Abstract
BACKGROUND
Digital subtraction angiography (DSA), the gold standard of cerebrovascular disease diagnosis, is limited in its diagnostic ability to evaluate arterial diameter. Intravascular ultrasonography (IVUS) has advantages in assessing stenosis and plaque nature and improves the evaluation and effectiveness of carotid artery stenting (CAS).
CASE SUMMARY
Case 1: A 65-year-old man presented with a five-year history of bilateral lower limb weakness due to stroke. Physical examination showed decreased strength (5-/5) in both lower limbs. Carotid artery ultrasound, magnetic resonance angiography, and computed tomography angiography (CTA) showed a right proximal internal carotid artery (ICA) stenosis (70%-99%), acute cerebral infarction, and severe right ICA stenosis, respectively. We performed IVUS-assisted CAS to measure the stenosis and detected a low-risk plaque at the site of stenosis prior to stent implantation. Post-stent balloon dilatation was performed and postoperative IVUS demonstrated successful expansion and adherence. CTA six months postoperatively showed no significant increase in in-stent stenosis. Case 2: A 36-year-old man was admitted with a right common carotid artery (CCA) dissection detected by ultrasound. Physical examination showed no positive neurological signs. Carotid ultrasound and CTA showed lumen dilation in the proximal CCA with an intima-like structure and bulging in the proximal segment of the right CCA with strip-like low-density shadow (dissection or carotid web). IVUS-assisted DSA confirmed right CCA dissection. CAS was performed and intraoperative IVUS suggested a large residual false lumen. Post-stent balloon dilatation was performed reducing the false lumen. DSA three months postoperatively indicated good stent expansion with mild stenosis.
CONCLUSION
IVUS aids decision-making during CAS by accurately assessing carotid artery wall lesions and plaque nature preoperatively, dissection and stenosis morphology intraoperatively, and visualizing and confirming CAS postoperatively.
Keywords: Intravascular ultrasonography, Carotid artery stenting, Carotid stenosis, Arteriosclerotic stenosis, Carotid artery dissection, Case report
Core Tip: Intravascular ultrasonography (IVUS) improves carotid artery stenting (CAS) evaluations and outcomes vs those of the traditional gold-standard, digital subtraction angiography (DSA). We present two cases of cerebrovascular diseases. Case 1 involves a 65-year-old patient with stroke. IVUS-assisted CAS was performed after right proximal internal carotid artery stenosis was diagnosed via DSA. Preoperative IVUS imaging showed a low-risk plaque and accurately measured the stenosis. Postoperative IVUS confirmed good stent expansion and adherence, and the patient had no discomfort at 4-mo follow-up. Case 2 involves a 36-year-old patient with common carotid artery dissection, confirmed via IVUS-assisted DSA, and CAS was performed. Intraoperative and postoperative IVUS imaging showed the size of the true and false lumen, assisted in treatment decision-making, and confirmed CAS outcomes. This paper highlights the crucial role of IVUS in decision-making during CAS. IVUS can facilitate the accurate assessment of carotid artery wall lesions and plaque nature preoperatively, measure stenosis and true and false lumen size intraoperatively, and confirm CAS effects postoperatively.
INTRODUCTION
Intravascular ultrasonography (IVUS) is a novel method for diagnosing vascular diseases. It involves placing a miniaturized ultrasound transducer into the lumen of a vessel using catheter technology to produce a cross-sectional image of blood vessel morphology[1].
