Abstract
Background:
There are an increasing number of patients with oral sensory complaints (OSCs) presenting to our dental clinic. For most dentists, it is difficult to distinguish burning mouth syndrome (BMS) from other oral mucosal diseases that may cause symptoms such as burning mouth. It is beneficial to effectively distinguish OSC patients to reduce misdiagnosis and eliminate burning symptoms as much as possible.
Methods:
Patients with oral burning sensations in the oral mucosal disease clinic were collected from the Peking University Hospital of Stomatology between September 1, 2014 and December 31, 2018. After excluding oral candidiasis, anemic stomatitis, dental material allergy, and other diseases from patients with oral sensory complaints, basic conditions such as gender, age, education level, job status, hyperglycemia, hypertension, hyperlipidemia, history of brain abnormalities, history of cervical spondylitis, history of thyroid disease, history of thyroid disease and insomnia were obtained. The BMS patients were compared with the control group. The t test and Chi-square test were used for statistical analysis to compare the clinical symptoms of these diseases and explore the risk factors for BMS.
Results:
In this case-control study, 395 patients (321 females and 74 males, mean age 55.26 ± 10.51 years) with oral sensory complaints and 391 healthy controls (281 females and 110 males, mean age 47.11 ± 13.10 years) were enrolled, among which, 8.4% (33/395) had oral candidiasis, 1.3% (5/395) had dental material allergy, 0.8% (3/395) had anemic stomatitis and 0.5% (2/395) had lichen planus. A total of 352 patients were eventually diagnosed with BMS. Anxiety and depression were more severe in BMS patients, as were the incidences of sleep disorders and brain abnormalities. Logistic regression analysis showed that age (odds ratio [OR] = 2.79, 95% confidence interval [CI]: 1.61–4.83, P < 0.001), total cholesterol level (OR = 2.92, 95% CI: 1.32–6.50, P = 0.009) and anxiety score (OR = 1.75, 95% CI: 1.01–2.77, P = 0.017) significantly increased the incidence of BMS. Patients with hyperglycemia (OR = 0.46, 95% CI: 0.23–0.89, P = 0.022), low body mass index (BMI: OR = 0.57, 95% CI: 0.34–0.93, P = 0.026) and low education level (OR = 3.43, 95% CI: 1.91–6.15, P < 0.001) were more likely to suffer from BMS.
Conclusions:
Oral candidiasis, anemic stomatitis, and dental material allergy with burning symptoms should be excluded from patients with BMS. It is recommended to conduct a questionnaire survey (including anxiety and depression), blood cell analysis, and salivary fungus culture for all patients with an oral burning sensation. It is necessary to conduct a patch test on patients with oral burning sensations and metal restorations.
Keywords: Oral sensory complaints, Burning mouth syndrome, Patch test, Candida, Anemia, Anxiety, Depression
Introduction
Burning mouth syndrome (BMS) is described as an intraoral burning or dysaesthetic sensation that recurs daily for more than 2 h per day for at least 3 months without any oral mucosal abnormalities.[1] The pain of BMS is usually bilateral and may be accompanied by subjective dry mouth, numbness, roughness, and taste changes. The most common site is the tongue. These abnormal sensations may be similar to those of oral diseases (oral candidiasis, lichen planus, metal allergy, etc.) or systemic diseases (drug induction, anemia, folic acid deficiency, diabetes mellitus, etc.), which can easily lead to misdiagnosis.[2] Laboratory and brain imaging studies have shown changes in the central and peripheral nervous systems of BMS patients.[3]
The reported prevalence ranged from 0.11% to 10.8%.[4,5] There are many reasons for such a wide range of morbidity, primarily due to the inconsistency of early diagnostic criteria, leading to higher morbidity. In general, burning is the most common type of oral mucosal pain in the study[6] and it is the most commonly used descriptive term for BMS patients. There are many additional descriptive terms, such as tingling, numbness, itching, and discharge.[7] Burning symptoms in BMS patients do not correspond to anatomical pathways. There are no mucosal lesions or known neurological or systemic diseases to explain the symptoms or characteristic laboratory abnormalities. Persistent oral and maxillofacial pain can lead to impaired eating ability, reduced social activity, and depression. Pain is the most common reason why patients seek treatment.
Misdiagnosis is also a problem. People often do not distinguish the cause, and all patients with burning sensations are diagnosed with BMS. However, some pains are caused by oral Candida infection, anemic stomatitis, dental material allergies, etc. Treating these diseases can effectively eliminate the burning sensation in the mouth. Therefore, those who suffer from BMS should be those who still have the burning sensation after excluding these diseases. We should not expand the diagnosis of BMS.
