
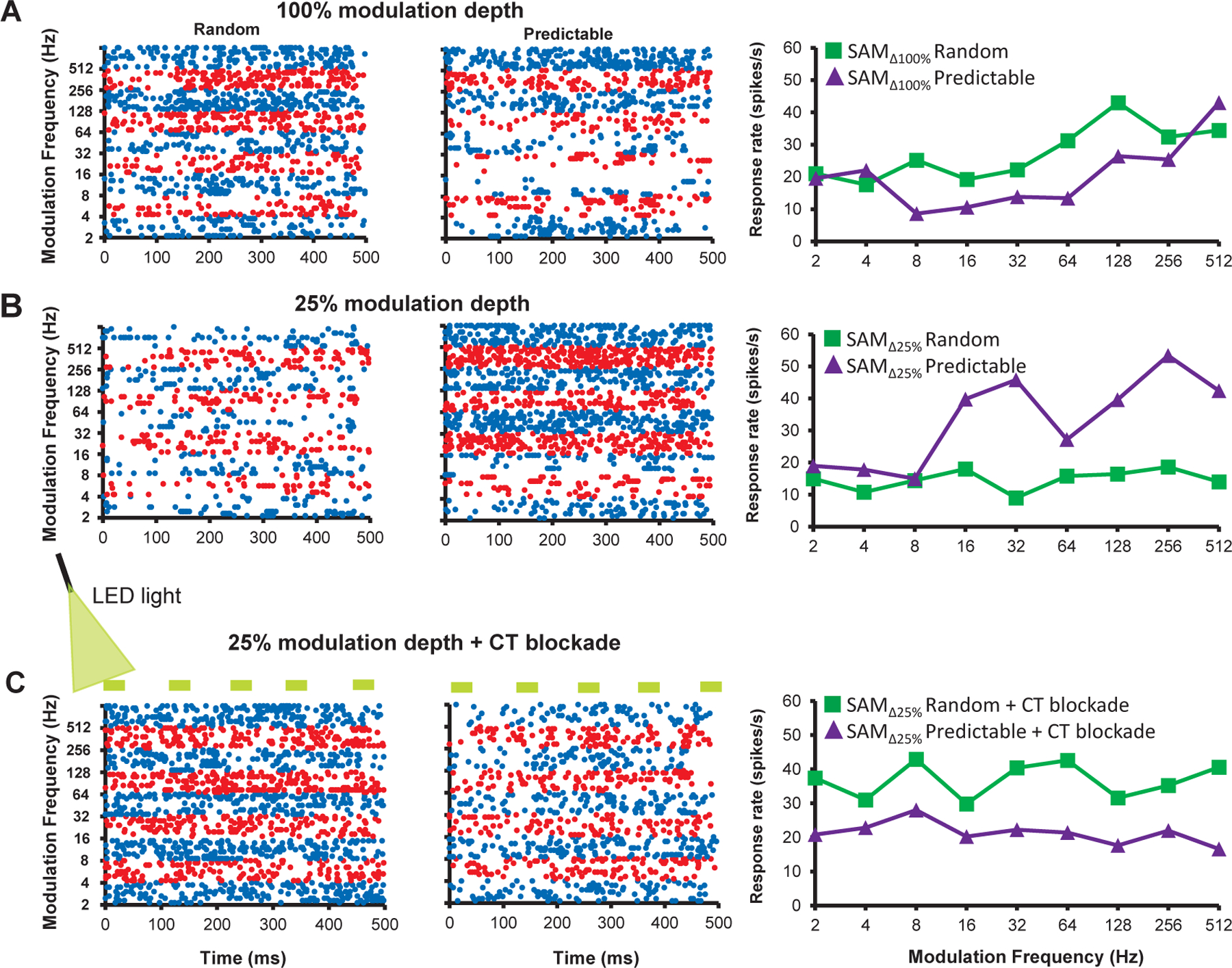
A. a representative MGB unit showing a higher discharge rate (spikes/sec) to randomly presented across than to predictable/repeating stimuli in dot raster and rate-modulation transfer functions (rMTFs). B. When modulation depth was decreased to , less distinct stimuli, the same MGB unit showed increased/greater responses to a predictable/repeating SAM, especially at higher . C. Optical blockade of CT input resulted in a return to strong random preference even in response to less distinct stimuli, in this same exemplar.
Since PR does not differentiate differences across , we calculated the predictable preference index (PPI), a quantitative measure derived from area under the curve (AUC) values across groups of modulation frequencies, PPI = [(AUCREP-AUCRAN)/(AUCREP+AUCRAN)]. Higher PPI values indicate increased preference for repeating trials, while lower PPI values indicate a preference for randomly presented trials. PPI values were lower for standard stimuli () across all tested (Fig. 5A). Seventy-nine percent of MGB units (56/71) showed increased PPI value with decreased modulation depth (), indicating repetition-enhancement. CT blockade during presentation of reversed the notable increase in PPI (repeated measures ANOVA, F(2, 165) = 39.512, p = 2.682E-11, Bonferroni corrected p-values (standard vs. less-salient = 0.000001; vs. blockade = 1.4624E-11; vs. blockade = 0.019 ) (Fig. 5A). Changes in PPI were determined for sets of increasing across different stimulus groups (Fig. 5B). significantly increased PPI values and these changes were more pronounced at higher . CT blockade significantly decreased PPI values across (Fig. 5B). At between 256–1024 Hz, PPI values were significantly decreased by CT blockade even when compared to standard, stimuli (Table 3 for repeated measures ANOVA, Bonferroni corrected p-values and comparisons at each range) (Fig. 5B).These results suggest that MGB responses to standard, stimuli show a degree of CT influences at the higher tested. For 13 single-units, the effects of CT blockade at was tested in resopnses to sequencial/repeating trails with and without CT blockade. There were no significant differences in spike rates ( vs. blockade = 17.62615 ± 3.52428 vs. 15.2132 ± 2.9107, p = 0.0529, T-test) and for PPI values between the two conditions across all ( vs. blockade = −0.03926 ± 0.0393 vs. −0.03136 ± 0.0316, p = 0.8611, T-test). This results supports the hypothesis that additional top-down resources were engaged by temporally less distinct SAM stimuli.
