Abstract
BACKGROUND
At present, neuroendoscopy technology has made rapid development, and great progress has been made in the operation of lesions in the saddle area of the skull base. However, the complications of cerebrospinal fluid and intracranial infection after the operation are still important and life-threatening complications, which may lead to poor prognosis.
AIM
To investigate the method of in situ bone flap combined with nasal septum mucosal flap for reconstruction of enlarged skull base defect by endonasal sphenoidal approach and to discuss its application effect.
METHODS
Clinical data of 24 patients undergoing transnasal sphenoidal endoscopic approach in the Department of Neurosurgery, Affiliated 2 Hospital of Nantong University from January 2019 to December 2022 were retrospectively analyzed. All patients underwent multi-layer reconstruction of skull base using in situ bone flap combined with nasal septum mucosa flap. The incidence of intraoperative and postoperative cerebrospinal fluid leakage and intracranial infection were analyzed, and the application effect and technical key points of in situ bone flap combined with nasal septum mucosa flap for skull base bone reconstruction were analyzed.
RESULTS
There were 5 cases of high flow cerebrospinal fluid (CSF) leakage and 7 cases of low flow CSF leakage. Postoperative cerebrospinal fluid leakage occurred in 2 patients (8.3%) and intracranial infection in 2 patients (8.3%), which were cured after strict bed rest, continuous drainage of lumbar cistern combined with antibiotic treatment, and no secondary surgical repair was required. The patients were followed up for 8 to 36 months after the operation, and no delayed cerebrospinal fluid leakage or intracranial infection occurred during the follow-up. Computed tomography reconstruction of skull base showed satisfactory reconstruction after surgery.
CONCLUSION
The use of in situ bone flap combined with vascular pedicled mucous flap to reconstruction of skull base defect after endonasal sphenoidal approach under neuroendoscopy has a lower incidence of cerebrospinal fluid leakage and lower complications, which has certain advantages and is worthy of clinical promotion.
Keywords: In situ bone flap, Nasal septum mucosa flap, Multilayer reconstruction, Skull base reconstruction, Neuroendoscopy, Endonasal sphenoidal approach
Core Tip: The use of "in situ bone flap" rigid reconstruction technology for the reconstruction of thesellar floor after nasal endoscopic surgery, which is an anatomical reduction repair technology, can not only reduce the incidence of postoperative cerebrospinal fluid leakage, but also has great advantages, worthy of clinical promotion.
INTRODUCTION
At present, neuroendoscopy technology has made rapid development, and great progress has been made in the operation of lesions in the saddle area of the skull base. With the continuous improvement of the anatomy of the skull base, the continuous popularization of endoscopic technology and the continuous improvement of endoscopic equipment, neuroendoscopy has developed from simple operation of pituitary tumor to perisaddle and suprasellar lesions[1-4]. Now, neuroendoscopic transnasal approach can carry out a variety of lesions in the sellar region, such as pituitary giant adenoma that breaks through the sellar septum and suprasellar craniopharyngioma[5-7]. However, the complications of cerebrospinal fluid and intracranial infection after the operation are still important and life-threatening complications, which may lead to poor prognosis[8-9]. Postoperative skull base reconstruction, restoration of skull base structure and prevention of cerebrospinal fluid leakage and intracranial infection are important problems that must be solved by neuroendoscopists[10-13]. At present, many experts have carried out a variety of technical studies on the reconstruction of the skull base after neuroendoscopic sellar approach, such as mucosal flap reconstruction, fat reconstruction, fascia lata reconstruction and other ways[14-17]. However, a number of studies have pointed out that these skull base reconstruction techniques have insufficient support and cannot achieve a good skull base support function, and it is necessary to carry out bone reconstruction of skull base defect with a commission[16-19]. From January 2019 to December 2022, we performed transnasal endoscopic surgery in sellar region, and used in situ bone flap and pedicled mucosal flap to perform skull base bone reconstruction for 24 patients with sellar region tumors, and achieved satisfactory results. In this study, clinical data of this group were retrospectively analyzed, and are reported as follows.
MATERIALS AND METHODS
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of Affiliated 2 Hospital of Nantong University. Informed consent has been obtained and this investigation has been conducted according to the principles expressed in the Declaration of Helsinki. And the authors have obtained written informed consent of all the patients or legal guardian.