In this study, we will distinguish all patients with the oral burning sensation in our outpatient clinics, treat patients diagnosed with oral Candida infection, anemia stomatitis, and dental material allergies first, and eliminate the possibility of pain caused by these factors. Subsequently, the patient was diagnosed with BMS. The relationship between BMS and multiple factors will also be analyzed.
Methods
Ethical approval
The protocol of this study was evaluated and approved by Peking University School and Hospital of Stomatology Ethics Committee (No. PKUSSIRB-201412029). All patients were informed about the details of rights, benefits, and obligations of the study, and they signed informed consent before participating in the survey.
Study design, inclusion, and exclusion criteria
The subjects of the experimental group were patients who went to the Department of Oral Medicine, Peking University Hospital of Stomatology from September 1, 2014 to December 31, 2018. The control group was enrolled in the physical examination center of Beijing Hospital.
The inclusion criteria for patients with oral sensory complaints in this study were as follows: (1) 18 to 85 years old; (2) burning pain in the mouth more than 2 h a day for more than 3 months; (3) other subjective symptoms (dry mouth, abnormal oral sensation and taste disorders); and (4) no obvious oral mucosal lesions.
Inclusion criteria for subjects in the control group were as follows: (1) 18 to 85 years old; (2) no abnormal sensation of the oral mucosa; and (3) no abnormal oral mucosa in an oral examination.
The exclusion criteria for both the experimental and control groups were as follows: (1) severe encephalopathy affecting the ability to cooperate and (2) pregnancy.
Information collection
Demographic and medical questionnaires were conducted in both groups. Information such as gender, age, BMI, education level, professional status, hyperlipidemia, hypertension, brain imaging abnormalities, cervical spondylosis, sleep disorders, triglyceride levels, total cholesterol levels, fasting blood sugar levels, anxiety and depression scores were collected. Age, BMI, and total cholesterol levels were also graded. Age was divided into a low and high group with a cutoff of 50 years; BMI was divided into a low index group and a high index group by 24 kg/m2; total cholesterol was divided into a normal blood lipid group and a high blood lipid group by 5.7 mmol/L; employment status was divided into an unemployed group and an employment group.
Oral general examination and oral mucosal examination were carried out, and information related to systemic diseases and all medications being taken was recorded.
To clarify the general condition of patients and investigate the potential causes, all patients underwent routine blood tests (including white blood cell count, red blood cell count, hemoglobin, platelet, blood sugar level, alanine aminotransferase, aspartate aminotransferase, total cholesterol, triglyceride, urea, and creatinine) to exclude all possible diseases. Salivary Candida culture was performed. Those with dental restorations were required to undergo a patch test. We also collected brain imaging information from BMS patients using CT and MRI examinations.
Assessment of anxiety or depression
The self-rating anxiety scale (SAS) and the self-rating depression scale (SDS) were used to assess the emotional state.[8] The SAS and SDS have 20 items each. Each score is multiplied by 1.25, and the integer part is the standard score. The standard scores below 50 were normal, 50–59 indicated mild anxiety or depression, 60–69 indicated moderate anxiety or depression, and more than 70 were severe anxiety or depression.[9]
Treatment of anemic stomatitis
Anemic stomatitis was diagnosed in patients with lower erythrocyte counts and hemoglobin levels and burning symptoms of oral mucosa and was transferred to the Department of Hematology for treatment. When erythrocyte counts and hemoglobin levels returned to normal, the symptoms of oral burning were evaluated. If the symptoms of burning persisted, BMS was diagnosed and the patient was included in the experimental group; otherwise, the subject was excluded from the study.
Treatment of oral candidiasis
Patients with positive salivary Candida cultures were treated with nystatin for 4 weeks. Then, the drug was stopped for one week, and salivary Candida culture was reexamined. If the result was negative and burning symptoms still existed, BMS was diagnosed; otherwise, the subject was excluded from the study.
Treatment of dental material allergy
A patch test was performed in patients with amalgam fillings and/or metal restorations. The types of tested substances included Benzocaine, rubber mixture, neomycin, p-phenylenediamine, potassium dichromate, imidazolium alkyl urea, ibuprofen, p-hydroxybenzoate mixture, formalin, epoxy resin, rosin, ethylenediamine, spices, nickel sulfate, and mercury reduction. For dental material allergy patients, nonmetallic fillers and/or nonmetallic restorations are replaced instead. If the burning symptoms persisted, BMS was diagnosed. If the burning symptoms disappeared, the subject was excluded from the study.
Statistical analysis
Data were processed and analyzed using SPSS 20.0 (IBM Co., Ltd., NY, USA). The odds ratio (OR) and 95% confidence interval (CI) were calculated in comparison to the variables between the BMS group and the control group. In addition, a t test was used to evaluate the differences among groups in age, demography, and medicine. A backward logistic regression model was applied to estimate the parameters with significant changes. Statistical significance was set as P < 0.05.
