Abstract
Diabetes is a major economic burden and an illness with a rising incidence worldwide. Type 2 diabetes mellitus (T2DM), the most prevalent kind of diabetes, is characterized by insulin resistance and insufficient insulin production. Recent research has implicated gut microbiota dysbiosis as a contributing factor to T2DM pathogenesis. The present study employed a methodology based on randomized controlled trials (RCTs) to assess the therapeutic efficacy of probiotics in the treatment of T2DM. A thorough search was done in PubMed and Medline for articles written in English and published between 2017 and 2023. Studies were chosen based on predetermined inclusion criteria, and the search technique adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) principles. This study also employed a robust assessment instrument, widely recognized in the medical and health sciences, to evaluate the potential presence of bias within the selected research studies. Out of 96 identified articles, 22 RCTs met the eligibility criteria. Both short-term (8 weeks or less) and long-term (12 weeks or more) probiotic administrations were made. The results of the meta-analysis demonstrated a significant improvement in the homeostatic model assessment of insulin resistance (HOMA-IR) following the probiotic intervention (P=0.02) and considerably decreased glycated hemoglobin HbA1c levels (P=0.004) and fasting blood glucose (FBG) levels (P<0.0001) in T2DM patients compared to placebo. This research offers proof that probiotics are clinically effective in the treatment of T2DM. Probiotic supplementation demonstrated favorable effects on glycemic control markers. However, the findings from RCTs were heterogeneous, and some studies showed inconsistent results. To clarify the processes underlying the probiotics' therapeutic benefits and to determine the best probiotic strains, doses, and therapy durations, more research is required. Nevertheless, probiotics offer a promising therapeutic approach for T2DM management and warrant consideration as a potential adjunct therapy in clinical practice.
Keywords: homeostasis model assessment for insulin resistance (homa-ir), glycated hemoglobin (hba1c), fasting blood glucose (fbg), diabetes mellitus type 2, glycated hemoglobin, randomized controlled trials, probiotics
Introduction and background
Increased blood glucose levels are a hallmark of the chronic metabolic condition known as diabetes mellitus. The manifestation of this condition occurs when the pancreatic gland fails to secrete an adequate amount of insulin or when the body exhibits an impaired ability to utilize the insulin that is produced. Diabetes mellitus, a metabolic disorder characterized by chronic hyperglycemia, has emerged as a prominent health concern in the contemporary era. Its prevalence has witnessed a rapid escalation on a global scale, rendering it one of the foremost health challenges of the 21st century. By 2045, 700 million individuals worldwide are expected to have diabetes mellitus, up from 463 million in 2019. The global health expenditure on diabetes management was nearly 760 billion USD in 2019, which will continue to increase with the escalating diabetes prevalence. Thus, diabetes mellitus is a quickly growing public health problem and economic burden [1]. Type 2 diabetes mellitus (T2DM) is the prevailing manifestation of diabetes, constituting approximately 90% of the global incidence of this disease. It is characterized by early insulin resistance-related hyperglycemia. As a result, there is a hypersecretion of insulin to overcome the inadequate insulin response. In the long run, this leads to inadequate insulin production, and the pancreatic cells fail to comply with the increased demand [1]. The resulting continuous hyperglycemia in the body affects the human vasculature directly as well as indirectly, leading to microvascular as well as macrovascular complications. The main factor of morbidity and death in T2DM is these comorbidities [2].
T2DM is typically a consequence of a mixture of different hereditary, metabolic, and ecological elements. The well-known risk factors contributing to T2DM include genetic susceptibility via solid family history, obesity, age, physical inactivity, and unhealthy dietary habits (high-calorie food, lack of a balanced diet, etc.) [3]. The gut microbiota has emerged as a potential, influential element in the underlying pathophysiology of obesity and T2DM over the past decade. The human gastrointestinal system harbors a vast population of bacteria, numbering in the millions, with a particular abundance observed in the distal gut region [4]. These microbes, which together weigh close to 1500 g, may be considered a microbial organ that carries out crucial tasks that the human body is unable to do on its own, e.g., digestion, extracting energy from food products, production of vitamins, xenobiotic metabolism, production of metabolites and antioxidative role performed through the production of reactive oxygen species scavengers, metal ion chelators, enzyme inhibitors, and reducers [4-6].
Species from all three domains of life, bacteria, eukaryotes, and archaea, comprise the gut microbiota [4]. The 16S ribosomal ribonucleic acid (rRNA) gene amplicon sequencing and shotgun metagenomics sequencing, two deoxyribonucleic acid (DNA)-based, culture-independent sequencing techniques, have improved our understanding of gut microbiota [7]. According to the metagenomic studies, 90% of the bacteria inhabiting the human intestine are members of Bacteroidetes and Firmicutes phyla. Furthermore, bacteria belonging to phyla like Proteobacteria, Actinobacteria, and Verrucomicrobia have a low abundance [8]. However, a condition known as dysbiosis can result from external causes such as food, the use of antibiotics, stress, gut inflammation, toxins, and other conditions [9]. According to studies conducted on both humans and animals, significant dysbiosis may be linked to obesity and T2DM. However, data from human investigations showed that T2DM patients' gut microbiota composition differs significantly from that of non-diabetic control people. Although the studies did not all agree on the precise microbiota makeup, they were all distinguished by a reduction in bacteria that produced butyrate [10-12].
Through a variety of mechanisms, including increased energy extraction from food, distorted fatty acid metabolism, particularly short-chain fatty acids (SCFAs), altered adipose tissue composition and sensitivity to insulin, metabolic endotoxemia, increased systemic inflammation, and intestinal permeability, human research and animal models have linked this change in gut microbiota to the pathogenesis of obesity, insulin resistance, and subsequently T2DM [10-13]. It is thus hypothesized that the gut microbiota significantly contributes to the onset and progression of T2DM [14].
