Abstract
Background
No study has compared EUS-guided radiofrequency ablation (EUS-RFA) plus systemic chemotherapy (CMT) with CMT alone for unresectable pancreatic ductal adenocarcinoma.
Methods
This study compared the results of treatment in patients receiving EUS-RFA plus concomitant CMT (group A; n = 14) with those receiving CMT (group B; n = 14) as a pilot study.
Results
From July 2017 to August 2018, 4 and 2 patients from groups A and B, respectively, withdrew from the study because of progression of the disease. In total, 10 and 12 patients from groups A and B, respectively, completed the study. All 30 EUS-RFA procedures were successful. Mean maximal tumor diameter before treatment of group A (n = 10) versus B (n = 12) was 62.2 ± 21.0 versus 50.5 ± 22.0 mm, respectively (P = not significant). After treatment, no statistically significant difference in mean maximal tumor diameter was found between both groups. However, in group B, mean maximal tumor diameter was significantly increased from 50.5 ± 22.0 to 56.3 ± 18.7 mm, respectively (P = 0.017). Tumor necrosis occurred in group A versus B at 10 of 10 (100%) versus 6 of 12 (50%) patients, respectively (P = 0.014). After treatment, group A patients could reduce the mean narcotic pain drug dosage at 26.5 mg of morphine equivalent per day (from 63.6 to 37.1 mg, P = 0.022), whereas group B patients could not reduce the dosage of pain-controlled medication. No statistically significant difference in 6-month mortality rate was found. In group A, 1 procedure-related nonsevere adverse event (n = 1 of 30 [3.3%]) occurred in 1 patient (n = 1 of 14 [7.1%]).
Conclusions
In this study, the mean tumor diameter of group B was significantly increased after the treatment. Group A had a significantly higher rate of necrosis of tumor and required less narcotic.
Key words: EUS-guided radiofrequency ablation (EUS-RFA), Chemotherapy (CMT), Pancreatic ductal adenocarcinoma (PDAC), American Society of Clinical Oncology, Pancreatic cancer
INTRODUCTION
Prognosis of pancreatic ductal adenocarcinoma (PDAC) is poor with a 5-year survival rate less than 10%.[1] The American Society of Clinical Oncology published 2 clinical guidelines to help clinicians make a clinical decision for locally advanced and metastatic PDAC.[2,3] The guidelines suggest that the goals of treatment for patients are to control disease progression, symptoms, and the maintenance of quality of life. All patients should be offered information about clinical trials in all kinds of treatments. Local ablative therapies including EUS–guided RFA (EUS-RFA) have been proposed for local control of PDAC. A recent systematic review by Saccomandi et al.[4] concluded that a lack of standardization of methods and outcomes leads to contrasting results on safety and feasibility. Uniform conclusions thus require further structured investigations.
Our group aimed to compare the efficacy of EUS-RFA plus chemotherapy (CMT) with that of CMT alone. Given that this type of study has never been conducted before, we conducted it as a pilot study. Given that this procedure has not been done in our country before, we therefore chose to conduct a study in patients with either metastatic or locally advanced PDAC.
METHODS
Hypothesis and research questions
The hypothesis of the study was that EUS-RFA plus CMT can decrease tumor diameter for patients with unresectable PDAC versus CMT alone. The secondary research question was to compare the rates of necrosis of tumor in both treatments. The study underwent a per-protocol analysis.
Patients
The design of the study was prospective, observational, and open labeled with a case-control cohort matched by diagnosis and staging of the disease. Target population comprised patients with either metastatic or locally advanced PDAC. Sample population comprised patients with either metastatic or locally advanced PDAC presented for palliative treatment who underwent EUS-RFA plus CMT or CMT alone at the King Chulalongkorn Memorial Hospital, Bangkok, Thailand, during the study period. Enrollment period started from July 2017 to August 2018. Inclusion criteria were patients with either metastatic or locally advanced PDAC proven by pathology, age older than 18 years, an Eastern Cooperative Oncology Group (ECOG) score of 2 or lower,[5] and agreement to participate in the study. Exclusion criteria were pregnancy, uncorrectable coagulopathy, massive ascites, and an ECOG score of 3 or more.