Results
A total of 395 patients with oral sensory complaints and 391 controls participated in the study. A total of 395 patients with oral sensory complaints included 8.4% (33/395) candidiasis, 1.3% (5/395) dental material allergy, 0.8% (3/395) anemic stomatitis and 0.5% (2/395) lichen planus. Finally, 352 patients were diagnosed with BMS.
There were 283 women and 69 men in the BMS group. The average age of the BMS group was 55.26 ± 10.51 years and that of the control group was 47.11 ± 13.10 years. All patients had a history of oral disease for more than 3 months (range: 3–192 months).
Candidiasis was detected in the saliva of 11.4% (45/395) of patients with OSC. After anti-Candida treatment, 8.3% (33/395) of the patients improved or recovered significantly, but 3.0% (12/395) of the patients did not improve significantly. These patients were also diagnosed with BMS. In the control group, salivary fungus cultures were detected in 156 subjects, and 13 were Candida-positive. There was no significant difference in the detection rate of salivary Candida between the experimental and control groups.
Among the 352 patients diagnosed with BMS, the burning sensation of oral mucosa was the most common sensory abnormality. Another 17.9% (63/352) of patients had dry mouth symptoms, and 6.8% (24/352) had taste disorders. A total of 10.22% (36/352) of patients complained of abnormal sensation in the whole oral mucosa. A total of 87.5% (308/352) of patients had abnormal sensation in the tongue, 23.0% (81/352) in the upper palate, 4.5% (16/352) in both cheeks, 9.1% (32/352) in the lip mucosa, 1.4% (5/352) in the gingiva and 0.3% (1/352) in the pharynx.
Of the 395 OSC patients, 38 patients with metal restoration underwent patch tests, of which nine were positive for mercury and nickel. The positive rate was 23.68% (9/38). After removing the old filler, five patients recovered from OSC, and 4 remained unchanged. In the 391 in control group, a total of 26 patients with metal restoration underwent a patch test, of which two were positive for mercury and nickel. The positive rate was 7.69% (2/26). Although the positive rate of the patch test in the experimental group was higher than that in the control group, there was no significant difference between the two groups through the chi-square test. The number of metal fillers in OSC patients was 2.03 ± 1.00 and 1.48 ± 0.51 in the control group. The result of the t-test was significantly different, P < 0.05.
Among the patients with abnormal oral sensation, three had no abnormal oral clinical examination. Anemia was found after blood cell analysis, and the referral hematology department was diagnosed with iron deficiency anemia. After more than 3 months of treatment, when the patient returned to visit, the abnormal blood cell analysis and oral paresthesia had disappeared.
All patients were examined regularly. During the follow-up, however, two patients were found to have white reticular stripes on their cheeks. Although the texture was very shallow, they were eventually diagnosed as having lichen planus.
After 71 patients with BMS went to the Department of Neurology, neurologists performed computed tomography (CT) or magnetic resonance imaging (MRI) brain imaging. A total of 39.4% (28/71) of the patients had abnormal brain imaging. Lacunar infarction occurred in 21.1% (15/71) of the patients. A total of 9.8% (7/71) of these patients had ischemic lesions. A total of 2.8% (2/71) of these patients had demyelination changes. The occurrence areas of brain malformations were 5.5% (11/71) in the parietal frontal lobe and 7.0% (5/71) in the basal ganglia. The remaining brain lesions were distributed in the center of the semiovale. Twenty-two patients in the control group were examined by computed tomography or magnetic resonance imaging. A total of 27.3% (6/22) of patients had abnormal brain imaging, lacunar infarction in three cases, demyelination in two cases, and an ischemic lesion in one case. According to the chi-square test, the incidence of brain imaging abnormalities in patients with BMS was significantly higher than that of the control group (P < 0.05).
A total of 32.4% (114/352) of BMS patients had sleep disorders, of which 20.5% (72/352) had used hypnotics to help them sleep. In the control group, 28.1% (110/391) of the subjects had sleep problems. The Chi-square test showed that there was no significant difference between the BMS group and the control group (P > 0.05).
The gender, age, body mass index, education level, working status, hyperlipidemia, hypertension, brain imaging abnormalities, sleep disorders, anxiety, depression, and fasting blood glucose of the BMS group and control group were analyzed using bivariate correlation analysis. Significant differences between groups were found in terms of gender, age, BMI, education level, job status, brain imaging abnormalities, total cholesterol, scores of anxiety, and depression. The results showed that BMS patients were older, more female, less educated, more unemployed, had a higher incidence of abnormal brain image abnormalities, and had a higher level of total cholesterol, anxiety, and depression. Table 1 shows the general situation and statistical results of the BMS group and the control group. There were significant differences in age and gender between the two groups. The BMS group was more common in women and the elderly.
