Abstract
Psychosocial stress is a top predictor of peripartum mood disorders in human mothers. In the present study, we developed a novel paradigm testing the effects of direct and vicarious social stress on maternal and mood-related behaviors in B6 mice. Using a novel housing paradigm, we examined the extent to which postpartum dams withdrew from litters following psychosocial stress. Repeated acute direct social stress involved exposing dams to a virgin male mouse for 7 min/day on postpartum days 5–7 during a brief (15-min) mother–pup separation. To remove the effects of direct stress, the vicarious social stress dams were housed in the same vivarium as direct social stressed dams, but without direct exposure to intruders. Control dams were given mock intruder exposure and housed in a separate vivarium room containing breeding mice. All dams experienced pup separation, and maternal care was investigated upon reunion. Direct and vicarious social stress induced significant deficits in maternal care and increased maternal anxiety relative to controls. Although vicarious stress effects were more likely to occur on days when there was acute stress exposure, direct stress sustained maternal deficits 24 h after the final stressor. Together, these data suggest psychosocial stress induces aberrant maternal phenotypes in mice.
Keywords: anxiety disorders, maternal behavior, mood disorders, postpartum depression, social stress
1 ∣. INTRODUCTION
Depression or anxiety symptoms that emerge during pregnancy or shortly after birth (i.e., peripartum mood disorders; PMD) are present in up to 20% of mothers (Pearlstein et al., 2009; Shorey et al., 2018). Mothers with PMD often experience strain in self-esteem, marital relationship, and relationship with their children (Beck & Gable, 2000; Brockington, 2004; Jolley & Betrus, 2007), posing significant social and health-related complications. Furthering complications for women who experience PMD is the fact that the DSM-V does not have a unique classification for PMD, instead listing only a peripartum onset specifier for depressive and bipolar disorders as well as obsessive compulsive disorder and brief psychotic disorder (American Psychiatric Association, 2013). There is currently no peripartum specifier for anxiety disorders despite abundant evidence that anxiety is frequently present, as is depression, in the peripartum period (Britton, 2008; Fawcett et al., 2019; Field, 2018; Paul et al., 2013; Tietz et al., 2014; Wenzel et al., 2005). Independent of specific clinical classification, mood symptoms (such as increased depression and/or anxiety in the peripartum period) reported by women with PMD tend to center around the infant. For example, research on PMDs have shown symptoms that include obsessive and compulsive thoughts/behaviors about the infant’s safety or withdrawal from the baby altogether (Beck & Gable, 2000, 2001). Furthermore, women with PMD experience anxiety/insecurity, emotional lability, sleep/eating disturbances, cognitive impairment, guilt/shame (Beck & Gable, 2000, 2001), and are less bonded with their babies (Tietz et al., 2014). Risk factors associated with the onset of PMD include psychosocial stress, low social support, low income/financial stress, or a low perception of one’s own health (Pawluski et al., 2017). However, psychosocial stress (e.g., low income, low levels of family support, experiencing discrimination/racism) is the top predictor in adult women with no previous history of mood disorders (Alipour et al., 2012; Austin et al., 2007; Murray et al., 1996; Nakić Radoš, 2018; Pawluski et al., 2017; Surkan et al., 2006). Moreover, lower perceived levels of social support are strongly associated with increased chance of displaying PMD symptoms (Reid & Taylor, 2015; Webster et al., 2011; Xie et al., 2009). In addition to the negative effects of PMD on the mothers, PMD is also associated with altered offspring development, including cognitive deficits, general health impairments, as well as an increased vulnerability for mood disorder diagnosis (Agrati et al., 2015; Aizer et al., 2016; Osborne et al., 2018; Pawluski et al., 2017).
Considering that psychosocial stress is linked to the onset of mood disorders in women with no history of anxiety or depression, it is likely the peripartum period confers a vulnerability for psychosocial stress-induced mood disorder onset. However, an understanding of how a physiological state, such as the peripartum period, would alter the impact of psychosocial stress on neural systems are largely missing from the literature. For example, although maternal and anxiety-like behaviors have been examined following chronic exposure to physical stressors (Bowman et al., 2002; Castagné et al., 2011; Chiba et al., 2012; Grippo et al., 2005; Jeong et al., 2013; Johnson et al., 2015; Ramos-Ortolaza et al., 2017; Rosinger et al., 2020; Scheich et al., 2017; Zhao et al., 2013), only a few studies have investigated the impact of psychosocial stress during the peripartum period on maternal and mood-like behaviors (Murgatroyd et al., 2015; Nephew & Bridges, 2011).
To better understand how social stress confers vulnerability to maternal dysfunction and mood disorder onset, we examined the effects of repeated acute social stress exposure on maternal behavior in postpartum B6 mice. Although peripartum hormones have an anxiolytic effect on lactating dams, an encounter with a male conspecific elicits a robust stress response in the postpartum period (Deschamps et al., 2003; Neumann et al., 2001). Male conspecifics that encounter pups will commit infanticide to induce a sexually receptive state in the dam and gain the opportunity for reproduction themselves (Elwood & Stolzenberg, 2020). Therefore, lactating dams must defend their nests against male intruders. In the present study, we repeatedly exposed postpartum B6 mice to novel male conspecifics and examined maternal and mood-like behaviors following the encounter. Maternal withdrawal is difficult to study in standard shoebox mouse cages as dams build large nests that tend to encompass half of the cage space. Thus, we developed a novel paradigm to investigate this critical aspect of maternal neglect/withdrawal associated with PMDs. Here, we housed subjects in two standard shoebox cages that were connected by a polyvinyl chloride (PVC) pipe allowing dams to travel freely between a cage containing their nests and an alternate cage space. To determine the extent to which intruder stress produced sustained behavioral modifications in dams, we also tested maternal behavior 24 h after the last intruder experience. In addition, we asked whether direct intruder experience was necessary to produce a social stress-induced neglect phenotype through measuring maternal and mood-like behaviors in dams that were housed next to intruder exposed females (indirect vicarious social stress). Although vicariously and directly stressed dams showed many similarities in their response to social stress, each group also displayed unique behavioral coping strategies. Last, it is important to note that we have no evidence that animals such as B6 mice have the subjective experience of depression as human beings do. Therefore, we are left to model what we can in contexts that result in variations of standard behavior and think strategically about how they map to humans (Bliss-Moreau, 2017; Bliss-Moreau & Rudebeck, 2021). Keeping this in mind, our work provides a novel model to understand how social stress exposure confers a vulnerability for maternal care dysfunction in B6 mice.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Subjects and housing
All mice were C57BL/6J adults obtained from our breeding colony, maintained on a 12/12 light/dark cycle (lights on at 0300 h), and given access ad libitum to food and water. Behavioral testing was conducted 1 h into the dark phase of the light/dark cycle under dim red light. All procedures were conducted in compliance with the University of California, Davis Institutional Animal Care and Use Committee. Nulliparous female mice (N = 28) were harem bred with stud males for 18 days, after which subjects were randomly assigned to one of three treatment groups: control (N = 12), vicarious (N = 6), or social stress (N = 10). Dams in the social stress treatment were exposed to three consecutive days of novel male intruder stress (7 min/day), and vicarious stress dams were housed in the same vivarium room as stress dams. The stress vivarium was 2.3876 m L × 2.5146 m W × 2.5399 m H. Cages in the stress vivarium were 7.62 cm apart. Each row was 152.4 cm long and house four experimental cages. The vertical distance between rows was 28.1 cm. Direct and vicarious stress cages were staggered across three rows on a single rack in the stress vivarium. Control females were housed in a vivarium breeding room that contained male and female breeders with their litters in which no social stress experiments were conducted. Control females were otherwise treated identically to the vicarious dams, including mock intruder exposure. Additionally, the control vivarium room and control cage rack dimensions were identical to the stress vivarium.