Patients meeting the inclusion criteria and presenting in King Chulalongkorn Memorial Hospital, Bangkok, were identified and enrolled. The study was conducted under the administration of the Pancreas Research Unit, Department of Medicine, Faculty of Medicine, Chulalongkorn University and Excellence Center for Gastrointestinal Endoscopy, King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand. Patients presenting to the endoscopy unit for management of PDAC received EUS-RFA plus CMT and were classified as group A. During the study period, patients presented to oncology service for palliative treatment with systemic CMT were recruited as a matched case-control cohort and classified as group B. Group B patients were matched with group A for sex, staging of the disease, ECOG score, and final diagnoses of the diseases. Regimens of CMT given to patients in both groups were at the discretion of oncologists.
The protocol was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, in July 2017. The institutional review board number is 432/60. Funding was obtained from the Pancreas Research Unit, Chulalongkorn University, and the Royal College of Physicians of Thailand. The study was registered at the Thai Clinical Trials Registry (http://www.clinicaltrials.in.th) with clinical trial number 20180706001.
Before initiation of treatment in both groups, blood for complete blood count, and liver and kidney samples for function tests were collected and analyzed. Baseline demographic data including age, sex, ECOG score, history of comorbid disease, presenting symptoms, pain score, type and dosage of pain medications, and results of radiological examination of the abdomen (either computed tomography [CT] or magnetic resonance image) were collected. Informed consent was obtained from patients after full explanations of the risk and benefit of participating in the study.
Procedure
In group A patients, the EUS-RFA procedure was performed after the diagnosis of PDAC was made. Agreement of patients to participate in the study was then requested and documented in the consent form. Concomitant CMT was given to group A patients during the same period. After EUS-RFA, all patients were admitted overnight in the hospital to observe possible postprocedure complications. Assigned physicians interviewed and examined the patients as standard treatment in our hospital. If any procedure-related adverse event was suspected, research team members would be notified immediately. The EUS-RFA for PDAC was then repetitively scheduled and performed every 2 to 4 weeks after the initial procedure. Continuation of repetitive EUS-RFA procedure will be held when endosonographic findings show no area of a viable tumor for EUS-RFA or some conditions precluding the procedure. These conditions included intervening vessels, worsening ECOG score (≥3), patients' wishes, significant progression of the disease, and patients' death. In group B, patients underwent CMT as primary treatment without EUS-RFA. Standard care of patients receiving CMT as treatment of PDAC was provided to the patients. In both groups of patients, oncologists had provided a standard of care to all patients similar to patients receiving standard CMT. Dosage and regimen of pain-controlled medication were individually adjusted by clinicians who took care of the patients.
Techniques of EUS-RFA
The patients were placed in the left lateral position. Conscious sedation with propofol was used. The linear echoendoscope (Pentax, EG3870UTK) with ultrasound processor was passed through the upper digestive tract of the patients. The distal end of the echoendoscope was placed in a proper position that allows the endosonographer to pass the EUS-RFA needle into target lesions.
Once target lesions were identified. Radiofrequency ablation generator was turned on, and EUS-RFA probe was attached with an internal cooling system. We used continuous mode, setting maximum power at 50 W. The 19-gauge needle-type EUS-RFA probe was inserted through the echoendoscope and pins it into the target lesion according to the standard technique of EUS-guided needle biopsy [Figure 1]. When the needle is at the right position, the endosonographer then controls the start and stop of the ablation by foot switch.
Figure 1.