Table 1.
Multiple comparisons of the characteristics of patients and controls.
| Parameters | BMS (n = 352) | Controls (n = 391) | Statistics | P |
| Age | 55.26 ± 10.51 | 47.11 ± 13.10 | 16.379∗ | <0.001 |
| <50 years | 98 (27.8) | 215 (55.0) | 55.991† | <0.001 |
| ≥50 years | 254 (72.2) | 176 (45.0) | 55.991† | <0.001 |
| Female | 283 (83.2) | 281 (71.8) | 7.370† | 0.007 |
| BMI (kg/m2) | 24.22 ± 3.14 | 23.31 ± 4.08 | 1.574∗ | 0.001 |
| <24 | 199 (56.5) | 192 (49.1) | 4.101† | 0.043 |
| ≥24 | 153 (43.5) | 199 (50.9) | 4.101† | 0.043 |
| Education, >high school | 85 (24.1) | 199 (50.9) | 56.12† | <0.001 |
| Job status in active | 89 (25.3) | 130 (33.2) | 5.650† | 0.017 |
| Hyperglycemia | 38 (10.8) | 28 (7.2) | 3.020† | 0.082 |
| Hypertension | 98 (27.8) | 92 (23.5) | 1.810† | 0.179 |
| History of brain abnormalities | 81 (23.0) | 22 (5.6) | 46.900† | <0.001 |
| History of cervical spondylitis | 207 (58.8) | 226 (57.8) | 0.080† | 0.781 |
| Sleep disorders | 114 (32.4) | 110 (28.1) | 1.590† | 0.207 |
| Triglyceride (mmol/L) | 1.42 ± 0.73 | 1.33 ± 0.86 | 4.290∗ | 0.181 |
| Total cholesterol (mmol/L) | 4.89 ± 0.92 | 4.64 ± 0.83 | 9.961∗ | <0.001 |
| <5.7 | 278 (78.9) | 365 (93.4) | 32.855† | <0.001 |
| ≥5.7 | 74 (21.1) | 26 (6.6) | 32.855† | <0.001 |
| Score of anxiety | 47.28 ± 11.03 | 40.39 ± 8.14 | 25.398∗ | <0.001 |
| <50 | 226 (64.2) | 332 (84.9) | 42.471† | <0.001 |
| ≥50 | 126 (35.8) | 59 (15.1) | 42.471† | <0.001 |
| Score of depression | 49.92 ± 13.63 | 41.64 ± 10.30 | 20.256∗ | <0.001 |
| <50 | 187 (53.1) | 323 (82.6) | 74.808† | <0.001 |
| ≥50 | 165 (46.9) | 68 (17.4) | 74.808† | <0.001 |
| FBG (mg/dL) | 5.47 ± 0.78 | 5.29 ± 0.76 | 3.471∗ | 0.001 |
Values are presented as means ± standard deviation or n (%).
t values.
χ2values; BMI: Body mass index; BMS: Burning mouth syndrome; FBG: Fasting blood glucose.
Univariate analysis showed that several features of the BMS group were significantly different from those of the control group (P < 0.05). The nine variables were BMI, education level, practice status, fasting blood sugar level, brain imaging abnormalities, thyroid disease, total cholesterol, anxiety score, and depression score. For the other variables investigated, there were no significant differences between the BMS group and the control group.
The binary logistic regression multivariate analysis was conducted with age, gender, education level, job status, BMI, total cholesterol, hyperglycemia, scores of anxiety, and depression as dependent variables. The results showed that the significant variables were age, education level, BMI, score and anxiety, and total cholesterol levels. Older patients were more likely to suffer BMS (OR = 2.791, P < 0.001); the lower the education level, the more susceptible they were to BMS (OR = 3.427, P < 0.001); the higher the total cholesterol level, the more susceptible they were to BMS (OR = 2.923, P = 0.009); the higher the anxiety level, the more susceptible they were to BMS (OR = 1.749, P < 0.017); and the lower the BMI, the more susceptible they were to BMS (OR = 0.566, P = 0.026). People with normal blood sugar were more susceptible to BMS (OR = 0.456, P = 0.022) [Table 2].
Table 2.