After 18 days of cohabiting with a B6 male breeder, all females were transferred to a modified home cage (HC) with Carefresh® bedding (Healthy Pet, Ferndale, WA), food and water ad libitum, and monitored daily for the presence of pups (designated postnatal day 0). On postpartum day (PPD) 3, an additional modified cage was attached to the HC, referred to as the escape cage (EC) (see Figure 1). The EC was designated as the cage without the dam’s nest and litter, but was otherwise identical to the HC. The modified caging was identical to standard shoebox caging except that modified cages included a PVC portal (4 cm diameter) that connected the two shoebox cages. Access to each compartment was controlled through the use of a PVC pipe cap. From PPD 3–9, subjects were able to move freely between the HC and EC with the exception of the 12-min period which access was limited exclusively to the HC (cage containing nest and litter on each test day). All dams were weighed on PPD 3. On PPD 9, dams and their litters were again weighed and transferred to a clean, standard shoebox cage. Pups were weighed daily from PPD 4–7 at the start of behavioral testing. Experimental timeline and intruder testing paradigm are shown in Figure 1.
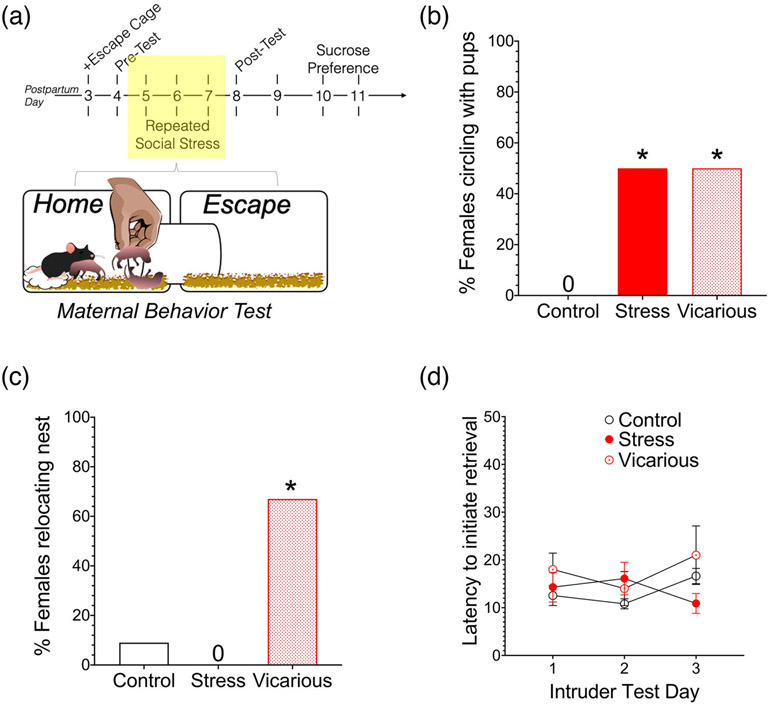
FIGURE 1.
Experimental timeline and intruder paradigm. (a) Dams were monitored for the presence of pups daily and the day of birth was deemed postpartum day 0. An additional shoebox (escape) cage was attached to the dam’s housing on postpartum day 3. After a 24 h habituation period, dams were screened for maternal care in the modified housing environment. On postpartum days 5–7, pups were removed from all dams for 15 min. During this time, direct stress dams were exposed to a novel male intruder for 7 min, whereas for vicarious and control dams, the cage tops were removed and replaced to simulate the introduction of an intruder. Maternal behaviors were observed upon mother–pup reunion for 1 h. On postpartum day 8, maternal behaviors were again observed following a 15 min maternal separation, however all dams experienced the simulated placement of an intruder. Finally, on postpartum days 10–11, dams were administered a sucrose preference test. (b) Illustration of the specific timeline for the intruder stress paradigm (postpartum days 5–8). Vicarious and control dams experienced an identical paradigm of simulated male mouse exposure, but were housed in different vivarium rooms
2.2 ∣. Behavioral testing
Behavioral testing began on PPD 4 with a brief maternal behavior pretest. All subjects were separated from their pups for 15 min. During the first 12 min, a PVC pipe cap was used to restrict the dam’s access to only the HC, and pups were kept warm on a heating pad set to low. After 12 min, the PVC pipe cap was removed, and all dams were given an additional 3 min to habituate back to both cages. Following the 15-min separation, pups were scattered in the dam’s HC. Subjects were observed for 15 min during which maternal behaviors were recorded. All dams retrieved all their pups to their nest location and nursed within 15 min.
2.3 ∣. Resident intruder paradigm
On PPD 5–7, behavioral testing began with closure of the PVC portal to the EC and removal of the dam’s litter. All dams were left undisturbed for 5 min after which a 7-min intruder (or mock for vicarious stress and control dams) exposure in the HC occurred. Mock exposure consisted of manipulating the cage in a way that mimicked the addition of an intruder mouse, without the actual introduction of an intruder. Following mock or intruder exposure, dams in all treatment groups were given an additional 3-min rehabituation period with open access to both HC and EC between intruder removal and pup replacement (15-min total pup separation on each test day). Dams assigned to the direct stress group were exposed to a sexually naïve, intruder B6 male in their HC for 7 min. Dams assigned to the vicarious stress group were housed in the same vivarium as direct stress dams, and present during intruder stress exposure, though were never directly exposed to a novel male conspecific in their own cage. Dams assigned to the control group were housed in a separate vivarium room that contained identical ambient stimuli (i.e., females, males, mating, pups, and parental caregiving), and mock intruder exposure, with the only exception being a lack of ambient intruder stress-associated stimuli. Control and vicarious dams were left undisturbed for a total of 15 min with the exception of the mock intruder exposure. During the 7 min intruder period, behavioral observations were recorded every 30 s (14 total per animal per day) for all groups, including controls. During the intruder exposure observers scored fighting behaviors, which included the following: bite, lunge, kick, boxing, or jumping at the male. Boxing occurred when the female stood on her hind legs and struck the male with her forepaws. Lunging occurred when the female made a quick dart toward the male. Intruders were removed early if the female bit the male seven or more times. No animals sustained any physical injuries as a result of intruder stress. Observers also scored social behaviors in dams including: avoidance (active move away from male), nose-to-nose contact, anogenital sniffing, grooming received/given, and sitting with male. Nose-to-nose contact was defined as any physical nose-to-nose contact between the female and intruder male. Anogenital sniffing was defined as any perioral contact with the male’s anogenital area. If the female was immobile, but in physical body contact with the male, the behavior was scored as side-by-side sitting. All dams, including control and vicarious dams that were not exposed to an intruder male, were observed for the following nonsocial behaviors: walking, sitting, digging, grooming, nonsocial sniffing, eating, or drinking.
2.4 ∣. Maternal behavior testing
At the time of intruder removal, the PVC portal was opened and all mice were left undisturbed for 3 min, after which a 1 h maternal behavior test began with the scattered placement of pups in the dam’s HC. Latency data were recorded for the following: retrieval of first pup, retrieval of all pups to nest, hover over all for at least 30 s, and entry to EC. The frequency of unsuccessful retrievals was also recorded. For example, some dams picked up displaced pups with their mouths but moved them around the cage rather than back to the nest. We define this circling behavior as any movement of pups by the dam to a location outside of a prior or future nest location. Frequently, this behavior was concurrent with picking up and putting down the pup in rapid succession outside of the nest or running through the cage with a pup in the mouth. Throughout the 1 h observation, spot checks were performed every 3 min, during which maternal behaviors were scored including: nursing, sniffing/licking, and nest building. Mothers nursed in a variety of postures. For example, crouched nursing is defined as an immobile, quiescent posture in which the dam’s back is arched over the pups with back legs splayed and all four paws on the ground. Dams also engaged in hovering behavior in which pups had access to the nipples while mothers were in engaged in grooming or nest building. These behaviors could be displayed toward the entire litter or a portion of the litter. Nonsocial behaviors were also recorded including: digging, self-grooming, eating, drinking, walking (active), or immobility (awake, inactive, away from nest). Note that the location of dam (in or out of nest/ in or out of HC) was recorded for each observation.