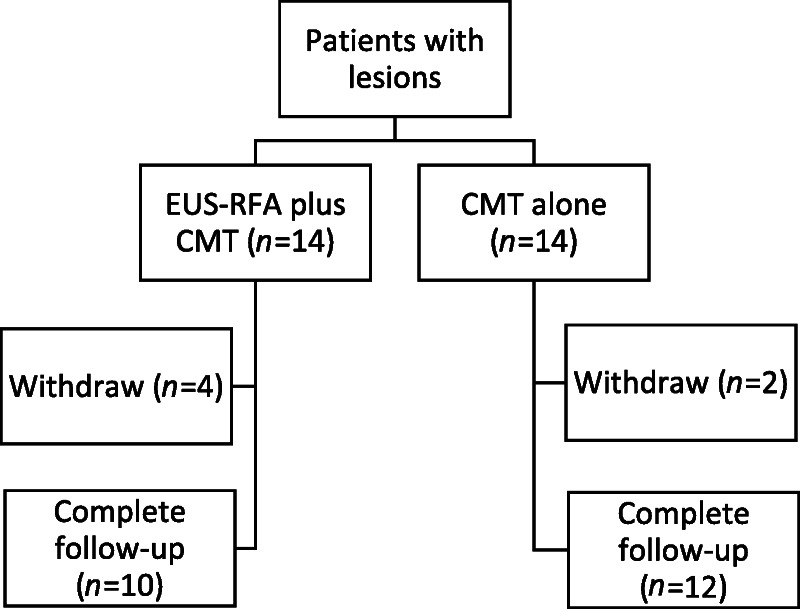
A diagram showing enrollment of eligible patients (n = 28). Fourteen of them received EUS-RFA plus CMT (group A), and another 14 received CMT alone (group B) as treatment of pancreatic ductal adenocarcinoma. In group A, 4 patients withdrew from the study after their first successful and uneventful EUS-RFA procedure because of progression of the disease with development of massive ascites (n = 1), development of pulmonary embolism requiring long-term anticoagulant (n = 1), and worsening ECOG score to ECOG 3 plus development of distant metastasis (n = 2). In group B, 2 patients withdrew after the first course of systemic CMT because of worsening ECOG score. In total, 22 patients (10 and 12 patients from groups A and B, respectively) completed the study. CMT: chemotherapy; ECOG: Eastern Cooperative Oncology Group; EUS-RFA: EUS–guided radiofrequency ablation.
For the techniques of King Chulalongkorn Memorial Hospital, the endosonographer ablates the lesion until it appears in white color, so-called bubble, resulting from the heat. The endosonographer who performs the procedure will notice that the white image covers the desired area and is at least 3 mm away from the bile ducts, pancreas, and blood vessels to avoid adverse events. After the endosonographer ablates the desired area until a white image is seen, the endosonographer will move the needle electrode to the next area and repeat the actions until no unablated area is left [Table 1].
Table 1.
Comparison of the baseline demographic data between patients receiving EUS-RFA plus CMT (group a) versus CMT alone (group B) as treatment for pancreatic ductal adenocarcinoma.
| Parameters | Subgroup | Total (n = 28) | Group A (EUS-RFA plus CMT) (n = 14) | Group B (CMT) (n = 14) | P Value |
|---|---|---|---|---|---|
| Gender | Female (%) | 19 (67%) | 10 (71) | 9 (64) | NS |
| Male (%) | 9 (32%) | 4 (28) | 5 (35) | ||
| Age | Mean ± SD | 65.3 ± 10 | 66.3 ± 10.8 | 64.3 ± 9.2 | NS |
| Median | 66 | 66 | 64.5 | ||
| ECOG score | I | 18 (64%) | 8 (57%) | 10 (71%) | NS |
| II | 10 (36%) | 6 (43%) | 4 (28%) | ||
| Diagnosis | PDAC | 28 (100%) | 14 (100%) | 14 (100%) | NS |
| Tumor stage | III | 2 (7%) | 1 (7%) | 1 (7%) | NS |
| III b | 6 (21%) | 3 (21%) | 3 (21%) | ||
| IV | 20 (72%) | 10 (72%) | 10 (72%) | ||
| Maximal tumor diameter (mm) | Mean ± SD | 54.8 ± 19.8 | 59.7 ± 18.6 | 50.0 ± 20.4 | NS |
| Median (IQR) | 53.1 (39.8–66.1) | 59 (49.8–66.5) | 43.9 (37.3–67.1) | ||
| Tumor volume (ml) | Mean ± SD | 76.8 ± 56.2 | 74.6 ± 50.7 | 78.9 ± 63.1 | NS |
| Median (IQR) | 59.5 (39.7–101) | 62.7 (27.4–113) | 56.7 (41.9–101) | ||
| Morphine equivalent dosage analgesia (mg/day) | Mean ± SD | 36.1 ± 32.5 | 51.3 ± 35.2 | 21.9 ± 22.8 | NS |
| Median (IQR) | 25 (5–60) | 60 (17.5–85.0) | 17.5 (0–33) |
No statistical difference between both groups in all parameters. CMT: chemotherapy; EUS-RFA: EUS–guided radiofrequency ablation.