It is possible to alter the gut microbiota for the better by employing probiotics, prebiotics, or fecal microbiota transplantation (FMT). The utilization of probiotics as functional foods and dietary supplements is gaining popularity [14]. They are characterized as being alive microorganisms that, if consumed properly, can enhance the host's health. The preclinical data from animal and human studies have shown an evident beneficial effect of probiotics in improving glycemia and other associated metabolic factors in T2DM patients [15-18]. Nevertheless, the outcomes of various randomized controlled trials (RCTs) have presented inconclusive results regarding the efficacy of probiotics in the management of T2DM [19-22]. Previous studies have conducted a multitude of systematic reviews and meta-analyses to elucidate the immediate impacts of probiotics on individuals diagnosed with T2DM; there is at least one systematic review on the long-term effect of this proposed treatment in humans [22-25]. This article aims to review the clinical evidence present in the form of RCTs on the clinical efficacy of probiotics’ use in T2DM patients while comparing its short-term effect (taken as 8 weeks or less) versus the long-term (taken as 12 weeks or more). Fasting blood glucose (FBG), glycated hemoglobin (HbA1c), and homeostatic model assessment of insulin resistance (HOMA-IR) are the three parameters used for the comparison in these studies.
Review
Method
Study Design
A search for relevant English-language full-text articles published from 2017 to 2023 was effectuated in online literature databases including Medline and PubMed. The study question was created using the Problem/Population, Intervention, Comparison, Outcome (PICO) format, as advised by Cochrane and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. A search approach that incorporates all pertinent articles was developed using terms from the Medical Subject Headings (MeSH) and keywords from the pertinent literature. Search parameters were [Probiotics OR symbiotic] AND [Type 2 Diabetes Mellitus OR Diabetes (T2DM) OR T2DM] AND [short-term effect OR long-term effect]. A cross-referencing procedure was conducted in the reference lists of the included articles to identify additional relevant research. The PRISMA standards were followed for conducting this study [27]. The scientists separately decided whether or not to include the titles and abstracts. A critical appraisal methodology was employed in the field of medical and health sciences to evaluate the presence of bias in the selected papers.
Study Inclusion and Exclusion Criteria
The authors independently examined the titles and abstracts to identify articles that would be possibly appropriate for full-text examination. The same process was followed throughout the whole text review. Reference lists from included papers were also checked to find other, perhaps suitable studies. Studies were only deemed qualified if they complied with the inclusion criteria (Table 1).
Table 1. Inclusion and exclusion standards for the present research.
T2DM: type 2 diabetes mellitus; HOMA-IR: homeostatic model assessment of insulin resistance; FBG: fasting blood glucose; HbA1c: hemoglobin A1c
| Components | Criteria for inclusion | Criteria for exclusion |
| Population | T2DM patients | Patients having disease other than T2DM |
| Intervention | Studies on the utilization of probiotics for the treatment/management of T2DM patients, with patients not on insulin | Anything relating to T2DM patient management not covered by the subjects listed |
| All sorts of study designs, including mixed methods, quantitative, and qualitative, had been subject to peer review. | Anything except works that had undergone peer review, including reviews, blogs, book chapters, website material, and more. | |
| Original articles | Reviews | |
| English-language studies | Publications in other languages | |
| Comparator/control | With a placebo control group | Studies with no control group |
| Outcome | HOMA-IR, FBG, and HbA1c | Studies reporting no outcome |
Data Extraction and Analysis
The authors independently gathered information on the author, publication year, country, patients, type of probiotics, duration, and outcomes. The two reviewers resolved discrepancies in data gathering using the original publications as a reference; if no consensus could be reached, they were referred to a third reviewer.
Results
A total of 96 articles were revealed after the original search, and 15 duplicate records were removed. Following an examination of the publications' titles and abstracts, 27 were eliminated from the study. The remaining 54 papers underwent thorough examination and further screening based on research. A total of 22 studies with investigations on probiotics and their role in the management of T2DM were found to be eligible (Figure 1).
Figure 1. PRISMA flowchart for choosing appropriate literature for meta-analysis.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; n: number of articles; T2DM: type 2 diabetes mellitus; NA: not applicable
Iran had the maximum number of chosen studies (n=6), followed by China (n=3), India (n=2), United States (n=2), and the United Kingdom (n=2), while one study each from Egypt, Turkey, Thailand, Japan, Sweden, Malaysia, and Brazil was selected (Figure 2).
Figure 2. Distribution of chosen studies by country.
Five of the selected studies compared short-term effects (taken as 8 weeks or less) of probiotics while 17 studies compared long-term effects (taken as 12 weeks or more) of probiotics. Kumar et al. used a probiotic capsule and reported that it reduced the levels of HbA1c, postprandial blood glucose, and FBG, whereas Şahin et al. used Bifidobacterium animalis subsp. lactis (BB-12) for 12 weeks and observed better glycemic control and better HbA1c reduction [28,29]. Similarly, Ismail et al. also utilized B. animalis dn-173 010 for 16 weeks and saw favorable effects on lipid profile, inflammatory markers, and glycemic management [30]. Another selected study by Toejing et al. also used single Lactobacillus paracasei HII01 strain for 12 weeks and suggested its possible use as an adjuvant therapy for T2DM [31]. Sanborn et al. used Lactobacillus rhamnosus GG for 12 weeks and reported alterations in blood sugar regulation [32]. Eight of the evaluated studies utilized a single probiotic strain to treat T2DM, whereas the other 13 trials used several probiotic strains. The characteristics of selected studies are presented in Table 2.
Table 2. Characteristics and findings of selected studies.
