Abstract
Background
Women who inject drugs in Ukraine are disproportionately burdened by HIV. To help address the needs of this population, a greater understanding of how interventions may uniquely benefit women who inject drugs is needed.
Methods
Data come from a randomized controlled trial of a social network intervention targeting people who inject drugs in Ukraine (N = 1195). Indexes, plus two of their injection network members, received HIV testing and counseling (control arm) or HIV testing and counseling plus a social network intervention (intervention arm), in which indexes were trained to influence network members’ risk behaviors. We used Cox regressions with interaction terms to assess differences in time to HIV seroconversion between arms by network gender composition and gender of the index. For significant interaction terms, we calculated simple effects, generated survival functions using Kaplan–Meier methods, and compared survival curves using log-rank tests.
Results
At 12 months, there were 45 seroconversions among women (40.0 [28.3, 51.7] per 100 person years) and 111 among men (28.4 [23.1, 33.6] per 100 person years) in the control arm; there were 27 seroconversions among women (17.1 [10.7, 23.6] per 100 person years) and 77 among men (18.7 [14.5, 22.9] per 100 person years) in the intervention arm. Network gender composition (but not gender of the index) moderated the intervention effect on HIV incidence (p < 0.05). Specifically, the intervention appeared to be even more protective against HIV acquisition as female gender composition increased. In the intervention arm, the HIV seroconversion hazard rate was 44% lower with 1 network female; 61% lower with 2 network females; and 72% lower with 3 network females.
Conclusions
A greater number of women in an injection network, coupled with the provision of risk-reduction strategies, is associated with HIV risk-mitigation, though the mechanisms through which this occurs remain unclear. Findings can support new research and practice directions that prioritize women who inject drugs and more thoughtfully support their health and wellbeing.
Keywords: Women who inject drugs, Ukraine, Social network intervention, Moderation, People who inject drugs, HIV, Gender
Introduction
Globally, people who inject drugs (PWID) are disproportionately burdened by HIV infection, but further disparities exist within the PWID population [1, 2]. An international systematic review of studies with PWID in North and South America, Europe, and Asia concluded that women who inject drugs have a significantly higher prevalence of HIV relative to men who inject drugs, despite comprising only one fifth of the global PWID population [3]. A similar review identified being female as a risk factor for HIV among PWID in Central Asia and Eastern Europe, including Ukraine [4].
Ukraine, a former Soviet republic in Eastern Europe, has the second-highest HIV incidence rate (39.0–42.5/100,000 in 2019) among the 53 countries comprising the WHO European Region [5]. Unsafe injection drug use (IDU) behaviors initially drove the HIV epidemic in Ukraine, and though heterosexual sex is now the dominant route of transmission, unsafe IDU alone continues to account for roughly one in four new HIV infections per year [6, 7]. HIV prevalence has been consistently high among all PWID in Ukraine but has remained higher among women compared to men, though women who inject drugs make up only one in four of Ukraine’s roughly 350,000 PWID [8]. HIV prevalence estimates among women compared to men who inject drugs in Ukraine have ranged from 14–40% versus 14–32% [3] and 22–31% versus 17–22% [9], with an HIV prevalence rate ratio of 1.25 (95% CI = 1.16, 1.34; 22.6% vs 18.1%) [10]. Moreover, women who inject drugs in Ukraine have been shown to make up a higher proportion of individuals newly diagnosed with HIV across diverse recruitment and testing strategies [11].
Factors across socioecological levels may amplify HIV risk for women who inject drugs in Ukraine. For example, individual-level factors include low condom use (44%) among women and men who inject drugs, and women’s enhanced biological susceptibility to contract HIV sexually [2, 12, 13]. Interpersonal and social network-related factors include syringe/needle-sharing with an injecting male partner, and violence and forced sex work by a male injecting partner [1, 2, 12, 14–17]. Community- and structural-level factors include sexist, patriarchal values and intersecting gender, HIV, and drug/IDU-related stigmas that cut across all other socioecological levels and undermine women’s agency and power further, affecting access to HIV and drug treatment [14, 16, 18–24].
Of note, however, is that protective factors have also been identified, with one social network factor in particular, gender homophily—the extent to which network members are the same gender—emerging as potentially critical to mitigate injection risk in this population. Having more women in one’s network has been shown to facilitate reductions in unsafe injection behaviors [25], increase awareness of HIV pre-exposure prophylaxis [26], lower frequency of arrests [27], and be associated with greater social support [28]. More systematic attention on network gender composition may therefore be pertinent to understanding and improving the health and psychosocial wellness of this specific sub-population.
Individual-level and social network/peer-led interventions have been implemented among PWID in Ukraine, with varying degrees of efficacy [29–32]. However, few interventions in Ukraine have been tailored to women who inject drugs, despite the disproportionate HIV risk faced by this group, and despite evidence that social network interventions may benefit women who inject drugs more than men who inject drugs [33]. Given the precarity of women who inject drugs’ social position and amplified HIV risks in Ukraine [7, 25, 34, 35], re-examining past intervention trial data to determine differences in efficacy by gender is a critical next step toward motivating and informing the development of interventions tailored to women who inject drugs in Ukraine, as well as comparable contexts in the region (such as other former Soviet blocs). Evaluating intervention trial data using nuanced approaches (e.g., considering multiple operational definitions of a potential effect modifier) is imperative to reach a fuller, more detailed understanding of intervention efficacy (e.g., identification of subgroups for whom the intervention was more or less effective). Moreover, maximizing use of available data is responsible, efficient, and cost-effective given how labor- and resource-intensive intervention trial implementation is [36].
A randomized controlled trial evaluated a social network intervention that trained PWID in Ukraine to educate their injection network members about safe injection and sexual practices, and this intervention was ultimately found to reduce HIV incidence [31]. However, this finding was based on the effect being averaged over all participants; other analyses, such as moderation or effect modification by gender, were not explored. The objectives of this secondary analysis were to determine whether the effect of the intervention on HIV seroconversion was moderated by network gender composition and by index gender (i.e., gender of the index). Though the data were collected roughly ten years ago, recent data show that women who inject drugs continue to be disproportionately burdened by HIV infection in Ukraine [9–11]. Also, while the extent to which the Russian invasion may change the epidemiologic context of HIV and IDU in Ukraine is unclear, disruption of HIV prevention efforts and exacerbation of gender disparities in HIV-related health outcomes can be expected [37], underscoring the urgency of understanding how best to intervene among women who inject drugs in Ukraine moving forward.
Methods
Data source, participants, and procedures
Data were drawn from a randomized controlled trial of a social network intervention targeting PWID in Odesa, Donetsk, and Mykolaiv, Ukraine. Detailed methods have been described elsewhere [31]. Briefly, the intervention sought to train peer leaders, or index participants, as educators to influence the injection and sexual risk behaviors of their injection network members. Index participants were recruited from the streets by outreach workers from nongovernmental organizations in each of the three cities. Eligibility criteria included being 16 years of age or older, self-reported drug injection in the past 30 days (verified by signs of recent venipuncture), willingness to participate in interviews and HIV testing, ability to provide informed consent, and willingness to recruit two members of their injection network for participation in the study. Network members recruited by index participants also had to meet these eligibility criteria (except for willingness to recruit two members). Recruitment lasted from July 2010 to November 2012.