In 2 to 4 weeks, the endosonographer made an appointment for the patient to repeat EUS-RFA. If unablated lesions were detected, the endosonographer would repeat the treatment. We would perform the examination by cross-sectional radiography using a multidetector CT, at 2 to 3 months after the beginning of a lesion [Table 1 and Figure 2].
Figure 2.
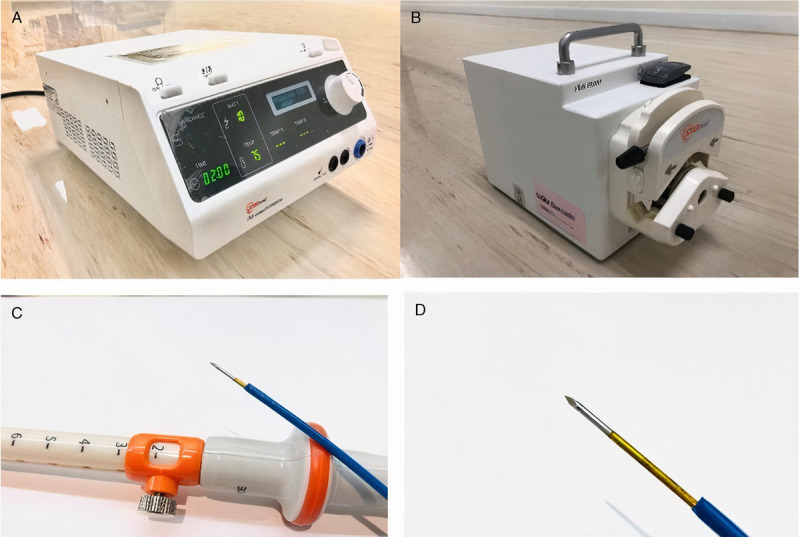
A19-guage EUS-guided radiofrequency probe (EUSRA; STARmed) with a generator that has an internal cooling system. The probe is an EUS-FNA needle type, not through-the-needle type. The probe is monopolar. The entire metal part of the needle is insulated except for the terminal end inside the patient's body. The naked terminal end is for the transmission of energy to target lesions. EUS-FNA: EUS–guided fine needle aspiration.
In this current study, for the EUS-RFA of PDAC, the author and the team at King Chulalongkorn Memorial Hospital provided treatment along with the CMT by physicians from the oncology department.
Devices
The RF generator and needle electrode inserted through the echoendoscope used in this current study is a 19-gauge EUSRA RF needle with its specific generator (STARmed, Koyang, Korea) [Figure 1]. The radio frequency emits heat, causing the tissue to be damaged and eventually ablated. The machine features an internal cooling system with a separate water pump because the radio frequency used in ablating tissues needs a cooling system.
Follow-up
Three months after the primary treatment, clinical visits of patients in both groups were scheduled. Results of clinical interview, physical examination, dosage of pain-controlled medications, blood tests, and either CT or magnetic resonance image of the abdomen were recorded and compared between both groups.