NM: not mentioned; T2DM: type 2 diabetes mellitus; FBG: fasting blood glucose; HOMA-IR: homeostatic model assessment of insulin resistance; HbA1c: hemoglobin A1c; SCFA: short-chain fatty acid
| Author (year) | Patients | Probiotics | Duration (weeks) | Outcomes |
| Kumar et al. (2022) [28] | 150 | NM | 12 | In the context of treating T2DM, the addition of probiotics to metformin as a supplementary therapeutic approach was found to result in reductions in FBG, postprandial blood glucose, and levels of HbA1c when compared to the use of metformin alone. The efficacy of probiotics in combination treatment has not been substantiated by significant findings. Nonetheless, the probiotics trial group exhibited a reduced incidence of gastrointestinal side effects associated with metformin therapy. |
| Şahin et al. (2022) [29] | 156 | Bifidobacterium animalis subsp. lactis (BB-12) | 12 | Patients receiving probiotic supplements showed improved glycemic control and HbA1c decrease, as well as improved treatment compliance and potential effects on the intestinal-pancreatic axis. |
| Ismail et al. (2021) [30] | 150 | Bifidobacterium animalis dn-173 010 | 16 | After 16 weeks, probiotic use in T2DM patients improved glycemic control, lipid profile, and inflammatory markers. |
| Toejing et al. (2021) [31] | 50 | Lactobacillus paracasei HII01 | 12 | L. paracasei HII01 reduced inflammatory indicators and hyperglycemia by properly regulating the stomach microbiota and so treating endotoxemia and damaged stomach, proposing a possible role as an adjuvant therapy for T2DM. |
| Sanborn et al. (2020) [32] | 200 | Lactobacillus rhamnosus GG | 12 | HbA1c was steady in individuals taking L. rhamnosus GG, whereas it rose in those receiving placebo at the follow-up. L. rhamnosus GG may offer a defense against alterations in blood sugar regulation. |
| Chen et al. (2023) [33] | 58 | Bifidobacterium animalis subsp. lactis M8, B. animalis subsp. lactis V9, Lactobacillus casei Zhang, L. plantarum P-8, and L. rhamnosus Probio-M9 | 12 | The findings of this study demonstrated that the co-administration of probiotics and metformin in individuals with T2DM resulted in an augmented hypoglycemic response. The observed impact is likely to have been facilitated through the modulation of the gastrointestinal microbiota, subsequently influencing the metabolism of bile acids and SCFAs. This study provides evidence supporting the benefits of combining metformin and probiotics as a treatment approach for individuals diagnosed with T2DM. |
| Hasanpour et al. (2023) [34] | 100 | Bifidobacterium longum, B. breve, Lactobacillus bulgaricus, L. rhamnosus, L. casei, L. acidophilus, and Streptococcus thermophilu | 6 | Consuming soymilk and probiotics may reduce several cardiovascular risk factors in T2DM patients. FBG and HOMA-IR were not considerably impacted, though. |
| Velayati et al. (2021) [35] | 50 | Lactobacillus rhamnosus, Bacillus coagulans, fructooligosaccharide and L. acidophilus | 12 | This study showed that symbiotic B. coagulans supplementation might reduce metabolic variables and inflammation in T2MD individuals. |
| Kanazawa et al. (2021) [36] | 88 | Bifidobacterium breve strain Yakult, Lacticaseibacillus paracasei strain Shirota, and galactooligosaccharides | 24 | Regarding incendiary markers, there was no method to distinguish between the treatment groups. The stomach environment was undoubtedly slightly altered by synbiotic treatment in obese individuals with T2DM. |
| Jiang et al. (2021) [37] | 101 | Bifidobacterium bifidum, Lactobacillus acidophilus, Streptococcus thermophilus | 12 | This clinical investigation found that administering probiotics in individuals with diabetic nephropathy improved their glycemic control, amplifying their therapeutic potential in clinical settings. |
| Ming et al. (2021) [38] | 300 | Bifidobacterium and berberine | 16 | According to this study, Bifidobacterium may improve the hypoglycemic effects of berberine. |
| Perraudeau et al. (2020) [39] | 76 | Clostridium butyricum, Akkermansia muciniphila, C. beijerinckii, Anaerobutyricum hallii and Bifidobacterium infantis | 12 | In participants with T2DM who were predominantly receiving metformin monotherapy, a unique five-strain probiotic formulation decreased total glucose in comparison to placebo. No changes in the body weight, HOMA-IR, or fasting glucose levels were seen, indicating that the majority of the impact was a decrease in FBG levels during the postprandial period. |
| Khalili et al. (2019) [40] | 40 | Lactobacillus casei | 8 | L. casei supplementation altered serum sirtuin 1 (SIRT1) and fetuin-A levels in people with T2DM in a manner that enhanced glycemic response. A novel recognised method of probiotic action in the treatment of diabetes was introduced by altering their amounts. |