2.5 ∣. Post-test
On PPD 8, behavioral testing began with the closure of the PVC portal limiting access to only the HC just as during previous intruder test days, and removal of pups from the nest. After 5 min, all subjects experienced the simulated intruder exposure in which the cage top was removed, a hand was placed into the cage to rustle the bedding and the cage top was replaced without the introduction of an intruder male. Seven minutes later the portal was opened and after 3 min of undisturbed exploration, pups were scattered in the dam’s nest cage and maternal behavior testing commenced for one hour as described above.
2.6 ∣. Sucrose preference testing
On PPD 10, dams were habituated to two sipper bottles containing water overnight. On PPD 11, one of the two bottles was replaced with a bottle that contained a 1% sucrose solution. The location of the replaced bottle was counterbalanced and the weight of both bottles was recorded. Twenty-four hours later, on PPD 12, bottle weights were recorded.
2.7 ∣. Statistical analyses
Composite scores were calculated to analyze the effects of stress on maternal care. Percentage of total pup contact was calculated by summing the total number of observations in which the subject was in physical contact with pups (e.g., nursing, sniffing/licking, nest building or drinking in contact) and dividing by the total number of possible observations (20) multiplied by 100. This score was calculated for both total and partial litter contact. Pup avoidance was calculated by summing the total number of observations in which dams were in the EC (or the cage not containing litter/nest) divided by the total number of possible observations (20) multiplied by 100. Disorganized or fragmented care was calculated by summing the number of spot checks where the dam transitioned from pup- to non-pup-directed behavior out of 20 (total number of behavior spot checks each day). For example, if at 3 min a dam was sniffing/licking the litter, and then at 6 min active out of the nest, the dam was awarded +1 point. This method has been previously used as a measure of maternal inconsistency (Bolton et al., 2017; Gallo et al., 2019; Rice et al., 2008). A maternal anxiety score was calculated, based on previous investigations, by summing the frequency of observations in which a dam was found to be digging or self-grooming (Nephew & Bridges, 2011), in addition to inactivity away from the nest (awake, inactive, and away from the nest/litter) during spot checks. Inactivity was included in our composite score of maternal anxiety-like behavior because the dams were tested during the beginning of the active phase, where inactivity away from the nest is an abnormal behavioral display not observed in typical mouse maternal care (König & Markl, 1987). Moreover, we use the term anxiety as a label for a prolonged negative affective state, as opposed to a label of emotion (Bliss-Moreau, 2017; Bliss-Moreau & Rudebeck, 2021). On the post-test day, preference for pups was calculated as number of contacts with full litter/contact with full litter + no litter contact. Sucrose preference was calculated as sucrose consumed/water + sucrose consumed (Grippo et al., 2005).
All data were analyzed by Graphpad Prism (version 8.4). Proportion data were analyzed using a Chi square test. Maternal behavior frequency data were analyzed in response to intruder exposure across 3 test days using a mixed-effects ANOVA, which allowed us to analyze behavior across time with a missing subject. Due to COVID-19 related complications, one control dam was unable to finish testing beyond intruder day 2 (i.e., intruder test 3, post-test, and sucrose preference). Importantly, the subject was not omitted due to any experimental related purpose. Post-hoc planned comparisons between control versus vicarious, and control versus stress dams were analyzed if a significant main effect was detected. For this and all post-hoc analyses, Dunnett’s correction was used to correct for multiple comparisons. Maternal behaviors 24 h following the last intruder stress were analyzed using a one-way ANOVA. The relationship between pup preference and sucrose preference was analyzed by Pearson correlation. All datasets were tested for the presence of outliers using the Grubbs test. Across 19 analyses, a total of 16 outlying data points in nine analyses were detected and removed. Data were considered significant if p < .05, and two-tailed tests were used for all comparisons.
3 ∣. RESULTS
3.1 ∣. Intruder test
On PPDs 5–7, females were exposed to direct or indirect intruder stress during which behaviors were scored (Figure 2(a)). Although direct social stress dams were able to physically interact with a sexually naïve male during the intruder test, vicarious stress and control dams were not. Thus, the ethogram used to examine behavior during the intruder test differed between social stress dams and vicarious or control groups. In contrast, vicarious and control dams only differ based on the vivarium room in which they were housed and tested. Therefore, the same ethogram was used to compare behavior during the mock intruder test in these two groups. Sixty percent of direct socially stressed dams exhibited fighting behavior in response to the first presentation of a male intruder (Figure 2(b)). Fighting tended to decrease by test day, however this effect did not reach statistical significance [X2(1,10) = 3.333, p = .07]. There were no differences between control and vicarious dams in anxiety-like behavior during intruder testing observations (Figure 2(c)). Finally, there were no differences in anxiety-like behavior across any of the treatment groups during mock intruder exposure on the post-test (Figure 2(d)).
FIGURE 2.
Intruder stress elicits aggressive behavior from lactating dams. (a) Timeline of experiment. Data illustrated in (b)-(c) represent behavior during the intruder phase of the repeated stress paradigm (shaded box). (b) Percentage of social stress dams engaging in fighting behavior on each intruder day. The reduction in fighting by day was marginally significant (p = .07). (c)The anxiety score represents a sum of wall climbing, treading, and self-grooming during the 7 min mock intruder test. Vicarious and control dams did not vary on intruder test days. (d) Similarly, no differences in anxiety were detected during the 7 min mock intruder exposure on the post-test day
3.2 ∣. Maternal behavior on intruder test days
On PPDs 5–7, females were exposed to a novel male intruder, followed by scoring maternal care for 1 h post pup reunion (Figure 3(a)). Although social stress had no effect on the total number of pups in each litter, the percentage of each litter surviving, or average pup weight during intruder testing (Table 1), caregiving behaviors were significantly compromised by both direct and vicarious social stress. For example, several of the dams in both stress (50%) and vicarious stress (50%) treatment carried pups in their mouth while running to locations in the cage outside of the nest area (i.e., circling behavior). In contrast, control dams carried scattered pups directly to the nest site. The proportion of circling behavior during retrievals within each group was compared by computing the total number of dams from each treatment group to display circling behavior on any intruder test day during pup reunion. The occurrence of this circling behavior significantly varied by group [X2(2) = 7.816, p = .02; Figure 3(b)]. In some cases, vicarious dams retrieved pups to more than one location and maintained two nest sites during observations. These dams eventually consolidated their nests to a new location outside of the HC. By intruder test day 3, half of the vicarious dams had moved their offspring to the EC (Figure 3(c)). Notably this behavior was not observed in social stress or control dams [X2(2) = 12.14, p = .002]. There were no significant differences among groups in the latency to retrieve the first pup [F(2,25) = 1.399, p = .265] (Figure 3(d)).
FIGURE 3.
Both direct and indirect social stress is associated with alterations in pup retrieval. (a) Timeline of experiment. Data illustrated in (b)–(d) represent maternal behavior following intruder exposure on PPDs 5–7 (shaded box). Percentage of dams that displayed circling behavior on any testing day. In contrast to control dams, both social stress and vicarious animals circled the cage with a pup in the mouth. (b) The percentage of dams relocating their nest following at least one intruder exposure. (c) Average time in seconds to retrieve each pup at the start of maternal behavior testing following each intruder exposure. However, there were no differences in retrieval rate across intruder test days. *Significantly different from control dams, p < .05
TABLE 1.