Sample size calculations
Based on the results of Kunzmann et al. that PDAC tumor diameter decreased by 10% (SD, 18%) after CMT treatment with gemcitabine,[6] we anticipated that EUS-RFA plus CMT could decrease tumor diameter by 30% after treatment with anticipated similar numbers of SD at 18%. With an error at 0.05, confidence interval at 95%, and power of 80%, the number of patients in each group was 14 (ratio 1:1). We used the per-protocol analysis. Because there was no information comparing both treatments before, we considered this study as the pilot study.
RESULTS
Demographic data
During the study period from August 2017 to August 2018, 28 patients from King Chulalongkorn Memorial Hospital, Bangkok, Thailand, with pathologically proven PDAC and who underwent EUS-RFA plus CMT or CMT alone were enrolled. Mean ± SD age of patients was 65.3 ± 10 years. Female-to-male ratio was 19:9. Fourteen of them underwent EUS-RFA plus CMT (group A), and another 14 underwent CMT alone (group B). Mean ± SD age in group A versus B was 66.3 ± 10.8 versus 64.3 ± 9.2 years, respectively (P = not significant [NS]). Table 1 shows the following ECOG scores: 8:6 versus 10:4 for stage I/II in group A versus B, respectively.
The final diagnosis in group A was PDAC (n = 14). This was similar to group B. Staging of patients in group A versus B was as follows: stages II (n = 1:1), IIIB (n = 3:3), and IV (10:10). Mean tumor diameter in group A versus B was 59.7 ± 18.6 versus 50.0 ± 20.4 mm, respectively (P = NS). Median preprocedure morphine equivalent dosage (interquartile range) of pain-controlled medication in group A versus B was 60 (17.5–85) versus 17.5 (0–33) mg, respectively (P = NS; Figures 3 and 4).
Figure 3.
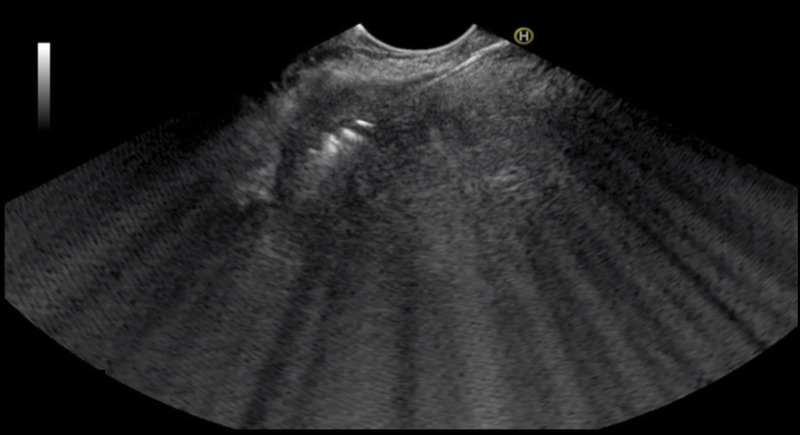
A malignant pancreatic mass with EUS-guided radiofrequency probe (echogenic linear line: arrow) in the middle of the mass. White echogenic bubble was noted around the tip of the probe. This white bubble was generated from the effect of heat from the probe to the target area.
Figure 4.
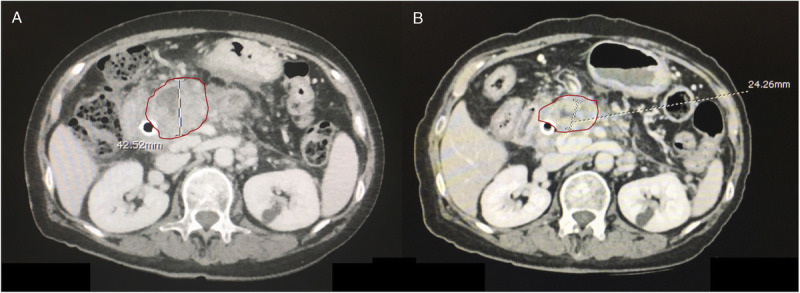
A, A pancreatic mass before the treatment in one of group A patients. B, The mass after treatment by the EUS-guided radiofrequency probe (EUSRA; STARmed) plus CMT for 3 months. As shown in both figures, the maximal diameter of the mass before and after treatment has decreased from 42.5 to 24.3 mm, respectively. CMT: chemotherapy.