Logistic regression analysis about risk factors of BMS.
| 95.0% CI for EXP (B) | |||||||
| Parameters | B | SE | Wald | Significant | Exp (B) | Lower | Upper |
| Age | 1.026 | 0.279 | 13.499 | 0 | 2.791 | 1.614 | 4.825 |
| BMI | −0.568 | 0.255 | 4.976 | 0.026 | 0.566 | 0.344 | 0.933 |
| TC | 1.073 | 0.408 | 6.923 | 0.009 | 2.923 | 1.315 | 6.501 |
| SA | 0.559 | 0.234 | 5.704 | 0.017 | 1.749 | 1.105 | 2.766 |
| SD | 0.310 | 0.171 | 3.294 | 0.070 | 1.363 | 0.976 | 1.904 |
| Hyperglycemia | −0.785 | 0.343 | 5.241 | 0.022 | 0.456 | 0.233 | 0.893 |
| Education level | 1.232 | 0.298 | 17.058 | 0 | 3.427 | 1.910 | 6.149 |
| Job status | 0.331 | 0.311 | 1.132 | 0.287 | 1.393 | 0.757 | 2.583 |
| Constant | −5.668 | 1.034 | 30.058 | 0 | 0.003 | – | – |
BMS: Burning mouth syndrome; CI: Confidence interval; SE: Standard error; BMI: Body mass index; TC: Total cholesterol; SA: Score of anxiety; SD: Score of depression; FBG: Fasting blood glucose; –: No data.
Discussion
The etiology of BMS is not very clear. It is thought to be multifactorial and related to various local, systemic, mental, and neurological causes.[10] The etiology of BMS has aroused considerable controversy in the medical literature, and several factors and different concepts have been proposed.[11] Perhaps the most relevant aspect is whether BMS should be considered a “unique entity” or a “symptom,” as reported in a recent review.[12] Under the latter hypothesis, the focus of the study was to identify the causal factors in BMS. Unfortunately, most of the research methods in local or systemic aspects were inadequate and do not support any pathogenic hypothesis.
Previous literature suggests that patients with OSC need to exclude known diseases before they can be diagnosed with BMS,[13] and oral candidiasis may cause tongue pain without objective abnormalities.[14] However, oral candidiasis with subjective symptoms but no objective signs are often ignored. In the past, this type of oral candidiasis was diagnosed as BMS. The same is true for anemia and dental material allergies. We should be soberly aware that not all OSCs are BMSs. This study indicates that 10.89% of OSCs are not BMS. Their oral burning sensations caused by Candida infection, anemia, dental material allergy, etc. can be eliminated by removing pathogenic factors. In other words, 10.89% of OSC is easier to relieve pain in the short term than BMS. We have a responsibility to help these people. Therefore, we suggest that the diagnosis path of OSC should be this: any diagnosis of OCS patients excluded Candida infection, anemia, dental material allergy, etc. first, and then consider the diagnosis of BMS, as shown in Figure 1.
Figure 1.
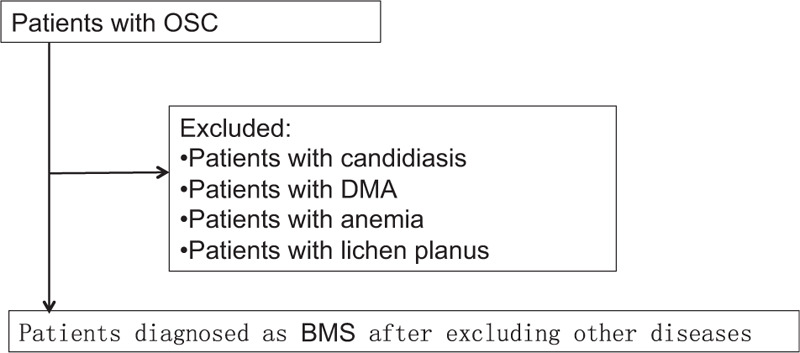
Diagnostic flow chart of BMS. BMS: Burning mouth syndrome; OSC: Oral Sensorial Complaints; DMA: Dental Material Allergy.
In this study, 33/395 (8.3%) patients with OSC had no mucosal lesions during the first clinical examination, but Candida was found in saliva after culture. After anti-Candida treatment, salivary Candida culture became negative, and abnormal oral sensation disappeared. This suggested that this patient previously diagnosed with BMS should be treated with anti-candidal drugs rather than with clonazepam.[15]
Candida was positive with a burning sensation but no oral mucosal lesions, which was not described in the previous classification of oral candidiasis. Nevertheless, this is real. Antifungal treatment can make Candida negative, and the burning sensation disappears. It also proves that the diagnosis of oral candidiasis, not BMS, is correct. We recommend that the clinical criteria of oral Candida infection should be increased by one: burning sensation or asymptomatic type.