In recent years, neurologists have shown increasing interest in utilizing the advantages of IVUS for the treatment of cerebrovascular diseases. Further, by advancing research and development, the widespread application of IVUS has become increasingly urgent owing to the high incidence and disability rate of stroke[2,3]. IVUS not only provides high-resolution images of vessel walls and lumens but it can also distinguish various arterial plaque components and irregular structures in vessel walls based on differences in echo intensity[4]. IVUS has become a powerful tool for diagnosing and treating endovascular lesions (such as arterial dissection and arterial stenosis), specifically for preoperative evaluation and postoperative optimization in carotid artery stenting (CAS)[5-7]. IVUS is used to preoperatively evaluate the characteristics of carotid plaques and measure the length and diameter of diseased blood vessels to select the appropriate stent[4,8]. Postoperative examinations of plaque protrusions using IVUS have been shown to be more accurate than those of digital subtraction angiography (DSA)[9]. IVUS is used postoperatively for several reasons, including: (1) To determine whether or not the stent fully expands and tightly adheres to the wall; (2) To observe complications (e.g., plaque protrusion or fragmentation)[10]; (3) To quickly manage issues to optimize postoperative results; and (4) To reduce the risk of stroke[11,12]. We present two patients who underwent IVUS-assisted CAS followed by a literature review to improve the management of CAS.
CASE PRESENTATION
Chief complaints
Case 1: The patient, a 65-year-old male, presented with prolonged bilateral weakness in the lower limbs for five years due to sequelae of a stroke that occurred in July 2022.
Case 2: In July 2022, a 36-year-old male patient was admitted due to a right common carotid artery (CCA) dissection detected by a carotid ultrasound one day previously, which also revealed an occlusion in the left CCA.
History of present illness
Case 1: Computed tomography (CT) angiography (CTA) performed 10 d prior to hospital admission showed severe right posterior cerebral artery stenosis and possible basilar artery occlusion. CT perfusion (CTP) imaging showed decreased blood perfusion and blood volume in the right anterior and middle cerebral artery territory.
Case 2: The patient felt no discomfort and was not treated with medication at that time.
History of past illness
Case 1: The patient had a history of hypertension, diabetes mellitus, and smoking for more than 10 years, and had undergone percutaneous coronary intervention two years previously for coronary heart disease.
Case 2: The patient had no previous history of chronic diseases, smoking, or drinking.
Personal and family history
Two patients’ personal and family histories were unremarkable.
Physical examination
Case 1: Physical examination at admission showed decreased muscle strength in the lower extremities (5-/5) but no other abnormal neurological signs.
Case 2: Physical examination at admission showed no positive neurological signs.
Laboratory examinations
Case 1: Laboratory analyses reported that syphilis and human immunodeficiency virus antibodies were negative, serum myoglobin, creatine phosphokinase, troponin I, B-type natriuretic peptide, and D-dimer levels were within normal limits, and thyroid and coagulation function indices showed no abnormalities. Abnormal laboratory findings are presented in Table 1.
Table 1.
Abnormal laboratory findings reported in cases 1 and 2
|
Parameter
|
Result
|
Reference range
|
Interpretation
|
| Patient 1 | |||
| White blood cell count, × 109/L | 10.53 | 3.5-9.5 | Increased |
| Hemoglobin, g/L | 125.0 | 130-170 | Decreased |
| Urine glucose | 2 + | Negative | Increased |
| Urine protein | 1 + | Negative | Increased |
| C-reactive protein, mg/L | 26.12 | 0-5 | Increased |
| Total protein, g/L | 63.7 | 65.0-85.0 | Decreased |
| Serum glycocholic acid, mg/L | 3.74 | 0.00-2.70 | Increased |
| Serum creatinine, mmol/l | 126.1 | 57-111 | Decreased |
| Urea, mmol/L | 9.8 | 3.6-9.5 | Increased |
| Anticardiolipin antibody IgG, gplu/mL | 20.60 | < 12 | Increased |
| Anticardiolipin antibody IgA, aplu/mL | 26.40 | < 12 | Increased |
| Patient 2 | |||
| Urine protein | Weakly positive | Negative | Increased |
| Red blood cells in urine | 48.40/μL | 0-18/μL | Increased |
| Fecal occult blood test | Weakly positive | Negative | Increased |
| Interleukin-6 at 3-mo follow-up, pg/mL | 13.56 | < 6.6 | Increased |
IgG: Immunoglobulin G; IgA: Immunoglobulin A.