Groups did not vary in the average number of pups born, surviving, or average pup weight throughout intruder testing
| Treatment | Mean ± SEM number of pups born |
Mean ± SEM percentage litter survival |
Mean ± SEM pup weight (g) |
|---|---|---|---|
| Control | 6.8 ± 0.474 | 98.81 ± 1.19 | 3.422 ± 0.370 |
| Vicarious | 6.3 ± 0.760 | 100 ± 0.00 | 3.794 ± 0.481 |
| Stress | 6.5 ± 0.734 | 95.78 ± 2.82 | 3.409 ± 0.345 |
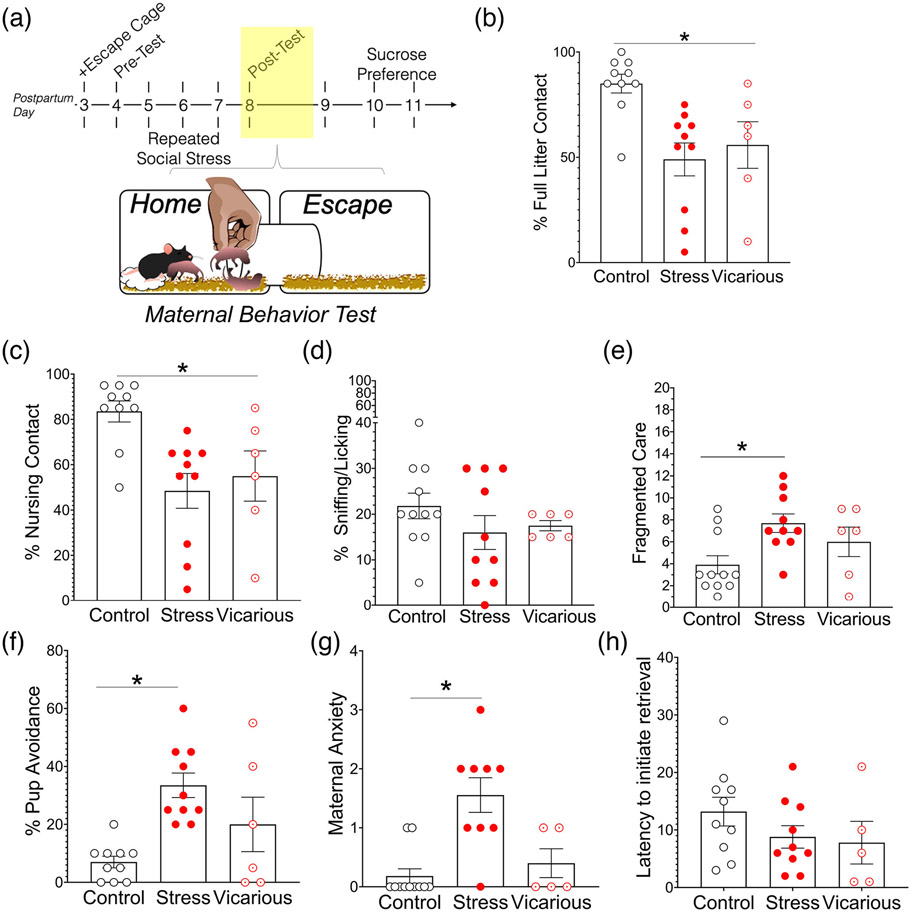
Once in the nest, the percentage of time spent with the litter in the nest varied by group [mixed-effects ANOVA, main effect of group F(2,25) = 4.58, p = .02]. Planned comparisons revealed significant differences between control dams and dams that experienced either direct (p = .02) or vicarious (p = .04) intruder stress (Figure 4(b)). Given that vicariously stressed dams tended to split their litters, we also analyzed percentage of time spent with any pups (e.g., in or out of the nest). When accounting for partial litter contact, groups varied significantly in time spent with pups [main effect of group [F(2,25) = 4.214, p = .0265]. However, post-hoc analyses indicated that control dams had significantly more pup contact than social stress dams (p = <.02) but not vicarious dams (p = .10; Figure 4(c)).
FIGURE 4.
Social stress impairs maternal care behavior during pup reunion. (a) Timeline of experiment. Data illustrated represent maternal behavior following intruder exposure on PPDs 5–7 (shaded box). (b) Percentage of observations in contact with entire litter during the 1 h observation on each day. (c) Percentage of observations in contact with any number of pups during the 1 h observation on each day. (d) Percentage of observations in nursing contact with pups on each day. (e) Percentage of observations in sniffing/licking contact with pups on each day. Data displayed as mean ± SEM, *Significant main effect of group: control dams were significantly different from all other groups, p < .05. αSignificant main effect of group: control dams were significantly different from social stress dams, p < .05
When mother–pup interactions did occur, the quality of care was significantly impacted by social stress. We report a main effect of group [F(2,25) = 4.402, p = .02] on the frequency of crouched nursing. Post-hoc analyses indicate that control dams had significantly more nursing contact with pups than dams exposed to direct social stress (p = .03). The reduced nursing contact displayed by vicarious dams did not reach statistical significance (p = .058; Figure 4(d)). Social stress also reduced sniffing/licking contact [main effect of group, [F(2,25) = 10.23, p = .0006] in direct stressed dams versus controls (p = .007; Figure 4(e)). Finally, social stress reduced the consistency of care provided and induced fragmented or disorganized mother–pup interactions [main effect of group (F(2,25) = 5.579, p = .009)], post-hoc analyses revealed both direct stress dams and vicarious stress dams displayed significantly more fragmented care than control dams (p = .01; p = .03, respectively) (Figure 5(b)).
FIGURE 5.
Social stress induces fragmented care during intruder test days. (a) Timeline of experiment. Data illustrated represent maternal behavior following intruder exposure on PPDs 5–7 (shaded box). (b) Intruder stress induced frequent behavioral switching between pup-directed and non-pup-directed behaviors or fragmented maternal care in direct and vicariously stressed dams. (c) The percentage of observations in which dams spent time in the escape cage (or the cage without nest and pups) varied by group and day (significant interaction effect). (d) Both direct and vicarious stress dams displayed significantly higher maternal anxiety during pup reunion than control dams. Data displayed as mean ± SEM; *main effect of group: control dams significantly different from all other groups, p < .05. βInteraction effect of group X day, p < .05. αControl dams significantly different from stress dams, adjusted for multiple comparisons, p < .05
Given that social stress reduced pup contact, we asked whether intruder stress induced pup avoidance. Percentage of observations spent in the EC varied by group and day [group × time interaction [F(4,49) = 6.491, p = .0003]. Interestingly, although control dams spent more time in the EC on the first test when compared with social stress dams (p = .02), by test 3, this relationship may have reversed (p = .052; Figure 5(c)). We measured maternal anxiety-like behavior during pup reunion (summation of self-grooming, digging, and immobility behaviors), and report a main effect of group [F(5,25) = 5.521, p = .0103]. Post-hoc analyses reveal both direct stress dams and vicarious dams displayed significantly higher anxiety-like behavior than controls (p = .03; p = .01, respectively) (Figure 5(d)).