The intervention arm received Ukraine’s standard of care HIV testing and counseling, an updated version of the Counseling and Education model developed during the National Institute on Drug Abuse’s cooperative agreement, and the social network intervention based on SHIELD (Self-Help in Eliminating Life-Threatening Diseases) [38–40], in which index participants were trained to teach their injection network members how to reduce HIV risk behaviors. Training occurred in five small-group sessions over two weeks and involved teaching peer leaders how to model and discuss safe behaviors via role-playing and other techniques. Network members assigned to the intervention arm received no training or additional intervention, as the social network intervention was based on index participants providing peer education to their injection network members. The control arm received HIV testing and counseling only. The present analysis included HIV negative participants only (N = 1200). The intervention arm consisted of 611 participants, including 190 indexes; the control arm consisted of 589 participants, including 171 indexes. All participants were interviewed and HIV-tested at baseline, 6 months, and 12 months.
Measures
The primary outcome was time to HIV seroconversion. HIV seroconversion at any time point between baseline and 12 months was considered an incident HIV infection, and date of seroconversion was estimated as the midpoint between the participant’s last HIV negative result and their HIV positive result.
Interviewers were instructed to code the gender of participants as male (coded “0”) or female (coded “1”) at baseline. Admittedly, this was not a true assessment of gender, as it was based on the perception and judgment of the interviewer and conflated, perhaps inadvertently, sex with gender. However, for the purposes of this analysis, we call this variable gender and consider those coded male to be men and those coded female to be women.
We examined gender as a potential moderator of the effect of the intervention on HIV seroconversion, which we operationalized and tested in two ways. First, network gender composition (i.e., number of women), including the index (range = 0–3), was assessed to determine if the effect of the intervention differed by the number of women comprising the network. This scenario would permit both female indexes and female network members to be included in the moderation effect. Second, moderation by index gender was assessed to determine if the effect of the intervention differed by whether or not the index was female, regardless of network gender composition. We examined number (rather than proportion) of women due to low variability in network size. Control variables were consistent with those in the original study and included age (continuous), city (Odesa vs Donetsk; Mykolaiv vs Donetsk), and injection frequency at baseline (continuous), though we log-transformed injection frequency to stabilize variance.
Analysis
We calculated descriptive statistics for sociodemographic characteristics and other variables of interest and used chi-square tests (for categorical covariates) and Kruskal–Wallis tests (for continuous covariates) to assess differences in sociodemographic and network characteristics between intervention and control arms.
We calculated hazard ratios (HR) with 95% confidence intervals (CI) by employing Cox regression analysis (with a frailty term to fit a random intercept for peer networks) to assess the extent to which time to HIV seroconversion between intervention and control arms differed by network gender composition and index gender. Model 1 included an interaction term between study arm (intervention vs control) and network gender composition, including index (female vs male); and Model 2 included an interaction term between study arm (intervention vs control) and index gender (female vs male). For significant interaction terms, we calculated simple effects to explore the nature of the interaction and generated survival functions stratified by experimental arm using Kaplan–Meier methods to plot and compare time to failure (i.e., HIV seroconversion). We compared corresponding survival curves (e.g., intervention participants with 2 network females compared to control participants with 2 network females) using log-rank tests.
We used several methods to assess the proportional hazards assumption. We graphed separate Kaplan–Meier survival functions for each categorical covariate. Visual inspection of the graphs showed parallel survival functions that diverged slowly over time and did not cross. We plotted scaled Schoenfeld residuals by time for all covariates and inspected the cumulative sum of residuals (score process pattern) with the corresponding results for a random selection of 20 out of 1000 simulated score process patterns. We then performed Kolmogorov-type supremum tests for all covariates to assess departure of the observed score process from the simulated ones and found none to be significant. Next, we generated time-dependent covariates by creating interaction terms between survival time and each covariate and added them to the models and found none to be significant. Finally, linear hypothesis testing and the score test yielded non-significant results. All of these procedures indicated that the proportional hazards assumption held. We calculated descriptive statistics in Stata (StataCorp, College Station, TX) and performed all other analyses in SAS (SAS Institute, Cary NC).
Results
Five network participants were missing data indicative to which network they belonged and were excluded, leaving 1,195 participants in the present analysis. Comparable proportions of participants were recruited in Odesa (n = 421; 35.2%) and Mykolaiv (n = 411; 34.4%), followed by Donetsk (n = 363; 30.4%). Mean age was 31.3 years (SD = 8.4), and three in four participants were men (n = 894). Three in four participants were Ukrainian (n = 900), and more than 20% were Russian (n = 258). No baseline differences in sociodemographic characteristics between conditions were found (Table 1).
Table 1.