Table 3:
Bonferroni-corrected p-values for PPI values of 56 units sensitive to modulation depth change
| range (Hz) | vs. * |
vs. * +CT blockade* |
vs. * +CT blockade* |
|---|---|---|---|
| 2~1024 | 0.000001 | 1.4624E-11 | 0.019 |
| 2~8 | 0.194306274 | 0.002414952 | 0.238624372 |
| 4~16 | 0.026271845 | 7.2624E-06 | 0.081380638 |
| 8~32 | 0.004712185 | 3.10386E-07 | 0.071969343 |
| 16~64 | 0.004245266 | 3.88949E-06 | 0.213901181 |
| 32~128 | 0.000870169 | 8.76613E-06 | 0.533565168 |
| 64~256 | 5.21613E-05 | 4.47651E-08 | 0.347311451 |
| 128~512 | 6.14401E-05 | 7.89532E-10 | 0.091124673 |
| 256~1024 | 3.8719E-05 | 4.2874E-11 | 0.039640353 |
Each row represents the Bonferroni-corrected p-values to corresponding range (in Hz) in the first column of the row. Comparisons are made between salient vs. less-salient (column 2); less-salient vs. less-salient + CT blockade (column 3); and salient vs. less-salient+ CT blockade (column 4) in the majority of neurons (n=56).
The 15 MGB units that did not show PPI changes in modulation depth paradoxically showed significantly increased PPI values with CT blockade, across examined (Table 4).
Table 4:
Bonferroni-corrected p-values for PPI values of 15 units insensitive to modulation depth change
| range (Hz) | vs. * |
vs. * +CT blockade* |
vs. * +CT blockade* |
|---|---|---|---|
| 2~1024 | 0.823374867 | 1.48214E-05 | 0.000142022 |
| 2~8 | 0.774652034 | 0.992047279 | 0.840986588 |
| 4~16 | 0.811395141 | 0.79258215 | 0.41545121 |
| 8~32 | 0.973549441 | 0.238930012 | 0.342751939 |
| 16~64 | 0.946456635 | 0.018936675 | 0.044413028 |
| 32~128 | 0.800376342 | 0.002708837 | 0.000256347 |
| 64~256 | 0.239459428 | 0.003792644 | 5.52114E-06 |
| 128~512 | 0.705864677 | 0.001001792 | 4.09608E-05 |
| 256~1024 | 0.997297984 | 0.000232669 | 0.000175392 |
Each row represents the Bonferroni-corrected p-values to corresponding range (in Hz) in the first column of the row. Comparisons are made between salient vs. less-salient (column 2); less-salient vs. less-salient + CT blockade (column 3); and salient vs. less-salient+ CT blockade (column 4) in the minority of neurons (n=15).
Trial by trial analysis
Based on the PPI results (Fig. 5) suggesting that sensory responses were adapting and top-down MGB inputs caused repetition-enhancement, we examined trial-by-trial data to 10 successive presentations of SAM stimuli, for the 21 MGB units with the highest PPI values (> 0.3) at that showed the largest changes (Fig. 7). Group data for repeating presentations of SAM stimuli (128 Hz and 256 Hz ) showed clear adaptation across trials for , while reducing SAM depth changed the slope to repetition-enhancement. CT blockade reversed the trial-by-trial repetition-enhancement in response to repeating stimuli (Fig. 7A&B). Trend line slopes for average spikes were significantly different across the three conditions for repeating presentation at 128 Hz (F(2,24) = 4.885. p = 0.0166). Differences were significant for individual trials 7, 8, 9 and 10 between less-distinct and less-distinct with CT blockade (Friedman test followed Wilcoxon test and respective p–values for each trial are mentioned: (trial 7, p = 0.0021; trial 8, p = 0.0011; trial 9, p = 0.0027; trial 10, p = 0.009) (Fig. 7A). Responses to a repeating SAM ( 256 Hz) significantly adapted to stimuli, while increasing responses across trials to , which was reversed by CT blockade (ANCOVA, two-tailed, F(2,24) = 6.527, p = 0.0055). Differences were significant for all trials but trial 2 between to with CT blockade (Friedman test followed Wilcoxon test and respective p –values for each trial are mentioned: (trial 1, p = 0.006; trial 3, p = 0.00018; trial 4, p = 0.00046; trial 5, p = 0.0002; trial 6, p = 0.0018; trial 7, p = 0.0034; trial 8, p = 0.0013; trial 9, p = 0.0004; trial 10, p = 0.038)) (Fig. 7B). The same trends were seen for trial-by-trial spike rate comparisons for 512 and 1024 Hz. The impact of onset responses on trial-by-trial rate data was examined by removing the first 50 ms. There were no significant differences in these data with or without inclusion of 50 ms onset across the three stimulus conditions (data not shown).
