Abstract
Background:
We evaluate the impact of post-operative 18-fluorodeoxyglucose positron emission tomography with computed tomography (PET/CT) for radiation planning on the detection of early recurrence (ER) and treatment outcomes in oral squamous cell carcinoma (OSCC).
Methods:
We retrospectively reviewed the records of patients treated with post-operative radiation between 2005 and 2019 for OSCC at our institution. Extracapsular extension and positive surgical margins were classified as high risk features; pT3–4, node positivity, lymphovascular invasion, perineural invasion, tumor thickness > 5mm, and close surgical margins were considered intermediate risk features. Patients with ER were identified. Inverse probability of treatment weighting (IPTW) was used to adjust for imbalances between baseline characteristics.
Results:
391 patients with OSCC were treated with post-operative radiation. 237 (60.6%) patients underwent post-operative PET/CT planning vs. 154 (39.4%) who were planned with CT only. Patients screened with post-operative PET/CT were more likely to be diagnosed with ER than those planned with CT only (16.5 vs. 3.3%, p<0.0001). Among patients with ER, those with intermediate features were more likely than those high risk features to undergo major treatment intensification, including re-operation, the addition of chemotherapy, or intensification of radiation by ≥10 Gy (91% vs. 9%, p<0.0001). Post-operative PET/CT was associated with improved disease-free and overall survival for patients with intermediate risk features (IPTW log-rank p=0.026 and p=0.047, respectively) but not high risk features (IPTW log-rank p=0.44 and p=0.96).
Conclusions:
Use of post-operative PET/CT is associated with increased detection of early recurrence. Among patients with intermediate risk features, this may translate to improved disease-free survival.
Introduction
The standard of care for surgically resected OSCC with intermediate or high-risk features is adjuvant radiotherapy or chemoradiotherapy.(1–4) For these patients, locoregional recurrence remains the most common mode of recurrence,(5) and treatment outcomes after salvage therapy are poor.(6–9)
Prior reports(7,10–13) have shown that a proportion of patients with OSCC will develop an early recurrence (ER) before post-operative radiation can start. Currently, the use of advanced imaging for PORT planning is inconsistent, and these gross recurrences can go undetected, translating into suboptimal treatment.
Detection of early recurrence affords an opportunity to modify treatment, including re-operation, increasing radiation dose, or adding chemotherapy.(7,10) In cases where the disease is incurable, patients can be transitioned to palliative intent therapy.(7)
In this paper, we report our experience employing post-operative positron emission tomography / computed tomography (PET/CT) for the detection of ER in a cohort of patients with surgically resected OSCC who were referred for post-operative radiotherapy (PORT).
Methods
This study was approved by the institutional review board (IRB# 16–1648). We reviewed the records of all patients with newly diagnosed OSCC treated with primary surgery and referred for adjuvant radiotherapy or chemoradiotherapy at our institution from 2005 to 2019. A minority (14.6%) of patients had surgery elsewhere and were treated at our institution for PORT. Patients were excluded if they received PORT at a different institution, if the index surgery was for recurrent disease, or if they had prior head and neck radiotherapy. Patients (n=7) found to have synchronous second primary cancers were included in the analysis of predictors of ER but were excluded from the survival analysis.
Post-Operative Risk Stratification
Patient characteristics, treatment details, clinicopathologic variables, and outcomes were recorded, including the dates of initial tissue diagnosis, surgical resection, simulation computed tomography scan (CT), post-operative PET/CT, as well as dates of local, regional, and distant recurrence. Tumor stage was based on the American Joint Committee on Cancer (AJCC) 7th edition staging manual. pT3–4 disease, two or more involved nodes, perineural invasion (PNI), lymphovascular invasion (LVI), close (≤ 5mm) surgical margins (SM), and tumor thickness > 5mm were considered intermediate risk factors (IRFs); extracapsular extension (ECE) and positive SM were considered high-risk factors (HRF). Patients with IRFs but not HRFs were considered to have intermediate risk disease. Patients with HRFs were considered to have high risk disease.
Post-operative Evaluation and Radiation Planning
Our institutional strategy for resected OSCC during this study period was to offer PORT or chemoradiation depending on clinicopathologic risk features. Early in the study period, patients underwent contrast-enhanced CT radiation planning without additional diagnostic imaging. After the introduction of the PET/CT simulator, PET/CTs were increasingly ordered as part of routine radiation planning. Adoption increased from <5% of cases before 2009 to >80% of cases after 2015. Our current standard practice is to obtain PET/CTs approximately four weeks after surgery for all patients referred for PORT.