Baseline sociodemographic and network characteristics of PWID in Ukraine (N = 1195)
| Control arm (n = 585) | Intervention arm (n = 610) | Overall/Total (N = 1195) | |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 31.4 (8.8) | 31.2 (7.9) | 31.3 (8.4) |
| Median (IQR) | 30 (25–37) | 30 (25–36) | 30 (25–37) |
| χ2 (p-value) | – | – | 0.01 (0.925) |
| Gender, n (%) | |||
| Female | 136 (23.2) | 165 (27.0) | 301 (25.2) |
| Male | 449 (76.8) | 445 (73.0) | 894 (74.8) |
| χ2 (p-value) | – | – | 2.29 (0.130) |
| Ethnicity, n (%) | |||
| Ukrainian | 427 (73.0) | 473 (77.5) | 900 (75.3) |
| Russian | 137 (23.4) | 121 (19.8) | 258 (21.6) |
| Other | 21 (3.6) | 16 (2.6) | 37 (3.1) |
| χ2 (p-value) | – | – | 3.50 (0.174) |
| City of residence, n (%) | |||
| Odesa | 205 (35.0) | 216 (35.4) | 421 (35.2) |
| Mykolaiv | 193 (33.0) | 218 (35.7) | 411 (34.4) |
| Donetsk | 187 (32.0) | 176 (28.9) | 363 (30.4) |
| χ2 (p-value) | – | – | 1.62 (0.445) |
| Relationship status, n (%) | |||
| Single | 288 (49.2) | 286 (46.9) | 574 (48.0) |
| Married | 85 (14.5) | 89 (14.6) | 174 (14.6) |
| Common law married, cohabiting | 93 (15.9) | 123 (20.2) | 216 (18.1) |
| Separated, divorced | 95 (16.2) | 92 (15.1) | 187 (15.6) |
| Widowed, other | 24 (4.1) | 20 (3.3) | 44 (3.7) |
| χ2 (p-value) | – | – | 4.16 (0.385) |
| Injection frequencya | |||
| Mean (SD) | 3.0 (0.9) | 3.1 (0.9) | 3.0 (0.9) |
| Median (IQR) | 3.0 (2.4–3.7) | 3.1 (2.3–3.7) | 3.1 (2.4–3.7) |
| χ2 (p-value) | – | – | 0.40 (0.525) |
| Gender of network membersb | |||
| Female | 107 (25.8) | 116 (27.6) | 223 (26.7) |
| Male | 307 (74.2) | 304 (72.4) | 611 (73.3) |
| χ2 (p-value) | – | – | 0.33 (0.566) |
| Number of women in network, including index | |||
| 0 | 252 (43.1) | 268 (43.9) | 520 (43.5) |
| 1 | 240 (41.0) | 220 (36.1) | 460 (38.5) |
| 2 | 81 (13.8) | 92 (15.1) | 173 (14.5) |
| 3 | 12 (2.1) | 30 (4.9) | 42 (3.5) |
| χ2 (p-value) | – | – | 9.26 (0.026)* |
| Number of women in network, excluding index | |||
| 0 | 310 (53.0) | 326 (53.4) | 636 (53.2) |
| 1 | 223 (38.1) | 223 (36.6) | 446 (37.3) |
| 2 | 52 (8.9) | 61 (10.0) | 113 (9.5) |
| χ2 (p-value) | – | – | 0.60 (0.742) |
| Index genderc | |||
| Female | 29 (17.0) | 49 (25.8) | 78 (21.6) |
| Male | 142 (83.0) | 141 (74.2) | 283 (78.4) |
| χ2 (p-value) | – | – | 4.14 (0.042)* |
PWID, people who inject drugs; SD, standard deviation; IQR, interquartile range
aLog-transformed
bOut of a total of 834 network members (control: n = 414; intervention: n = 420)
cOut of a total of 361 indexes (control: n = 171; intervention: n = 190)
*p < 0.05
Both the control and intervention arms had networks comprised of comparable proportions of women when excluding indexes (χ2[2] = 0.60, p = 0.742) but not when including indexes (χ2[3] = 9.26, p = 0.026). In the control arm, 1 in 6 indexes were women; in the intervention arm, 1 in 4 indexes were women (χ2[1] = 4.14, p = 0.042). Since our aim was to target gender as a moderator of intervention effects, the models we tested inherently adjusted for this difference between study arms. At 12 months, there were 260 HIV seroconversions over 1,073.4 person years, including 72 women (26.7 [20.5, 32.8] per 100 person years) and 188 men (23.4 [20.1, 26.7] per 100 person years). In the control arm, there were 45 seroconversions among women (40.0 [28.3, 51.7] per 100 person years) and 111 among men (28.4 [23.1, 33.6] per 100 person years). In the intervention arm, there were 27 seroconversions among women (17.1 [10.7, 23.6] per 100 person years) and 77 among men (18.7 [14.5, 22.9] per 100 person years) (Table 2).
Table 2.
Main and interaction effects of female gender and intervention arm on HIV incidence among PWID in Ukraine (N = 1195)
| HR (95% CI) | aHRa (95% CI) | |
|---|---|---|
| Model 1 | ||
| Network gender composition | 1.20 (0.96, 1.51) | 1.13 (0.93, 1.38) |
| Study arm (intervention vs control) | 0.75 (0.51, 1.09) | 0.79 (0.56, 1.11) |
| Interaction | 0.72 (0.52, 1.02) ~ | 0.71 (0.52, 0.97)* |
| Model 2 | ||
| Index gender (female vs male) | 1.17 (0.56, 1.77) | 1.10 (0.61, 1.63) |
| Study arm (intervention vs control) | 0.61 (0.45, 0.82)** | 0.62 (0.47, 0.83)** |
| Interaction | 0.88 (0.47, 1.64) | 0.85 (0.47, 1.56) |
HIV, human immunodeficiency virus; PWID, people who inject drugs; HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval
aControlling for age, city, log-injection frequency, and main effects of study arm and gender variable
~ p < 0.10 *p < 0.05, **p < 0.01, ***p < 0.001
Network gender composition
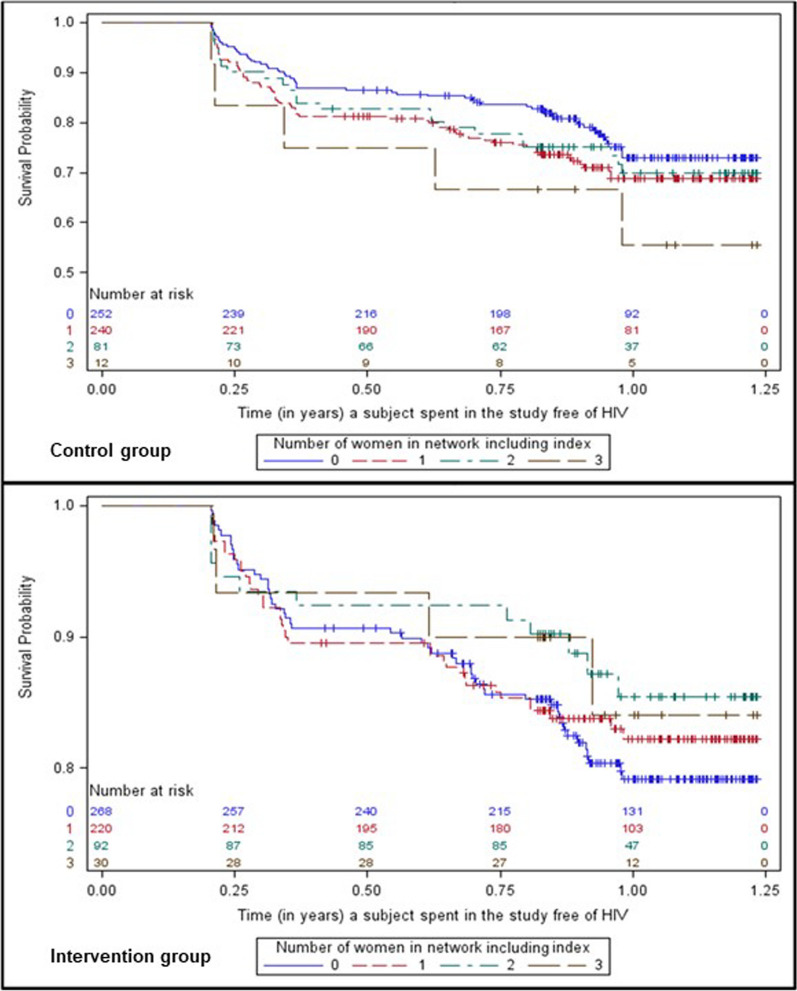
The main effect of network gender composition was non-significant in unadjusted and adjusted models (p = 0.109 and p = 0.222, respectively), as was the main effect of study arm (p = 0.135 and p = 0.170, respectively) (Table 2). The interaction was significant in the adjusted model (p = 0.031). Simple effects indicated that, in the control arm, for each additional network female, the HIV seroconversion hazard rate was 13% higher (HR = 1.13; 95% CI = 0.93, 1.38; p = 0.222). In the intervention arm, for each additional network female, the HIV seroconversion hazard rate was 20% lower (HR = 0.80; 95% CI = 0.63, 1.03; p = 0.078). In the intervention arm, the HIV seroconversion hazard rate was 21% lower with 0 network females (HR = 0.79, 95% CI = 0.56, 1.11), which was non-significant (p = 0.170); 44% lower with 1 network female (HR = 0.56, 95% CI = 0.43, 0.73), which was significant (p < 0.001); 61% lower with 2 network females (HR = 0.39, 95% CI = 0.25, 0.63), which was significant (p < 0.001); and 72% lower with 3 network females (HR = 0.28, 95% CI = 0.13, 0.59), which was significant (p < 0.001). Survival curves for time to HIV seroconversion by number of network females stratified by study arm are presented in Fig. 1. Log-rank tests indicated that time to HIV seroconversion was significantly longer for intervention participants with 1 or 2 network females compared to control participants with 1 or 2 network females (p = 0.002 and p = 0.0.015, respectively), and marginally longer for intervention participants with 3 network females compared to control participants with 3 network females (p = 0.057).