| Razmpoosh et al. (2019) [41] | 60 | Lactobacillus, Bifidobacterium, and Streptococcus | 6 | This study found that using multi-strain probiotic supplements significantly reduced fasting plasma glucose levels when compared among groups, although more research is required to validate the findings. |
| Madempudi et al. (2019) [42] | 79 | Lactobacillus casei UBLC42, L. acidophilus UBLA34, L. plantarum UBLP40, Bacillus coagulans Unique IS2, Bifidobacterium breve UBBr01 and fructo-oligosaccharides, L. salivarius UBLS22 | 12 | The reduction in HbA1c values showed that UB0316 (probiotic) considerably improved glycemic management. Additionally, the probiotic-treated patients' weight significantly decreased as compared to the control group. |
| Sabico et al. (2019) [43] | 150 | Bifidobacterium bifidum W23, Lactobacillus brevis W63, L. acidophilus W37, L. salivarius W24, L. casei W56, Lactococcus lactis W58, Lactococcus lactis W19, and B. lactis W52 | 24 | In T2DM patients, six months of multi-strain probiotic treatment as a monotherapy dramatically decreased HOMA-IR. Consequently, multi-strain probiotics are a successful adjunctive treatment for diabetes. |
| Mafi et al. (2018) [44] | 60 | Lactobacillus with Bifidobacterium | 12 | Supplementing with probiotics improved indicators of cardiometabolic risk and glycemic control. |
| Mobini et al. (2017) [45] | 46 | Lactobacillus reuteri DSM 17938 | 12 | The administration of L. reuteri DSM 17938 for a duration of 12 weeks did not result in any significant changes in HbA1c levels among individuals with T2DM who were undergoing insulin treatment. Nevertheless, the impact of L. reuteri on the insulin response was observed to be significant only in a limited subset of individuals, leading us to hypothesize that this discrepancy could potentially be attributed to the diverse compositions of gut microbiota. |
| Sabico et al. (2017) [46] | 78 | Bifidobacterium lactis W52, B. bifidum W23, Lactobacillus brevis W63, L. acidophilus W37, L. salivarius W24, L. casei W56, Lactococcus lactis W58, and Lactococcus lactis W19 | 12 | A multi-strain probiotic supplement was taken by T2DM patients who had not taken any medication for 12 or 13 weeks. This greatly improved HOMA-IR and very slightly decreased abdominal obesity. |
| Firouzi et al. (2017) [47] | 136 | Lactobacillus and Bifidobacterium | 12 | In persons with type 2 diabetes, probiotics only slightly decreased HbA1c and fasting insulin levels. |
| Feizollahzadeh et al. (2017) [48] | 40 | Lactobacillus plantarum A7 | 8 | Utilising probiotic soy milk did not decrease inflammation or serum adiponectin; however, it could alter a patient's lipid profile if they had T2DM. |
| Tonucci et al. (2017) [49] | 50 | Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus acidophilus La-5 | 6 | Probiotics reduced T2DM patients' glucose levels across the board, but consumption of mature milk appears to be linked to additional metabolic changes, including a decline in pro-inflammatory cytokines and an increase in acidic corrosive enzymes. |
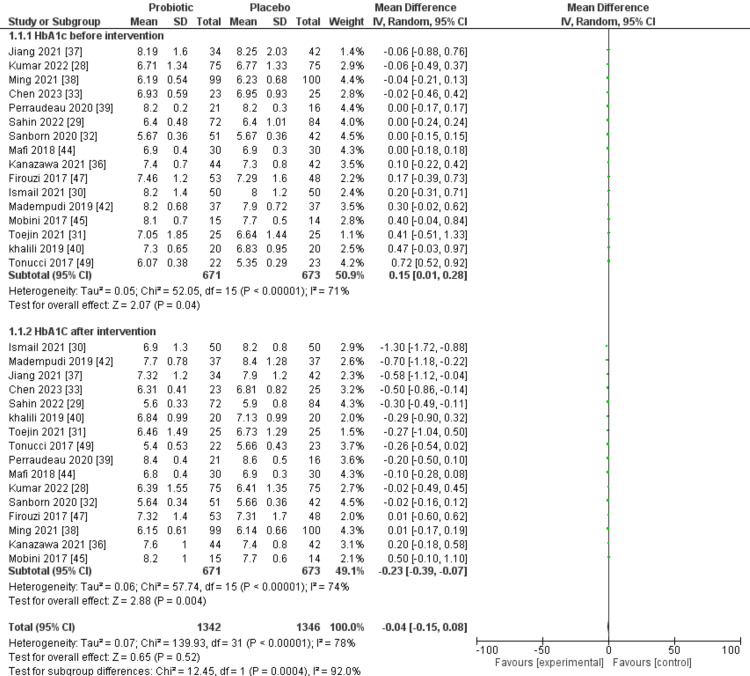
In 16 of the selected trials, the results of glycated hemoglobin (HbA1c) were observed both prior to and following the implementation of the intervention. Across all selected trials, the probiotic group consisted of 671 patients, while the placebo group comprised 673 individuals. The forest plot for HbA1c before intervention showed 71% I2 value for heterogeneity among studies. Before the intervention, there was just a little difference (P=0.04) between the probiotic and placebo groups; however, the forest plot showed a highly significant difference (P=0.004) with more beneficial effects observed in patients in the probiotic group. Figure 3 displays the findings of a meta-analysis comparing the effects of probiotic and placebo interventions on HbA1c levels.
Figure 3. A forest plot comparing probiotic and placebo groups before and after the intervention to assess HbA1c.
SD: standard deviation; IV: interval; CI: confidence interval; HbA1c: hemoglobin A1c, df: difference
In 20 of the chosen trials, the results of FBG were observed both before and after the intervention. Across all selected trials, the probiotic group consisted of 799 patients, while the placebo group comprised 811 patients. The forest plot for FBG before the intervention showed 93% I2 value for heterogeneity among studies. Overall, there was a slight difference (P=0.04) between placebo and probiotic groups prior to the intervention; however, the forest plot showed a highly significant difference (P<0.0001) with more beneficial effects observed in patients in the probiotic group. Figure 4 shows outcomes of a meta-analysis comparing the effects of probiotic and placebo interventions on FBG before and after the intervention.
Figure 4. A forest plot comparing probiotic and placebo groups before and after the intervention to assess FBG.