3.3 ∣. Maternal behavior 24-h after intruder stress
Twenty-four hours following the last intruder stress, dams were briefly separated from pups and maternal behavior was measured upon reunion (Figure 6(a)). Three days of social stress had a sustained effect on litter contact [one-way ANOVA, main effect of group (F(2,23) = 7.403, p = .0033)] with both direct social stress dams (p = .0024) and indirect vicarious stressed dams (p = .0310) showing significantly less full litter contact than control dams (Figure 6(b)). There was also a main effect of social experience on nursing contact [F(2,23) = 7.048, p = .0041] with both direct social stress (p = .003) and indirect vicarious stressed dams (p = .0348) showing less nursing contact than controls (Figure 6(c)). However, there were no sustained effects of stress on sniffing/licking contact (p = .33; Figure 6(d)). Mother–pup interactions continued to be inconsistent in direct stressed dams [F(2,24) = 4.704, p = .02] when compared with control dams (p = .01; Figure 6(e)). Direct social stress also sustained pup avoidance [main effect of group (F(2,23) = 8.755), p = .0015] in social stress compared with control dams (p = .0007; Figure 6(f)). We also report a main effect of group on maternal anxiety-like behavior during pup reunion on the post-test [F(2,22) = 12.12, p = .0003], specifically in social stress dams compared with controls (p = .0002; Figure 6(g)). Finally, we report no differences in latency to initiate pup retrieval during the post-test (p = .295; Figure 6(h)).
FIGURE 6.
Social stress induces deficits in maternal care 24 h following final stress. (a) Timeline of experiment. Data illustrated represent maternal behavior 24 h after intruder exposure on PPD 8 (shaded box). (b) and (c) Sustained effect of intruder stress on percentage of observations in contact with full litter or in nursing contact with litter. (d) Prior intruder stress had no effect on the percentage of observations in sniffing/licking contact with pups. (e) and (f) Socially stressed dams continued to show fragmented care and pup avoidance. (g) Socially stressed dams also showed heightened anxiety-like behaviors on the post-test. (h) Groups did not vary in their latency to initiate pup retrieval. Data displayed as mean ± SEM. *Significantly different from control group, p < .05
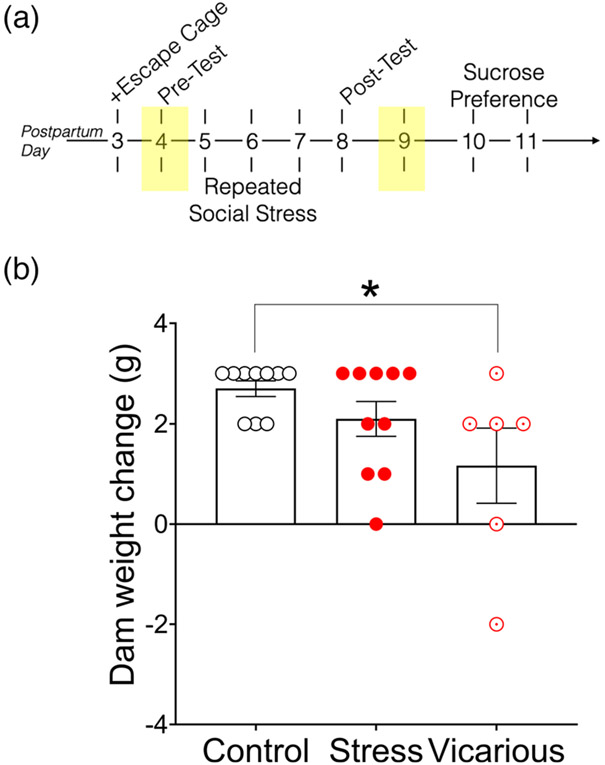
3.4 ∣. Maternal weight change
All dams were weighed at the start and end of behavioral testing (Figure 7(a)). Weight change across testing was marginally significantly different by social experience group [F(2,24) = 4.165, p = .05]. Vicarious dams showed significantly less weight gain than control dams (p = .029; Figure 7(b)).
FIGURE 7.
Social stress influenced maternal weight gain. (a) Timeline of experiment. Dam weights were measured on PPDs 4 and 9 (shaded boxes). (b) Groups varied in weight change across the experimental period. Data displayed as mean ± SEM. *Significant difference between groups, p = .05
3.5 ∣. Maternal sucrose anhedonia
On PPDs 10 and 11, all dams underwent sucrose preference testing as a measure of anhedonia (Figure 8(a)). Groups did not significantly vary in their preference for sucrose (p = .10; Figure 8(b)). However, the variability in sucrose preference within groups significantly varied (p = .04). Thus, we asked whether preference for sucrose was correlated with another rewarding stimulus (i.e., preference for pups during the post-test). Across all mice there was a significant correlation between preference for pups in the post-test and sucrose preference [r = .42, p = .036; Figure 8(c)].
FIGURE 8.
Social stress influenced anhedonic behavior. (a) Timeline of experiment. Dams were habituated to two water bottles on PPD9 and sucrose preference was measured over the following 24-h period (shaded boxes). (b) and (c) Groups did not vary in sucrose preference (p = .36), although there was a relationship between sucrose preference and the percentage of observations in contact with pups during the post-test. Data displayed as mean ± SEM
4 ∣. DISCUSSION
Social stress during lactation impairs maternal care in several ways (Table 2). To begin, direct stress and vicarious stress dams showed deficits in maternal care directly following intruder stress; both showed abnormal pup retrieval, spent less time with the full litter, showed inconsistent/fragmented pup care, and increased anxiety-like behavior compared with control dams. Direct and vicariously stressed dams also had unique responses to acute social stress. For example, in comparison with controls, direct stress exposure reduced nursing and licking, and increased pup avoidance, while vicarious stress induced nest relocation. For direct stressed dams, some of these deficits (i.e., anxiety, reduced nursing, reduced litter contact, pup avoidance, and fragmented care) were sustained for at least 24 h after intruder stress ceased. The maintained pup avoidance seen relative to nonstressed controls highlights the influence of direct social stress on such behaviors. In the vicarious stress group, dams continued to display reduced full litter contact and nursing behaviors 24 h after the final intruder stress. Together these data suggest that the effects of direct social stress create a potentially long-term change in stress reactivity. However, this would need to be directly tested by measuring maternal care throughout the rest of the rearing period. Given that infant withdrawal is a key symptom of PMD (Beck & Gable, 2000, 2001), with known influences on both the mother and infant (Agrati et al., 2015; Aizer et al., 2016; Osborne et al., 2018; Pawluski et al., 2017), data from the present study suggest that our model has preliminary translatable value. One possibility for our results is that as a consequence of repeated stress, interaction with pups is no longer rewarding (Robinson et al., 2011). Although we did not detect significant differences in sucrose anhedonia following repeated stress, the significant amount of within-group variability in sucrose preference prompted us to investigate the relationship between pup preference and sucrose preference. We found that pup preference 24 h following intruder stress was positively correlated with sucrose preference on postnatal day 11. Thus, dams that showed a reduced preference for pups in the post-test also showed a reduced preference for sucrose several days later. However, this effect was seen across all mice, and the fact that sucrose and pup preference is correlated suggests that reduced pup preference could also be a measure of anhedonia, which is consistent with the literature. It is also possible that there are certain predispositions in maternal behavior toward reduced hedonic valence throughout lactation.
TABLE 2.
Summary of the significant behavioral effects of direct stress and vicarious stress in comparison with control conditions
| Direct stress phenotype | Vicarious stress phenotype | |
|---|---|---|
| Immediately following acute stress | Circling | Circling |
| n.s. | Nest relocation | |
| Reduced litter contact | Reduced litter contact | |
| Reduced nursing | n.s. | |
| Reduced licking | n.s. | |
| Fragmented care | Fragmented care | |
| Pup avoidance | n.s. | |
| Increased maternal anxiety | Increased maternal anxiety | |
| 24 h after last acute stress | Reduced litter contact | Reduced litter contact |
| Reduced nursing | Reduced nursing | |
| Fragmented care | n.s. | |
| Pup avoidance | n.s. | |
| Increased maternal anxiety | n.s. |
Acute stress refers to behavioral changes immediately following intruder exposure. Twenty-four hours after stress refers to behavioral changes observed 24 h later during the post test. Behaviors are color coded based on experimental group compared with control dams. Bold text indicates both direct and vicarious stress dams’ behavior at a significantly different rate than controls. Italicized text indicates direct stress dams’ behavior at a significantly higher rate than controls. Underlined text indicates vicarious dams’ behavior at a significantly higher rate than controls; n.s., nonsignificant difference.