A patch test is a common skin test for identifying allergens in allergic contact dermatitis. It can also be used to determine whether patients are allergic to dental metal materials. The common manifestations of oral allergies are congestion, edema, and white keratosis in the oral mucosa, which can be diagnosed as lichenoid lesion.[16] Kedward et al[16] found that 23 out of 47 BMS patients were allergic to nickel sulfate and 2 patients were allergic to mercury. Büyüköztürk et al[17] reported a case of nickel-sensitized patients with systemic symptoms caused by nickel-containing dental alloys. However, some people believe that nickel can be detected by patch tests; nevertheless, there is no evidence that individual patients increase the risk of nickel allergy due to exposure to nickel-containing dental restorations.[18]
In this study, patients with metal restorations had burning sensations but no oral mucosal congestion, edema, or other lesions. The positive patch test indicated that the burning sensation may be caused by metal restoration. After replacing the metal restoration with a nonmetal restoration, the disappearance of the burning sensation further proves that the diagnosis was correct. Removal of metal allergens is also an effective treatment method. It is not recommended to diagnose such patients as BMS. We have a breakthrough in our understanding: metal allergies can also only have a burning sensation without oral mucosal damage.
Physical examination alone is not enough to diagnose anemia; however, it usually provides important clues to help determine the cause of anemia.[19] Some patients with anemia may have symptoms such as fatigue, mental retardation, pallor of the skin and mucosa, atrophy of the tongue papilla, and flaky redness of the oral mucosa, called anemic stomatitis. Most patients had a burning sensation in the mouth. In this study, 3 patients with abnormal oral sensations were diagnosed with iron deficiency anemia after blood examination, although no abnormality was found in the oral examination, and they recovered after anemia treatment. This indicates that a small number of patients with anemia have only an oral burning sensation but no oral mucosal lesions, which can easily lead us to misdiagnose them as BMS.
Functional brain imaging revealed typical neurological and brain changes in BMS patients. The brain activity of BMS patients was weaker than that of the control group, especially the bilateral thalamus. Neurophysiological and neurotransmitter PET showed that the basal ganglia, especially brain dopaminergic networks, played an important role in pain management and regulation in BMS patients.[20–22] The experimental evidence also supports the notion of a relationship between the striatal dopamine system and trigeminal neuralgia. Damage to the dopamine pathway in the substantia nigra striatum can also lead to pain in the trigeminal nerve area.[22] Santos et al[23] found that the occurrence of depressive symptoms was related to the location of cerebral ischemia. The incidence of depression was higher in patients with basal ganglia and thalamic ischemia. It was speculated that some parts of the brain, such as basal ganglia ischemia, may affect the secretion of neurotransmitters such as dopamine, leading to anxiety and depression and then somatization symptoms.
This study found that the prevalence of brain diseases in BMS patients was significantly higher than that in the control group. In the course of collecting patients’ medical histories, we found that most patients had brain imaging examinations in other hospitals to exclude brain abnormalities. When brain abnormalities are detected by imaging examination, they can aggravate anxiety and depression, leading to worsening abnormal sensation of the mouth. In this study, the proportion of brain imaging examinations in the control group was relatively low, and further research is needed to increase the sample size. OSC may be an early sign of brain diseases, which can prompt early brain imaging examination, early detection of central nervous system-related diseases, and early prevention.
BMS was considered to be psychiatric pain until neurological disorders were discovered. This is because of the high scores of the Psychological Assessment Scale, especially depression and anxiety. Recent studies have shown that anxiety may be more important than depression,[24] which is the same conclusion as this study. It is well known that patients with BMS mental disorders are secondary nonspecific phenomena often encountered by patients with chronic pain. Whether anxiety and depression lead to BMS or vice versa, is unclear.[25] Some scholars believe that the anxiety and depression of BMS patients are more serious. In this study, the self-rating anxiety and depression scale was used to screen the anxiety and depression status of patients in a short time and to provide a timely referral to the Department of Psychiatry for diagnosis and treatment. Previous studies used various anxiety and depression scales, such as the Baker Anxiety Scale,[26] General Hospital Anxiety Hospital, and Depression Scale,[27] and the results were consistent with those of this study.
BMS usually occurs gradually. In this study, BMS patients were mostly housewives or retired, with dissatisfactory economic status. Half of the patients had a lower education level. Most of them were women with lower socioeconomic status in rural towns. Poor capability to cope with negative life events (such as major events in the family, dietary changes, anxiety, and depression) may lead to somatization symptoms, which may also be the cause of oral sensory complaints. Most of the patients in this study had severe psychological fear of cancer before seeking medical treatment. After consulting with the doctor, the tension was relieved, and the sleep condition improved.
