Abbreviations
- AG
atrophic gastritis
- AUC
area under the curve
- CA19‐9
carbohydrate antigen 19‐9
- CA72‐4
carbohydrate antigen 72‐4
- CEA
carcinoembryonic antigen
- CRC
colorectal cancer
- Ct
threshold count
- GA
gastric adenocarcinoma
- GD
gastric dysplasia
- IM
intestinal metaplasia
- mRNF180
methylated ring finger protein 180
- mSEPT9
methylated SEPTIN9
- RNF180
ring finger protein 180
- RS9 panel
a panel of mRNF180 and mSEPT9
- ROC
receiver operating characteristic
- SEPT9
SEPTIN9
Dear Editor,
Early diagnosis is critical for successful treatment of gastric adenocarcinoma (GA). However, the sensitivities of tumor markers carcinoembryonic antigen (CEA), cancer antigen 19‐9 (CA19‐9) and CA72‐4 for GA detection are approximately 20% [1], and the sensitivities of all markers combined for early gastric cancer detection is still very low [2]. DNA methylation plays a major role in tumorigenesis and therefore has obvious potential as a non‐invasive biomarker for cancer detection [3]. Through genome‐wide methylation analysis and histological verification, we previously identified ring finger protein 180 (RNF180) as a novel preferentially methylated gene in GA [4, 5].
To increase the sensitivity for detecting GA, we combined mRNF180 and other methylated DNA markers. According to previous studies, the sensitivity and specificity of circulating methylated SEPTIN9 (mSEPT9) are estimated to be 50%‐70% and ≥ 90%, respectively, to detect colorectal cancer (CRC) [6, 7]. GA and CRC share similar biological features. Notably, GA and CRC share many similar aberrant promoter DNA methylations, resulting in sharing many consistent gene methylation biomarkers [8]. Therefore, we attempted to establish a panel of mRNF180 and mSEPT9 (RS9 panel) for the early detection of GA. The study protocols are included in the Supplementary Materials.
To assess the diagnostic potential of the RS9 panel, we prospectively examined 324 plasma specimens from 195 GA patients and 129 controls in the training cohort (Supplementary Figure S1, Supplementary Table S1). All subjects underwent upper endoscopy before blood collection. Receiver operating characteristic (ROC) analysis revealed that methylation levels of mSEPT9 and mRNF180 performed well to distinguish GA from control patients, as evidenced by high area under the curve (AUC) values of mSEPT9 (AUC: 0.723, 95% confidence interval [CI]: 0.669–0.776) and mRNF180 (AUC: 0.748, 95% CI: 0.695–0.800) (Supplementary Figure S2). ROC analysis using a combination of mSEPT9 and mRNF180 revealed that the RS9 panel was effective in discriminating cancer patients from controls (AUC: 0.791, 95% CI: 0.743–0.839) (Supplementary Figure S2C). We then selected cut‐off values of mSEPT9 and mRNF180 from the ROC curve that provided higher sensitivity while ensuring specificity was above 85%. The present results align with previous studies, and mSEPT9 showed high tumor specificity. Therefore, the cut‐off threshold cycle (Ct) value of mSEPT9 was based on a previous study [6] (mSEPT9 cut‐off 45.0). The cut‐off ΔCt value of mRNF180 was rounded to make this methylation marker panel easier to follow. A blood sample was considered methylation positive if at least one, two or all three PCR replicates were positive for target gene methylation. Using Youden's index, the best‐rounded cut‐off plasma levels of mRNF180 and mSEPT9 were 9.0 and 45.0, respectively (Supplementary Figure S2D).
To validate the performance of the RS9 panel, 1,381 subjects were included in a multicenter prospective validation cohort (Supplementary Figure S1). The control group included 527 subjects without neoplastic lesions. A total of 689 participants were diagnosed with gastric neoplastic lesions, including GA (n = 650) and gastric dysplasia (GD) (n = 39) (Supplementary Table S2). The remaining 165 subjects of the validation cohort had other malignant tumors (Supplementary Table S3).
We examined whether the methylation status of mSEPT9 and mRNF180 was related to the clinicopathological features of GA patients (Supplementary Table S4). Only sex correlated with mRNF180 status (P = 0.003). Female GA patients had a higher positive rate of mRNF180 than male patients. The mSEPT9 status was associated with age, T stage, N stage and pathological TNM stage (all P < 0.05). Patients with a positive mSEPT9 status were typically older, with advanced T, N and pathological stages.
Compared with controls, the methylation levels of mRNF180 and mSEPT9 were higher in cancer patients, even in stage I GA (all P < 0.001; Figure 1A‐B). In control participants, no significant difference was observed in the methylation levels of either mRNF180 or mSEPT9 among non‐neoplastic subgroups (P = 0.503 and P = 0.391, respectively) (Figure 1A‐B). ROC analyses revealed that methylation levels of both mSEPT9 and mRNF180 had good performances in discriminating patients with or without GA, as demonstrated by high sensitivity, specificity, and AUC values of mRNF180 (sensitivity: 46.2%, 95% CI, 42.3%–50.1%; specificity: 87.3%, 95% CI, 84.1%–89.9%; AUC: 0.723, 95% CI, 0.694–0.752), mSEPT9 (sensitivity: 40.0%, 95% CI, 36.2%–43.9%; specificity: 96.0%, 95% CI, 93.9%–97.5%; AUC: 0.741, 95% CI, 0.713–0.769), and the RS9 panel (sensitivity: 62.2%, 95% CI, 58.3%–65.9%; specificity: 84.8%, 95% CI, 81.4%–87.7%; AUC: 0.804, 95% CI, 0.779–0.829) (Figure 1C‐D).