Fig. 1.
Kaplan–Meier product-limit survival estimates of time to HIV seroconversion by number of females in one’s injection network, including index. HIV, human immunodeficiency virus
Index gender
The main effect of index gender was non-significant in both unadjusted and adjusted models (p = 0.656 and p = 0.451, respectively), while the main effect of study arm was significant in both (both p = 0.001). The interaction term between study arm and index gender was non-significant in the adjusted model (p = 0.605). Consequently, we did not calculate simple effects or generate survival curves.
Sensitivity analysis 1: testing network gender composition excluding the index
Given the significant interaction effect when network gender composition including the index was examined, we sought to conduct a sensitivity analysis to examine whether this pattern of results would hold if we excluded the index. We found that the main effect of network gender composition excluding the index was non-significant in unadjusted and adjusted models (p = 0.147 and p = 326, respectively), as was the main effect of study arm (p = 0.082 and p = 0.096, respectively). However, their interaction was significant in both the unadjusted (p = 0.032) and adjusted models (p = 0.034), lending further support for the role of network gender composition. Simple effects indicated that, in the control arm, for each additional network female, the HIV seroconversion hazard rate was 13% higher (HR = 1.13; 95% CI = 0.88, 1.45), but this was non-significant (p = 0.326). In the intervention arm, for each additional network female, the HIV seroconversion hazard rate was 26% lower (HR = 0.74; 95% CI = 0.54, 1.01), which approached statistical significance (p = 0.059). In the intervention arm, the HIV seroconversion hazard rate was 24% lower with 0 network females (HR = 0.76, 95% CI = 0.55, 1.05), which was non-significant (p = 0.096); 51% lower with 1 network female (HR = 0.49, 95% CI = 0.36, 0.68), which was significant (p < 0.001); and 68% lower with 2 network females (HR = 0.32, 95% CI = 0.17, 0.61), which was significant (p < 0.001).
Sensitivity analysis 2: moderation by network gender, stratified by gender
To further examine our findings regarding network gender composition, we conducted a sensitivity analysis to test whether male and female network members varied in response to the intervention by the number of females present in the injection network. We repeated our Model 1 analysis, in which we incorporated a product term between study arm and network gender composition in our Cox regression, but we also stratified by gender. We calculated simple effects regardless of the significance of the product terms to examine trends.
For men (n = 894) and women (n = 301), product terms were non-significant in both unadjusted (p = 0.195 and p = 0.820) and adjusted (p = 0.330 and p = 0.981) models, respectively. Among men in the intervention arm, the HIV seroconversion hazard rate was 21% lower with 0 network females (HR = 0.79, 95% CI = 0.55, 1.14), which was non-significant (p = 0.213); 37% lower with 1 network female (HR = 0.63, 95% CI = 0.42, 0.92), which was significant (p = 0.018); and 51% lower with 2 network females (HR = 0.49, 95% CI = 0.22, 1.09), which was marginally significant (p = 0.079). Among women in the intervention arm, the HIV seroconversion hazard rate was 62% lower with 1 network female (HR = 0.38, 95% CI = 0.19, 0.78), which was significant (p = 0.009); 61% lower with 2 network females (HR = 0.39, 95% CI = 0.19, 0.77), which was significant (p = 0.007); and 61% lower with 3 network females (HR = 0.39, 95% CI = 0.10, 1.52), which was non-significant (p = 0.173; Fig. 2).
Fig. 2.
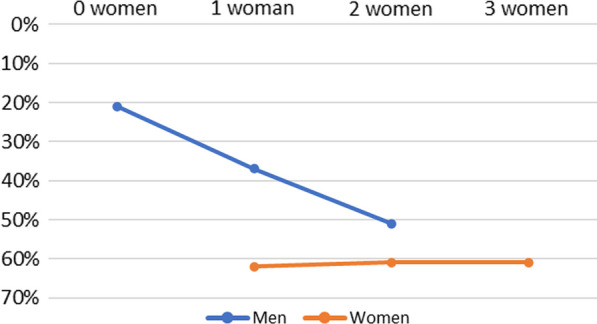
Reductions in HIV seroconversion risk by number of women in one’s injection network for intervention vs control participants, stratified by gender. HIV, human immunodeficiency virus
Discussion
In this study, we explored the extent to which network gender composition and index gender moderated the effects of a social network intervention on HIV seroconversion among PWID in Ukraine. Though women comprised only one quarter of the sample, our findings revealed significant moderation by network gender composition but not by index gender. Our first sensitivity analysis also revealed significant moderation by network gender composition even though we excluded the index, while our second sensitivity analysis showed a sustained, constant trend of reduced risk among women and a decreasing trend of reduced risk among men as the number network females increased. These findings contribute to the broader literature that has shown social network approaches to be effective in documenting and reducing unsafe behaviors among PWID across diverse contexts, including Ukraine [30, 38, 41–50]. More importantly, these findings extend prior research, particularly research in Eastern Europe, by contributing to the knowledge base on social network interventions and on women who inject drugs specifically, which can help inform the tailoring of future interventions to this group and motivate research to examine mechanisms (e.g., gendered power dynamics) linking gender composition in injection networks to HIV risk [25, 27, 28].
Our main analysis (and first sensitivity analysis) revealed a more robust intervention effect on HIV seroconversion among participants with more women in their injection network, which recalls prior research showing a protective effect for PWID with a greater proportion of women in their networks [25, 27, 28]. One potential explanation for this finding is that participants in these networks were less exposed to gendered (i.e., unequal) power relations and consequently had increased agency to adopt the intervention on account of their being fewer men in the network [25]. Similar to those of women who inject drugs in other contexts [1, 7, 17, 51–57], the injection behaviors and sexual relationships of women who inject drugs in Ukraine are complexly interconnected, with male injecting partners often wielding power over women that increases their HIV risk through unsafe practices (such as syringe/needle-sharing or condomless sex) or limited access to drug treatment (such as opioid agonist treatment) [14, 15]. Arguably, the control participants in networks with comparable gender composition would have likewise experienced reduced exposure to gendered power relations and therefore increased agency. However, they were not presented with safer, alternative risk-reduction practices upon which to enact their agency as were the participants in the intervention arm.