Case 2: Laboratory analysis showed normal levels of serum electrolytes, cholesterol, myoglobin, creatine phosphokinase, troponin I, B-type natriuretic peptide, glycosylated hemoglobin, D-dimer, antistreptolysin O, rheumatoid factor, C-reactive protein, anti-cyclic citrullinated peptide, anti-nuclear, extractable nuclear antigen, anti-ds-DNA, and antineutrophil cytoplasmic and anticardiolipin antibodies. Red and white blood cell counts and the erythrocyte sedimentation rate were within normal limits. Serum liver, kidney, thyroid, and coagulation function indices showed no abnormalities.
Imaging examinations
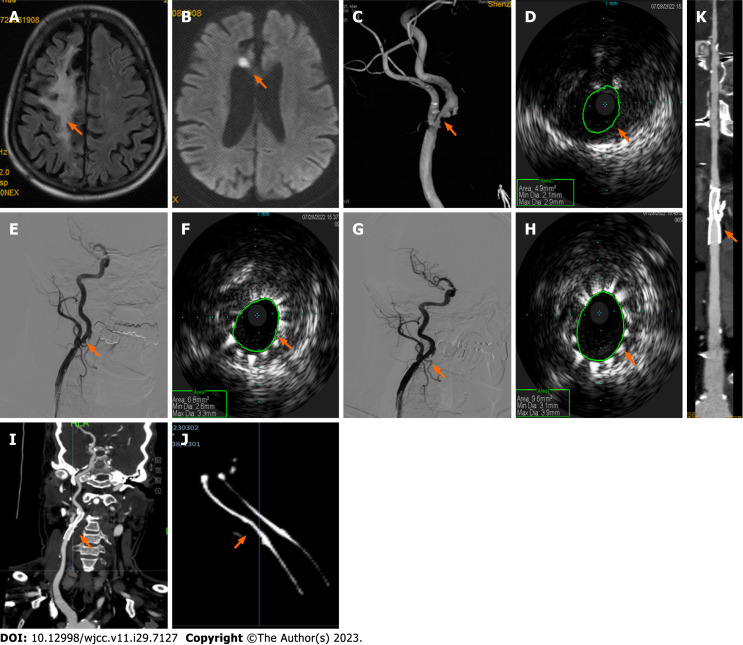
Case 1: Carotid artery ultrasound showed bilateral carotid intima-media thickening, plaque formation, and right proximal internal carotid artery (ICA) stenosis (70%-99%). Brain magnetic resonance imaging (MRI) revealed a paraventricular white matter lesion in the right frontal lobe (Figure 1A) and a small acute cerebral infarction at the genu of the corpus callosum on diffusion-weighted imaging (Figure 1B). Head and neck CTA showed severe stenosis of the initial segment of the right ICA and right posterior cerebral artery, and CTP imaging reported decreased blood perfusion in the right anterior and middle cerebral artery territory. DSA revealed: (1) The persistent trigeminal artery compensated for the basilar artery supply; (2) Bilateral fetal-type posterior cerebral artery; (3) 70% stenosis of the initial segment of the right ICA (Figure 1C); (4) 80% stenosis of the local P2 segment of the right posterior cerebral artery; and (5) Mild stenosis of the CCA and initial segment of the external carotid arteries.
Figure 1.
Neuroimaging results of patient one during intravascular ultrasound-assisted carotid artery stenting. A and B: Brain magnetic resonance imaging demonstrates a paraventricular white-matter lesion in the right frontal lobe (A, arrow) on fluid-attenuated inversion recovery imaging, and a small acute infarction at the genu of the corpus callosum on diffusion weighted imaging (B, arrow); C: Angiography shows 70% stenosis of the initial segment of the right internal carotid artery (ICA) (arrow); D and E: Preoperative intravascular ultrasound (IVUS) displays severe stenosis with plaque formation and obvious calcification under plaque (D, arrow) and a well-positioned stent (E, arrow); F-H: Subsequent IVUS imaging confirmed improvement of the narrowest part (F, arrow), thus post-stent balloon dilatation was performed. Postoperative angiography shows a right ICA residual stenosis of 20% (G, arrow), and IVUS confirmed good stent expansion and adherence (H, arrow); I-K: Computed tomography angiography six months postoperatively revealed mild in-stent stenosis in the right initial segment of the ICA (I, multiplanar reconstruction; J, amplification imaging with adjusting parameters; K, curve planar reformation; arrows).