MGB subdivisions
PPI values across were examined for all 80 units based on their location within the major MGB subdivisions (Fig. 6). PPI values were significantly increased in ventral and dorsal MGB when modulation depth was reduced from to (Fig.6). Corticothalamic blockade reversed the PPI changes in the dorsal division with a trend toward reversal in the ventral MGB (repeated measures ANOVA F(1.714, 132) = 8.562, p = 0.0006, Bonferroni corrected p-values across all in ventral division ( to ; to blockade = 0.0859; to blockade = 0.5902); Bonferroni corrected p-values across all in dorsal division ( to ; to blockade = 0.0012; to blockade = 0.5146); Fig. 6). None of these changes were significant in the medial division of the MGB (Bonferroni corrected p-values across all in medial division ( to ; to blockade = 0.9971; to blockade = 0.3117; Fig. 6).
Spike-rate changes with altered SAM modulation depth and CT blockade
Across 80 neurons there were significant changes between and in total spikes in response to both random and repeated trials of stimuli across , (Table 5). No significant differences in total spikes between and blockade were noted for randomly presented trials (Table 5). For repeating trials across , a switch from to showed no significant differences in total spikes (731.3 ± 46.3 vs. 693.5 ± 45.1) (Table 5). However, a significant decrease in total spikes was noted when repeating trials across were switched from to to blockade (Table 5).
Table 5:
An average of total spike count and Bonferroni-corrected p-values across all neurons to standard and weakly modulated stimuli presented in random or repeating order.
| Total spike count | p-value* | |||||
|---|---|---|---|---|---|---|
| Presentation order |
blockade |
vs. |
vs. blockade |
vs. blockade |
||
| Random | 839.2±54.6 | 675.6±45.1 | 708.6±50.3 | 0.000005 | 0.549 | 0.001 |
| Repeating | 731.3±46.3 | 693.5±45.1 | 625.8±50.2 | 0.618 | 0.011 | 0.0024 |
Each row represents the average total spike count to vs. and the
Bonferroni-corrected p-values following the repeated measures ANOVA for comparisons. Comparisons were made between vs. , and vs. vs. vs. blockade, and vs. blockade in all the of neurons (n=80).
Discussion
Previous studies found that both aging and decreased modulation depth, presumptively reducing the salience/fidelity of the ascending temporal code, increased responses to a repeating modulated signal, suggesting engagement of top-down, cognitive and mnemonic resources (Cai et al., 2016b; Kommajosyula et al., 2019). The present study used optogenetic CT blockade to test whether repetition-enhancement in response to less distinct temporal stimuli was due to the increased involvement of top-down CT resources. In order to maintain speech understanding, older individuals have been shown to increase use of cognitive and memory resources (Bidelman et al., 2019a; Roque et al., 2019). The impact of aging can be simulated in humans and in animal models by decreasing the temporal clarity of the stimulus. Reducing modulation depth of a SAM stimulus changes the rate and synchrony of the up-stream code introducing temporal jitter (Pichora-Fuller et al., 2007; Malone et al., 2010; Dimitrijevic et al., 2016; Mamo et al., 2016). A less temporally distinct ascending acoustic code is thought to engage top-down cognitive resources by generating predictions to support decoding of modulated speech-like signals (Peelle & Wingfield, 2016; Pichora-Fuller et al., 2017; Caspary & Llano, 2018; Recanzone, 2018). Consistent with human and animal studies, the present study finds that weakening periodicity cues by decreasing modulation depth ( to ) decreased the percentage of neurons showing temporal phase-locking to the SAM envelope(Pichora-Fuller et al., 2007; Malone et al., 2010; Parthasarathy & Bartlett, 2011; Mamo et al., 2016; Kommajosyula et al., 2019; McClaskey et al., 2019). Previously we found that jittering the SAM envelope with a 1.0kHZ centered noise produced similar levels of repetition-enhancement to the used in the present study (Kommajosyula et al., 2019).) CT blockade did not alter temporal locking of units to the . The lack of CT blockade changes on temporal locking contrasts to changes observed in SAM rate coding suggesting that CT projections do not play a significant role in temporal coding using this stimulus paradigm (Bartlett & Wang, 2007; Felix et al., 2018).
In response to repeating modulated stimuli, decreasing temporal clarity by decreasing modulation depth changed single unit rate responses from adapting to responses showing repetition-enhancement to the repeating modulated SAM stimulus. The switch to increasing responses to less temporally distinct repeating stimuli was blocked/reversed by optical inhibition of CT projections, thought to provide top-down resources to the MGB (Homma et al., 2017; Parras et al., 2017). A majority of MGB units showed the largest increases in repetition enhancement at higher SAM rates (> 128 Hz).
Temporal distinction and top-down resource usage
The present study used , as a surrogate for a diminished acoustic cue that is poorly detected and discriminated in the ascending code in human and animal models of aging (Strouse et al., 1998; Nelson & Carney, 2006; Harris & Dubno, 2017). These findings are also consistent with studies modeling aging in young humans with normal hearing and studies of auditory processing of less-distinct stimuli that reveal perceptual deficits due to decrease precision of temporal coding (Shannon et al., 1995; Krishna & Semple, 2000; Pichora-Fuller et al., 2007; Malone et al., 2010; Jorgensen & Dau, 2011; Parthasarathy & Bartlett, 2011; Dimitrijevic et al., 2016; Anderson et al., 2020; Erb et al., 2020).
Previous studies suggest that salience is multidimensional, nonlinear and context-dependent (Kayser et al., 2005; Huang & Elhilali, 2017). Based on the context, cortical structures generate predictions of the upcoming sensory stimuli as postulated by predictive coding theory (Mumford, 1992; Koelsch et al., 2019). If the prediction and ascending sensory signals do not match, a prediction error should be generated (Auksztulewicz & Friston, 2016). Prediction error is a mechanism to strengthen the internal representation of less temporally distinct stimuli which may lead to generation of a better prediction upon the next repetition (Rao & Ballard, 1999). Studies have suggested increased use of predictive coding in order to cope with less-distinct stimuli or aging accompanied by a less temporally distinct signal to noise ratio (Heinemann et al., 2011; Peelle & Wingfield, 2016; Bidelman et al., 2019a; Bidelman et al., 2019b; Presacco et al., 2019; Price et al., 2019; Saderi et al., 2020). Electrophysiological and fMRI studies suggest a role for repetition suppression/adaptation to repeating stimuli in support of image sharpening and perceptual priming (Gross et al., 1967; Dolan et al., 1997; James et al., 2000; Grill-Spector et al., 2006; Näätänen et al., 2007). The present findings suggest that for a sensory signal whose features are unclear, adaptation would be counterproductive, whereas repetition-enhancement could potentially facilitate identification of the unclear signal and its characteristics.