Other explanations for the moderation effect could be related to features of women’s injection networks. Research in other contexts has indicated that the injection networks of women who inject drugs tend to be relatively small and built on trust and close bonds with others [58–61], which may also be characteristic of women who inject drugs in Ukraine [29, 30, 62]. Moreover, women—particularly in traditional, patriarchal societies like Ukraine—tend to be socialized toward engaging in more prosocial behavior than men [63, 64], a tendency also evident in injection networks [59, 60, 65]. Taken together, women in injection networks with more women may be more likely to give (i.e., on the part of the prosocial index member providing education) and receive help (i.e., on the part of network members who are closely bonded with and trust the index), translating to greater uptake of risk-reduction strategies. This is an area for future research.
Our second sensitivity analysis showed support for what we found in our main analysis, demonstrating that men benefited more from the intervention as the number of women in their injection network increased. While women were shown to have benefited regardless of whether other women were in their network, it should be recalled that our sample size of women was a third of our sample size of men, and therefore more prone to parameter estimation error. Nevertheless, findings from both the main and sensitivity analyses do suggest that women uniquely benefited from the intervention and may have even played a role in helping facilitate men’s benefit as well. Again, whether this was due to women’s unique contribution to the network or women’s self-selecting into networks that are inherently safer is unclear, but this lack of clarity on the mechanism does not negate the fact that the presence of women in the network seems to signal greater potential for safer behaviors and reduced HIV risk. Future research should closely examine the mechanisms explaining the role of women in injection risk networks (i.e., whether women promote safer behavior, and/or they self-select into safer networks).
Importantly, we did not assess the full extent of the size or gender composition of participants’ real-world injection networks, as the very nature of the intervention dictated that participants only recruit two of their injection network members. Therefore, participants’ actual injection networks could have been much larger and differently composed than what was indicated in our findings. Unmeasured network links could produce a confounding effect, especially if women’s injection networks vary substantially from those of men [66]. Additionally, the number of women in a given injection network will at least depend on network size, which is associated with HIV risk [67, 68]. Replication trials that fully assess network size, gender composition, and gender dynamics are needed.
There was no moderation effect by index gender. Given women’s more subjugated social position relative to men in Ukraine [14, 21–23, 69–71], including in injection dyads/networks [14, 16], it may be reasonable to expect that having a female index (i.e., be taught risk-reduction strategies by a woman) would be less protective than having a male index. However, our findings did not reveal this to be the case. Given the small number of female indexes in our sample, more targeted research focused on recruiting networks with women indexes are needed to ensure sufficient power to better understand the role of index gender in social network interventions to reduce HIV risk among PWID.
Limitations
Findings should be considered in light of several limitations. Participants were not asked to report their sex assigned at birth or their gender identity. Rather, interviewers recorded a participant as “male” or “female” based on appearance. Therefore, some participants’ gender identity could have been misreported. Second, as noted previously, conclusions about the overall size or gender composition of participants’ injection networks beyond what was represented in the study cannot be drawn, as participants recruited only two (i.e., not all) members of their injection networks to join the study, and information about their broader networks was not collected. Indeed, had participants’ full injection networks been included, then our findings may certainly differ. Researchers may wish to consider the feasibility of including a sample’s full injection network in future intervention studies. Though the proportion of women who participated in the study was comparable to nationwide population estimates of PWID [35], the unique challenges and disproportionate HIV burden faced by women warrant oversampling of this population to ensure their needs are better understood and addressed.
Research and practice implications
As this is now one of several studies demonstrating protective effects of having more women in injection networks [25, 27, 28], investigations of the mechanisms (e.g., unequal power relations and other gender dynamics; collective/within-group social support) through which this occurs are needed. Similarly, given that our findings indicated a dose–response relationship of sorts (i.e., the protective effect increased with each additional network female), understanding what the critical share or proportion of network females is needed for maximum, sustained impact on the injection network would be helpful. Because HIV incidence was high in the intervention group as well, despite the intervention’s demonstrated efficacy, full uptake of risk-reduction strategies was not achieved. Teasing out the extent to which there were gender differences in successfully teaching (on the part of indexes) and taking-up risk-reduction strategies (on the part of the network members) would help refine and inform future interventions for women who inject drugs.
Considerations for implementation of this or comparable social network interventions may include a preliminary assessment of gender composition of the injection networks in targeted communities. Informed by these data, organizations may then roll out the intervention to networks with a critical share of network females for optimal intervention impact (though research has yet to determine what this critical share might be, as noted previously). Of course, this is under the assumption that recruited networks in our study, including their size and gender composition, roughly approximate actual networks. Secondly, while a logical implication of our findings would be to alter the gender composition of injection networks, this is likely not an appropriate or feasible course of action. Instead, different sorts of interventions, such as gender-transformative interventions [72, 73], may actually be needed for networks in which the proportion of women is minimal, or for networks with strong gendered or sexist power dynamics. Perhaps in conjunction with, or subsequent to, implementation of gender-transformative programming, our social network or comparable interventions may be implemented. Finally, our finding that the intervention seemed to uniquely benefit women reflects the unique lived experiences of women, including their drug-using experiences, warranting the development of more interventions that are women-focused and women-tailored [74–77]. Of course, future implementation science research would need to be conducted to examine the feasibility, acceptability, and sustainability of the aforementioned intervention strategies.
Implications for further intervention implementation in Ukraine are less clear. As noted previously, these data were collected roughly ten years ago and the applicability to the current Ukrainian context is unknown. Some research conducted during the intervening period suggests that the epidemiologic context of HIV and injection drug use did remain comparable over time [9–11]. However, there is evidence that major recent and current events like the COVID-19 pandemic and the Russian invasion have disrupted HIV prevention and care, drug treatment, and overall healthcare, and will continue to do so [78–82]. These disruptions will likely exacerbate gender disparities in HIV and injection drug-related health outcomes further, as we have seen in previous instances of Russian aggression toward Ukraine [37]. It is perhaps more likely that extant HIV-related disparities faced by women who inject drugs will only grow rather than shrink in response to these events. Therefore, social network interventions like the one examined here, or intentionally women-focused interventions, will continue to remain relevant and needed for women who inject drugs in Ukraine. While the practicalities of successfully implementing any public health intervention in Ukraine in the midst of warfare are formidable, taking the lessons learned from existing data will be valuable as the research and service infrastructure in the country regrow.
Acknowledgements
The authors wish to acknowledge the dedicated team and participants from the original trial.
Author contributions
JMW contributed to the design of the analytic approach, and primarily led the development of the manuscript. RB was the principal investigator of the original trial and contributed to the development of the manuscript. LRS, CEDS, and TLP assisted with interpretation of results and contributed to the development of the manuscript. SS conducted the statistical analysis. EVP garnered funding to support the analysis in this study, conceptualized the aims and approach for this analysis, and co-led the development of the manuscript.
Funding
This research was supported by NIDA R01DA042666 (PI: Pitpitan), and preparation of this manuscript was supported by the San Diego Center for AIDS Research (P30AI036214). The original trial was supported by NIDA R01DA026739 (PI: Booth).
Availability of data and materials
The data that support the findings of this study are available from the authors upon reasonable request and with the permission of the PI.
Declarations
Ethics approval and consent to participate
The original trial involved study procedures that were approved by the Institutional Review Board (IRB) of the University of Colorado Denver and by the IRB of the Ukrainian Institute on Public Health Policy.