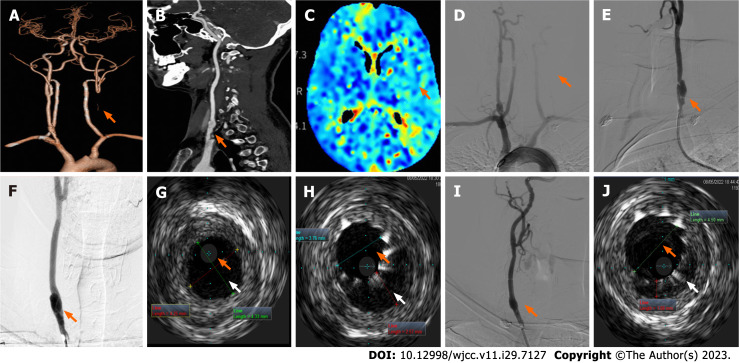
Case 2: Electrocardiogram, ambulatory blood pressure monitoring, echocardiogram, and ultrasound of the renal arteries and abdominal aorta results were unremarkable. Arterial ultrasounds of the lower extremities showed inhomogeneous thickening of the intima in artery segments on both sides and stenosis of the right common femoral artery and left superficial femoral artery (nearly occluded). Chest CT imaging reported scattered, patchy high-density lesions in both lungs (pneumonia was considered). Carotid ultrasound revealed a left CCA occlusion, right carotid artery stenosis, and local lumen dilation in the proximal CCA with an intima-like structure. Brain MRI showed no obvious abnormalities. Head and neck CTA showed left CCA occlusion (Figure 2A) and local bulging in the inferior segment of the right CCA with a strip-shaped low-density shadow (dissection or carotid web were considered, Figure 2B). Brain CTP images (Figure 2C) revealed that blood flow perfusion in the left frontal lobe, parietal lobe, right temporal lobe, and insula was lower than that on the contralateral side. Aortic arch angiography revealed an initial segment of the left CCA occlusion (Figure 2D). Angiography of the right CCA reported an initial segment of dissection (Figures 2E and F).
Figure 2.
Neuroimaging results of patient two during intravascular ultrasound-assisted carotid artery stenting. A and B: Head and neck computed tomography angiography showing left common carotid artery (CCA) occlusion (A, arrow) and local bulging in the inferior segment of the right CCA with strip-shaped, low-density shadow (B, arrow); C: Brain computed tomography perfusion images showed that blood flow perfusion in the left frontal lobe, parietal lobe, right temporal lobe, and insula was lower than that on the opposite side (arrow); D-F: Digital subtraction angiography shows the initial segment of the left CCA occlusion (D, arrow) and the initial segment of the right CCA dissection (posteroanterior film, E; left oblique film, F); G: Preoperative intravascular ultrasonography (IVUS) probe check dissection; H: IVUS images after stent placement show insufficient true lumen (orange arrow) with visible intimal flaps, and a relatively large pseudo-lumen (white arrow) with a large intermural hematoma; I: Angiography after post-balloon dilatation showed a smooth intra-arterial lumen; J: Postoperative IVUS images showed that the true lumen enlarged (orange arrow), the pseudo-lumen diminished (white arrow), and confirmed satisfactory stent expansion and adherence.
FINAL DIAGNOSIS
Case 1: Based on the patient’s medical history and laboratory and imaging findings, he was given a final diagnosis of stenosis of the right ICA, right posterior cerebral artery, CCA, and external carotid arteries.
Case 2: Combined with the patient’s medical history, the final diagnosis was CCA occlusion of the left and right initial segment.