The present findings and two prior studies strongly support the idea of CT-mediated transmission of intracortical signals leading to repetition-enhancement (Cai et al., 2016b; Kommajosyula et al., 2019). Nearly 80% (56/71) of the neurons showed increases in PR, indicating relative increases in unit responses to a repeating stimulus, especially at higher . MGB units showing the largest repetition enhancement effects (PPI > 0.3) showed increases in firing rates with each successive repeating trial of less-distinct stimuli at higher (Fig. 7). SSA studies using short tone-burst stimuli show significantly less adaptation across trials in awake animals, suggesting that top-down projections may reduce SSA in IC and MGB as suggested in the present study and (Antunes et al., 2010; Richardson et al., 2013; Ayala et al., 2015; Duque & Malmierca, 2015; Cai et al., 2016a; Yaron et al., 2020). The increase in discharge rate with repetition is best explained by a buildup in the strength of the top-down/CT-mediated contribution to the MGB response (Fig. 8B). This is supported by significant decreases in the preference ratios (Figs. 3&5), and trial-by-trial enhancement (Fig. 7) which could be blocked during repeating stimuli. The level of adaptation seen with CT blockade during less-distinct stimuli was comparable or greater than seen with the stimuli (Figs. 4&5) suggesting blockade of an some on-going level of top-down resource engagement even during a temporally clear stimulus. We suggest that CT blockade reduces the ability to convey cortical estimates of the stimulus to MGB neurons, rendering the MGB neurons less sensitive to mismatch/prediction error. (Fig. 8C).
Figure 8. Salience based generation of predication errors in auditory thalamus:
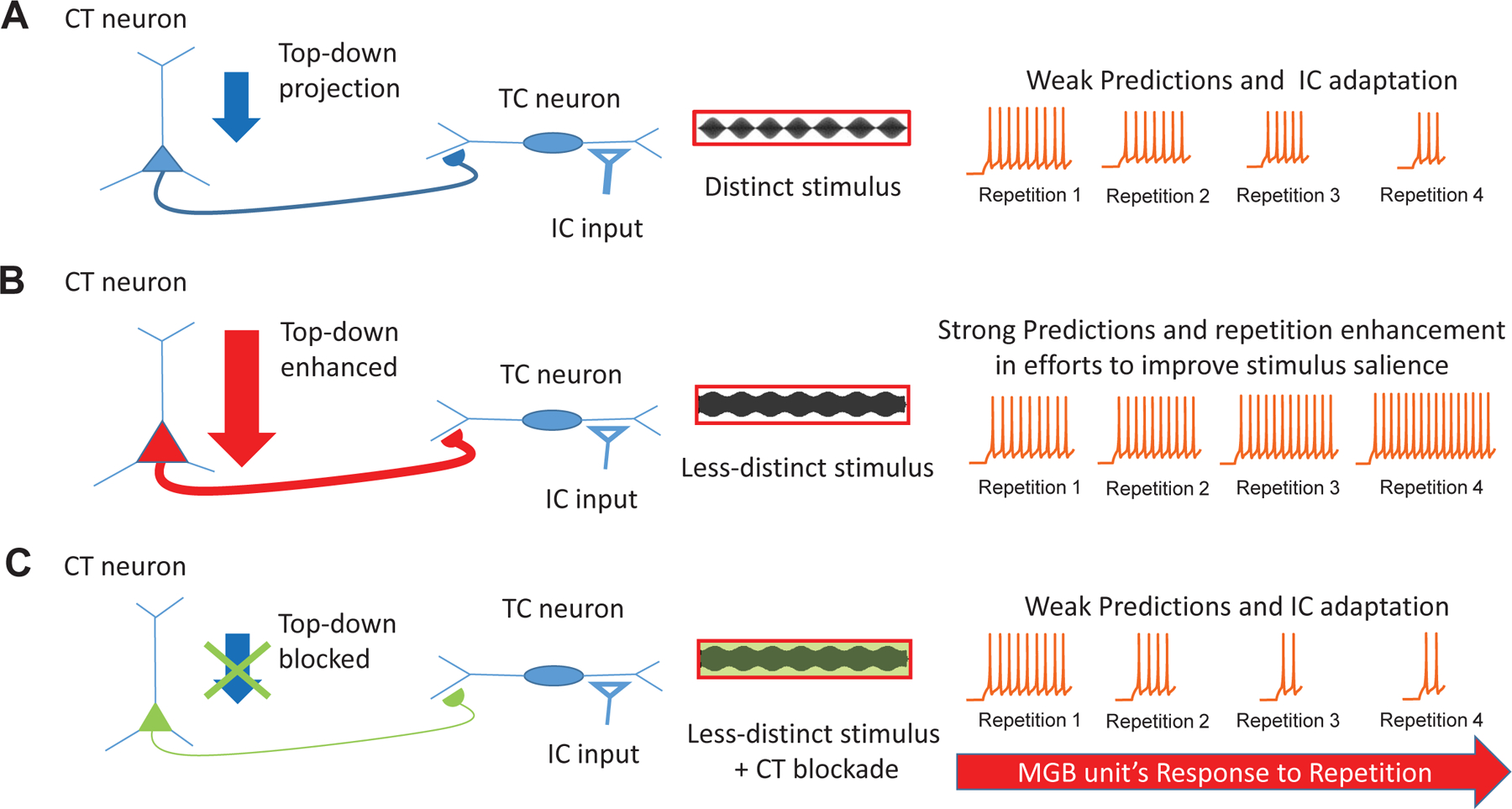
An upcoming sensory signal from inferior colliculus (IC) at the level of medial geniculate body (MGB) could interact with a top-down prediction from cortex, and generate prediction error component. The upcoming sensory signals (spikes) generated in response to distinct stimuli, are matched by the top-down predictions and hence little to less generation of prediction error component upon repetition of the distinct stimuli (A). The spike signals to weakly modulated stimuli fail to match the predictions, hence generation of prediction error increases upon repetition until the occurrence of a correct prediction based on the new internal representation formed by feedback from previous prediction error signals. This phenomenon is observed as an increase in response to each repetition (repetition enhancement) (B). CT blockade with weakly modulated stimuli, leads to blockade of delivery of predictions to MGB, and possibly erroneous prediction error signals and adaptive spike responses (C).