Trial design
The study was a retrospective clinical study in patients undergoing neuroendoscopic sellar surgery with in situ bone flap combined with vascular pedicled mucosal flap reconstruction. The trial was done and analyzed according to the CONSORT guidelines.
General data
In the observation group, there were 16 males and 8 females; The average age was (40.1 ± 17.2) years, ranging from 21 to 67 years. All of them were confirmed by postoperative pathological examination, and the pathological types and distribution of tumors were shown in Table 1.
Table 1.
Pathological types and distribution of tumors in this group
| Pathological type of tumor | Number of cases |
| Pituitary adenoma | 17 |
| Craniopharyngioma | 4 |
| Rathke cleft cysts | 3 |
Surgical methods
In all patients, the skull base was reconstructed using the multi-layer reconstruction technique of the in situ bone flap with vascular pedicled mucosal flap. Skull base multi-layer reconstruction technique: The tumor cavity after tumor surgery was supported by gelatin sponge or hemostatic gauze (Johnson, United States), and the absorbable artificial dura(Tianyifu, China) was laid flat under the dural defect, and the epidural absorbable artificial dura(Tianyifu, China) was laid flat, followed by the in-situ bone flap, which covered the nasal mucosa flap. There were 2 patients who did not use a pedicled nasal septum mucosa flap, due to the failure to make a suitable mucosal flap during the operation in one, and the other case failed to obtain a mucosal flap due to preoperative nasal surgery.
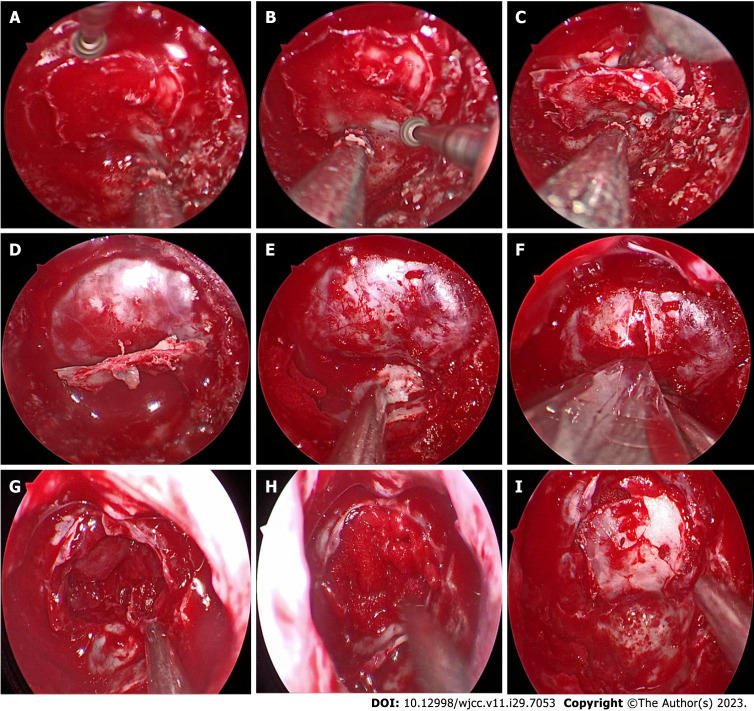
(1) Making "pedicled nasal septum mucosal flap: Making nasal septum mucosal flap with vascular pedicle and placing it in the posterior nasal passage for use[16,20]; and (2) Fabrication of in situ bone flap of sellar floor: After grinding the anterior wall of sphenoid sinus, the posterior sphenoid platform, tuberculum sellae, sellar floor, bilateral optic neural tube, internal carotid artery eminence and other structures were exposed. The margin of the open bone window was designed according to the surgical plan. A small emery grinding head (diameter 2.5 mm) was used to gradually thin the bone along the internal carotid artery eminance, the root of the optic nerve canal, and the back of the sphenoid bone platform to form an "inverted U-shaped bone trough". During this period, the depth of the "bone trough" was checked periodically. Then we thin the bone slightly under the situ bone flap of sellar floor. Finally, we grind out a ring of bone grooves, carefully push the "fracture" at the top of the bone groove with a small stripper, and then use the peel to lift the "inverted U-shaped" bone flap to make the "in situ bone flap". The bone flap was left on the bottom wall of the sphenoid sinus for standby application (Figure 1). Intraoperative navigation and Doppler ultrasound are helpful in determining bilateral internal carotid artery position.