TREATMENT
Case 1: IVUS-assisted CAS utilizing an Eagle Eye Platinum IVUS diagnostic instrument (Volcano Corporation, United States) with a phase array probe (external diameter: 3.5 F; 18.6 MHz) was performed under local anesthesia on day six of hospitalization. First, we performed arteriography of the diseased carotid artery. A 6 F long sheath head end was placed in the right distal CCA, and the road map was redesigned. The end of the catheter reached the C2 segment of the right ICA and a distal protection device was deployed. Next, an IVUS imaging catheter was placed and the road map guided the catheter through the site of the right ICA stenosis. Finally, IVUS imaging confirmed severe right ICA stenosis with plaque formation and noticeable calcification (Figure 1D).
We inserted a 5 mm × 30 mm balloon through the narrowest part of the right ICA and ensured correct positioning. Subsequent angiography demonstrated improved right ICA stenosis. A 7 mm × 40 nm WallstentTM stent (Boston Scientific Corporation) was then positioned (Figure 1E). Subsequent IVUS confirmed unsatisfactory improvement of the narrowest part of the right ICA (Figure 1F). Therefore, post-stent balloon dilatation was performed.
Case 2: A long sheath was placed in the right distal CCA and a distal protection device was deployed. The IVUS probe was subsequently placed to check the dissection (Figure 2G), and a WallstentTM stent (Boston Scientific Corporation) was deployed at the site of dissection. IVUS images obtained immediately after stent placement showed insufficient dilation of the true lumen with a large intermural hematoma (Figure 2H) and post-balloon dilatation was performed.
OUTCOME AND FOLLOW-UP
Case 1: Postoperative angiography revealed the right ICA stenosis had noticeably improved (residual stenosis, 20%; Figure 1G), and IVUS confirmed good stent expansion and adherence (Figure 1H) without signs of plaque protrusions. CTA at six months postoperatively revealed mild in-stent stenosis in the right initial segment of ICA, with no discomfort reported by the patient (Figures 1I-K). The patient was satisfied with the treatment he received and his recovery.
Case 2: Final angiography showed a smooth intra-arterial lumen (Figure 2I), and postoperative IVUS images showed the true lumen increased in size with good stent expansion and adherence (Figure 2J). The patient experienced no postoperative discomfort, and head CT imaging on postoperative day three showed no definite lesions. DSA three months postoperatively indicated good stent adhesion in the right CCA with mild stenosis (approximately 25%) in the proximal stent. The patient was satisfied with the treatment he received and his recovery.
DISCUSSION
These cases demonstrate that IVUS can accurately determine the cross-sectional area of the narrowest point in the carotid artery and assess the area of the false lumen in arterial dissections, as well as plaque properties. This allows the operator to use IVUS to evaluate the impact of stent placement during the procedure. Specifically, IVUS can effectively detect unsatisfactory stent expansion and improve arterial stenosis by using post-balloon dilatation. The operator can effectively detect the postoperative residual false lumen using IVUS and accurately guide post-balloon dilatation. These advantageous characteristics of IVUS may help to improve patient prognosis.
IVUS enables a more accurate determination of stent size, expansion, and fit[13]. It also provides real-time intraluminal imaging, which is unavailable with DSA, making it a useful tool for endovascular treatment and pre- or postoperative assessments[14]. IVUS can also provide diagnostic information, specific intraluminal and transmural vessel data with high accuracy, aid the selection of appropriate angioplasty and intravascular devices, and assess intervention effects[12].
The role of IVUS in preoperative CAS
Using IVUS, we can precisely measure the cross-sectional area of the narrowest segment of the artery and false lumen and clarify the nature of the endovascular lesions. IVUS accurately reveals the cross-sectional morphology and changes after balloon dilatation[15]. IVUS plays an important role in preoperative balloon angioplasty and CAS, especially when managing challenging plaques, such as lipidic or fragile plaques[8], and in identifying the nature of stenotic lesions, such as dissection or carotid web. Furthermore, IVUS can be used to calculate the stenosis ratio (by measuring the minimum lumen area and distal reference lumen area), help select the stent diameter and landing zone, assess stent adhesion and expansion, and clarify whether the lesion can be completely covered by the stent. IVUS during CAS provides a more detailed assessment of events, such as atherosclerotic plaque protrusion through the stent cells[16]. Expert consensus provides standards for IVUS acquisition as well as for measuring and reporting boundary, lumen, atheromatous plaque, calcium deposit, reference segment, and lesion length measurements[17]. We measured the minimum and reference lumen area using IVUS and calculated the rate of stenosis to help select the appropriate stent. Identifying carotid artery dissections using IVUS for CAS, determining the size and length of lesions, and guiding the positioning and placement of stents, helps inform operators to make better decisions and improve patency rates[8,18]. In cases where other imaging methods indicate significant variations in the severity or nature of vasculopathy, using IVUS can support the treatment decision-making process[4].