FIGURE 1.
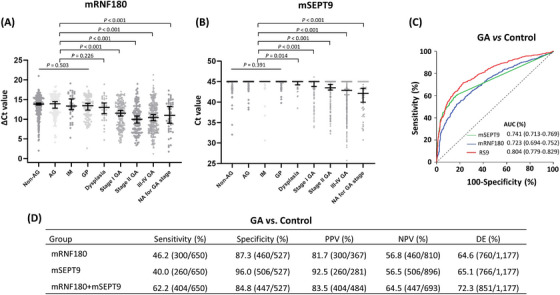
Testing and validation of mSEPT9 and mRNF180 in the validation cohort. (A, B) Methylation levels of RNF180 and SEPT9 in the plasma by the disease status in the prospective validation cohort. Dot plots illustrating DNA methylation levels of subjects with gastric neoplastic lesions or non‐neoplastic disease (mRNF180 and mSEPT9, respectively) are shown; (C) ROC curve analysis of mSEPT9, mRNF180, and the RS9 panel in GA patients and controls; (D) Diagnostic performance of mSEPT9, mRNF180, and the RS9 panel in GA patients and controls. Abbreviations: AG, atrophic gastritis; AUC, area under the curve; Ct, threshold cycle; DE, diagnostic efficiency; GA, gastric adenocarcinoma; GP, gastric polyp; IM, intestinal metaplasia; NA, not available; Non‐AG, non‐atrophic gastritis; NPV: negative predictive value; PPV: positive predictive value.
In the pathological subgroups, the sensitivity of the RS9 panel was increased in parallel with the progression of neoplastic lesions from GD to stage IV GA (χ2 for linear association = 29.7, P < 0.001) (Supplementary Figure S3A). In age subgroups, older participants had higher sensitivity to the RS9 panel than younger participants (χ2 for linear association = 12.0, P = 0.001) (Supplementary Figure S3B). Sensitivities for female and male GA patients were 70.2% and 59.1%, respectively (P = 0.003) (Supplementary Figure S3C). The analysis revealed no significant differences in the sensitivity distributions of the RS9 panel between subgroups based on Lauren subtypes (Supplementary Figure S3D). Subjects with intestinal metaplasia (IM) or gastric polyps had higher false‐positive rates of the RS9 panel than those with atrophic gastritis (AG) or non‐AG, but the differences were not statistically significant (Supplementary Figure S3E). Overall, the RS9 panel demonstrated relatively high organ specificity among several solid malignancies, except for GA, including breast (12.5%), lung (17.2%), liver (20.0%), esophageal (21.0%), pancreatic (25.0%) and colorectal cancers (63.6%) (Supplementary Figure S3F).
Information about conventional protein markers was unavailable for all patients. Data for the RS9 panel, three conventional protein markers (CEA, CA19‐9, and CA72‐4) were available for 533 patients with GA and GD, and 127 representative controls. Compared with protein markers, the RS9 panel showed similar specificity and significantly higher sensitivity (Supplementary Tables S5‐S7). Each of these three protein markers had a sensitivity of 20.6%, 20.8% and 22.7%, respectively, for GA detection and 5.5%, 6.7% and 12.3%, respectively, for GD and stage I GA detection (Supplementary Table S7). The sensitivity of the RS9 panel was more than treble that of these three protein markers. Notably, the positive detection rate of GD and stage I GA was 22.1% (95% CI, 16.1%–29.4%) in combination with the three protein markers, whereas the RS9 panel was two times more sensitive (47.9%, 95% CI, 40.0%–55.8%) (Supplementary Table S7).
The proposed RS9 panel exhibited a high degree of specificity but only a moderate degree of sensitivity. Unlike earlier liquid biopsy panels for GA [9], this panel incorporated only two methylation genes, which simplified the design of the detection panel. We believe this panel provides an alternative for patients who are unable or unwilling to undergo initial endoscopic screening, similar to the plasma mSEPT9 test for CRC screening. Although the same topic study has been published previously [10], this study had several strengths, including 1) the first large‐scale multicenter study; 2) analysis of the performance of the RS9 panel in different TNM stages; and 3) analysis of the performance of RS9 panel in malignant tumors other than GA. This RS9 panel for GA diagnosis was approved by the National Medical Products Administration in China, and post‐marketing surveillance data will clarify its performance in various clinical scenarios, including population GA screening and postsurgical surveillance.