SD: standard deviation; IV: interval; CI: confidence interval; FBG: fasting blood glucose; df: difference
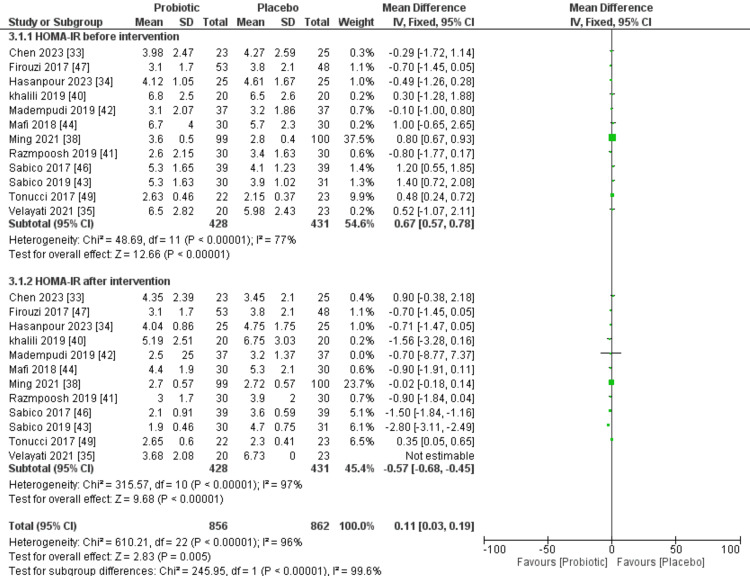
Twelve of the selected studies presented HOMA-IR outcomes before and after the intervention. In each of the selected trials, the probiotic group consisted of 428 patients, while the placebo group comprised 431 patients. The forest plot for HOMA-IR before the intervention showed 77% I2 value for heterogeneity among studies. Overall, there was a non-significant difference (P=0.07) between placebo and probiotic groups prior to the intervention; however, the forest plot showed a highly significant difference (P=0.02) with more beneficial effects observed in patients in the probiotic group. Figure 5 shows the outcomes of the meta-analysis comparing the effects of probiotic and placebo interventions on HOMA-IR.
Figure 5. A forest plot comparing probiotic and placebo groups before and after the intervention to assess HOMA-IR.
SD: standard deviation; IV: interval; CI: confidence interval; HOMA-IR: homeostatic model assessment of insulin resistance; df: difference
Discussion
In the current investigation, we systematically reviewed the literature and checked the effects of probiotics in T2DM patients. This study comprised 22 RCTs totaling 2218 patients. The findings suggested that probiotics may lower baseline levels of HbA1c, FBG, and HOMA-IR. Gut microbiota significantly contributes to T2DM [50]. For treating T2DM and associated consequences, probiotic and synbiotic supplementation has drawn a lot of interest. For instance, according to a research by Łagowska et al., the administration of synbiotics and probiotics has been observed to have a beneficial effect on reducing blood glucose levels, HOMA-IR, and insulin levels in pregnant women diagnosed with gestational diabetes mellitus [51]. Synbiotics and probiotics both lower HOMA-IR and serum insulin levels, yet just probiotics have the ability to bring down glucose. Another study recommends that synbiotic and probiotic supplementation might be useful in bringing down FBG levels in people with high baseline FBG [52]. To evaluate the impacts of synbiotics, Tabrizi et al. carried out an investigation of seven RCTs and found that synbiotics improved the patients' condition with T2DM [53]. Rittiphairoj et al. discovered that probiotics may reduce blood sugar in T2DM patients without changing HbA1c levels in another meta-analysis of 28 trials [22]. In this study, only long-term focus areas revealed beneficial effects of probiotic supplementation. According to Du et al., synbiotic and probiotic supplementation enhanced glycemic control variables in people with prediabetes [54].
In T2DM patients, the HbA1c level can accurately indicate how well the patients' blood glucose is being controlled. Patients' HbA1c levels are determined by both fasting and postprandial blood glucose, with the latter considerably increasing HbA1c [55]. The impact of probiotics on HbA1c percentage was quite significant in our meta-analysis. It could be brought on by an unidentified postprandial blood glucose level that was left out of the evaluation due to insufficient clinical information. In one of the researches, it was found that those with prediabetes and T2DM who got probiotics and metformin together rather than metformin alone saw significant reductions in HbA1c levels and gastrointestinal intolerance [29]. Zhang et al. reported similar results in a meta-analysis [56]. Intestinal microorganisms produce short-chain fatty acids, byproducts that manage immune reaction, gluconeogenesis, modify gastrointestinal hormonal secretion, decrease gut permeability, maintain intestinal anaerobic environment, and mediate glucose homeostasis by furnishing colonocytes with energy [50,57]. Probiotics may exercise their glycemic-moderating effects via reducing inflammation and oxidative stress in hyperglycemic individuals. From a sub-atomic perspective, oxidative stress and inflammation have been linked to increased insulin resistance, decreased glucose resilience, and mitochondrial cell dysfunction [58,59]. Last but not the least, probiotics and synbiotics have been found to have antioxidant properties [60]. Additionally, antioxidants are believed to be able to control insulin resistance [61].