In group A, regimens of CMT were as follows: gemcitabine alone (n = 6), Nab-paclitaxel plus gemcitabine (n = 3), and mFOLFIRINOX (n = 1); 4 patients withdrew from the study after their first successful and uneventful EUS-RFA procedure because of progression of the disease with development of massive ascites (n = 1), development of pulmonary embolism requiring long-term anticoagulant (n = 1), and worsening ECOG score to ECOG 3 plus development of distant metastasis (n = 2; Figure 5).
Figure 5.
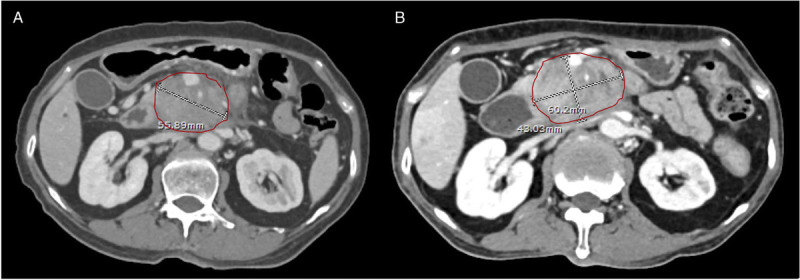
A, A pancreatic mass before the treatment in one of group B patients. B, The mass after treatment by CMT alone for 3 months. As shown in both figures, the maximal diameter of the mass before and after treatment has increased from 55.9 to 60.2 mm, respectively. CMT: chemotherapy.
In group B, regimens of CMT were as follows; gemcitabine alone (n = 8), Nab-paclitaxel plus gemcitabine (n = 2), cisplatin plus gemcitabine (n = 1), and capecitabine (n = 1); 2 patients withdrew after the first course of systemic CMT because of worsening ECOG score. In total, 22 patients (10 and 12 patients from groups A and B, respectively) completed the study [Figure 6].
Figure 6.
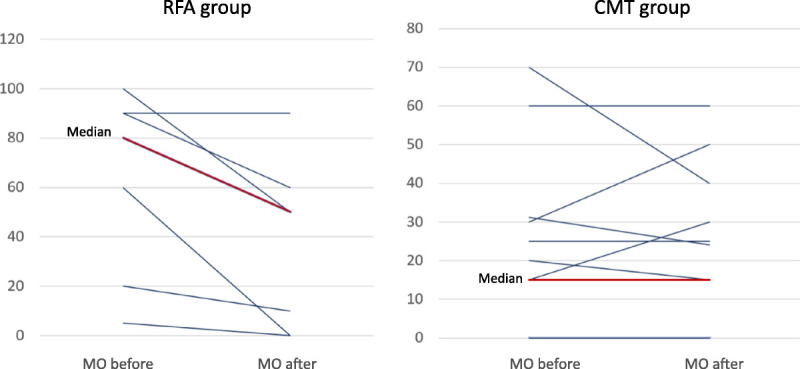
Comparison of the dosage of analgesia in morphine equivalent dosage between before and after treatment in patients receiving EUS-guided radiofrequency ablation plus CMT (group A) versus CMT alone (group B). Before treatment, group A (n = 7) versus B (n = 12) used median morphine equivalent dosage at 80 (20–90) and 17.5 (0–30.9) mg/d, respectively. After treatment, group A patients could reduce mean narcotic pain drug dosage at 26.5 mg of morphine equivalent per day (from 63.6 to 37.1 mg; P = 0.022), whereas group B patients could not reduce dosage of pain-controlled medication. CMT: chemotherapy.