In conclusion, we developed and validated a blood methylation biomarker panel assay using mRNF180 and mSEPT9 and established a reliable technique for diagnosing GA. The methylation status of mRNF180 and mSEPT9 can be used to detect GA at an early treatable stage.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Yongzhan Nie, Xianchun Gao, and Xiqiang Cai had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Yongzhan Nie, Kaichun Wu, and Daiming Fan were responsible for the study conception and design. Guobing Xu, Na Liu, Peng Gao, Jingyu Deng, Hongzhi Xu, Zhanlong Shen, Zhen Wu, Changqi Cao, Fenrong Chen, Nannan Zhang, Yongxi Song, Mingjun Sun, Chengyin Liu, Weili Han, Jianhua Dou, Huahong Xie, Liping Yao, Zhiguo Liu, Gang Ji, Xin Wang, and Qingchuan Zhao were responsible for managing patients and data acquisition. Analysis and interpretation of the data were conducted by Yongzhan Nie, Xianchun Gao, Xiqiang Cai, and Lei Shang. Drafting the manuscript was performed by Yongzhan Nie, Kaichun Wu, Xianchun Gao, Xiqiang Cai, Zhen Wu, and Chengyin Liu. Critical revision of the manuscript for important intellectual content was done by Yongzhan Nie, Kaichun Wu, Xiaoliang Han, Jianlin Ren, Han Liang, Zhenning Wang, Jinhai Wang, Qi Wu, and Qiaoyi Liang. Obtaining funding was the responsibility of Yongzhan Nie, Kaichun Wu, and Xin Wang. Administrative, technical or material support was provided by Jun Yu, Qiaoyi Liang, Xiaoliang Han, Guangpeng Zhou, and Zhen Wu. Supervision: Yongzhan Nie, Kaichun Wu, Jun Yu, and Xiaoliang Han.
FUNDING INFORMATION
This work was supported by the National Key R&D Program of China (Grant no. 2016YFC1303200, 2022YFC2505100, 2017YFC0908300, and 2018YFC1313101), Tianjin Key Medical Discipline (Specialty) Construction Project (Grant no. TJYXZDXK‐009A), and Beijing Municipal Science & Technology Commission, Administrative Commission of Zhongguancun Science Park (Grant no. Z201100005420007).
COMPETING INTERESTS
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Zhen Wu, Chengyin Liu, Dr. Guangpeng Zhou and Dr. Xiaoliang Han are current employees of BioChain (Beijing) Science & Technology, Inc., which has licensed the technology of the blood‐based SEPT9 assay from Epigenomics AG (Berlin, Germany). The other authors have no potential conflicts of interest to report.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethical committees of all participating hospitals (QX20161008‐1). All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study was obtained from all patients.
CONSENT FOR PUBLICATION
Not applicable
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank Drs. Tao Jiang, Xianli He, Feng Zhao, Haiqiang Tao, and Wen Fei for data collection of other malignant tumors (breast, lung, liver, esophageal and pancreatic cancers). We thank Dr. Jielai Xia for his advice on statistical analysis.
Contributor Information
Yongzhan Nie, Email: yongznie@fmmu.edu.cn, Email: kaicwu@fmmu.edu.cn.
Xiaoliang Han, Email: shan@biochain.com.
Jun Yu, Email: junyu@cuhk.edu.hk.
Kaichun Wu, Email: kaicwu@fmmu.edu.cn.
DATA AVAILABILITY STATEMENT
All data analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
REFERENCES
- 1. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17(1):26‐33. [DOI] [PubMed] [Google Scholar]
- 2. Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, et al. Diagnostic and prognostic value of CEA, CA19‐9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17(1):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibrahim J, Peeters M, Van Camp G, Op de Beeck K. Methylation biomarkers for early cancer detection and diagnosis: Current and future perspectives. Eur J Cancer. 2023;178:91‐113. [DOI] [PubMed] [Google Scholar]
- 4. Cheung KF, Lam CN, Wu K, Ng EK, Chong WW, Cheng AS, et al. Characterization of the gene structure, functional significance, and clinical application of RNF180, a novel gene in gastric cancer. Cancer. 2012;118(4):947‐59. [DOI] [PubMed] [Google Scholar]
- 5. Han F, Sun LP, Liu S, Xu Q, Liang QY, Zhang Z, et al. Promoter methylation of RNF180 is associated with H.pylori infection and serves as a marker for gastric cancer and atrophic gastritis. Oncotarget. 2016;7(17):24800‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Church TR, Wandell M, Lofton‐Day C, Mongin SJ, Burger M, Payne SR, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63(2):317‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, et al. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55(7):1337‐46. [DOI] [PubMed] [Google Scholar]
- 8. Vedeld HM, Goel A, Lind GE. Epigenetic biomarkers in gastrointestinal cancers: The current state and clinical perspectives. Semin Cancer Biol. 2018;51:36‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. So JBY, Kapoor R, Zhu F, Koh C, Zhou L, Zou R, et al. Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high‐risk population. Gut. 2021;70(5):829‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu J, Song J, Wang T, Zhu W, Zuo L, Wu J, et al. A combination of methylation and protein markers is capable of detecting gastric cancer detection by combined markers. Epigenomics. 2021;13(19):1557‐70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
All data analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.