Consent for publication
All authors provide consent for the publication of this manuscript.
Competing interests
The authors have no conflicts of interest or competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azim T, Bontell I, Strathdee SA. Women, drugs and HIV. Int J Drug Policy. 2015;26(Suppl 1):S16–21. doi: 10.1016/j.drugpo.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts A, Mathers B, Degenhardt L. Reference group to the United Nations on HIV and injecting drug use. Women who inject drugs: a review of their risks, experiences and needs. 2010.
- 3.Des Jarlais DC, Feelemyer JP, Modi SN, Arasteh K, Hagan H. Are females who inject drugs at higher risk for HIV infection than males who inject drugs: an international systematic review of high seroprevalence areas. Drug Alcohol Depend. 2012;124(1–2):95–107. doi: 10.1016/j.drugalcdep.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jolley E, Rhodes T, Platt L, Hope V, Latypov A, Donoghoe M, et al. HIV among people who inject drugs in Central and Eastern Europe and Central Asia: a systematic review with implications for policy. BMJ Open. 2012;2(5):e001465. doi: 10.1136/bmjopen-2012-001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control & World Health Organization Regional Office for Europe. HIV/AIDS surveillance in Europe 2020 (2019 data). 2020.
- 6.World Health Organization Regional Office for Europe. Good practices in Europe: HIV prevention for People Who Inject Drugs implemented by the International HIV/AIDS Alliance in Ukraine. 2014.
- 7.Burruano L, Kruglov Y. HIV/AIDS epidemic in Eastern Europe: recent developments in the Russian Federation and Ukraine among women. Gend Med. 2009;6(1):277–289. doi: 10.1016/j.genm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 8.UNAIDS Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2017. 2017. [PubMed]
- 9.Dumchev K, Sazonova Y, Salyuk T, Varetska O. Trends in HIV prevalence among people injecting drugs, men having sex with men, and female sex workers in Ukraine. Int J STD AIDS. 2018;29(13):1337–1344. doi: 10.1177/0956462418784096. [DOI] [PubMed] [Google Scholar]
- 10.Leung J, Peacock A, Colledge S, Grebely J, Cunningham EB, Hickman M, et al. A global meta-analysis of the prevalence of HIV, hepatitis C virus, and hepatitis B virus among people who inject drugs-do gender-based differences vary by country-level indicators? J Infect Dis. 2019;220(1):78–90. doi: 10.1093/infdis/jiz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smyrnov P, Williams LD, Korobchuk A, Sazonova Y, Nikolopoulos GK, Skaathun B, et al. Risk network approaches to locating undiagnosed HIV cases in Odessa, Ukraine. J Int AIDS Soc. 2018;21(1):e25040. doi: 10.1002/jia2.25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iversen J, Page K, Madden A, Maher L. HIV, HCV, and health-related harms among women who inject drugs: implications for prevention and treatment. J Acquir Immune Defic Syndr. 2015;69(Suppl 2):S176–81. doi: 10.1097/QAI.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzin I, Martzynovska V, Antonenko Z. HIV infection in Ukraine Public Health Center of the Ministry of Health of Ukraine. 2020;Newsletter No. 51.
- 14.Hoff E, Marcus R, Bojko MJ, Makarenko I, Mazhnaya A, Altice FL, et al. The effects of opioid-agonist treatments on HIV risk and social stability: a mixed methods study of women with opioid use disorder in Ukraine. J Subst Abuse Treat. 2017;83:36–44. doi: 10.1016/j.jsat.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corsi KF, Dvoryak S, Garver-Apgar C, Davis JM, Brewster JT, Lisovska O, et al. Gender differences between predictors of HIV status among PWID in Ukraine. Drug Alcohol Depend. 2014;1(138):103–108. doi: 10.1016/j.drugalcdep.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owczarzak J, Phillips SD, Cho W. 'Pure' drug users, commercial sex workers and 'ordinary girls': gendered narratives of HIV risk and prevention in post-Soviet Ukraine. Cult Health Sex. 2018;20(11):1171–1184. doi: 10.1080/13691058.2017.1421708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irfan SD, Khan MNM, Khan SI. Tales of gender-based oppression and violence: risks and vulnerabilities of women who inject drugs (WWID) in Dhaka. Bangladesh Int J Drug Policy. 2021;92:103144. doi: 10.1016/j.drugpo.2021.103144. [DOI] [PubMed] [Google Scholar]
- 18.Spicer N, Harmer A, Aleshkina J, Bogdan D, Chkhatarashvili K, Murzalieva G, et al. Circus monkeys or change agents? Civil society advocacy for HIV/AIDS in adverse policy environments. Soc Sci Med. 2011;73(12):1748–1755. doi: 10.1016/j.socscimed.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Spicer N, Bogdan D, Brugha R, Harmer A, Murzalieva G, Semigina T. 'It's risky to walk in the city with syringes': understanding access to HIV/AIDS services for injecting drug users in the former Soviet Union countries of Ukraine and Kyrgyzstan. Global Health 2011 Jul 13;7:22–8603–7–22. [DOI] [PMC free article] [PubMed]
- 20.Owczarzak J, Kazi AK, Mazhnaya A, Alpatova P, Zub T, Filippova O, et al. "You're nobody without a piece of paper:" visibility, the state, and access to services among women who use drugs in Ukraine. Soc Sci Med. 2021;269:113563. doi: 10.1016/j.socscimed.2020.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maume D, Hewitt B, Ruppanner L. Gender equality and restless sleep among partnered Europeans. J Marriage Fam. 2018;80(4):1040–1058. [Google Scholar]
- 22.Yakushko O. Ambivalent sexism and relationship patterns among women and men in Ukraine. Sex Roles 2005 05;52(9):589–596.