In addition to the fact that probiotics reduced blood sugar, our selected studies showed that these also have a role in reducing inflammation. Obesity is a risk factor for developing insulin resistance in T2DM patients, but intriguingly, without changing the body mass index (BMI), probiotics were found to decrease insulin resistance [47,49]. Our meta-analysis revealed a minimal disparity between the probiotic and placebo cohorts prior to the implementation of the FBG intervention. However, upon examination of the forest plot, a substantial discrepancy emerged, indicating a significantly greater magnitude of positive outcomes among patients assigned to the probiotic group. Prior to the implementation of the intervention, there was no discernible distinction between the groups receiving probiotics and those receiving a placebo in terms of the HOMA-IR. However, upon analysis using a forest plot, a remarkably significant disparity was observed, with a greater number of favorable outcomes observed among patients in the probiotic group. In one of the studies, Ismail et al. used B. animalis dn-173 010 and reported beneficial effects on inflammatory markers, lipid profile and glycemic control, after 16 weeks [30]. Another study by Toejing et al. also used single L. paracasei HII01 strain for 12 weeks and reported its potent role as an adjuvant treatment in T2DM [31]. Sanborn et al. used L. rhamnosus GG for 12 weeks and reported alterations in blood sugar regulation [32]. In a research by Andreasen et al., individuals received an intravenous injection of Escherichia coli lipopolysaccharide (LPS) two days after the probiotic intervention [62]. We hypothesize that the improvement in the gut microbiota and the decrease in the blood's exposure to LPS contributed to how effectively probiotics cured insulin resistance in vivo. Additionally, the reduction in insulin resistance may contribute to the decline in FBG and HbA1c%. These factors lead us to hypothesize that T2DM was not caused by patients' abnormal gut flora.
Strengths and Limitations of the Study
This study followed a systematic approach, including a well-defined search strategy, clear inclusion and exclusion criteria, and adherence to PRISMA standards. This enhances the reliability and reproducibility of the findings. This study also considered a diverse set of studies published over a span of years, contributing to a comprehensive overview of the current literature on probiotics and T2DM. Furthermore, by differentiating between short-term and long-term interventions, the study provides insights into the potential timing-dependent impacts of probiotics on T2DM indicators. However, this study also has some limitations that include heterogeneity among studies that affected the ability to draw consistent conclusions. There might be a bias towards publishing studies with positive outcomes, potentially leading to an overrepresentation of studies showing significant effects of probiotics on T2DM management. The inclusion of relatively short follow-up periods, especially in the short-term interventions lasting six weeks or less, poses a limitation in evaluating the long-term effects of probiotics on T2DM indicators. T2DM is a chronic condition that develops over years, so short-term assessments may not capture the full spectrum of how probiotics affect the disease, emphasizing the necessity for longer term studies to comprehensively assess their sustained impact on T2DM management. Variability in study participant numbers in research on probiotics and T2DM can impact statistical power, with smaller samples potentially missing important effects, and generalizability, as smaller samples may not represent the broader population accurately. Larger, well-powered studies are essential for robust and widely applicable findings in this field. The wide-ranging use of various probiotic strains across studies presents a challenge in pinpointing the most effective strains for T2DM management. This diversity complicates efforts to establish clear recommendations regarding which specific strains are the most beneficial in the context of T2DM, underscoring the need for further research and standardization in probiotic interventions for T2DM.
Conclusions
This systematic review indicates that probiotics have the potential to lower baseline levels of HbA1c, FBG, and HOMA-IR in T2DM patients. Furthermore, the study highlights the significant contribution of gut microbiota to the development of T2DM, with dysbiosis potentially linked to obesity and insulin resistance. Selected studies suggest that specific strains of probiotics, such as L. rhamnosus GG and B. animalis, could serve as adjuvant therapies for T2DM management. In conclusion, our findings suggest that incorporating probiotics into T2DM management strategies could offer potential benefits in terms of glycemic control, insulin sensitivity, and inflammation reduction. More research studies, particularly randomized controlled trials, are required to establish conclusive evidence and determine optimal probiotic strains and dosages.
Acknowledgments
This statement serves to express acknowledgment to multiple individuals whose collective contributions have profoundly shaped and enriched this work. A substantial portion of the article is contributed by the first author, Ismat E. Ayesha, who designed the research question, established inclusion and exclusion criteria, created the search strategy, and conducted the data collection. She was also involved in the correction, formulation of figures and tables, as well as drafting the entire article from introduction to conclusion. Neetha R. Monson thoroughly explored relevant databases, screened titles and abstracts, extracted data, and removed duplicates. She also contributed to formulating the PRISMA flowchart. Nimra Klair contributed by performing quality assessments, and by proofreading according to recommended guidelines. Utkarsh Patel drafted the abstract section and checked for possible errors. Ayushi Saxena and Dhara Patel were involved in assessing the risk of bias and editing the final manuscript. The completion of this systematic review in such an exemplary manner was only possible due to the continuous support and guidance of our mentor, Sathish Venugopal, who was readily available at every step, providing excellent advice.
The authors have declared that no competing interests exist.