The role of IVUS during CAS
In the second case presented (case 2), intermural hematoma within the dissection was detected by IVUS after stent implantation, followed by post-balloon dilatation. Finally, IVUS indicated the stent was well placed and attached to the wall, noticeably reducing the false lumen. IVUS has unique advantages for the treatment of aortic dissection. IVUS-assisted CAS may be an effective treatment option to prevent intraoperative complications and further stroke recurrence for isolated spontaneous CCA dissection[19]. IVUS compensates for the insufficiency in the assessment of atherosclerotic plaque composition and intravascular arterial morphology, which are difficult to evaluate using DSA[9]. IVUS can also provide information on stent placement to facilitate the successful completion of surgery.
The role of IVUS in postoperative CAS
Postoperatively, IVUS helps to visualize stent placement, adherence, expansion, and complications such as stent edge dissection and determines whether the stent fully covers the lesion to optimize immediate results[20]. In this study, it was demonstrated that IVUS can be utilized to assess stent placement and perform balloon post-dilation to improve vascular stenosis.
Security of IVUS
IVUS is safe to perform during CAS and may not require mechanical protection for small vascular foci, as high-risk lesions can be safely assessed using IVUS under flow blockade[21]. In-stent protrusions (ISP) may occur following CAS, which increases the risk of postoperative embolization. ISP may be related to vulnerable plaques and fragments, and IVUS contributes to the assessment of stent protrusion during CAS[8]. IVUS performs better than DSA in detecting ISP, which is imperative to ensure the proper treatment of ISP which can reduce stroke complications[22].
Development tendency of IVUS
A new type of virtual histology IVUS (VH-IVUS) was recently developed based on grayscale IVUS to detect atherosclerosis. VH-IVUS can identify various tissues with high accuracy, making it useful in identifying high-risk plaques[23]. As manual border detection is time-consuming, VH-IVUS integrates automatic border detection technology and can be used as a diagnostic tool for evaluating the suitability of carotid artery stenosis for CAS. As surgeons become more skilled in operating IVUS, utilizing it to assist stent placement and confirm post-stenting will help to ensure precise positioning and improve patency rates[18]. Continuous improvement has been made to the frequency, bandwidth, and resolution of IVUS probes[24]. The future development trend of IVUS is to innovate materials and improve manufacturing processes and equipment performance. IVUS transducers are miniaturized to meet the imaging requirements for smaller blood vessels[25]. Despite its high value in diagnosis and adjuvant treatment, IVUS is not recommended as a routine intraprocedural evaluation tool, as it is invasive and expensive. Some experts believe the use of IVUS in predicting microembolization remains limited[7].
CONCLUSION
IVUS was utilized preoperatively to evaluate the nature of plaques, measure lesions, reference vessels, and develop a preconditioning strategy. Intraoperative use of IVUS has incomparable advantages over other detection techniques in the identification of artery dissection and assessment of postoperative stent placement and expansion, which may improve patient prognosis. With the continued development of IVUS technology, and as doctors become more familiar with the application and operation of the technology, IVUS is expected to be widely used in interventional diagnosis and treatment.
ACKNOWLEDGEMENTS
We would like to thank the “Double-First Class” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science) for the support.
Footnotes
Informed consent statement: The patient has provided informed consent for publication of the case.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 8, 2023
First decision: August 31, 2023
Article in press: September 25, 2023
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chow WK, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Peng-Cheng Fu, Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen 518000, Guangdong Province, China.