Figure 1.
Surgical procedure for skull base reconstruction. A: The shape of inverted U was used to open the bottom bone of the saddle; B: Thin the bone beneath the bone flap; C: Break above the bone flap, thin bone along the underside, then fold it down; D: Inverted U-shaped flap formation; E: Lower the bone flap and place it underneath for use; F: Cut the dural membrane at the base of the saddle; G: The structure of the saddle revealed after the tumor was removed; H: The structure of tumor Cavity of the saddle was filled; I: The bone flap is rebacked to reconstruct the sole of the saddle.
Postoperative observation and follow-up
The postoperative complications (cerebrospinal fluid leakage, intracranial infection, etc.) were observed. Head computed tomography (CT) scan was performed postoperative day to determine the surgical area. Follow-up examination was performed 3 mo, 6 mo, once a year later. Periodic telephone follow-up was conducted to see if there were any delayed complications associated with skull base reconstruction.
RESULTS
For this group of patients, no significant demographic differences were found in mean age, sex, or medical history and presentation of symptoms. Patients in this group were followed for an average of 22.6 mo, ranging from 8 to 36 mo. In this study, we mainly studied the methods and procedures of skull base reconstruction, and we have made a full description and picture display. In this paper, we did not give too much description and statistics on the vision protection, nerve function and endocrine status of patients. There was no statistical analysis on whether the intraoperative tumor was complete resection and the final recurrence rate.
In this group, there were 24 patients, 22 of whom were reconstructed with in situ bone flap and pedicled mucosal flap. There were 2 patients who did not use a pedicled nasal septum mucosa flap, due to the failure to make a suitable mucosal flap during the operation in one, and the other case failed to obtain a mucosal flap due to preoperative nasal surgery. There were 5 cases of high flow cerebrospinal fluid (CSF) leakage and 7 cases of low flow CSF leakage. Postoperative cerebrospinal fluid leakage occurred in 2 patients (8.3%) and intracranial infection in 2 patients (8.3%), which were cured after strict bed rest (one case was in bed for 5 d and the other case for 7 d), continuous drainage of lumbar cistern combined with antibiotic treatment, and no secondary surgical repair was required. The patients were followed up for 8 to 36 mo after the operation (fourteen patients were followed up for more than 24 mo, three were lost, and the rest were still being followed up), and no delayed cerebrospinal fluid leakage or intracranial infection occurred during the follow-up. CT reconstruction of skull base showed satisfactory reconstruction after surgery.
DISCUSSION
The application of neuroendoscopy in neurosurgery has made great progress. At present, neuroendoscopy can be used to perform surgery for many diseases, including transcranioscopy and transnasal endoscopy[21-26]. However, the problem of skull base reconstruction and cerebrospinal fluid in transnasal neuroendoscopic surgery has always been one of the difficult problems for neurosurgeons. With the continuous efforts of neurosurgeons to study various skull base reconstruction techniques, the incidence of cerebrospinal fluid leakage has been significantly improved[16,19], including vascular pedicled mucosal flap technique[27-30], artificial dura replacement material[31-32], or multi-layer reconstruction of autologous fat and fascia lata[16,33]. It has been reported that the incidence of cerebrospinal fluid leakage using the nasal septum pedicled mucosal flap technique is less than 5%[16].
However, at present, there are still restrictions in the control of cerebrospinal fluid in different hospitals and regions, the incidence is uneven, and the surgical effect is not optimistic. At present, the reconstruction of the skull base after neuroendoscopy is still mainly soft reconstruction, and a few of them use rigid reconstruction with alternative materials, which has not been well promoted and applied[18,31]. At present, the reconstruction is usually filled with gelatin material inside the sella, tile inside the artificial dura, supported by external hemostatic material or covered with mucosal flap. In fact, the surgical area is connected with intracranial pressure after operation, especially in patients with cerebrospinal fluid leakage, when the intracranial pressure increases with brain pulse/posture/cough, or due to factors such as tamponade or displacement of supporting materials and autologous tissue atrophy, the reconstruction is not enough to support, cerebrospinal fluid leakage and reconstruction failure, if complicated with intracranial infection, there is a life-threatening risk.