Significant changes in PPI were found in the ventral and dorsal MGB divisions, but not the medial subdivision of the MGB (Fig. 4). The absence of significant changes in the medial subdivision reflect the differential inputs, intrinsic properties and/or connectivity patterns of dorsal MGB neurons, such that they receive different and more widespread CT projections (Smith et al., 2007). However, some caution should be exercised in the interpretation of the subdivision findings since recorded neurons were not dye marked and absolute location was only approximated using a template (see methods).
In conclusion, we found that less temporally distinct stimuli increased the preference for repeating modulated signals, i.e. emergence of repetition-enhancement, while blockade of CT projections led to reversal of this effect. In traditional predictive coding theory, an error signal between cortical prediction and incoming sensory inputs generates spiking activity that diminishes as the sensory and prediction templates match, with the mechanisms of this operation not fully understood. The present results are consistent with the idea that a less-distinct acoustic signal leads to the generation of a prediction component similar to what might be seen with phonemic restoration (Bologna et al., 2018; Jaekel et al., 2018). Cortiothalamic feedback to MGB may serve to amplify weak but predictable features in order to generate a more reliable stimulus template for subsequent predictions, leading to improved detection of changes. We suggest that CT blockade led to a decrease in higher order/top-down information received by MGB neurons, leading to a decrease in corticothalamic mediated repetition-enhancement.
Key points:
Aging has been shown to increase temporal jitter in the ascending acoustic code prompting use of cognitive/attentional mechanisms to help better understand communication-like signals.
Auditory thalamus receives extensive projections from cortex that are implicated in delivering higher-order cortical computations to enhance thalamic responses.
The present study modeled aging in young rats by using temporally less distinct stimuli shown to alter the pattern of MGB unit responses from response adaptation to repetition-enhancement. Enhanced responses to repeating less temporally distinct modulated stimuli were reversed when inputs from cortex to auditory thalamus were blocked. Collectively, these data argue that low salience temporal signals engage cortical processes to enhance coding of weakly modulated signals in auditory thalamus.
Acknowledgements:
This work was supported by National Institute on Deafness and Other Communication Disorders DC000151 to D.M.C. We thank the National Institute on Aging for providing FBN rats; Kevin Brownell for data reduction, Dr. Kristin Delfino for statistical analysis; Dr. Ken Hancock for design and continued development of our stimulus/acquisition system, Lydia Howes for proof reading and Dr. Laurel Carney for suggestions on an earlier version of the manuscript.
Footnotes
Conflict of interest:
The authors declare no competing financial interests
References
- Anderson LA & Malmierca MS. (2013). The effect of auditory cortex deactivation on stimulus-specific adaptation in the inferior colliculus of the rat. The European journal of neuroscience 37, 52–62. [DOI] [PubMed] [Google Scholar]
- Anderson S & Karawani H. (2020). Objective evidence of temporal processing deficits in older adults. Hear Res 397, 108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Roque L, Gaskins CR, Gordon-Salant S & Goupell MJ. (2020). Age-Related Compensation Mechanism Revealed in the Cortical Representation of Degraded Speech. J Assoc Res Otolaryngol 21, 373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes FM, Nelken I, Covey E & Malmierca MS. (2010). Stimulus-Specific Adaptation in the Auditory Thalamus of the Anesthetized Rat. PloS one 5, e14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auksztulewicz R & Friston K. (2016). Repetition suppression and its contextual determinants in predictive coding. Cortex 80, 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YA, Udeh A, Dutta K, Bishop D, Malmierca MS & Oliver DL. (2015). Differences in the strength of cortical and brainstem inputs to SSA and non-SSA neurons in the inferior colliculus. Scientific reports 5, 10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL. (2013). The organization and physiology of the auditory thalamus and its role in processing acoustic features important for speech perception. Brain Lang 126, 29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, Guillery RW & Smith PH. (2000). Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience 100, 811–828. [DOI] [PubMed] [Google Scholar]
- Bartlett EL & Wang X. (2005). Long-lasting modulation by stimulus context in primate auditory cortex. Journal of neurophysiology 94, 83–104. [DOI] [PubMed] [Google Scholar]
- Bartlett EL & Wang X. (2007). Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol 97, 1005–1017. [DOI] [PubMed] [Google Scholar]
- Başkent D, Clarke J, Pals C, Benard MR, Bhargava P, Saija J, Sarampalis A, Wagner A & Gaudrain E. (2016). Cognitive Compensation of Speech Perception With Hearing Impairment, Cochlear Implants, and Aging: How and to What Degree Can It Be Achieved? Trends Hear 20, 2331216516670279. [Google Scholar]