Low-risk and intermediate-risk volumes were treated to 50–56 Gy, and 60 Gy in 1.8 – 2 Gy daily fractions, respectively. Areas of ECE or positive SM were treated to 66 Gy at the discretion of the treating radiation oncologist. Concurrent chemotherapy was recommended for fit patients who had HRFs, or in some cases, patients with multiple IRFs. Radiation doses exceeding 66 Gy were reserved for patients deemed to have ER with gross disease on physical exam or imaging.
The details of radiation planning studies, subsequent investigations, and patient disposition were recorded. Patients were considered to have ER if 1) confirmed by cytology or histology; 2) they were determined to have ER by multidisciplinary consensus based on imaging or physical exam findings, resulting in a change in management.
Follow-up
Patients were routinely seen every 3–4 months for the first two years and every six months between years two through five. A post-treatment PET/CT was typically performed three months after completion of PORT. Subsequent imaging was performed at the discretion of the treating physicians.
Statistics
Propensity Scores for the use of PET/CT and Inverse Probability of Treatment (IPT) Weights
IPTW was used for both logistic regression and cox proportional hazards analyses. To estimate IPTW weights, we developed a logistic regression model for allocation to post-operative PET/CT, including age, sex, IRFs, HRFs, days from surgery to simulation, and the number of positive lymph nodes. This model was used to estimate a propensity score for the use of post-operative PET/CT. IPTWs were calculated as 1/(propensity score) for patients who received post-operative PET/CT and 1/(1-propensity score) for patients who did not undergo postoperative PET/CT.
Missing Data
For logistic regression and survival analyses, missing data were imputed using the MICE package (version 3.14). A summary of variables with missing values is shown in the supplement.
Predictors of Early Recurrence
To identify predictors of ER, we developed univariable and multivariable logistic regression models, including post-operative PET/CT, IRFs and HRFs, free flap reconstruction, referral pattern, and time to simulation. Logistic regression models were developed using IPT weights as described above. Adjusted odds ratios (aOR) are reported.
Survival Analyses
After excluding seven patients with second primary tumors, 384 patients comprised the cohort used for survival analyses. (Figure S1). Kaplan-Meier models were used for univariable survival analyses, and Cox proportional hazards models were used for multivariable survival analyses. IPT weighting was used based on a logistic regression model of post-operative PET use to resolve bias. Adjusted hazard ratios (HRs) are reported.
Time-to-event data were analyzed from the start of radiation to avoid immortal time bias.
To model DFS for patients with early evidence of recurrence, we defined DFS from the start of radiotherapy as a composite endpoint of 1) any subsequent locoregional recurrence after radiotherapy or 2) any distant metastasis, or 3) death. Patients with distant metastatic disease identified before radiotherapy were considered to have an event at time zero. Patients were otherwise censored at the time of last follow-up.
For the freedom from locoregional failure (FFLRF) analysis, events were defined as any local or regional recurrence after the start of PORT. Patients were censored at last follow-up or death.
For the freedom from distant metastasis analysis (FFDM), events were defined as any development of distant metastases. Patients who were found to have distant metastases before the start of PORT were considered to have an event at time zero. Patients were censored at the time of last follow-up or death.
For the OS, death was considered an event. Patients were censored at the time of last follow-up.
Results
Patient Characteristics
Between February 2005 and July 2019, 391 patients underwent surgery and PORT or chemoradiation for OSCC at our institution. Patient and tumor characteristics are shown in Table 1. Treatment details are included in the supplement. (Table S1)
Table 1:
Patient and Tumor Characteristics
| CT simulation (n=154) | PET/CT simulation (n=237) | |
|---|---|---|
| Sex (%) | ||
| Male | 87 (56.5) | 152 (64.1) |
| Female | 67 (43.5) | 85 (35.9) |
| Median Age (range) | 64 (21 – 89) | 60 (27 – 92) |
| Site (%) | ||
| alveolar ridge | 30 (19.5) | 40 (16.9) |
| buccal | 26 (16.9) | 25 (10.5) |