Jing-Yi Wang, Faculty of Chinese Medicine, Macau University of Science and Technology, Macau 999078, China.
Ying Su, Graduate School, Guangzhou Medical University, Guangzhou 511495, Guangdong Province, China.
Yu-Qi Liao, School of Medicine, Shenzhen University, Shenzhen 518000, Guangdong Province, China.
Shao-Ling Li, School of Medicine, Shenzhen University, Shenzhen 518000, Guangdong Province, China.
Ge-Lin Xu, Department of Neurology, The First Affiliated Hospital of Shenzhen University, Shenzhen 518000, Guangdong Province, China.
Yan-Jiao Huang, Medical Department, Baise People’s Hospital, Baise 533000, Guangxi Zhuang Autonomous Region, China.
Ming-Hua Hu, Hunan Provincial Key Laboratory of the Research and Development of Novel Pharmaceutical Preparations, Changsha Medical University, Changsha 410219, Hunan Province, China.
Li-Ming Cao, Clinical College of the Shenzhen Second People’s Hospital, Anhui Medical University, Shenzhen 518000, Guangdong Province, China. caolm-2007@163.com.
References
- 1.Hassani S, Nogueira RG, Al-Bayati AR, Sachdeva R, McDaniel M, Haussen DC. Intravascular Ultrasound in Carotid Web. J Neurointerv Surg. 2020;12:531–534. doi: 10.1136/neurintsurg-2019-015387. [DOI] [PubMed] [Google Scholar]
- 2.Escolar E, Weigold G, Fuisz A, Weissman NJ. New imaging techniques for diagnosing coronary artery disease. CMAJ. 2006;174:487–495. doi: 10.1503/cmaj.050925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zacharatos H, Hassan AE, Qureshi AI. Intravascular ultrasound: principles and cerebrovascular applications. AJNR Am J Neuroradiol. 2010;31:586–597. doi: 10.3174/ajnr.A1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X. [A preliminary study of intravascular ultrasound in carotid arterial stenting] Chin J Neuromed. 2017:16. [Google Scholar]
- 5.Li S, Wan J, Ge J, Dai J, He B, Wang B, Han Z. [Application of intravascular ultrasound in intravascular stenting for carotid artery stenosis] Acta Universitatis Medicinalis Secondae Shanghai. 2004:559–561. [Google Scholar]
- 6.Joan MM, Moya BG, Agustí FP, Vidal RG, Arjona YA, Alija MP, Paredero VM. Utility of intravascular ultrasound examination during carotid stenting. Ann Vasc Surg. 2009;23:606–611. doi: 10.1016/j.avsg.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Hitchner E, Zayed MA, Lee G, Morrison D, Lane B, Zhou W. Intravascular ultrasound as a clinical adjunct for carotid plaque characterization. J Vasc Surg. 2014;59:774–780. doi: 10.1016/j.jvs.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiocchi M, Morosetti D, Chiaravalloti A, Loreni G, Gandini R, Simonetti G. Intravascular ultrasound assisted carotid artery stenting: randomized controlled trial. Preliminary results on 60 patients. J Cardiovasc Med (Hagerstown) 2019;20:248–252. doi: 10.2459/JCM.0b013e32835898f1. [DOI] [PubMed] [Google Scholar]
- 9.Shinozaki N, Ogata N, Ikari Y. Plaque protrusion detected by intravascular ultrasound during carotid artery stenting. J Stroke Cerebrovasc Dis. 2014;23:2622–2625. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Morr S, Vakharia K, Fanous AA, Waqas M, Siddiqui AH. Utility of Intravascular Ultrasound During Carotid Angioplasty and Stenting with Proximal Protection. Cureus. 2019;11:e4935. doi: 10.7759/cureus.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wehman JC, Holmes DR Jr, Ecker RD, Sauvageau E, Fahrbach J, Hanel RA, Hopkins LN. Intravascular ultrasound identification of intraluminal embolic plaque material during carotid angioplasty with stenting. Catheter Cardiovasc Interv. 2006;68:853–857. doi: 10.1002/ccd.20871. [DOI] [PubMed] [Google Scholar]