- Bidelman GM, Mahmud MS, Yeasin M, Shen D, Arnott SR & Alain C. (2019a). Age-related hearing loss increases full-brain connectivity while reversing directed signaling within the dorsal-ventral pathway for speech. Brain Struct Funct 224, 2661–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Price CN, Shen D, Arnott SR & Alain C. (2019b). Afferent-efferent connectivity between auditory brainstem and cortex accounts for poorer speech-in-noise comprehension in older adults. Hear Res 382, 107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow J, Morrill RJ, Dekloe J & Hasenstaub AR. (2019). Movement and VIP Interneuron Activation Differentially Modulate Encoding in Mouse Auditory Cortex. eNeuro 6, ENEURO.0164–0119.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna WJ, Vaden KI Jr, Ahlstrom JB & Dubno JR. (2018). Age effects on perceptual organization of speech: Contributions of glimpsing, phonemic restoration, and speech segregation. The Journal of the Acoustical Society of America 144, 267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R & Caspary DM. (2015). GABAergic inhibition shapes SAM responses in rat auditory thalamus. Neuroscience 299, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Montgomery SC, Graves KA, Caspary DM & Cox BC. (2018). The FBN rat model of aging: investigation of ABR waveforms and ribbon synapse changes. Neurobiology of aging 62, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Richardson BD & Caspary DM. (2016a). Responses to Predictable versus Random Temporally Complex Stimuli from Single Units in Auditory Thalamus: Impact of Aging and Anesthesia. Journal of Neuroscience 36, 10696–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Richardson BD & Caspary DM. (2016b). Responses to Predictable versus Random Temporally Complex Stimuli from Single Units in Auditory Thalamus: Impact of Aging and Anesthesia. J Neurosci 36, 10696–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG & Hughes LF. (2008). Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. The Journal of experimental biology 211, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM & Llano DA. (2018). Aging Processes in the Subcortical Auditory System. In The Oxford Handbook of the Auditory Brainstem, ed. Kandler K. Oxford University Press. [Google Scholar]
- Davis MH, Ford MA, Kherif F & Johnsrude IS. (2011). Does semantic context benefit speech understanding through "top-down" processes? Evidence from time-resolved sparse fMRI. J Cogn Neurosci 23, 3914–3932. [DOI] [PubMed] [Google Scholar]
- de Gardelle V, Waszczuk M, Egner T & Summerfield C. (2013). Concurrent repetition enhancement and suppression responses in extrastriate visual cortex. Cerebral cortex 23, 2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Hintz F, Kiebel SJ & von Kriegstein K. (2012). Dysfunction of the auditory thalamus in developmental dyslexia. Proceedings of the National Academy of Sciences of the United States of America 109, 13841–13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic A, Alsamri J, John MS, Purcell D, George S & Zeng F-G. (2016). Human Envelope Following Responses to Amplitude Modulation: Effects of Aging and Modulation Depth. Ear and hearing 37, e322–e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Fink GR, Rolls E, Booth M, Holmes A, Frackowiak RSJ & Friston KJ. (1997). How the brain learns to see objects and faces in an impoverished context. Nature 389, 596–599. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD & Morgan DE. (1984). Effects of age and mild hearing loss on speech recognition in noise. The Journal of the Acoustical Society of America 76, 87–96. [DOI] [PubMed] [Google Scholar]
- Duque D & Malmierca MS. (2015). Stimulus-specific adaptation in the inferior colliculus of the mouse: anesthesia and spontaneous activity effects. Brain structure & function 220, 3385–3398. [DOI] [PubMed] [Google Scholar]
- Erb J, Schmitt LM & Obleser J. (2020). Temporal selectivity declines in the aging human auditory cortex. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix RA 2nd, Gourévitch B & Portfors CV. (2018). Subcortical pathways: Towards a better understanding of auditory disorders. Hearing research 362, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons PJ & Gordon-Salant S. (1994). Age effects on measures of auditory duration discrimination. Journal of speech and hearing research 37, 662–670. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO & Fabbricatore AT. (2006). Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self-administration. Neuroscience 137, 1075–1085. [DOI] [PubMed] [Google Scholar]
- Goldberg JM & Brown PB. (1969). Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sould localization. Journal of neurophysiology 32, 613–636. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R & Martin A. (2006). Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10, 14–23. [DOI] [PubMed] [Google Scholar]
- Gross CG, Schiller PH, Wells C & Gerstein GL. (1967). Single-unit activity in temporal association cortex of the monkey. Journal of neurophysiology 30, 833–843. [DOI] [PubMed] [Google Scholar]
- Guo W, Clause AR, Barth-Maron A & Polley DB. (2017). A Corticothalamic Circuit for Dynamic Switching between Feature Detection and Discrimination. Neuron 95, 180–194.e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R & Boyden ES. (2011). A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Frontiers in systems neuroscience 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC & Dubno JR. (2017). Age-related deficits in auditory temporal processing: unique contributions of neural dyssynchrony and slowed neuronal processing. Neurobiology of aging 53, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J (2003). Corticofugal modulation on both ON and OFF responses in the nonlemniscal auditory thalamus of the guinea pig. J Neurophysiol 89, 367–381. [DOI] [PubMed] [Google Scholar]
- Heinemann LV, Kaiser J & Altmann CF. (2011). Auditory repetition enhancement at short interstimulus intervals for frequency-modulated tones. Brain research 1411, 65–75. [DOI] [PubMed] [Google Scholar]
- Homma NY, Happel MFK, Nodal FR, Ohl FW, King AJ & Bajo VM. (2017). A Role for Auditory Corticothalamic Feedback in the Perception of Complex Sounds. J Neurosci 37, 6149–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N & Elhilali M. (2017). Auditory salience using natural soundscapes. The Journal of the Acoustical Society of America 141, 2163–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH & Wingfield A. (2012). Central presbycusis: a review and evaluation of the evidence. Journal of the American Academy of Audiology 23, 635–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekel BN, Newman RS & Goupell MJ. (2018). Age effects on perceptual restoration of degraded interrupted sentences. The Journal of the Acoustical Society of America 143, 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS & Goodale MA. (2000). The effects of visual object priming on brain activation before and after recognition. Current Biology 10, 1017–1024. [DOI] [PubMed] [Google Scholar]