| floor of mouth | 17 (11.0) | 18 (7.6) |
| hard palate | 4 (2.6) | 2 (0.8) |
| lip | 0 (0.0) | 3 (1.3) |
| overlapping | 8 (5.2) | 9 (3.8) |
| retromolar trigone | 12 (7.8) | 15 (6.3) |
| tongue | 56 (36.4) | 125 (52.7) |
| not reported | 1 (0.6) | 0 (0.0) |
| pT (%) | ||
| 1 | 30 (19.5) | 35 (14.8) |
| 2 | 57 (37.0) | 98 (41.4) |
| 3 | 9 (5.8) | 26 (11.0) |
| 4a | 55 (35.7) | 77 (32.5) |
| 4b | 2 (1.3) | 1 (0.4) |
| not reported | 1 (0.6) | 0 (0.0) |
| pN (%) | ||
| 0 | 56 (36.6) | 72 (30.5) |
| 1 | 40 (26.1) | 59 (25.0) |
| 2a | 4 (2.6) | 4 (1.7) |
| 2b | 46 (30.1) | 83 (35.2) |
| 2c | 6 (3.9) | 18 (7.6) |
| 3 | 1 (0.7) | 0 (0.0) |
| not reported | 1 (0.6) | 2 (0.8) |
| PNI | 76 (49.4) | 134 (56.5) |
| LVI | 37 (24.0) | 42 (17.9) |
| Grade | ||
| 1 | 8 (5.9) | 17 (7.4) |
| 1–2 | 0 (0.0) | 6 (2.6) |
| 2 | 103 (76.3) | 166 (71.9) |
| 2–3 | 1 (0.7) | 7 (3.0) |
| 3 | 23 (17.0) | 35 (15.2) |
| Margin | ||
| Neg | 51 (33.1) | 59 (24.9) |
| Close | 71 (46.1) | 153 (64.6) |
| Pos | 32 (20.8) | 25 (10.5) |
| ECE | 41 (26.6) | 77 (32.5) |
| Thickness mm, median (IQR) | 11.2 (1.3 – 44) | 12 (1 – 52) |
| Num pos nodes, median (IQR) | 1 (0–25) | 1 (0–43) |
| Time to simulation, median (IQR) | 37 (13 – 138) | 37 (14 – 118) |
| Internal vs External Surgery | 16 (10.4) | 41 (17.3) |
Median follow-up was 4.1 years (95% CI 3.6–4.5) for the overall cohort, 6.4 years (95% CI 5.5–7.1) for the CT planning group, and 2.8 years (95% CI 2.2–3.4) for the PET/CT planning group.
Pre-radiation work-up and evaluation of patients in the CT and PET/CT groups
There was no difference in time to simulation (median 37 days vs. 37 days, Wilcoxan p=0.39) between patients planned with and without PET/CT. The most common reasons for delayed radiation were infection, flap complication, and delayed referral to radiation oncology. (Table S2).
Among patients referred for biopsy based on examination or imaging for radiation planning, 17/42 (40.4%) were positive. In total, 17/44 (39%) of patients with ER were confirmed by histopathology. Twenty-seven patients were diagnosed with ER on the basis of imaging review alone, including five patients who had non-diagnostic biopsies.
Representative Case
Representative images are shown (Figure 1) from a patient with left oral tongue and floor of mouth SCC, pT4a1N2b (AJCC 7th edition), treated with partial glossectomy and anterolateral thigh free-flap reconstruction. The post-operative PET/CT simulation showed a 7mm left level IV lymph node with SUV of 4.7, which was biopsied and confirmed to represent early recurrence. Also noted were two sub-centimeter areas of hypermetabolism adjacent to the flap with intense FDG avidity (10.3) that were poorly visualized on the CT due to beam hardening artifacts from dental amalgam. This area corresponded with an area of necrotic recurrence after the completion of chemoradiation.
Figure 1:

Representative images from a radiation planning PET/CT simulation (A–D) and follow-up CT Neck with contrast (E-F) from a patient with an AJCC 7th edition T4aN2bM0 SCC of the left oral tongue and floor of mouth treated with partial glossectomy and floor of mouth resection, left modified radical neck dissection, and anterolateral thigh free flap reconstruction. Postoperative PET/CT simulation revealed multiple foci of FDG avidity in the tumor bed and a second focus in the left neck, corresponding to a small, subcentimeter lymph node. Biopsy of the inferior lymph node was positive for SCC, and the patient was treated with chemoradiation to 70 Gy.
Treatment modification for ER
Treatment was modified for ER for 44 patients. Systemic therapy was added for seven patients (16%); radiotherapy dose was escalated by <10 Gy for 29 patients (66%) and by ≥10 Gy in 8 patients (18%); 4 patients underwent re-operation (9%); 5 patients (11%) were transitioned to early chemotherapy or palliative intent therapy. 13/44 (30%) of patients had a major intensification of treatment, defined as the addition of systemic therapy, re-operation, or intensification of radiation dose by ≥10 Gy. Major treatment intensification was more common among patients with intermediate than high risk features (91% vs. 9%, p<0.0001). Five patients, all of whom with high risk features, were transitioned to palliative intent therapy. (Table S3)
Logistic Regression for IPT Weights
A multivariable logistic regression model was developed for the use of post-operative PET/CT and used to estimate IPT weights. (Table S4). The median IPTW was 1.68 (range 1.1–7.2).