If rigid reconstruction of autogenous material can be achieved, the relative stability will be improved, because rigid reconstruction can provide enough mechanical support to achieve near-anatomical "reset". Unlike materials such as mucosal flap, hard materials, such as bone materials, are not malleable. The anatomical structure variation of skull base bone and the uneven thickness and roughness of skull base bone make it difficult to obtain autorigid material effectively. Moreover, even if fixed material is available, it is difficult to fix it, which makes hard repair difficult[31].
Leng et al[12] first proposed the concept of skull base rigid reconstruction in 2008 and proposed gasket seal technology. None of the patients who underwent skull base rigid reconstruction had cerebrospinal fluid leakage after surgery and achieved good reconstruction results. In addition, many experts have proposed other rigid reconstruction technologies, including glass, artificial bone, titanium plate and other technologies. But these technologies also have corresponding drawbacks, can not be shaped or installed in place problems.
In view of the above situation, our team further studied the concept of rigid reconstruction, proposed and improved the "in situ bone flap technology", designed the bone flap of sellar floor, and made the in situ bone flap with a small grinding head under neuroendoscopy. During the operation, the upper and lateral edges of the bone flap were ground open, the lower bone was thinned, and then the bone flap was turned down and placed on the floor of the sphenoid sinus. After the operation, the bone flap was reconstructed in the sella, and the multi-layer repair method of the nasal septum mucosal flap with pedicle was used. All the patients in this group achieved satisfactory reconstruction results, two patients had craniocerebral infection after surgery, and were treated with active anti-infection. Postoperative cerebrospinal fluid leakage occurred in 2 patients (8.3%), and were cured after strict bed rest, continuous drainage of lumbar cistern combined with antibiotic treatment, and no secondary surgical repair was required. Even though there were many cases of high-flow cerebrospinal fluid leakage and low-flow cerebrospinal fluid leakage during the operation, the reconstruction results were still satisfactory. No complications related to the "in situ flap" were observed during postoperative follow-up.
Through the study of this group, we found that the "in situ bone flap" reconstruction technique provides a rigid reconstruction of the saddle bottom, and the results are safe and effective. We conclude that the "in situ bone flap" technique has the following advantages: (1) The application of this "in situ bone flap" technology, the bone flap comes from the operative area, does not need to remove the surgical area, reduces the process of entering and leaving the narrow space of the nasal cavity, reduces the damage of the nasal mucosa, and reduces the operation time[34,35]; (2) The bone flap is the bone of the surgical site, and the size and shape of the bone flap match the bone window of the skull base, which is a kind of anatomical reduction operation and fully meets the requirements of skull base reconstruction[36-39]; (3) After rigid bone reconstruction, the anatomical layers are clearly defined, and there are no disadvantages such as rejection and foreign body, which greatly reduces adhesion and postoperative scar formation in the operative area , and facilitates the expedient healing and re-operation of tumor recurrence[30,40,41]; and (4) Through rigid bone reconstruction, the direct impact of cerebrospinal fluid on mucosal flap is slowed down, the reconstruction is enhanced, and the medium is provided for the growth and adhesion of mucosal flap, which has a positive effect on the fixation and survival of mucosal flap after surgery[42,43].
CONCLUSION
In summary, the use of "in situ bone flap" rigid reconstruction technology for the reconstruction of thesellar floor after nasal endoscopic surgery, which is an anatomical reduction repair technology, can not only reduce the incidence of postoperative cerebrospinal fluid leakage, but also has great advantages, worthy of clinical promotion. At present, the number of patients in this group who have been treated with this technique is still small, and the follow-up time is still short. Further observation is needed to verify whether there are complications in the follow-up. Cerebrospinal fluid leakage after endoscopic nasal approach is an important complication that restricts this surgical method. According to the actual situation of the operation, relevant materials and techniques are adopted according to local conditions, and specific individual treatment plans are formulated for patients, so as to achieve the best surgical treatment effect.
ARTICLE HIGHLIGHTS
Research background
At present, neuroendoscopy technology has made rapid development, and great progress has been made in the operation of lesions in the saddle area of the skull base. However, the complications of cerebrospinal fluid and intracranial infection after the operation are still important and life-threatening complications, which may lead to poor prognosis.
Research motivation
At present, cerebrospinal fluid leakage is still one of the difficulties after transnasal endoscopic sellar surgery. Our center has also conducted a variety of reconstruction methods for training and clinical use, and this article is a summary of one of them.
Research objectives
To investigate the method of in situ bone flap combined with nasal septum mucosal flap for reconstruction of enlarged skull base defect by endonasal sphenoidal approach and to discuss its application effect.