- 23.Lee JO, Yoon Y, Idrisov B, Kiriazova T, Makarenko O, Sereda Y, et al. Violence, HIV risks, and polysubstance use among HIV-positive people who inject drugs in Ukraine. AIDS Behav. 2021;25(7):2120–2130. doi: 10.1007/s10461-020-03142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran LT, Peacock A, Colledge S, Memedovic S, Grebely J, Leung J, et al. Injecting risk behaviours amongst people who inject drugs: a global multi-stage systematic review and meta-analysis. Int J Drug Policy. 2020;84:102866. doi: 10.1016/j.drugpo.2020.102866. [DOI] [PubMed] [Google Scholar]
- 25.Smith LR, Strathdee SA, Metzger D, Latkin C. Evaluating network-level predictors of behavior change among injection networks enrolled in the HPTN 037 randomized controlled trial. Drug Alcohol Depend. 2017;1(175):164–170. doi: 10.1016/j.drugalcdep.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felsher M, Koku E, Bellamy SL, Mulawa MI, Roth AM. Predictors of willingness to diffuse PrEP information within ego-centric networks of women who inject drugs. AIDS Behav. 2021;25(6):1856–1863. doi: 10.1007/s10461-020-03115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curry AD, Latkin CA. Gender differences in street economy and social network correlates of arrest among heroin injectors in Baltimore. Maryland J Urban Health. 2003;80(3):482–493. doi: 10.1093/jurban/jtg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowlton A, Hua W, Latkin C. Social support among HIV positive injection drug users: implications to integrated intervention for HIV positives. AIDS Behav. 2004;8(4):357–363. doi: 10.1007/s10461-004-7320-7. [DOI] [PubMed] [Google Scholar]
- 29.Booth RE, Lehman WE, Dvoryak S, Brewster JT, Sinitsyna L. Interventions with injection drug users in Ukraine. Addiction. 2009;104(11):1864–1873. doi: 10.1111/j.1360-0443.2009.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth RE, Lehman WE, Latkin CA, Dvoryak S, Brewster JT, Royer MS, et al. Individual and network interventions with injection drug users in 5 Ukraine cities. Am J Public Health. 2011;101(2):336–343. doi: 10.2105/AJPH.2009.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Booth RE, Davis JM, Dvoryak S, Brewster JT, Lisovska O, Strathdee SA, et al. HIV incidence among people who inject drugs (PWIDs) in Ukraine: results from a clustered randomised trial. Lancet HIV. 2016;3(10):e482–e489. doi: 10.1016/S2352-3018(16)30040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booth RE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Predictors of HIV sero-status among drug injectors at three Ukraine sites. AIDS. 2006;20(17):2217–2223. doi: 10.1097/QAD.0b013e328010e019. [DOI] [PubMed] [Google Scholar]
- 33.Interventions to reduce sex-related HIV risks for women drug injectors: a systematic review. American Public Health Association 133rd Annual Meeting & Exposition; 2005.
- 34.Auerbach JD, Smith LR. Theoretical foundations of research focused on HIV prevention among substance-involved women: a review of observational and intervention studies. J Acquir Immune Defic Syndr. 2015;69(Suppl 2):S146–54. doi: 10.1097/QAI.0000000000000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Springer SA, Larney S, Alam-Mehrjerdi Z, Altice FL, Metzger D, Shoptaw S. Drug treatment as HIV prevention among women and girls who inject drugs from a global perspective: progress, gaps, and future directions. J Acquir Immune Defic Syndr. 2015;69(Suppl 2):S155–161. doi: 10.1097/QAI.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitpitan EV, MacKinnon DP, Eaton LA, Smith LR, Wagman J, Patterson TL. Using novel approaches to evaluate behavioral interventions: overlooked significant HIV prevention effects in the HPTN 015 Project EXPLORE. J Acquir Immune Defic Syndr. 2021;87(5):1128–1135. doi: 10.1097/QAI.0000000000002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filippovych S. Impact of armed conflicts and warfare on opioid substitution treatment in Ukraine: responding to emergency needs. Int J Drug Policy. 2015;26(1):3–5. doi: 10.1016/j.drugpo.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol. 2003;22(4):332–339. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- 39.Latkin CA, Forman V, Knowlton A, Sherman S. Norms, social networks, and HIV-related risk behaviors among urban disadvantaged drug users. Soc Sci Med. 2003;56(3):465–476. doi: 10.1016/s0277-9536(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 40.Latkin CA, Hua W, Davey MA. Factors associated with peer HIV prevention outreach in drug-using communities. AIDS Educ Prev. 2004;16(6):499–508. doi: 10.1521/aeap.16.6.499.53794. [DOI] [PubMed] [Google Scholar]
- 41.Latkin CA. Outreach in natural settings: the use of peer leaders for HIV prevention among injecting drug users' networks. Public Health Rep. 1998;113(Suppl 1):151–159. [PMC free article] [PubMed] [Google Scholar]
- 42.Theall KP, Sterk CE, Elifson KW, Kidder D. Factors associated with positive HIV serostatus among women who use drugs: continued evidence for expanding factors of influence. Public Health Rep. 2003;118(5):415–424. doi: 10.1093/phr/118.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobin KE, Kuramoto SJ, Davey-Rothwell MA, Latkin CA. The STEP into action study: a peer-based, personal risk network-focused HIV prevention intervention with injection drug users in Baltimore. Maryland Addiction. 2011;106(2):366–375. doi: 10.1111/j.1360-0443.2010.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier VM, Friedman SR, Des Jarlais DC. Transitions to injecting drug use among noninjecting heroin users: social network influence and individual susceptibility. J Acquir Immune Defic Syndr. 2006;41(4):493–503. doi: 10.1097/01.qai.0000186391.49205.3b. [DOI] [PubMed] [Google Scholar]
- 45.De P, Cox J, Boivin JF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh D, Krishnan A, Gibson B, Brown SE, Latkin CA, Altice FL. Social network strategies to address HIV prevention and treatment continuum of care among at-risk and HIV-infected substance users: a systematic scoping review. AIDS Behav. 2017;21(4):1183–1207. doi: 10.1007/s10461-016-1413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Booth RE, Lehman WEK, Latkin CA, Brewster JT, Sinitsyna L, Dvoryak S. Use of a peer leader intervention model to reduce needle-related risk behaviors among drug injectors in Ukraine. J Drug Iss. 2009;39(3):607–625. [Google Scholar]
- 48.Latkin CA, Donnell D, Metzger D, Sherman S, Aramrattna A, Davis-Vogel A, et al. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Soc Sci Med. 2009;68(4):740–748. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman IF, Latkin CA, Kukhareva PV, Malov SV, Batluk JV, Shaboltas AV, et al. A peer-educator network HIV prevention intervention among injection drug users: results of a randomized controlled trial in St. Petersburg Russia. AIDS Behav. 2013;17(7):2510–2520. doi: 10.1007/s10461-013-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mihailovic A, Tobin K, Latkin C. The influence of a peer-based HIV prevention intervention on conversation about HIV prevention among people who inject drugs in Baltimore, Maryland. AIDS Behav. 2015;19(10):1792–1800. doi: 10.1007/s10461-015-1048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fairbairn N, Small W, Van Borek N, Wood E, Kerr T. Social structural factors that shape assisted injecting practices among injection drug users in Vancouver, Canada: a qualitative study. Harm Reduct J 2010;7:20–7517–7–20. [DOI] [PMC free article] [PubMed]