The results of the present study are the first to indicate that direct repeated acute social stress can induce active withdrawal from infants in the absence of social stress (illustrated by increased avoidance on the post-test, +24 h from last male intruder session). Our novel modified housing paradigm enabled withdrawal behavior as it offered dams a location to escape from the cage containing their litter and nest. Interestingly, although direct stressed dams avoided their pups significantly more than the vicarious or controls on the post-test, they were less avoidant than controls on the first day of intruder testing. One possibility is that acute mild stress improved maternal care (Bailoo et al., 2014; Boccia & Pedersen, 2001). In support of this idea, repeated direct social stress ultimately induced significant differences in pup avoidance by the post-test, supporting the influence of mild social stress improving, but ongoing social stress impairing maternal care. These deficits were present only in the direct social stress group during the full hour of maternal care observations post-reunion 1 day past final stress exposure, lending preliminary support of an association between repeated acute social stress and ongoing maternal care deficits.
Additionally, there were alterations in retrieval strategies in both direct and indirectly stressed dams on intruder exposure days. Part of the impaired retrieval pattern included circling behavior, which involved carrying the mouse around the cage in circles before placing the pup outside of the nest, frequently accompanied by audible pup vocalizations. To our knowledge, circling behavior has only been reported once in the literature, using a rat model (Salas et al., 1984). Based on this previous study, and our data, it suggests an indecisive and compulsive behavioral response specific to threat ambiguity in the peripartum period. In further support of threat ambiguity driving subtle differences in behavior, unlike direct stress dams, half of the vicarious dams ultimately relocated their nests to the EC, which was not seen in any directly stressed dams. In rats, nest relocation has been shown to be contingent upon ambiguous threat (Brewster & Leon, 1980; Pinel et al., 1990). Thus, the perception of stress and threat was likely slightly different between direct and vicarious intruder exposed dams. Although vicarious stress dams were exposed to an ambiguous threat, direct exposure to an intruder male was an ethologically relevant maternal challenge. Maternal defense or maternal aggression behavior, which occurs in response to a novel male conspecific, is an essential aspect of maternal care (Bosch & Neumann, 2010,2012; Flannelly et al., 1986; Gammie et al., 2004; Hasen & Gammie, 2005; Klampfl et al., 2018). Accordingly, the proportion of lactating dams in our study that engaged in defensive behavior is consistent with what has been reported for B6 dams in the literature (Ogawa & Makino, 1984). Although maternal defense provides critical protection to infants, it also activates the stress response (Hasen & Gammie, 2005; Klampfl et al., 2013; Klampfl et al., 2018). This repeated activation of the stress response seems to be detrimental to successful maternal care. In support of this idea, we found that maternal anxiety was amplified as a result of repeated acute direct intruder stress. Specifically, maternal anxiety during pup reunion was elevated during intruder test days (higher in both direct and vicarious stressed dams compared with controls), as well as during the post-test pup reunion, where direct stressed dams continued to display higher maternal anxiety than controls. Independent of individual mouse experience, this “anxiety-like” behavioral difference suggests a prolonged negative affective state in direct response to a social stress context during lactation.
Our findings that social stress induced a maternal withdrawal and neglect-like outcome in female mice is consistent with work investigating the effects of social stress in lactating rats. For example, in a series of manuscripts, Nephew and colleagues used a similar intruder stress paradigm to socially stress lactating rat dams for one hour each day across PPDs 2–16 (Murgatroyd et al., 2015; Nephew & Bridges, 2011). Social stress reduced mother–infant contact (nursing and grooming), increased maternal anxiety, and had no impact on sucrose preference on PPD 9 (Murgatroyd et al., 2015). Thus, the results reported here are generally consistent with the findings of Nephew and colleagues, however there is one important distinction. Although we report maternal impairments 24 h beyond final intruder stress, Nephew and colleagues report a transient effect of chronic intruder stress on maternal care, which peaked on PPD 9 but was absent by PPD 16, despite ongoing daily stressors continuing through PPD16. This discrepancy could be related to species differences or a number of methodological differences. For example, we employed an acute repeated (7 min exposure for 3 days in the absence of pups) rather than chronic (1-h exposure for 2 weeks in the presence of pups) stress paradigm. It is also possible that the dams from Nephew and colleagues study habituated to the intruder stress after 2 weeks of chronic exposure compared with our 3 days of acute intruder exposure. Finally, the maternal deficits reported here are also consistent with work examining how nonsocial stress impacts PMD-like outcomes in rodents (Brummelte & Galea, 2010; Carini & Nephew, 2013; Gobinath et al., 2018; Heun-Johnson & Levitt, 2016; Murgatroyd & Nephew, 2013; Nephew & Bridges, 2011; Nephew et al., 2018; Neumann et al., 2005).
It is important to note that our study has limitations on the conclusions we can draw. We report a novel effect of indirect vicarious stress exposure during lactation on the quality of maternal care provided to offspring. However, it is difficult to assess the source of variability within the vicarious dams’ behavior. We hypothesize this effect is driven by exposure to ambient cues related to intruder stress, considering the control and vicarious dams were exposed to otherwise identical sensory stimuli (environments containing females, males, breeding, and pup stimuli). Additionally, experimenters treated both vicarious and control cages identically during mock intruder sessions. Therefore, the only key difference between vicarious and control dams was the proximity to intruder stress cues. We did not measure ultrasonic vocalizations, nor pheromone release. Therefore, identification of the specific stimulus or combination of stimuli that drove vicarious stress-induced impaired maternal care will be an important question for future work.
In conclusion, our study presents a novel paradigm for investigating social stress effects on impaired maternal care outcomes in female mice. Human PMD literature highlights social stress during the peripartum period as a top risk factor. Therefore, it is important to develop an animal model that addresses the role of social stress specifically, in precipitating the onset of maternal care impairments that resemble symptoms in human literature. Importantly, the social stress-induced behavioral outcomes we report in B6 dams are consistent with the PMD behaviors reported in the human literature, such as a diminished ability of the mother to attend her baby’s needs (Murray et al., 1996), and feeling withdrawn from the baby (i.e., avoidant) (Beck & Gable, 2000, 2001). Therefore, our model has preliminary translational value, though more research is needed. Finally, the finding that vicarious social stress exposure can impact mood-like outcomes is significant because it may allow for increased opportunities to study social stress-induced mood-like behaviors in female mice. Presently, social stress-induced mood disorder onset has almost exclusively been studied in male rodents despite the fact that women are more likely to be diagnosed with a mood disorder (Laman-Maharg & Trainor, 2017). However, the introduction of an intruder mouse elicits approach and affiliative responses in virgin female mice, lactating dams exclusively engage in defense behavior. Thus, the fact that indirect social stress can impact mood-like outcomes, at least in lactating dams, is important. Future work will need to determine whether this vicarious effect can occur in nonlactating dams.
ACKNOWLEDGMENT
We would like to thank Cory Ardekani for proofreading the manuscript.