EUS-RFA procedure
In group A, all EUS-RFA procedures had been successfully technically performed. In total, 30 procedures were performed in 14 patients, with median number of procedures at 2.5 times per patient (range, 1–4 times) and median procedural time at 4.6 minutes per patient (range, 1.5–16.0 minutes). Median procedural time per procedure was 2.3 minutes (range, 1.1–6.7 minutes). The EUS-RFA procedures were done by transgastric only versus a combination of transgastric and transduodenal approach in 13 and 1 patients, respectively. Mean number of EUS-RFA needle passes per procedure was 5.6 ± 2.9 times. Of 30 procedures, target lesions were located in the head (n = 5 [16.7%]), body (n = 11 [36.7%]), neck (n = 12 [40%]), and uncinate process (n = 2 [6.6%]).
Radiological outcomes
Mean maximal tumor diameters before treatment of group A (n = 10) versus B (n = 12) was 62.2 ± 21.0 versus 50.5 ± 22.0 mm, respectively (P = NS). After treatment, no statistically significant difference in mean maximal tumor diameter was found in group A, 62.3 ± 21 versus 65.2 ± 23 (P = 0.122), respectively. However, in group B, mean maximal tumor diameter was significantly increased from 50.5 ± 22.0 to 56.3 ± 18.7 mm, respectively (P = 0.017). Tumor necrosis occurred in 10 of 10 (100%) versus 6 of 12 (50%) patients, respectively (P = 0.014).
Clinical outcomes
For the analysis of the effect of pain control by EUS-RFA with or without CMT, 3 and 1 patients in groups A and B, respectively, were excluded from the analysis because they underwent EUS-guided celiac plexus neurolysis, and another 4 and 2 patients from groups A and B were also excluded because they withdrew from the study, as described in the last paragraph of the Demographic Data section. Consequently, before treatment, group A (n = 7) versus B (n = 12) used median morphine equivalent dosage at 80 (20–90) and 17.5 (0–30.9) mg/d, respectively. After treatment, group A patients could reduce mean narcotic pain drug dosage at 26.5 mg of morphine equivalent per day (from 63.6 to 37.1 mg, P = 0.022), whereas group B patients could not reduce the dosage of pain-controlled medication. No statistically significant difference in 6-month mortality rate was found between groups A (n = 10) and B (n = 12), 70% versus 70%, respectively (P = NS).
Adverse events
In group A, 30 EUS-RFA procedures were performed in 14 patients. No procedure-related serious adverse event was found. One procedure-related adverse event (n = 1 of 30 [3.3%]) occurred in 1 of 14 patients (7.1%). The event occurred within 24 hours after the procedure. The adverse event was mild after EUS-RFA pancreatitis was successfully treated with conservative treatment with a length of hospital stay of 2 days.
The adverse events were classified as nonsevere ones based on Lexicon classification by the ASGE.[7] No delay scheduled CMT from all of these events was performed.
DISCUSSIONS
Regarding radiological outcome, evaluation of treatment in different studies of EUS-RFA for PDAC used different criteria. Song et al.[8] used necrotic areas demonstrated by contrast-enhanced EUS. Crinò et al.[9] used contrast-enhanced CT to show an ablated area. The study by Scopelliti et al.[10] is the only study that used criteria commonly used by oncologists (Response Evaluation Criteria in Solid Tumors [RECIST] criteria) in only 50% of patients. Nevertheless, it might be unfair to evaluate the outcome of EUS-RFA in PDAC using the RECIST criteria that determine response based on only the diameter of a tumor, as in post–EUS-RFA PDAC; the diameter of the tumor might not be decreasing, but some areas of the tumor became necrosis.[11] Future study is warranted to answer this question in the era of varieties of local ablative treatment of cancers including PDAC.