References
- 1.International Diabetes Federation. Belgium. Belgium: International Diabetes Federation; 2019. IDF Diabetes Atlas. [Google Scholar]
- 2.Microvascular and macrovascular complications of diabetes. Fowler MJ. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 3.Risk factors for type 2 diabetes mellitus. Fletcher B, Gulanick M, Lamendola C. http://journals.lww.com/jcnjournal/abstract/2002/01000/risk_factors_for_type_2_diabetes_mellitus.3.aspx. J Cardiovasc Nurs. 2002;16:17–23. doi: 10.1097/00005082-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Allin KH, Nielsen T, Pedersen O. Eur J Endocrinol. 2015;172:0–77. doi: 10.1530/EJE-14-0874. [DOI] [PubMed] [Google Scholar]
- 5.How the microbiota shapes rheumatic diseases. Van de Wiele T, Van Praet JT, Marzorati M, Drennan MB, Elewaut D. Nat Rev Rheumatol. 2016;12:398–411. doi: 10.1038/nrrheum.2016.85. [DOI] [PubMed] [Google Scholar]
- 6.Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Nutrition. 2012;28:539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Human microbiome acquisition and bioinformatic challenges in metagenomic studies. D'Argenio V. Int J Mol Sci. 2018;19:383. doi: 10.3390/ijms19020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A cross talk between dysbiosis and gut-associated immune system governs the development of inflammatory arthropathies. Kalinkovich A, Livshits G. Semin Arthritis Rheum. 2019;49:474–484. doi: 10.1016/j.semarthrit.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Hegde S, Lin YM, Golovko G, et al. Sci Rep. 2018;8:13044. doi: 10.1038/s41598-018-31033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gut metagenome in European women with normal, impaired and diabetic glucose control. Karlsson FH, Tremaroli V, Nookaew I, et al. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 11.Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. Larsen N, Vogensen FK, van den Berg FW, et al. PLoS One. 2010;5:0. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A metagenome-wide association study of gut microbiota in type 2 diabetes. Qin J, Li Y, Cai Z, et al. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 13.Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Musso G, Gambino R, Cassader M. Diabetes Care. 2010;33:2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Future for probiotic science in functional food and dietary supplement development. Neef A, Sanz Y. Curr Opin Clin Nutr Metab Care. 2013;16:679–687. doi: 10.1097/MCO.0b013e328365c258. [DOI] [PubMed] [Google Scholar]
- 15.Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Ann Nutr Metab. 2013;63:1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 16.Preclinical relevance of probiotics in type 2 diabetes: a systematic review. Marques AM, Sarandy MM, Novaes RD, Gonçalves RV, Freitas MB. Int J Exp Pathol. 2020;101:68–79. doi: 10.1111/iep.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Bioimpacts. 2014;4:83–88. doi: 10.5681/bi.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effect of probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: a randomized controlled single-blind pilot study. Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. J Cardiovasc Pharmacol Ther. 2015;20:289–298. doi: 10.1177/1074248414555004. [DOI] [PubMed] [Google Scholar]
- 19.The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Ivey KL, Hodgson JM, Kerr DA, Lewis JR, Thompson PL, Prince RL. Eur J Clin Nutr. 2014;68:447–452. doi: 10.1038/ejcn.2013.294. [DOI] [PubMed] [Google Scholar]
- 20.The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Ivey KL, Hodgson JM, Kerr DA, Thompson PL, Stojceski B, Prince RL. Nutr Metab Cardiovasc Dis. 2015;25:46–51. doi: 10.1016/j.numecd.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3642943/ Iran J Med Sci. 2013;38:38–43. [PMC free article] [PubMed] [Google Scholar]
- 22.Probiotics contribute to glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T. Adv Nutr. 2021;12:722–734. doi: 10.1093/advances/nmaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: a meta-analysis of RCTs. He J, Zhang F, Han Y. Medicine (Baltimore) 2017;96:0. doi: 10.1097/MD.0000000000009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: a meta-analysis of randomized, controlled trials. Li C, Li X, Han H, Cui H, Peng M, Wang G, Wang Z. Medicine (Baltimore) 2016;95:0. doi: 10.1097/MD.0000000000004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effects of probiotics on type II diabetes mellitus: a meta-analysis. Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF. J Transl Med. 2020;18:30. doi: 10.1186/s12967-020-02213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Chichester, UK: Wiley; 2008. Cochrane Handbook for Systematic Reviews of Interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Moher D, Shamseer L, Clarke M, et al. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Probiotics efficacy and safety as add-on therapy to metformin in type 2 diabetes mellitus. Kumar VM, Ahmed Z, Rahman SAU. Indian J Public Health Res Dev. 2022;13:317–321. [Google Scholar]
- 29.Metformin with versus without concomitant probiotic therapy in newly diagnosed patients with type 2 diabetes or prediabetes: a comparative analysis in relation to glycemic control, gastrointestinal side effects, and treatment compliance. Şahin K, Şahintürk Y, Köker G, et al. Turk J Gastroenterol. 2022;33:925–933. doi: 10.5152/tjg.2022.211063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Impact of probiotic intake on the glycemic control, lipid profile and inflammatory markers among patients with type 2 diabetes mellitus. Ismail A, Darwish O, Tayel D, Elneily D, Elshaarawy G. Clin Diabetol. 2021;10:468–475. [Google Scholar]
- 31.Influence of Lactobacillus paracasei HII01 supplementation on glycemia and inflammatory biomarkers in type 2 diabetes: a randomized clinical trial. Toejing P, Khampithum N, Sirilun S, Chaiyasut C, Lailerd N. Foods. 2021;10:1455. doi: 10.3390/foods10071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lactobacillus rhamnosus GG and HbA1c in middle age and older adults without type 2 diabetes mellitus: a preliminary randomized study. Sanborn VE, Azcarate-Peril MA, Gunstad J. Diabetes Metab Syndr. 2020;14:907–909. doi: 10.1016/j.dsx.2020.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Adjunctive Probio-X treatment enhances the therapeutic effect of a conventional drug in managing type 2 diabetes mellitus by promoting short-chain fatty acid-producing bacteria and bile acid pathways. Chen Y, Shen X, Ma T, et al. mSystems. 2023;8:0. doi: 10.1128/msystems.01300-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The effects of soymilk plus probiotics supplementation on cardiovascular risk factors in patients with type 2 diabetes mellitus: a randomized clinical trial. Hasanpour A, Babajafari S, Mazloomi SM, Shams M. BMC Endocr Disord. 2023;23:36. doi: 10.1186/s12902-023-01290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Does symbiotic supplementation which contains Bacillus coagulans Lactobacillus rhamnosus, Lactobacillus acidophilus and fructooligosaccharide has favourite effects in patients with type-2 diabetes? A randomised, double-blind, placebo-controlled trial. Velayati A, Kareem I, Sedaghat M, et al. Arch Physiol Biochem. 2021:1–8. doi: 10.1080/13813455.2021.1928225. [DOI] [PubMed] [Google Scholar]