Clinicopathologic Risk Factors for ER
We developed univariable and multivariable logistic regression models of ER (Table 2). In total, 44/391 patients (11.3%) were found to have ER. 39/237 (16.5%) of patients in the PET/CT planning group were found to have ER compared with 5/154 (3.3%) of patients in the CT planning group (aOR 8.06, 95% CI 4.09–18.02, p<0.0001, Figure 2A). ER was correlated with the number of IRFs (aOR 1.66, 95% CI 1.36–2.04, p<0.0001) and presence of HRFs (aOR 4.99, 95% CI 2.92–8.88, p<0.0001, Figure 2B). On multivariable logistic regression analysis, detection of ER was improved with PET/CT simulation, and associated with ECE and time to simulation. (Figure 2A–C).
Table 2:
Logistic Regression Model of Early Recurrence
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Risk Factor | ORR | 95% CI | p-value | ORR | 95% CI | p-value |
| Post-op PET vs CT | 8.06 | 4.09 – 18.02 | <0.0001 | 7.91 | 2.88 – 26.8 | 0.00023 |
| ECE | 5.30 | 3.17 – 9.04 | <0.0001 | 5.21 | 2.14 – 13.4 | 0.00038 |
| Margins | ||||||
| Negative | Ref | - | - | Ref | - | - |
| Close | 0.75 | 0.41 – 1.40 | 0.35 | 0.79 | 0.33 – 1.99 | 0.61 |
| Positive | 2.32 | 1.18 – 4.59 | 0.015 | 2.06 | 0.69 – 6.19 | 0.19 |
| T3–4 vs. T1–2 | 2.87 | 1.71 – 4.96 | <0.0001 | 1.98 | 0.87 – 4.58 | 0.11 |
| N2–3 vs. N0–1 | 2.86 | 1.72 – 4.84 | <0.0001 | 1.00 | 0.37 – 2.61 | 1.00 |
| Tumor Thickness (mm) | 1.04 | 1.01 – 1.07 | 0.0045 | 1.01 | 0.96 – 1.06 | 0.77 |
| Num Nodes (per node) | 1.09 | 1.04 – 1.14 | 0.00011 | 1.07 | 0.99 – 1.18 | 0.14 |
| PNI | 1.89 | 1.12 – 3.27 | 0.019 | 1.67 | 0.73 – 3.98 | 0.23 |
| LVI | 2.11 | 1.22 – 3.56 | 0.0060 | 1.44 | 0.59 – 3.43 | 0.41 |
| Time to sim (per day) | 1.02 | 1.01 – 1.03 | 0.0015 | 0.36 | 0.14 – 0.92 | 0.029 |
| Referral pattern (internal referral vs. tertiary referral) | 0.46 | 0.26 – 0.84 | 0.0086 | 0.36 | 0.14 – 0.92 | 0.029 |
| Free Flap | 1.24 | 0.75 – 2.07 | 0.41 | - | - | - |
| Oral Tongue vs other | 0.76 | 0.45 – 1.25 | 0.29 | - | - | - |
Figure 2:

Incidence of early recurrence detected during radiation planning stratified by use of post-operative PET/CT (A), number and type of clinicopathologic risk factors (B), time from initial surgery to simulation (C). IRF = Intermediate risk factor, including pT3–4, pN2–3, margin (≤ 5mm), thickness > 5mm, PNI or LVI. HRF = high-risk factor, including ECE or positive margins.
Use of post-operative PET/CT is associated with longer DFS and OS for patients with Intermediate Risk Features
Among all patients in the survival analysis cohort, there were no differences in DFS (IPTW log-rank p=0.26) or OS (IPTW log-rank p=0.21) between patients in the CT vs. PET/CT simulation groups. There was an interaction between the simulation strategy and clinicopathologic risk group for DFS (p=0.026) and a trend for OS (p=0.11), suggesting that the effect of PET/CT was dependent on clinicopathologic risk class. Among patients with intermediate risk features (n=235), post-operative PET/CT was associated with improved DFS (IPTW log-rank p=0.026) and OS (IPTW log-rank p=0.047). No differences were detected for patients with high risk features (p=0.44 and p=0.96). (Figure 3A–D) Freedom from locoregional and distant failure by simulation type are shown in the supplement (Figure S2).