FUNDING
This work was supported by the National Institute of Child Health and Human Development (R01HD087709-01A1).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Agrati D, Browne D, Jonas W, Meaney M, Atkinson L, Steiner M, & Fleming AS (2015). Maternal anxiety from pregnancy to 2 years post-partum: transactional patterns of maternal early adversity and child temperament. Archives of Women’s Mental Health, 18(5), 693–705. 10.1007/s00737-014-0491-y [DOI] [PubMed] [Google Scholar]
- Aizer A, Stroud L, & Buka S (2016). Maternal stress and child outcomes: Evidence from siblings. Journal of Human Resources, 51(3), 523–555. 10.3368/jhr.51.3.0914-6664R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipour Z, Lamyian M, & Hajizadeh E (2012). Anxiety and fear of childbirth as predictors of postnatal depression in nulliparous women. Women and Birth, 25(3), e37–e43. 10.1016/j.wombi.2011.09.002 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Anxiety Disorders, Depression Disorders. Diagnostic and statistical manual of mental disorders: Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. [Google Scholar]
- Austin M-P, Tully L, & Parker G (2007). Examining the relationship between antenatal anxiety and postnatal depression. Journal of Affective Disorders, 101(1-3), 169–174. 10.1016/j.jad.2006.11.015 [DOI] [PubMed] [Google Scholar]
- Bailoo JD, Jordan RL, Garza XJ, & Tyler AN (2014). Brief and long periods of maternal separation affect maternal behavior and offspring behavioral development in C57BL/6 mice: Maternal separation and offspring emotionality. Developmental Psychobiology, 56(4), 674–685. 10.1002/dev.21135 [DOI] [PubMed] [Google Scholar]
- Beck CT, & Gable RK (2000). Postpartum depression screening scale: Development and psychometric testing. Nursing Research, 49(5), 272–282. 10.1097/00006199-200009000-00006 [DOI] [PubMed] [Google Scholar]
- Beck CT, & Gable RK (2001). Further validation of the postpartum depression screening scale. Nursing Research, 50(3), 155–164. 10.1097/00006199-200105000-00005 [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E. (2017). Constructing nonhuman animal emotion. Current Opinion in Psychology, 17, 184–188. 10.1016/j.copsyc.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, & Rudebeck PH (2021). Animal models of human mood. Neuroscience & Biobehavioral Reviews, 120, 574–582. 10.1016/j.neubiorev.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, & Pedersen CA (2001). Brief vs. long maternal separations in infancy: Contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology, 26(7), 657–672. 10.1016/S0306-4530(01)00019-1 [DOI] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Ivy A, & Baram TZ (2017). New insights into early-life stress and behavioral outcomes. Current Opinion in Behavioral Sciences, 14, 133–139. 10.1016/j.cobeha.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, & Neumann ID (2010). Vasopressin released within the central amygdala promotes maternal aggression. European Journal of Neuroscience, 31(5), 883–891. 10.1111/j.1460-9568.2010.07115.x [DOI] [PubMed] [Google Scholar]
- Bosch OJ, & Neumann ID (2012). Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior, 61(3), 293–303. 10.1016/j.yhbeh.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, & Luine VN (2002). Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience, 113(2), 401–410. 10.1016/s0306-4522(02)00156-2 [DOI] [PubMed] [Google Scholar]
- Brewster J, & Leon M (1980). Relocation of the site of mother-young contact: Maternal transport behavior in Norway rats. Journal of Comparative and Physiological Psychology, 94(1), 69–79. 10.1037/h0077644 [DOI] [Google Scholar]
- Britton JR (2008). Maternal anxiety: Course and antecedents during the early postpartum period. Depression and Anxiety, 25(9), 793–800. 10.1002/da.20325 [DOI] [PubMed] [Google Scholar]
- Brockington I. (2004). Diagnosis and management of post-partum disorders: A review. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 3(2), 89–95. [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, & Galea LAM (2010). Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Hormones and Behavior, 58(5), 769–779. 10.1016/j.yhbeh.2010.07.012 [DOI] [PubMed] [Google Scholar]
- Carini LM, & Nephew BC (2013). Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Hormones and Behavior, 64(4), 634–641. 10.1016/j.yhbeh.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, & Porsolt RD (2011). Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Current Protocols in Neuroscience, 55(1). 10.1002/0471142301.ns0810as55 [DOI] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, & Kunugi H (2012). Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 39(1), 112–119. 10.1016/j.pnpbp.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, & Walker C-D (2003). Pups Presence Eliminates the Stress Hyporesponsiveness of Early Lactating Females to a Psychological Stress Representing a Threat to the Pups. Journal of Neuroendocrinology, 15(5), 486–497. 10.1046/j.1365-2826.2003.01022.x [DOI] [PubMed] [Google Scholar]
- Elwood RW, & Stolzenberg DS (2020). Flipping the parental switch: From killing to caring in male mammals. Animal Behaviour, 165, 133–142. 10.1016/j.anbehav.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett EJ, Fairbrother N, Cox ML, White IR, & Fawcett JM (2019). The Prevalence of Anxiety Disorders During Pregnancy and the Post-partum Period. The Journal of Clinical Psychiatry, 80(4), 10.4088/jcp.18r12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. (2018). Postnatal anxiety prevalence, predictors and effects on development: A narrative review. Infant Behavior and Development, 51, 24–32. 10.1016/j.infbeh.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Kemble ED, Caroline Blanchard D, & Blanchard RJ (1986). Effects of septal-forebrain lesions on maternal aggression and maternal care. Behavioral and Neural Biology, 45(1), 17–30. 10.1016/S0163-1047(86)80002-4 [DOI] [PubMed] [Google Scholar]
- Gallo M, Shleifer DG, Godoy LD, Ofray D, Olaniyan A, Campbell T, & Bath KG (2019). Limited Bedding and Nesting Induces Maternal Behavior Resembling Both Hypervigilance and Abuse. Frontiers in Behavioral Neuroscience, 13, 10.3389/fnbeh.2019.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, & Rhodes JS (2004). Corticotropin-Releasing Factor Inhibits Maternal Aggression in Mice. Behavioral Neuroscience, 118(4), 805–814. 10.1037/0735-7044.118.4.805 [DOI] [PubMed] [Google Scholar]
- Gobinath AR, Richardson RJ, Chow C, Workman JL, Lieblich SE, Barr AM,& Galea LAM (2018). Voluntary running influences the efficacy of fluoxetine in a model of postpartum depression. Neuropharmacology, 128, 106–118. 10.1016/j.neuropharm.2017.09.017 [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, & Van de Kar LD (2005). Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology, 179(4), 769–780. 10.1007/s00213-004-2103-4 [DOI] [PubMed] [Google Scholar]
- Hasen NS, & Gammie SC (2005). Differential fos activation in virgin and lactating mice in response to an intruder. Physiology & Behavior, 84(5), 681–695. 10.1016/j.physbeh.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Heun-Johnson H, & Levitt P (2016). Early-life stress paradigm transiently alters maternal behavior, dam-pup interactions, and offspring vocalizations in mice. Frontiers in Behavioral Neuroscience, 10. 10.3389/fnbeh.2016.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JY, Lee DH, & Kang SS (2013). Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinology and Metabolism, 28(4), 288. 10.3803/EnM.2013.28.4.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Zuloaga DG, Bidiman E, Marzulla T, Weber S, Wahbeh H, & Raber J (2015). ApoE2 exaggerates PTSD-related behavioral, cognitive, and neuroendocrine alterations. Neuropsychopharmacology, 40(10), 2443–2453. 10.1038/npp.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley SN, & Betrus P (2007). Comparing postpartum depression and major depressive disorder: Issues in assessment. Issues in Mental Health Nursing, 28(7), 765–780. 10.1080/01612840701413590 [DOI] [PubMed] [Google Scholar]