Sardella et al[28] found that 15% of patients had high glucose levels, but they did not find a statistically significant difference in hyperglycemia compared with the control group. Morr Verenzuela et al[29] also found that almost 24% of patients had increased blood glucose levels. This study found that fasting blood glucose in the BMS group was higher than that in the control group. This suggests that hyperglycemia may be involved in neurosensory changes, leading to an abnormal sensation in patients. One of the clinical symptoms of diabetes is nerve numbness in the skin, which may cause a burning sensation or numbness of the oral mucosa in patients with poor blood sugar control. The control group of diabetic patients had better blood sugar control. Conversely, the appearance of abnormal sensation in oral mucosa prompts early detection of hyperglycemia or diabetes.
Previous studies did not suggest that total cholesterol in BMS patients was higher than that in the control group. However, Sangwan et al[30] found that patients with hyperlipidemia were more likely to suffer from periodontal disease than the general population. It is suggested that further studies may need to explore the correlation between BMS and chronic periodontitis. In this study, we found that the level of total cholesterol in BMS patients was significantly higher than that in the control group. It is speculated that hyperlipidemia increases blood viscosity and reduces blood flow speed, thereby increasing the risk of cerebral ischemic diseases leading to central nervous-type BMS.
The diagnosis of BMS is difficult and is a diagnosis of exclusion. Before diagnosis, we should try to find as many local, systemic, and psychological factors as possible. The treatment of BMS is also difficult. However, if we can find that the oral burning sensation is caused by oral Candida infection, anemia, or dental metal material allergies, it will become certain and easy to eliminate the burning sensation.
Conflicts of interest
None.
Footnotes
How to cite this article: Jin JQ, Cui HM, Han Y, Su S, Liu HW. Multifactor analysis of patients with oral sensory complaints in a case-control study. Chin Med J 2020;133:2822–2828. doi: 10.1097/CM9.0000000000001190
Jian-Qiu Jin and Hong-Mei Cui contributed equally to this work.
References
- 1. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie A, Kramer JM. Recent advances in the etiology and treatment of burning mouth syndrome. J Dent Res 2018; 97:1193–1199. doi: 10.1177/0022034518782462. [DOI] [PubMed] [Google Scholar]
- 3.Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain 2003; 101:149–154. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 4.Kohorst JJ, Bruce AJ, Torgerson RR, Schenck LA, Davis MDP. The prevalence of burning mouth syndrome: a population-based study. Br J Dermatol 2015; 172:1654–1656. doi: 10.1111/bjd.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki N, Mashu S, Toyoda M, Nishibori M. Oral burning sensation: prevalence and gender differences in a Japanese population. Pain Pract 2010; 10:306–311. doi: 10.1111/j.1533-2500.2010.00361.x. [DOI] [PubMed] [Google Scholar]
- 6.Fischoff DK, Spivakovsky S. Little evidence to support or refute interventions for the management of burning mouth syndrome. Evid Based Dent 2017; 18:57–58. doi: 10.1038/sj.ebd.6401244. [DOI] [PubMed] [Google Scholar]
- 7.Braud A, Touré B, Agbo-Godeau S, Descroix V, Boucher Y. Characteristics of pain assessed with visual analog scale and questionnaire in burning mouth syndrome patients: A pilot study. J Orofac Pain 2013; 27:235–242. doi: 10.11607/jop.1038. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Li S, Shen C, Shan J, Fan Y. Expression and significance of phosphodiesterase 4B gene in peripheral blood of patients with oral lichen planus. Int J Dermatol 2019; 58:302–310. doi: 10.1111/ijd.14203. [DOI] [PubMed] [Google Scholar]
- 9.Milanović SM, Erjavec K, Poljičanin T, Vrabec B, Brečić P. Prevalence of depression symptoms and associated socio-demographic factors in primary health care patients. Psychiatr Danub 2015; 27:31–37. [PubMed] [Google Scholar]
- 10.Scardina GA, Ruggieri A, Provenzano A, Messina P. Burning mouth syndrome is acupuncture a therapeutic possibility. Br Dental J 2010; 209:E2.doi: 10.1038/sj.bdj.2010.582. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen AML, Smidt D, Nauntofte B, Christiani CJ, Jerlang BB. Burning mouth syndrome: etiopathogenic mechanisms, symptomatology, diagnosis and therapeutic approaches. Oral Biosci Med 2004; 1:3–19. [Google Scholar]