Research methods
Clinical data of 24 patients undergoing transnasal sphenoidal endoscopic approach in the Department of Neurosurgery, Affiliated 2 Hospital of Nantong University from January 2019 to December 2022 were retrospectively analyzed. All patients underwent multi-layer reconstruction of skull base using in situ bone flap combined with nasal septum mucosa flap. The incidence of intraoperative and postoperative cerebrospinal fluid leakage and intracranial infection were analyzed, and the application effect and technical key points of in situ bone flap combined with nasal septum mucosa flap for skull base bone reconstruction were analyzed.
Research results
There were 5 cases of high flow cerebrospinal fluid (CSF) leakage and 7 cases of low flow CSF leakage. Postoperative cerebrospinal fluid leakage occurred in 2 patients (8.3%) and intracranial infection in 2 patients (8.3%), which were cured after strict bed rest, continuous drainage of lumbar cistern combined with antibiotic treatment, and no secondary surgical repair was required. The patients were followed up for 8 to 36 months after the operation, and no delayed cerebrospinal fluid leakage or intracranial infection occurred during the follow-up. Computed tomography reconstruction of skull base showed satisfactory reconstruction after surgery.
Research conclusions
In summary, the use of "in situ bone flap" rigid reconstruction technology for the reconstruction of thesellar floor after nasal endoscopic surgery, which is an anatomical reduction repair technology, can not only reduce the incidence of postoperative cerebrospinal fluid leakage, but also has great advantages, worthy of clinical promotion. At present, the number of patients in this group who have been treated with this technique is still small, and the follow-up time is still short.
Research perspectives
According to the actual situation of the operation, relevant materials and techniques are adopted according to local conditions, and specific individual treatment plans are formulated for patients, so as to achieve the best surgical treatment effect.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Affiliated 2 Hospital of Nantong University. Informed consent has been obtained and this investigation has been conducted according to the principles expressed in the Declaration of Helsinki. And the authors have obtained written informed consent of all the patients or legal guardian.
Conflict-of-interest statement: All authors has nothing to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society of Peripheral Neurosurgery.
Peer-review started: June 27, 2023
First decision: September 4, 2023
Article in press: September 25, 2023
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mostafavinia A, Iran; Tsou HK, Taiwan S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
Contributor Information
Ming Qian, Department of Neurosurgery, Affiliated Hospital 2 of Nantong University, Nantong 226000, Jiangsu Province, China.
Xi Chen, Department of Nursing, Affiliated Hospital 2 of Nantong University, Nantong 226001, Jiangsu Province, China.
Long-Yao Zhang, Department of Neurosurgery, Affiliated Hospital 2 of Nantong University, Nantong 226000, Jiangsu Province, China.
Zhi-Feng Wang, Department of Neurosurgery, Affiliated Hospital 2 of Nantong University, Nantong 226000, Jiangsu Province, China.
Yi Zhang, Department of Neurosurgery, Affiliated Hospital 2 of Nantong University, Nantong 226000, Jiangsu Province, China.
Xue-Jian Wang, Department of Neurosurgery, Affiliated Hospital 2 of Nantong University, Nantong 226000, Jiangsu Province, China; Department of Neurosurgery, Nantong Clinical Medical College, Kangda College, Nanjing Medical University, Nantong 226000, Jiangsu Province, China. 6841441@163.com.
Data sharing statement
The data related to the article can be obtained from the corresponding author via email: 6841441@163.com.