Figure 3:
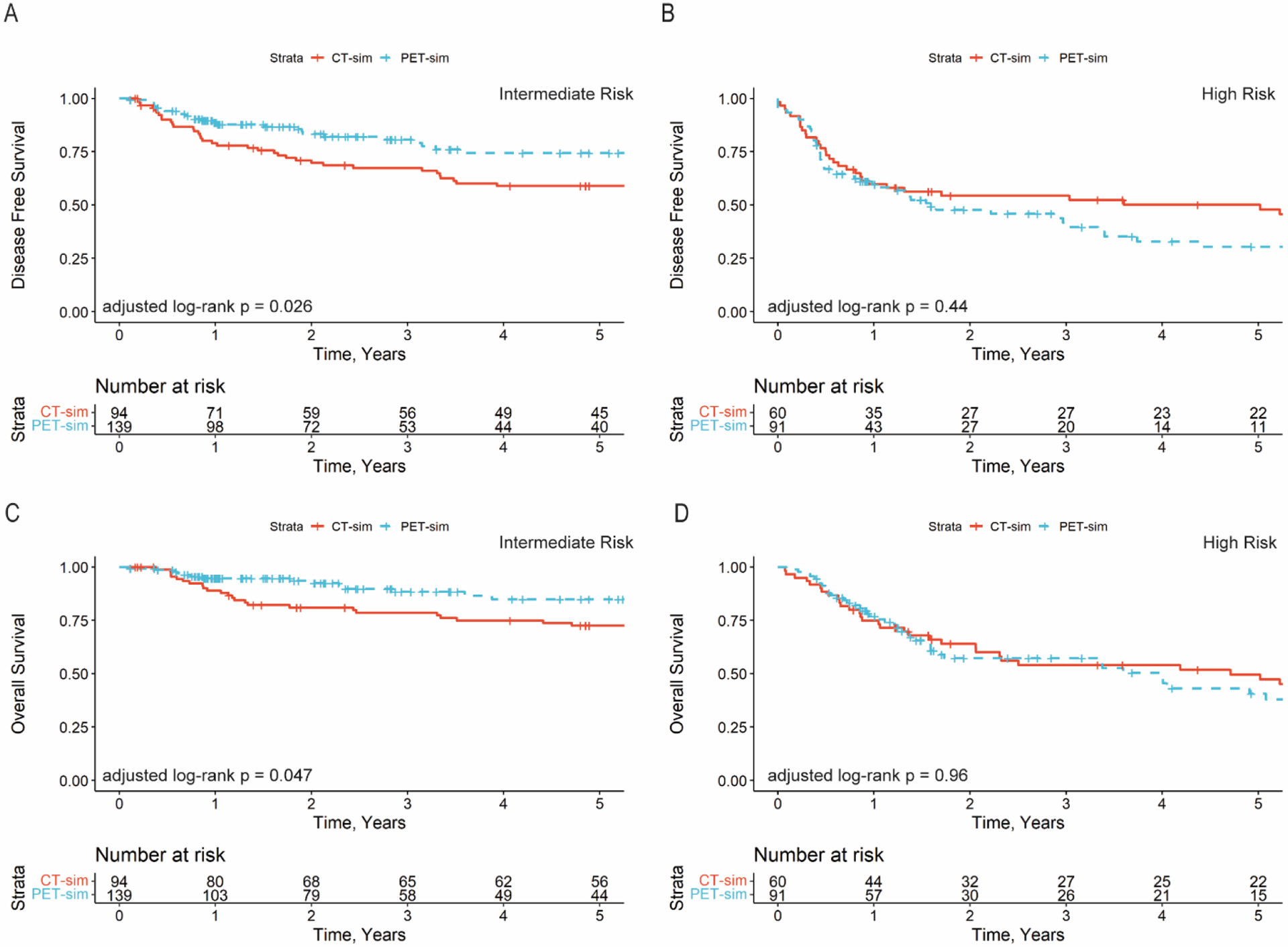
Disease-free survival for patients with intermediate risk features (A) and high risk features (B). Overall survival for patients with intermediate risk features (C) and high risk features (D). IRF = Intermediate risk features. HRD = high risk features.
On multivariable cox proportional hazards analysis, post-operative PET/CT remained associated with improved DFS (Table 3) and OS (Table S5) for patients with intermediate risk disease.