- 36.Effects of synbiotic supplementation on chronic inflammation and the gut microbiota in obese patients with type 2 diabetes mellitus: a randomized controlled study. Kanazawa A, Aida M, Yoshida Y, et al. Nutrients. 2021;13:558. doi: 10.3390/nu13020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Probiotics ameliorates glycemic control of patients with diabetic nephropathy: a randomized clinical study. Jiang H, Zhang Y, Xu D, Wang Q. J Clin Lab Anal. 2021;35:0. doi: 10.1002/jcla.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Effectiveness and safety of Bifidobacterium and berberine in human hyperglycemia and their regulatory effect on the gut microbiota: a multi-center, double-blind, randomized, parallel-controlled study. Ming J, Yu X, Xu X, et al. Genome Med. 2021;13:125. doi: 10.1186/s13073-021-00942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. Perraudeau F, McMurdie P, Bullard J, et al. BMJ Open Diabetes Res Care. 2020;8:0. doi: 10.1136/bmjdrc-2020-001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Khalili L, Alipour B, Jafar-Abadi MA, et al. Iran Biomed J. 2019;23:68–77. doi: 10.29252/.23.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. Diabetes Metab Syndr. 2019;13:175–182. doi: 10.1016/j.dsx.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: a double blind, randomized, placebo controlled study. Madempudi RS, Ahire JJ, Neelamraju J, Tripathi A, Nanal S. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0225168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Sabico S, Al-Mashharawi A, Al-Daghri NM, et al. Clin Nutr. 2019;38:1561–1569. doi: 10.1016/j.clnu.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z. Food Funct. 2018;9:4763–4770. doi: 10.1039/c8fo00888d. [DOI] [PubMed] [Google Scholar]
- 45.Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Mobini R, Tremaroli V, Ståhlman M, et al. Diabetes Obes Metab. 2017;19:579–589. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 46.Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: a randomized clinical trial. Sabico S, Al-Mashharawi A, Al-Daghri NM, Yakout S, Alnaami AM, Alokail MS, McTernan PG. J Transl Med. 2017;15:249. doi: 10.1186/s12967-017-1354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY. Eur J Nutr. 2017;56:1535–1550. doi: 10.1007/s00394-016-1199-8. [DOI] [PubMed] [Google Scholar]
- 48.Effect of Probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Feizollahzadeh S, Ghiasvand R, Rezaei A, Khanahmad H, Sadeghi A, Hariri M. Probiotics Antimicrob Proteins. 2017;9:41–47. doi: 10.1007/s12602-016-9233-y. [DOI] [PubMed] [Google Scholar]
- 49.Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clin Nutr. 2017;36:85–92. doi: 10.1016/j.clnu.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Gut microbiota and type 2 diabetes mellitus: association, mechanism, and translational applications. Zhang L, Chu J, Hao W, et al. Mediators Inflamm. 2021;2021:5110276. doi: 10.1155/2021/5110276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Improvement of glucose metabolism in pregnant women through probiotic supplementation depends on gestational diabetes status: meta-analysis. Łagowska K, Malinowska AM, Zawieja B, Zawieja E. Sci Rep. 2020;10:17796. doi: 10.1038/s41598-020-74773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Effect of probiotics and synbiotics on blood glucose: a systematic review and meta-analysis of controlled trials. Nikbakht E, Khalesi S, Singh I, Williams LT, West NP, Colson N. Eur J Nutr. 2018;57:95–106. doi: 10.1007/s00394-016-1300-3. [DOI] [PubMed] [Google Scholar]
- 53.The effects of synbiotic supplementation on glucose metabolism and lipid profiles in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Tabrizi R, Moosazadeh M, Lankarani KB, Akbari M, Heydari ST, Kolahdooz F, Asemi Z. Probiotics Antimicrob Proteins. 2018;10:329–342. doi: 10.1007/s12602-017-9299-1. [DOI] [PubMed] [Google Scholar]
- 54.Probiotics, prebiotics, and synbiotics supplementation in prediabetes: protocol for a systematic review and meta-analysis. Du X, Xie C, Shi L, Gao H, Yang C, Liu Q. Medicine (Baltimore) 2020;99:0. doi: 10.1097/MD.0000000000019708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Zhou J, Martin RJ, Tulley RT, et al. Am J Physiol Endocrinol Metab. 2008;295:0–6. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Zhang Q, Wu Y, Fei X. Medicina (Kaunas) 2016;52:28–34. doi: 10.1016/j.medici.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 57.The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Markowiak-Kopeć P, Śliżewska K. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Effects of folic acid supplementation on inflammatory markers: a grade-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Asbaghi O, Ashtary-Larky D, Bagheri R, et al. Nutrients. 2021;13:2327. doi: 10.3390/nu13072327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Folic acid supplementation improves glycemic control for diabetes prevention and management: a systematic review and dose-response meta-analysis of randomized controlled trials. Asbaghi O, Ashtary-Larky D, Bagheri R, et al. Nutrients. 2021;13:2355. doi: 10.3390/nu13072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Characterization and antioxidant property of probiotic and synbiotic yogurts. Madhu AN, Amrutha N, Prapulla SG. Probiotics Antimicrob Proteins. 2012;4:90–97. doi: 10.1007/s12602-012-9099-6. [DOI] [PubMed] [Google Scholar]
- 61.Effects of chromium supplementation on glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Asbaghi O, Fatemeh N, Mahnaz RK, et al. Pharmacol Res. 2020;161:105098. doi: 10.1016/j.phrs.2020.105098. [DOI] [PubMed] [Google Scholar]
- 62.Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Andreasen AS, Larsen N, Pedersen-Skovsgaard T, et al. Br J Nutr. 2010;104:1831–1838. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]