- 12.Kan P, Binning MJ, Siddiqui AH. Intravascular ultrasound-guided thrombus retrieval with a multipurpose-angled catheter during carotid artery stenting. J Neuroimaging. 2012;22:394–399. doi: 10.1111/j.1552-6569.2011.00651.x. [DOI] [PubMed] [Google Scholar]
- 13.Clark DJ, Lessio S, O'Donoghue M, Schainfeld R, Rosenfield K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv. 2004;63:355–362. doi: 10.1002/ccd.20188. [DOI] [PubMed] [Google Scholar]
- 14.Hachinohe D, Mitomo S, Candilio L, Latib A. A Practical Approach to Assessing Stent Results with IVUS or OCT. Methodist Debakey Cardiovasc J. 2018;14:32–41. doi: 10.14797/mdcj-14-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobis JM, Mahon DJ, Moriuchi M, Honye J, McRae M. Intravascular ultrasound imaging following balloon angioplasty. Int J Card Imaging. 1991;6:191–205. doi: 10.1007/BF01797851. [DOI] [PubMed] [Google Scholar]
- 16.Volkov SV, Mytsyk SA, Naumov SM, Korobkov AO, Gontarenko VN. Intravascular ultrasound-guided internal carotid artery stenting. Angiol Sosud Khir. 2019;25:41–52. doi: 10.33529/ANGIO2019419. [DOI] [PubMed] [Google Scholar]
- 17.American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents developed in collaboration with the European Society of Cardiology endorsed by the Society of Cardiac Angiography and Interventions. Eur J Echocardiogr. 2001;2:299–313. [PubMed] [Google Scholar]
- 18.Hussain AS, Hussain NS. Intravascular Ultrasound for Intracranial and Extracranial Carotid Artery Stent Placement. Cureus. 2016;8:e732. doi: 10.7759/cureus.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanada T, Wada H, Sato H, Shirai W, Kinoshita M, Tokumitsu N. Carotid artery stenting assisted with intravascular ultrasonography for isolated spontaneous common carotid artery dissection. J Surg Case Rep. 2021;2021:rjab232. doi: 10.1093/jscr/rjab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Tsujita K, Maehara A, Mintz GS, Weisz G, Dangas GD, Lansky AJ, Kreps EM, Rabbani LE, Collins M, Stone GW, Moses JW, Mehran R, Leon MB. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc Interv. 2009;2:997–1004. doi: 10.1016/j.jcin.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Musialek P, Pieniazek P, Tracz W, Tekieli L, Przewlocki T, Kablak-Ziembicka A, Motyl R, Moczulski Z, Stepniewski J, Trystula M, Zajdel W, Roslawiecka A, Zmudka K, Podolec P. Safety of embolic protection device-assisted and unprotected intravascular ultrasound in evaluating carotid artery atherosclerotic lesions. Med Sci Monit. 2012;18:MT7–M18. doi: 10.12659/MSM.882452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okazaki T, Sakamoto S, Shinagawa K, Ichinose N, Ishii D, Matsushige T, Kiura Y, Kurisu K. Detection of in-stent protrusion (ISP) by intravascular ultrasound during carotid stenting: Usefulness of stent-in-stent placement for ISP. Eur Radiol. 2019;29:77–84. doi: 10.1007/s00330-018-5636-3. [DOI] [PubMed] [Google Scholar]
- 23.Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, Murata A, Takeda Y, Ito T, Ehara M, Matsubara T, Terashima M, Suzuki T. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 24.Peng J, Qin Z, Diao X, Jin C, Wang T, Chen S. [Progress in the research on key technologies for medical ultrasound, part one: ultrasonic transducer and ultrasonic coded excitation] Shanghai of Biomedical Engineering. 2013;34:21–27. [Google Scholar]
- 25.Dong QL. [Current status and research progress of intravascular ultrasound probes] Cardiovascular Dis Elect J Integrated Tradit Chin Western Med. 2016;4 [Google Scholar]