References
- 1.Marigil Sanchez M, Karekezi C, Almeida JP, Kalyvas A, Castro V, Velasquez C, Gentili F. Management of Giant Pituitary Adenomas: Role and Outcome of the Endoscopic Endonasal Surgical Approach. Neurosurg Clin N Am. 2019;30:433–444. doi: 10.1016/j.nec.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Zhang B, Shao D, Ji S, Li Y, Xie S, Jiang Z. Analysis of neuroendoscopy for the treatment of macroadenomas and giant pituitary adenomas. Front Surg. 2022;9:956345. doi: 10.3389/fsurg.2022.956345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Yu H, Cai Z, Wang Z, Ma B, Zhang Y, Ye Z. Anatomical study on Meckel cave with endoscopic endonasal, endo-maxillary sinus, and endo-pterygoid process approaches. PLoS One. 2014;9:e91444. doi: 10.1371/journal.pone.0091444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceylan S, Emengen A, Caklili M, Ergen A, Yılmaz E, Uzuner A, Icli D, Cabuk B, Anik I. Operative nuances and surgical limits of the endoscopic approach to clival chordomas and chondrosarcomas: A single-center experience of 72 patients. Clin Neurol Neurosurg. 2021;208:106875. doi: 10.1016/j.clineuro.2021.106875. [DOI] [PubMed] [Google Scholar]
- 5.Rahimli T, Hidayetov T, Rajabov T. Endoscopic Endonasal Approach to Multilobular Giant Pituitary Adenoma with Cavernous Sinus Invasion and Petroclival Extension. World Neurosurg. 2021;147:128–129. doi: 10.1016/j.wneu.2020.11.055. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Zhang X, Hu F, Yu Y, Gu Y, Xie T, Ge J. Image-Guided Endoscopic Endonasal Transmaxillary Transpterygoid Approach to Meckel's Cave. Turk Neurosurg. 2016;26:309–314. doi: 10.5137/1019-5149.JTN.6430-12.0. [DOI] [PubMed] [Google Scholar]
- 7.Liu JK, Hattar E, Eloy JA. Endoscopic Endonasal Approach for Olfactory Groove Meningiomas: Operative Technique and Nuances. Neurosurg Clin N Am. 2015;26:377–388. doi: 10.1016/j.nec.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Xuejian W, Fan H, Xiaobiao Z, Yong Y, Ye G, Tao X, Junqi G. Endonasal endoscopic skull base multilayer reconstruction surgery with nasal pedicled mucosal flap to manage high flow CSF leakage. Turk Neurosurg. 2013;23:439–445. doi: 10.5137/1019-5149.JTN.6176-12.0. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj J, Chandra PS. Recent Developments in Endoscopic Endonasal Approach for Pituitary Adenomas. Neurol India. 2020;68:S79–S84. doi: 10.4103/0028-3886.287671. [DOI] [PubMed] [Google Scholar]
- 10.Nie S, Li K, Huang Y, Zhao J, Gao X, Sun J. Endoscopic endonasal transsphenoidal surgery for treating pituitary adenoma via a sub-septum mucosa approach. Int J Clin Exp Med. 2015;8:5137–5143. [PMC free article] [PubMed] [Google Scholar]
- 11.Fong RP, Babu CS, Schwartz TH. Endoscopic endonasal approach for craniopharyngiomas. J Neurosurg Sci. 2021;65:133–139. doi: 10.23736/S0390-5616.21.05097-9. [DOI] [PubMed] [Google Scholar]
- 12.Leng LZ, Brown S, Anand VK, Schwartz TH. "Gasket-seal" watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62:ONSE342–3; discussion ONSE343. doi: 10.1227/01.neu.0000326017.84315.1f. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. 2013;80:563–568. doi: 10.1016/j.wneu.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D, Tao S, Zhang D, Qin M, Bao Y, Wu A. "Five-layer gasket seal" watertight closure for reconstruction of the skull base in complex bilateral traumatic intraorbital meningoencephaloceles: a case report and literature review. Brain Inj. 2018;32:804–807. doi: 10.1080/02699052.2018.1440631. [DOI] [PubMed] [Google Scholar]
- 15.Samadian M, Moghaddasi H, Vazirnezami M, Haddadian K, Rezaee O, Armanfar M, Khormaee F. Transcranial approach for spontaneous CSF rhinorrhea due to Sternberg's canal intrasphenoidal meningoencephalocele: case report and review of the literature. Turk Neurosurg. 2012;22:242–245. doi: 10.5137/1019-5149.JTN.2902-10.1. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Zhang X, Hu F, Yu Y, Gu Y, Xie T, Ge J. Middle Turbinate Mucosal Flap in Endoscopic Skull Base Reconstruction. Turk Neurosurg. 2016;26:200–204. doi: 10.5137/1019-5149.JTN.6250-12.0. [DOI] [PubMed] [Google Scholar]