- Jorgensen S & Dau T. (2011). Predicting speech intelligibility based on the signal-to-noise envelope power ratio after modulation-frequency selective processing. The Journal of the Acoustical Society of America 130, 1475–1487. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG & Caspary DM. (2014). Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. The Journal of physiology 592, 5065–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Asinof SK & Isaacson JS. (2017). Network-Level Control of Frequency Tuning in Auditory Cortex. Neuron 95, 412–423.e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Lippert M & Logothetis NK. (2005). Mechanisms for allocating auditory attention: an auditory saliency map. Current biology : CB 15, 1943–1947. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Vuust P & Friston K. (2019). Predictive Processes and the Peculiar Case of Music. Trends Cogn Sci 23, 63–77. [DOI] [PubMed] [Google Scholar]
- Kommajosyula SP, Cai R, Bartlett E & Caspary DM. (2019). Top-down or bottom up: decreased stimulus salience increases responses to predictable stimuli of auditory thalamic neurons. J Physiol 597, 2767–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna BS & Semple MN. (2000). Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J Neurophysiol 84, 255–273. [DOI] [PubMed] [Google Scholar]
- Luce PA & Pisoni DB. (1998). Recognizing spoken words: the neighborhood activation model. Ear and hearing 19, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumani A & Zhang H. (2010). Responses of neurons in the raťs dorsal cortex of the inferior colliculus to monaural tone bursts. Brain Res 1351, 115–129. [DOI] [PubMed] [Google Scholar]
- Mafi AM, Hofer LN, Russ MG, Young JW & Mellott JG. (2020). The Density of Perineuronal Nets Increases With Age in the Inferior Colliculus in the Fischer Brown Norway Rat. Frontiers in aging neuroscience 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Anderson LA & Antunes FM. (2015). The cortical modulation of stimulus-specific adaptation in the auditory midbrain and thalamus: a potential neuronal correlate for predictive coding. Frontiers in systems neuroscience 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone BJ, Scott BH & Semple MN. (2010). Temporal Codes for Amplitude Contrast in Auditory Cortex. The Journal of Neuroscience 30, 767–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo SK, Grose JH & Buss E. (2016). Speech-evoked ABR: Effects of age and simulated neural temporal jitter. Hearing research 333, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardia KV & Jupp PE. (2000). Directional Statistics New York: Wiley. [Google Scholar]
- McClaskey CM, Dias JW & Harris KC. (2019). Sustained envelope periodicity representations are associated with speech-in-noise performance in difficult listening conditions for younger and older adults. Journal of neurophysiology 122, 1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA & Pape HC. (1990). Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. The Journal of physiology 431, 319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihai PG, Moerel M, de Martino F, Trampel R, Kiebel S & von Kriegstein K. (2019). Modulation of tonotopic ventral medial geniculate body is behaviorally relevant for speech recognition. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller NG, Strumpf H, Scholz M, Baier B & Melloni L. (2013). Repetition suppression versus enhancement--iťs quantity that matters. Cerebral cortex 23, 315–322. [DOI] [PubMed] [Google Scholar]
- Mumford D (1992). On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biological cybernetics 66, 241–251. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T & Alho K. (2007). The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology 118, 2544–2590. [DOI] [PubMed] [Google Scholar]
- Natan RG, Rao W & Geffen MN. (2017). Cortical Interneurons Differentially Shape Frequency Tuning following Adaptation. Cell Rep 21, 878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC & Carney LH. (2006). Cues for masked amplitude-modulation detection. The Journal of the Acoustical Society of America 120, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC & Carney LH. (2007). Neural rate and timing cues for detection and discrimination of amplitude-modulated tones in the awake rabbit inferior colliculus. Journal of neurophysiology 97, 522–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J (2014). Putting the listening brain in context. Language and Linguistics Compass 8, 646–658. [Google Scholar]
- Ono K, Kudoh M & Shibuki K. (2006). Roles of the auditory cortex in discrimination learning by rats. The European journal of neuroscience 23, 1623–1632. [DOI] [PubMed] [Google Scholar]
- Orman SS & Humphrey GL. (1981). Effects of changes in cortical arousal and of auditory cortex cooling on neuronal activity in the medial geniculate body. Experimental brain research 42, 475–482. [DOI] [PubMed] [Google Scholar]
- Parras GG, Nieto-Diego J, Carbajal GV, Valdes-Baizabal C, Escera C & Malmierca MS. (2017). Neurons along the auditory pathway exhibit a hierarchical organization of prediction error. Nature communications 8, 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A & Bartlett EL. (2011). Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience 192, 619–630. [DOI] [PubMed] [Google Scholar]
- Paxinos W & Watson C. (1998). The Rat Brain in Stereotaxic Coordinates Academic Press, San Diego. [Google Scholar]