Table 3:
Cox Proportional Hazards Model, Disease Free Survival with IPTW Patients with Intermediate Risk Disease
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Risk Factor | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Post-op PET vs CT | 0.58 | 0.35 – 0.95 | 0.032 | 0.46 | 0.26 – 0.84 | 0.011 |
| Age (per year) | 1.02 | 1.00 – 1.04 | 0.035 | 1.03 | 1.00 – 1.05 | 0.020 |
| pT3–4 vs. T1–2 | 1.30 | 0.78 – 2.17 | 0.31 | 1.34 | 0.75 – 2.39 | 0.32 |
| pN2–3 vs. N0–1 | 1.86 | 1.08 – 3.21 | 0.026 | 1.05 | 0.35 – 3.14 | 0.94 |
| Thickness (per mm) | 0.99 | 0.96 – 1.02 | 0.63 | 0.97 | 0.94 – 1.01 | 0.20 |
| Nodes Pos (per node) | 1.30 | 1.13 – 1.50 | 0.00037 | 1.43 | 0.93 – 2.19 | 0.10 |
| PNI | 1.52 | 0.92 – 2.52 | 0.10 | 1.31 | 0.77 – 2.23 | 0.32 |
| LVI | 1.21 | 0.64 – 2.28 | 0.55 | 1.59 | 0.77 – 2.23 | 0.32 |
| Close Margins vs. Negative Margins | 1.50 | 0.86 – 2.61 | 0.15 | 1.62 | 0.92 – 2.85 | 0.092 |
| Time to sim (per day) | 1.01 | 0.99 – 1.02 | 0.45 | 1.01 | 0.99 – 1.02 | 0.54 |
A potential limitation of our analysis is the inclusion of patients treated for ER without biopsy confirmation due to inaccessibility or a need to start patients without delay. To address this, we investigated the outcomes of patients with ER. Among patients with ER, freedom from locoregional failure was not different for patients with and without biopsy confirmation (log-rank p=0.15). 45.5% of patients (20/44) developed subsequent locoregional failures after salvage therapy. 75% (15/20) of these locoregional failures involved the area of abnormality identified on radiation planning imaging.
The patterns of early recurrence (Table S6) and management of early recurrence (Table S7) are shown in the supplement.
Discussion
PET/CT is a standard part of radiation treatment planning for patients treated with upfront chemoradiation, but these scans are not routinely used for radiation planning after surgery. This paper reports our institutional experience utilizing PET/CT as part of routine PORT planning for OSCC. Post-operative PET/CT was associated with more frequent detection of ER compared with CT simulation. While patients with intermediate risk features had a lower incidence of ER than patients with high risk features, these patients were more likely to undergo major treatment intensification when ER was identified. In this intermediate risk group, post-operative PET/CT was associated with improved DFS and OS. We hypothesize that these differences in outcomes were driven by the improved detection of ER and major escalation of treatment in these patients.
Post-operative PET/CT can improve risk stratification and decisions regarding post-operative therapy. Among patients with intermediate risk features, 91% underwent major treatment intensification. Even among patients with high risk features who already have indications for chemoradiation, identification of ER can be beneficial. The benefit of adding concurrent chemotherapy to radiation is uncertain for patients with HRFs who are elderly or frail, as these patients were not well represented in the RTOG 9501 and EORTC 22931 trials.(3,4) For such patients, additional prognostic information can help facilitate informed decision-making. Additionally, a small proportion of patients were found to have distant metastases during radiation planning, resulting in early initiation of systemic therapy or transition to palliative care.
We identified an increased risk of ER among patients with long intervals between surgery and simulation. This is consistent with prior reports correlating the total treatment package time with outcomes.(14–17) In these patients, we posit that microscopic residual disease may progress to gross residual disease before radiation can be administered. Such patients may require re-operation, higher doses of radiation, or concurrent systemic therapy. Thus, it is critical to identify gross recurrence before PORT begins. In practice, this can be complex because delays to radiation are often associated with patient frailty, poor wound healing, and complicated perioperative courses.
Prior reports from our institution(12) and others (7,10,11,13) have identified an approximately 15–30% risk of ER when utilizing advanced imaging, consistent with the findings in this report. Hosni et al. reported that 15% of patients simulated with advanced imaging, including CT or MRI, were found to have an ER, although PET/CT was not routinely used.(7) Lee et al. reported a 20% risk of ER and a similar association with poor DFS and OS.(13) Shintani et al. performed a single-arm prospective trial evaluating PET/CT after surgery for head and neck cancers, including non-squamous tumors. In their trial of 91 patients, 27 (30%) had suspicious findings on PET, and 24 (26.4%) were referred for biopsy, of which 45.8% of biopsies were positive.(10) 15.4% of patients ultimately had a change in treatment. Similarly, Liao et al. reported a 24% risk of ER among patients who underwent repeat PET/CT.(11) In our cohort, we identified a 16% risk of ER among patients re-evaluated with PET/CT after surgery. Further, as in our study, nearly 50% of positive post-operative imaging findings were confirmed with pathologic analysis, and nearly all of these patients had a significant change in their treatment plan.