- 17.Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, Esposito F, Carrau RL, Barkhoudarian G, Kelly DF. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2018;130:861–875. doi: 10.3171/2017.11.JNS172141. [DOI] [PubMed] [Google Scholar]
- 18.Munich SA, Fenstermaker RA, Fabiano AJ, Rigual NR. Cranial base repair with combined vascularized nasal septal flap and autologous tissue graft following expanded endonasal endoscopic neurosurgery. J Neurol Surg A Cent Eur Neurosurg. 2013;74:101–108. doi: 10.1055/s-0032-1330118. [DOI] [PubMed] [Google Scholar]
- 19.Strickland BA, Lucas J, Harris B, Kulubya E, Bakhsheshian J, Liu C, Wrobel B, Carmichael JD, Weiss M, Zada G. Identification and repair of intraoperative cerebrospinal fluid leaks in endonasal transsphenoidal pituitary surgery: surgical experience in a series of 1002 patients. J Neurosurg. 2018;129:425–429. doi: 10.3171/2017.4.JNS162451. [DOI] [PubMed] [Google Scholar]
- 20.Takahara K, Ueda R, Toda M. Endoscopic Endonasal Reconstruction Using a Pedicled Middle Turbinate Flap for Spontaneous Cerebrospinal Fluid Rhinorrhea. J Craniofac Surg. 2022;33:e318–e320. doi: 10.1097/SCS.0000000000008214. [DOI] [PubMed] [Google Scholar]
- 21.Oertel J, Gaab MR, Tschan CA, Linsler S. Mononostril endoscopic transsphenoidal approach to sellar and peri-sellar lesions: Personal experience and literature review. Br J Neurosurg. 2015;29:532–537. doi: 10.3109/02688697.2015.1014997. [DOI] [PubMed] [Google Scholar]
- 22.Solari D, Morace R, Cavallo LM, Amoroso F, Cennamo G, Del Basso DE Caro M, Cappabianca P. The endoscopic endonasal approach for the management of craniopharyngiomas. J Neurosurg Sci. 2016;60:454–462. [PubMed] [Google Scholar]
- 23.Gellner V, Tomazic PV. Limits of the endoscopic transnasal transtubercular approach. J Neurosurg Sci. 2018;62:297–300. doi: 10.23736/S0390-5616.18.04347-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang XJ, Wang ZF, Chen JR, Li F, Shi C, Chen H, Shang RF, Ni J. The removal of brainstem hemorrhages (BH) using the whole-course, endoscopic retrosigmoid approach. Int J Clin Exp Med. 2002;13:5506–5511. [Google Scholar]
- 25.Wang XJ, Li XD, Wang ZF. Clinical Study of Neuroendoscopic Treatment of Subacute Subdural Hematoma (SSH) Arch neurol neurosci. 2020;8 [Google Scholar]
- 26.Wang XJ, Yin YH, Wang ZF, Zhang Y, Sun C, Cui ZM. Efficacy evaluation of neuroendoscopy vs burr hole drainage in the treatment of chronic subdural hematoma: An observational study. World J Clin Cases. 2022;10:12920–12927. doi: 10.12998/wjcc.v10.i35.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutlay M, Durmaz O, Kırık A, Yaşar S, Özer İ, Ezgü MC, Kural C, Temiz Ç, Durmaz A, Daneyemez MK, Izci Y. Sellar Defect Reconstruction with Vascularized Superior Turbinate Mucosal Flaps in Endonasal Endoscopic Transsellar Approach. World Neurosurg. 2020;133:e503–e512. doi: 10.1016/j.wneu.2019.09.082. [DOI] [PubMed] [Google Scholar]
- 28.Kim GG, Hang AX, Mitchell CA, Zanation AM. Pedicled extranasal flaps in skull base reconstruction. Adv Otorhinolaryngol. 2013;74:71–80. doi: 10.1159/000342282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JK, Schmidt RF, Choudhry OJ, Shukla PA, Eloy JA. Surgical nuances for nasoseptal flap reconstruction of cranial base defects with high-flow cerebrospinal fluid leaks after endoscopic skull base surgery. Neurosurg Focus. 2012;32:E7. doi: 10.3171/2012.5.FOCUS1255. [DOI] [PubMed] [Google Scholar]
- 30.Baussart B, Racy E, Gaillard S. Double pedicled nasoseptal flap for skull base repair after endoscopic expanded endonasal approach. Acta Neurochir (Wien) 2022;164:1111–1114. doi: 10.1007/s00701-021-05094-6. [DOI] [PubMed] [Google Scholar]