- Peelle JE & Wingfield A. (2016). The Neural Consequences of Age-Related Hearing Loss. Trends Neurosci 39, 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González D & Malmierca MS. (2014). Adaptation in the auditory system: an overview. Front Integr Neurosci 8, 19–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Alain C & Schneider BA. (2017). Older Adults at the Cocktail Party. In The Auditory System at the Cocktail Party, ed. Middlebrooks JC, Simon JZ, Popper AN & Fay RR, pp. 227–259. Springer International Publishing, Cham. [Google Scholar]
- Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BW, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, Naylor G, Phillips NA, Richter M, Rudner M, Sommers MS, Tremblay KL & Wingfield A. (2016). Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL). Ear and hearing 37 Suppl 1, 5s–27s. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Macdonald E, Pass HE & Brown S. (2007). Temporal jitter disrupts speech intelligibility: a simulation of auditory aging. Hear Res 223, 114–121. [DOI] [PubMed] [Google Scholar]
- Presacco A, Simon JZ & Anderson S. (2019). Speech-in-noise representation in the aging midbrain and cortex: Effects of hearing loss. PloS one 14, e0213899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CN, Alain C & Bidelman GM. (2019). Auditory-frontal Channeling in alpha and beta Bands is Altered by Age-related Hearing Loss and Relates to Speech Perception in Noise. Neuroscience 423, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP & Ballard DH. (1999). Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci 2, 79–87. [DOI] [PubMed] [Google Scholar]
- Recanzone G (2018). The effects of aging on auditory cortical function. Hear Res 366, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Hancock KE & Caspary DM. (2013). Stimulus-specific adaptation in auditory thalamus of young and aged awake rats. J Neurophysiol 110, 1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque L, Karawani H, Gordon-Salant S & Anderson S. (2019). Effects of Age, Cognition, and Neural Encoding on the Perception of Temporal Speech Cues. Frontiers in neuroscience 13, 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM & Welker E. (1991). Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Hear Res 56, 179–190. [DOI] [PubMed] [Google Scholar]
- Rybalko N, Suta D, Nwabueze-Ogbo F & Syka J. (2006). Effect of auditory cortex lesions on the discrimination of frequency-modulated tones in rats. The European journal of neuroscience 23, 1614–1622. [DOI] [PubMed] [Google Scholar]
- Saalmann YB & Kastner S. (2011). Cognitive and perceptual functions of the visual thalamus. Neuron 71, 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saderi D, Buran BN & David SV. (2020). Streaming of repeated noise in primary and secondary fields of auditory cortex. J Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR & Hurley L. (2018). Circuits for Modulation of Auditory Function. In The Mammalian Auditory Pathways, pp. 235–267. Springer. [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J & Ekelid M. (1995). Speech recognition with primarily temporal cues. Science (New York, NY) 270, 303–304. [DOI] [PubMed] [Google Scholar]
- Shinn-Cunningham BG & Wang D. (2008). Influences of auditory object formation on phonemic restoration. The Journal of the Acoustical Society of America 123, 295–301. [DOI] [PubMed] [Google Scholar]
- Smith PH, Bartlett EL & Kowalkowski A. (2007). Cortical and Collicular Inputs to Cells in the Rat Paralaminar Thalamic Nuclei Adjacent to the Medial Geniculate Body. Journal of Neurophysiology 98, 681–695. [DOI] [PubMed] [Google Scholar]
- Sottile SY, Hackett TA, Cai R, Ling L, Llano DA & Caspary DM. (2017). Presynaptic Neuronal Nicotinic Receptors Differentially Shape Select Inputs to Auditory Thalamus and Are Negatively Impacted by Aging. J Neurosci 37, 11377–11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN & Grantham DW. (1998). Temporal processing in the aging auditory system. The Journal of the Acoustical Society of America 104, 2385–2399. [DOI] [PubMed] [Google Scholar]
- Tabas A & von Kriegstein K. (2021). Adjudicating Between Local and Global Architectures of Predictive Processing in the Subcortical Auditory Pathway. Front Neural Circuits 15, 644743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Yang W & Suga N. (2012). Modulation of thalamic auditory neurons by the primary auditory cortex. Journal of neurophysiology 108, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L & Nelken I. (2003). Processing of low-probability sounds by cortical neurons. Nat Neurosci 6, 391–398. [DOI] [PubMed] [Google Scholar]
- Vaden KI Jr., Kuchinsky SE, Ahlstrom JB, Teubner-Rhodes SE, Dubno JR & Eckert MA. (2016). Cingulo-Opercular Function During Word Recognition in Noise for Older Adults with Hearing Loss. Experimental aging research 42, 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kriegstein K, Patterson RD & Griffiths TD. (2008). Task-dependent modulation of medial geniculate body is behaviorally relevant for speech recognition. Current biology : CB 18, 1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF & Caspary DM. (2009). Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience 164, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RM. (1970). Perceptual restoration of missing speech sounds. Science (New York, NY) 167, 392–393. [DOI] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ & Larue DT. (2001). Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol 430, 27–55. [PubMed] [Google Scholar]
- Winer JA, Miller LM, Lee CC & Schreiner CE. (2005). Auditory thalamocortical transformation: structure and function. Trends Neurosci 28, 255–263. [DOI] [PubMed] [Google Scholar]
- Wingfield A (1975). Acoustic redundancy and the perception of time-compressed speech. Journal of speech and hearing research 18, 96–104. [DOI] [PubMed] [Google Scholar]
- Yaron A, Jankowski MM, Badrieh R & Nelken I. (2020). Stimulus-specific adaptation to behaviorally-relevant sounds in awake rats. PloS one 15, e0221541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Johnson JS, O'Connor KN & Sutter ML. (2011). Coding of amplitude modulation in primary auditory cortex. J Neurophysiol 105, 582–600. [DOI] [PMC free article] [PubMed] [Google Scholar]