In our current practice, patients are routinely referred for post-operative PET/CT, and cases are reviewed in a consensus conference. Patients with indeterminate imaging are referred for pathology confirmation. In cases where the imaging is compelling, some biopsies may be omitted due to patient preference and concerns about delaying treatment. It is essential to engage the multidisciplinary team for decision-making in these cases, including radiation oncology, head and neck surgery, reconstructive surgery, and medical oncology. Future studies incorporating imaging and novel prognostic markers, such as circulating and salivary tumor DNA, may further refine risk stratification and improve diagnostic accuracy.(18) ERs are usually unanticipated by both the patient and the treating team and occur after expectations for PORT have been set.
There are several limitations to this analysis. ERs were identified based on a review of the medical record and documented changes in the treatment plan. One-third of ER cases were biopsy-proven, potentially leading to false positives. In practice, this was due to a preference to avoid further treatment delay for biopsy in patients with rapidly progressive disease. We performed a sensitivity analysis to assess whether treatment outcomes for patients with ER were different depending on whether they were biopsy-confirmed or not. Salvage locoregional control was moderate in both groups and was not statistically different based on biopsy status. Furthermore, 75% of subsequent locoregional recurrences correlated with the radiographic abnormality seen on post-operative imaging, regardless of biopsy status.
Because the use of post-operative PET/CT increased over the study period, confounding from other improvements to treatment over time may have contributed to the improvement in DFS and OS seen in the intermediate-risk group. To address a potential imbalance in baseline risk, we used IPTW and performed a multivariable analysis controlling for known clinicopathologic risk factors. Further, we did not observe any improvement in outcomes among patients with high-risk disease who had post-operative PET/CT, which would have been expected if this effect was confounded by other improvements in treatment outcomes over time. The treatment strategy for resected OSCC has remained stable during the study period; indications for PORT and post-operative chemoradiation have been established since 2005.(2–4) While immunotherapy has altered the landscape of recurrent and metastatic HNSCC and is a potential confounder for the OS analysis, it should not impact DFS, since there is no standard of care role for immunotherapy before recurrence or distant metastases.(19) The interpretation of PET/CT findings may be subjective; in our current practice, cases are centrally reviewed prospectively at a consensus conference. Because routine CT-simulations are not officially reviewed by a radiologist, it is possible that part of the improved detection of ER by PET/CT was due to dedicated review by a nuclear medicine physician.
Conclusion
ERs occur in a significant proportion of patients after surgery and portend a high risk of subsequent recurrence. PET/CT may improve the detection of ER and lead to treatment modifications in a substantial proportion of patients. In patients with intermediate risk features, this may translate into improved DFS and OS.
Supplementary Material
Highlights.
Use of PET/CT for post-operative radiation planning is associated with increased detection of early recurrence
Patients with intermediate risk clinicopathologic risk factors disproportionately benefit from post-operative PET/CT due to a higher likelihood of major treatment intensification
Use of post-operative PET/CT is associated improved disease-free survival among patients with intermediate risk clinicopathologic risk factors
Funding
This work was supported in part by the NIH/National Cancer Institute (NCI) Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748).
Disclosures / Conflicts of Interest
Yao Yu: Travel: Elekta
Heiko Schoder: None
Kaveh Zakeri: None
Linda Chen: None
Jung Kang: None
Sean McBride: Consulting or Advisory: Janssen, Astra Zeneca; Research Funding: Genentech, AstraZeneca
C. Jillian Tsai: Honoraria: Varian Medical Inc; Consulting or Advisory: Varian Medical Systems
Daphna Gelblum: None
Jay Boyle: None
Jennifer R Cracchiolo: None
Marc A Cohen: None
Bhuvanesh Singh: None
Ian Ganly: None
Snehal Patel: Patent PCT/US2016/026717; equity in Summit Biomedical Imaging; patent US 10,016,238 B2 Apparatus; equity in ColdSteel Laser Inc; patent PCT/US2014/073053; patent PCT/US2015/065816 ; Patent PCT/US2016/066969