- 52.Meyers SA, Smith LR, Luisa Mittal M, Strathdee SA, Garfein RS, Guise A, et al. The role of gender and power dynamics in injection initiation events within intimate partnerships in the US-Mexico border region. Cult Health Sex. 2020;22(9):1080–1095. doi: 10.1080/13691058.2019.1651903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young AM, Larian N, Havens JR. Gender differences in circumstances surrounding first injection experience of rural injection drug users in the United States. Drug Alcohol Depend. 2014;1(134):401–405. doi: 10.1016/j.drugalcdep.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gicquelais RE, Werb D, Marks C, Ziegler C, Mehta SH, Genberg BL, et al. Prevalence and correlates of providing and receiving assistance with the transition to injection drug use. Epidemiol Rev. 2020;42(1):4–18. doi: 10.1093/epirev/mxaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mburu G, Limmer M, Holland P. HIV risk behaviours among women who inject drugs in coastal Kenya: findings from secondary analysis of qualitative data. Harm Reduct J 2019;16(1):10–019–0281-y. [DOI] [PMC free article] [PubMed]
- 56.Morris MD, Montgomery ME, Briceno A, Evans JL, Andrew EVW, Page K, et al. A study of sexual relationship power among young women who inject drugs and their sexual partners. Subst Use Misuse. 2018;53(8):1281–1287. doi: 10.1080/10826084.2017.1404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoicescu C, Cluver LD, Spreckelsen TF, Mahanani MM, Ameilia R. Intimate partner violence and receptive syringe sharing among women who inject drugs in Indonesia: a respondent-driven sampling study. Int J Drug Policy. 2019;63:1–11. doi: 10.1016/j.drugpo.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Cruz MF, Mantsios A, Ramos R, Case P, Brouwer KC, Ramos ME, et al. A qualitative exploration of gender in the context of injection drug use in two US-Mexico border cities. AIDS Behav. 2007;11(2):253–262. doi: 10.1007/s10461-006-9148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnhart KJ, Dodge B, Sayegh MA, Herbenick D, Reece M. Shared injection experiences: interpersonal involvement in injection drug practices among women. Subst Abus. 2021;2:1–7. doi: 10.1080/08897077.2021.1903650. [DOI] [PubMed] [Google Scholar]
- 60.Syvertsen JL, Robertson AM, Strathdee SA, Martinez G, Rangel MG, Wagner KD. Rethinking risk: gender and injection drug-related HIV risk among female sex workers and their non-commercial partners along the Mexico-US border. Int J Drug Policy. 2014;25(5):836–844. doi: 10.1016/j.drugpo.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Felsher M, Tobin KE, Sulkowski M, Latkin C, Falade-Nwulia O. HCV communication within ego-centric networks of men and women who inject drugs. Drug Alcohol Depend. 2021;229(Pt B):109157. doi: 10.1016/j.drugalcdep.2021.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Booth RE, Kennedy J, Brewster T, Semerik O. Drug injectors and dealers in Odessa, Ukraine. J Psychoactive Drugs. 2003;35(4):419–426. doi: 10.1080/02791072.2003.10400488. [DOI] [PubMed] [Google Scholar]
- 63.Espinosa MP, Kovářík J. Prosocial behavior and gender. Front Behav Neurosci. 2015;14(9):88. doi: 10.3389/fnbeh.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nielson MG, Padilla-Walker L, Holmes EK. How do men and women help? Validation of a multidimensional measure of prosocial behavior. J Adolesc. 2017;56:91–106. doi: 10.1016/j.adolescence.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Simpson KA, Kral AH, Goldshear JL, Wenger L, Strike CS, Bluthenthal RN. Reasons for assisting with injection initiation: results from a large survey of people who inject drugs in Los Angeles and San Francisco, California. Drug Alcohol Depend. 2020;1(209):107885. doi: 10.1016/j.drugalcdep.2020.107885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman SR, Cooper HLF, Osborne AH. Structural and social contexts of HIV risk among African Americans. Am J Public Health. 2009;99(6):1002–1008. doi: 10.2105/AJPH.2008.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cepeda JA, Odinokova VA, Heimer R, Grau LE, Lyubimova A, Safiullina L, et al. Drug network characteristics and HIV risk among injection drug users in Russia: the roles of trust, size, and stability. AIDS Behav. 2011;15(5):1003–1010. doi: 10.1007/s10461-010-9816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cepeda JA, Solomon SS, Srikrishnan AK, McFall AM, Kumar MS, Vasudevan CK, et al. Injection drug network characteristics are important markers of HIV risk behavior and lack of viral suppression. J Acquir Immune Defic Syndr. 2017;75(3):257–264. doi: 10.1097/QAI.0000000000001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran TD, Nguyen H, Fisher J. Attitudes towards intimate partner violence against women among women and men in 39 low- and middle-income countries. PLoS ONE. 2016;11(11):e0167438. doi: 10.1371/journal.pone.0167438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.UN Women. Where we are: Europe and Central Asia: Ukraine. Available at: https://eca.unwomen.org/en/where-we-are/ukraine. Accessed October 24, 2021.
- 71.The World Bank. Country gender assessment for Ukraine. 2016.
- 72.Casey E, Carlson J, Two Bulls S, Yager A. Gender transformative approaches to engaging men in gender-based violence prevention: a review and conceptual model. Trauma Viol Abuse. 2018;19(2):231–246. doi: 10.1177/1524838016650191. [DOI] [PubMed] [Google Scholar]
- 73.Gibbs A, Jacobson J, Kerr Wilson A. A global comprehensive review of economic interventions to prevent intimate partner violence and HIV risk behaviours. Glob Health Act. 2017 doi: 10.1080/16549716.2017.1290427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pinkham S, Stoicescu C, Myers B. Developing effective health interventions for women who inject drugs: key areas and recommendations for program development and policy. Adv Prev Med. 2012;2012:269123. doi: 10.1155/2012/269123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harris MTH, Laks J, Stahl N, Bagley SM, Saia K, Wechsberg WM. Gender dynamics in substance use and treatment: a women's focused approach. Med Clin North Am. 2022;106(1):219–234. doi: 10.1016/j.mcna.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones HE, Kirtadze I, Otiashvili D, Murphy K, O'Grady KE, Zule W, et al. Feasibility and initial efficacy of a culturally sensitive women-centered substance use intervention in Georgia: Sex risk outcomes. Subst Abuse Treat Prev Policy 2015;10:47–015–0043–0. [DOI] [PMC free article] [PubMed]
- 77.Wechsberg WM. Promising international interventions and treatment for women who use and abuse drugs: focusing on the issues through the InWomen's Group. Subst Abuse Rehabil. 2012;3(Suppl 1):1–4. doi: 10.2147/SAR.S21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meteliuk A, de Leon G, Samy J, Madden LM, Pykalo I, Fomenko T, Filippovych M, et al. Rapid transitional response to the COVID-19 pandemic by opioid agonist treatment programs in Ukraine. J Subst Abuse Treat. 2021;121:108164. doi: 10.1016/j.jsat.2020.108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dumchev K, Kiriazova T, Chernova O. Impact of the COVID-19 epidemic on drug markets, substance use patterns, and delivery of harm reduction and treatment services in Ukraine. 2021;Retrieved from https://uiphp.org.ua/media/k2/attachments/2021-02-01_Ukraine_Covid.pdf.
- 80.Altice FL, Bromberg DJ, Dvoriak S, Meteliuk A, Pykalo I, Islam Z, et al. Extending a lifeline to people with HIV and opioid use disorder during the war in Ukraine. Lancet Public Health. 2022;7(5):e482–e484. doi: 10.1016/S2468-2667(22)00083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kai J, Parczewski M, van de Vijver D. The war refugees from Ukraine: an HIV epidemic is fleeing as well. AIDS. 2022;36(12):1745–1746. doi: 10.1097/QAD.0000000000003271. [DOI] [PubMed] [Google Scholar]
- 82.Kazatchkine M. Ukrainian war: an economic crisis in Eastern Europe and Central Asia will lead to a health crisis. BMJ. 2022;24(376):o793. doi: 10.1136/bmj.o793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request and with the permission of the PI.