- 31.Oyama K, Ditzel Filho LF, Muto J, de Souza DG, Gun R, Otto BA, Carrau RL, Prevedello DM. Endoscopic endonasal cranial base surgery simulation using an artificial cranial base model created by selective laser sintering. Neurosurg Rev. 2015;38:171–178. doi: 10.1007/s10143-014-0580-4. [DOI] [PubMed] [Google Scholar]
- 32.Mori K, Yamamoto T, Nakao Y, Esaki T. Development of artificial cranial base model with soft tissues for practical education: technical note. Neurosurgery. 2010;66:339–341. doi: 10.1227/01.neu.0000369664.24998.b6. [DOI] [PubMed] [Google Scholar]
- 33.Thorp BD, Sreenath SB, Ebert CS, Zanation AM. Endoscopic skull base reconstruction: a review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak. Neurosurg Focus. 2014;37:E4. doi: 10.3171/2014.7.FOCUS14350. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Hei Y, Soto JM, Jin T, Jiang X, Feng D, Liu W, Gao D. Clinical Efficacy of the Multilayered Skull Base Reconstruction Using In Situ Bone Flap in Endoscopic Endonasal Approach for Craniopharyngioma. J Neurol Surg B Skull Base. 2022;83:e291–e297. doi: 10.1055/s-0041-1726128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin B, Wang XS, Huo G, Mou JM, Yang G. Reconstruction of skull base bone defects using an in situ bone flap after endoscopic endonasal transplanum-transtuberculum approaches. Eur Arch Otorhinolaryngol. 2020;277:2071–2080. doi: 10.1007/s00405-020-05911-1. [DOI] [PubMed] [Google Scholar]
- 36.Guinto G, Guinto Y. Reconstruction techniques in skull base surgery. World Neurosurg. 2015;83:17–18. doi: 10.1016/j.wneu.2013.01.077. [DOI] [PubMed] [Google Scholar]
- 37.Gagliardi F, Boari N, Mortini P. Reconstruction techniques in skull base surgery. J Craniofac Surg. 2011;22:1015–1020. doi: 10.1097/SCS.0b013e31821015b5. [DOI] [PubMed] [Google Scholar]
- 38.Yang YC, Shen Y, Wang XD, Jiang Y, Qiu QH, Li J, Yu SQ, Ke X, Liu F, Xu YT, Lou HF, Wang HT, Yu GD, Xu R, Meng J, Meng CD, Sun N, Chen JJ, Zeng M, Xie ZH, Sun YQ, Tang J, Zhao KQ, Zhang WT, Shi ZH, Xu CL, Yang YL, Lu MP, Ye HP, Wei X, Sun B, An YF, Sun YN, Gu YR, Zhang TH, Ba L, Yang QT, Ye J, Xu Y, Li HB Chinese Rhinology Research Collaborative Group. [Expert consensus on the prevention and treatment of adverse reactions in subcutaneous immunotherapy(2023, Chongqing)] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2023;58:643–656. doi: 10.3760/cma.j.cn115330-20221111-00679. [DOI] [PubMed] [Google Scholar]
- 39.Cappabianca P, Di Somma A, de Notaris M. Rerum magistra experientia est: the evolution of modern endoscopic endonasal skull base surgery and reconstruction techniques. World Neurosurg. 2014;82:e67–e69. doi: 10.1016/j.wneu.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 40.Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122:452–459. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]
- 41.Gabriele M, Antonio G, Nicole C, Angelo M, Daniele M. Single Versus Double Hadad-Bassagasteguy Flap in Expanded Endoscopic Skull-Base Surgery. Indian J Otolaryngol Head Neck Surg. 2022;74:394–401. doi: 10.1007/s12070-021-02961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah RN, Surowitz JB, Patel MR, Huang BY, Snyderman CH, Carrau RL, Kassam AB, Germanwala AV, Zanation AM. Endoscopic pedicled nasoseptal flap reconstruction for pediatric skull base defects. Laryngoscope. 2009;119:1067–1075. doi: 10.1002/lary.20216. [DOI] [PubMed] [Google Scholar]
- 43.Kong DS, Kim HY, Kim SH, Min JY, Nam DH, Park K, Dhong HJ, Kim JH. Challenging reconstructive techniques for skull base defect following endoscopic endonasal approaches. Acta Neurochir (Wien) 2011;153:807–813. doi: 10.1007/s00701-011-0941-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data related to the article can be obtained from the corresponding author via email: 6841441@163.com.