Loren Michel: Research Funding: Exelixis; Travel: Immunomedics
Lara Dunn: Consulting: Regeneron, CUE Biopharma, Merck; Research Funding: Regeneron, Eisai, CUE-101
Eric Sherman: Consulting: Cota Healthcare, Goldilocks, Eisai, Regeneron, UpToDate, Lilly, Blueprint Medicines; Research Funding: Plexxikon, Regeneron
David Pfister: Consulting or Advisory: Boehringer Ingelheim, Incyte; Research Funding: AstraZeneca, Atara Biotherapeutics, Bayer, Boehringer Ingelheim, Eisai, Exelixis, Genentech/Roche, Hoopika Pharma, Lilly, Medimmune, MeiraGtx, Merck, Novartis, Regeneron Richard Wong: None
Nadeem Riaz: Honoraria: PeerView; Consulting or Advisory: Mirati Therapeutics, Repare Therapeutics; Speakers Bureau: Illumina; Research Funding: Bristol Myers Squibb, Pfizer, Repare Therapeutics; Travel, Accommodations, Expenses: Varian Medical Systems
Nancy Lee: Consulting or Advisory: Merck, Pfizer, Merck Serono, Sanofi, Mirati Therapeutics, Roche/Genentech; Research Funding: AstraZeneca, Pfizer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zanoni DK, Montero PH, Migliacci JC, Shah JP, Wong RJ, Ganly I, et al. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019. Mar;90:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck. 2005. Oct;27(10):843–50. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2004. May 6;350(19):1937–44. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004. May 6;350(19):1945–52. [DOI] [PubMed] [Google Scholar]
- 5.Leeman JE, Li J gao, Pei X, Venigalla P, Zumsteg ZS, Katsoulakis E, et al. Patterns of Treatment Failure and Postrecurrence Outcomes Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol. 2017. Nov 1;3(11):1487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsoulakis E, Leeman JE, Lok BH, Shi W, Zhang Z, Tsai JC, et al. Long-term outcomes in oral cavity squamous cell carcinoma with adjuvant and salvage radiotherapy after surgery. The Laryngoscope. 2018;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosni A, Huang SH, Chiu K, Xu W, Su J, Bayley A, et al. Predictors of Early Recurrence Prior to Planned Postoperative Radiation Therapy for Oral Cavity Squamous Cell Carcinoma and Outcomes Following Salvage Intensified Radiation Therapy. Int J Radiat Oncol. 2019. Feb;103(2):363–73. [DOI] [PubMed] [Google Scholar]
- 8.Koo BS, Lim YC, Lee JS, Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. 2006. Sep;42(8):789–94. [DOI] [PubMed] [Google Scholar]
- 9.Lok BH, Chin C, Riaz N, Ho F, Hu M, Hong JC, et al. Irradiation for locoregionally recurrent, never-irradiated oral cavity cancers. Head Neck. 2014. Aug 28;37(11):1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shintani SA, Foote RL, Lowe VJ, Brown PD, Garces YI, Kasperbauer JL. Utility of PET/CT Imaging Performed Early After Surgical Resection in the Adjuvant Treatment Planning for Head and Neck Cancer. Int J Radiat Oncol. 2008. Feb;70(2):322–9. [DOI] [PubMed] [Google Scholar]
- 11.Liao CT, Fan KH, Lin CY, Wang HM, Huang SF, Chen IH, et al. Impact of a second FDG PET scan before adjuvant therapy for the early detection of residual/relapsing tumours in high-risk patients with oral cavity cancer and pathological extracapsular spread. Eur J Nucl Med Mol Imaging. 2012. Jun 1;39(6):944–55. [DOI] [PubMed] [Google Scholar]
- 12.Dutta PR, Riaz N, McBride S, Morris LG, Patel S, Ganly I, et al. Postoperative PET/CT and target delineation before adjuvant radiotherapy in patients with oral cavity squamous cell carcinoma. Head Neck. 2016. Apr 1;38(S1):E1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DY, Abraham J, Ross E, Ridge JA, Lango MN, Liu JC, et al. Rapid recurrence in head and neck cancer: Underappreciated problem with poor outcome. Head Neck. 2021. Jan;43(1):212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997. Aug 1;39(1):137–48. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal DI, Liu L, Lee JH, Vapiwala N, Chalian AA, Weinstein GS, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002. Feb;24(2):115–26. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol Off J Am Soc Clin Oncol. 2003. Feb 1;21(3):555–63. [DOI] [PubMed] [Google Scholar]
- 17.I Ghanem A, Woody NM, Schymick MA, Joshi NP, Geiger JL, Jillian Tsai C, et al. Influence of Treatment Package Time on outcomes in High-Risk Oral Cavity Carcinoma in patients receiving Adjuvant Radiation and Concurrent Systemic Therapy: A Multi-Institutional Oral Cavity Collaborative study. Oral Oncol. 2022. Mar;126:105781. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015. Jun 24;7(293):293ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a rand omised, open-label, phase 3 study. Lancet Lond Engl. 2019. Nov 23;394(10212):1915–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