- 12.Chimenos-Küstner E, de Luca-Monasterios F, Schemel-Suárez M, Rodríguez de Rivera-Campillo ME, Pérez-Pérez AM, López-López J. Burning mouth syndrome and associated factors: a case-control retrospective study. Med Clin (Barc) 2017; 148:153–157. doi: 10.1016/j.medcli.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Osaki T, Yoneda K, Yamamoto T, Ueta E, Kimura T. Candidiasis may induce glossdynia without objective manifestation. Am J Med Sci 2000; 319:100–105. doi: 10.1097/00000441-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Peng F, Schwartz RA, Chen Z, Zhang JZ. High prevalence of contact hypersensitivity to metals and preservatives in Chinese patients with atopic dermatitis. Chin Med J 2019; 132:2881–2882. doi: 10.1097/CM9.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, Xu H, Chen FM, Liu JL, Jiang L, Zhou Y, et al. Efficacy evaluation of clonazepam for symptom remission in burning mouth syndrome: a meta-analysis. Oral Dis 2016; 22:503–511. doi: 10.1111/odi.12422. [DOI] [PubMed] [Google Scholar]
- 16.Kedward AL, Crawley JM, Armstrong KD, Marley J, Cowan G, Lamey PJ. The role of patch testing in oral disease. Dermatitis 2010; 21:294–296. [PubMed] [Google Scholar]
- 17.Büyüköztürk S, Gelincik A, Ünal D, Demirtürk M, Çelik DD, Erden S, et al. Oral nickel exposure may induce Type I hypersensitivity reaction in nickel-sensitized subjects. Int Immunopharmacol 2015; 26:92–96. doi: 10.1016/j.intimp.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Setcos JC, Babaei-Mahani A, Silvio LD, Mjör IA, Wilson NH. The safety of nickel containing dental alloys. Dent Mater 2006; 22:1163–1168. doi: 10.1016/j.dental.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 19. Martin Greenberg, Michael Glick, Jonathan Ship. Burket's Oral Medicine. 11th ed. Hamilton: BC Decker Inc, 2008. [Google Scholar]
- 20.Chimenos-Küstner E, de Luca-Monasterios F, Schemel-Suárez M, Rodríguez de Rivera-Campillo ME, Pérez-Pérez AM, López-López J. Burning mouth syndrome and associated factors: A case-control retrospective study. Med Clin (Barc) 2017; 148:153–157. doi: 10.1016/j.medcli.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Liu BL, Yao H, Zheng XJ, Du GH, Shen XM, Zhou YM, et al. Low regional cerebral blood flow in burning mouth syndrome patients with depression. Oral Dis 2015; 21:602–607. doi: 10.1111/odi.12322. [DOI] [PubMed] [Google Scholar]
- 22.Jaaskelainen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J. Role of the dopaminergic system in chronic pain-afluorodopa-PET study. Pain 2001; 90:257–260. doi: 10.1016/s0304-3959(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 23.Santos M, Gold G, Kövari E, Herrmann FR, Bozikas VP, Bouras C, et al. Differential impact of lacunes and microvascular lesions on poststroke depression. Stroke 2009; 40:3557–3562. doi: 10.1161/STROKEAHA.109.548545. [DOI] [PubMed] [Google Scholar]
- 24.Jääskeläinen SK, Woda A. Burning mouth syndrome Cephalalgia 2017; 37:627–647. doi: 10.1177/0333102417694883. [DOI] [PubMed] [Google Scholar]
- 25.Taiminen T, Kuusalo L, Lehtinen L, Forssell H, Hagelberg N, Tenovuo O, et al. Psychiatric (axis I) and personality (axis II) disorders in patients withburning mouth syndrome or atypical facial pain. Scand J Pain 2011; 2:155–160. doi: 10.1016/j.sjpain.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Amenábar JM, Pawlowski J, Hilgert JB, Hugo FN, Bandeira D, Lhüller, et al. Anxiety and salivary cortisol levels in patients with burning mouth syndrome: case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105:460–465. doi: 10.1016/j.tripleo.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Soto Araya M, Rojas Alcayaga G, Esguep A. Association between psychological disorders and the presence of Oral lichen planus, Burning mouth syndrome and Recurrent aphthous stomatitis. Med Oral 2004; 9:1–7. [PubMed] [Google Scholar]
- 28.Sardella A, Lodi G, Demarosi F, Uglietti D, Carrassi A. Causative or precipitating aspects of burning mouth syndrome: a case-control study. J Oral Pathol Med 2006; 35:466–471. doi: 10.1111/j.1600-0714.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 29.Morr Verenzuela CS, Davis MDP, Bruce AJ, Torgerson RR. Burning mouth syndrome: results of screening tests for vitamin and mineral deficiencies, thyroid hormone, and glucose levels-experience at Mayo Clinic over a decade. Int J Dermatol 2017; 56:952–956. doi: 10.1111/ijd.13634. [DOI] [PubMed] [Google Scholar]
- 30.Sangwan A, Tewari S, Singh H, Sharma RK, Narula SC. Periodontal status and hyperlipidemia: statin users versus non-users. J Periodontol 2013; 84:3–12. doi: 10.1902/jop.2012.110756. [DOI] [PubMed] [Google Scholar]