- Klampfl SM, Neumann ID, & Bosch OJ (2013). Reduced brain corticotropin-releasing factor receptor activation is required for adequate maternal care and maternal aggression in lactating rats. European Journal of Neuroscience, 38(5), 2742–2750. 10.1111/ejn.12274 [DOI] [PubMed] [Google Scholar]
- Klampfl SM, Schramm MM, Gaßner BM, Hübner K, Seasholtz AF, Brunton PJ, Bayerl DS, & Bosch OJ (2018). Maternal stress and the MPOA: Activation of CRF receptor 1 impairs maternal behavior and triggers local oxytocin release in lactating rats. Neuropharmacology, 133, 440–450. 10.1016/j.neuropharm.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B,& Markl H (1987). Maternal care in house mice. Behavioral Ecology and Sociobiology, 20(1), 1–9. 10.1007/bf00292161 [DOI] [Google Scholar]
- Laman-Maharg A, & Trainor BC (2017). Stress, sex, and motivated behaviors: Stress, sex, and motivated behaviors. Journal of Neuroscience Research, 95(1–2), 83–92. 10.1002/jnr.23815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, & Nephew BC (2013). Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology, 38(2), 219–228. 10.1016/j.psyneuen.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Taliefar M, Bradburn S, Carini LM, Babb JA, & Nephew BC (2015). Social stress during lactation, depressed maternal care, and neuropeptidergic gene expression: Behavioural Pharmacology, 26, 642–653. 10.1097/FBP.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, & Cooper P (1996). The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Development, 67(5), 2512. 10.2307/1131637 [DOI] [PubMed] [Google Scholar]
- Nakić Radoš S. (2018). Anxiety during pregnancy and postpartum: Course, predictors and comorbidity with postpartum depression. Acta Clinica Croatica, 57(1), 39–51. 10.20471/acc.2018.57.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, & Bridges RS (2011). Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress, 14(6), 677–684. 10.3109/10253890.2011.605487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Febo M, Huang W, Colon-Perez LM, Payne L, Poirier GL, Greene O, & King JA (2018). Early life social stress and resting state functional connectivity in postpartum rat anterior cingulate circuits. Journal of Affective Disorders, 229, 213–223. 10.1016/j.jad.2017.12.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID,Toschi N, Ohl F,Torner L,& Krömer SA (2001). Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. European Journal of Neuroscience, 13(5), 1016–1024. 10.1046/j.0953-816x.2001.01460.x [DOI] [PubMed] [Google Scholar]
- Neumann I, Kromer S, & Bosch O (2005). Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology, 30(8), 791–806. 10.1016/j.psyneuen.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Ogawa S, & Makino J (1984). Aggressive behavior in inbred strains of mice during pregnancy. Behavioral and Neural Biology, 40(2), 195–204. 10.1016/S0163-1047(84)90303-0 [DOI] [PubMed] [Google Scholar]
- Osborne S, Biaggi A, Chua TE, Du Preez A, Hazelgrove K, Nikkheslat N, Previti G, Zunszain PA, Conroy S, & Pariante CM (2018). Antenatal depression programs cortisol stress reactivity in offspring through increased maternal inflammation and cortisol in pregnancy: The Psychiatry Research and Motherhood – Depression (PRAM-D) Study. Psychoneuroendocrinology, 98, 211–221. 10.1016/j.psyneuen.2018.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul IM, Downs DS, Schaefer EW, Beiler JS, & Weisman CS (2013). Postpartum Anxiety and Maternal-Infant Health Outcomes. Pediatrics, 131(4), e1218–e1224. 10.1542/peds.2012-2147 [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Lonstein JS, & Fleming AS (2017). The neurobiology of postpartum anxiety and depression. Trends in Neurosciences, 40(2), 106–120. 10.1016/j.tins.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Howard M, Salisbury A, & Zlotnick C (2009). Postpartum depression. American Journal of Obstetrics and Gynecology, 200(4), 357–364. 10.1016/j.ajog.2008.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel J, Petrovic D, & Jones C (1990). Defensive burying, nest relocation, and pup transport in lactating female rats. The Quarterly Journal of Experimental Psychology, 42(4), 401–411. [PubMed] [Google Scholar]
- Ramos-Ortolaza DL, Doreste-Mendez RJ, Alvarado-Torres JK, & Torres-Reveron A (2017). Ovarian hormones modify anxiety behavior and glucocorticoid receptors after chronic social isolation stress. Behavioural Brain Research, 328, 115–122. 10.1016/j.bbr.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KM, & Taylor MG (2015). Social support, stress, and maternal post-partum depression: A comparison of supportive relationships. Social Science Research, 54, 246–262. 10.1016/j.ssresearch.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, & Baram TZ (2008). A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology, 149(10), 4892–4900. 10.1210/en.2008-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, & Williams SK (2011). Mesolimbic dopamine transients in motivated behaviors: Focus on maternal behavior. Frontiers in Psychiatry, 2. 10.3389/fpsyt.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger ZJ, De Guzman RM, Jacobskind JS, Saglimbeni B, Malone M, Fico D, Justice NJ, Forni PE, & Zuloaga DG (2020). Sex-dependent effects of chronic variable stress on discrete corticotropin-releasing factor receptor 1 cell populations. Physiology & Behavior, 219, 112847. 10.1016/j.physbeh.2020.112847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M, Torrero C, & Pulido S (1984). Long-term alterations in the maternal behavior of neonatally undernourished rats. Physiology & Behavior, 33(2), 273–278. 10.1016/0031-9384(84)90111-2 [DOI] [PubMed] [Google Scholar]
- Scheich B, Vincze P, Szőke É, Borbély É, Hunyady Á, Szolcsányi J, Dénes Á, Környei Zs., Gaszner B & Helyes Zs. (2017). Chronic stress-induced mechanical hyperalgesia is controlled by capsaicin-sensitive neurones in the mouse. European Journal of Pain, 21(8), 1417–1431. 10.1002/ejp.1043 [DOI] [PubMed] [Google Scholar]
- Shorey S, Chee CYI, Ng ED, Chan YH, Tam WWS, & Chong YS (2018). Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. Journal of Psychiatric Research, 104, 235–248. 10.1016/j.jpsychires.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Peterson KE, Hughes MD, & Gottlieb BR (2006). The role of social networks and support in postpartum women’s depression: A multiethnic urban sample. Maternal and Child Health Journal, 10(4), 375–383. 10.1007/s10995-005-0056-9 [DOI] [PubMed] [Google Scholar]
- Tietz A, Zietlow A-L, & Reck C (2014). Maternal bonding in mothers with postpartum anxiety disorder: The crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Archives of Women’s Mental Health, 17(5), 433–442. 10.1007/s00737-014-0423-x [DOI] [PubMed] [Google Scholar]
- Webster J, Nicholas C, Velacott C, Cridland N, & Fawcett L (2011). Quality of life and depression following childbirth: Impact of social support. Midwifery, 27(5), 745–749. 10.1016/j.midw.2010.05.014 [DOI] [PubMed] [Google Scholar]
- Wenzel A, Haugen EN, Jackson LC, & Brendle JR (2005). Anxiety symptoms and disorders at eight weeks postpartum. Journal of Anxiety Disorders, 19(3), 295–311. 10.1016/j.janxdis.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Xie R-H, He G, Koszycki D, Walker M, & Wen SW (2009). Prenatal Social Support, Postnatal Social Support, and Postpartum Depression. Annals of Epidemiology, 19(9), 637–643. 10.1016/j.annepidem.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Zhao X, Seese RR, Yun K, Peng T, & Wang Z (2013). The role of galanin system in modulating depression, anxiety, and addiction-like behaviors after chronic restraint stress. Neuroscience, 246, 82–93. 10.1016/j.neuroscience.2013.04.046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.