Regarding a secondary outcome of tumor necrosis, results from this current study showed the advantage of EUS-RFA plus CMT over CMT alone. If we look at the results of past studies on tumor necrosis from EUS-RFA, we will find the following information: among all thermal ablations of PDAC techniques, EUS-RFA interestingly can provide necrosis to PDAC in several studies.[8–10,12,13] Two studies using machines similar to those of our center reported necrosis in all 12 patients with PDAC and 3 patients with pancreatic metastasis.[8,9] EUS–guided radiofrequency ablation for PDAC has been studied in organs, animals, and in vivo PDAC.[6,14–17] Results from several studies using EUS-RFA to ablate PDAC showed the benefit of tumor necrosis without serious adverse events similar to this current study.[8–10,12,13]
Techniques of EUS-RFA by EUSRA RF electrode (STARmed) for PDAC were different from studies to studies. In this current one, we chose multiple EUS-RFA procedures because we felt that the tumors might increase in diameter when time goes by and the adverse event rate is relatively small. In other studies, Song et al.[8] used an 18-gauge RFA needle, with the radiofrequency generator activated to deliver 20- to 50-W ablation power with ablation time of 10 seconds for one site until hyperechoic zone seen around the electrode tip. The probe was then moved to cover other areas of tumor. Crinò et al.[9] used 30-W power to ablate until the hyperechoic zone was visualized. The generator was stopped when there was an increase in the value of impedance by the generator. Scopelliti et al.[10] used an 18-gauge RFA electrode with 1-cm tip for energy delivery. Energy was set at 30 W for lesions larger than 3 cm and at 20 W for smaller lesions. Ablation time was not limited, but energy was stopped when a sharp rise in electric impedance occurred or an impedance value of 500 Ω on a scale between 1 and 999 Ω reached. In fact, it is still not possible to recommend which regimen is the best for ablating PDAC because of the limitation of current data.
It is interesting that in this present study, after treatment, group A patients could reduce narcotic pain drug dosage significantly, whereas group B patients could not reduce the dosage of pain-controlled medication. In a recent study done by Bang et al.,[13] the authors used 1F HabibTM EUS-RFA catheter (EMcision Ltd, London, United Kingdom) via a 19-gauge FNA needle to ablate the area of celiac plexus or visualized ganglia to reduce pain severity comparing with EUS-guided celiac plexus neurolysis. Results showed that the EUS-RFA cohort experienced significantly less pain and less severe gastrointestinal symptoms and had better emotional functioning compared with the EUS-CPN group. Future study is definitely warranted to explain this phenomenon and also compare between EUS-RFA of celiac ganglia and EUS-RFA of PDAC.
Strengths of this current study are that it is thus far the only available comparative study between standard and new treatments and that data were prospectively collected. Moreover, the character of patients in both groups was quite similar. Limitations of this current study are the small sample size of patients in both groups and nonrandomization of enrolled patients, a majority of patients being metastatic PDAC, no data of long-term follow-up, and no standard technique to measure the tumor size.
In summary, this study compares the results of EUS-RFA plus CMT with those of CMT alone in patients with either metastatic (majority) or locally advanced PDAC. Results showed the advantages of EUS-RFA plus CMT, such as a slower growing rate of the size of primary lesion, a higher rate of tumor necrosis, and a decrease in pain score compared with CMT alone. It also confirmed the feasibility and safety of the EUS-RFA procedure as shown in several previous studies. Future studies with a larger number of patients, in a randomized controlled trial fashion, and longer time of follow-up is needed. Perhaps, next study should focus on locally advanced PDAC rather than metastatic ones.
Footnotes
Published online: 23 October 2023
Contributor Information
Pradermchai Kongkam, Email: kongkam@hotmail.com.
Kasenee Tiankanon, Email: bi_z10@hotmail.com.
Thanawat Luangsukrerk, Email: drthanawatl@gmail.com.
Virote Sriuranpong, Email: vsmdcu40@gmail.com.
Chonnipa Nantavithya, Email: c.nantavithya@gmail.com.
Trirat Jantarattana, Email: Trirat.Ja@chula.ac.th.
Arlyn Cañones, Email: arcanonesmd@gmail.com.
Stephen J. Kerr, Email: stephen.k@hivnat.org.
Kittithat Tantitanawat, Email: kittithat.tantitanawat@gmail.com.
Phonthep Angsuwatcharakon, Email: borndeb@gmail.com.
Wiriyaporn Ridtitid, Email: wiriyaporn_r@yahoo.com.
Pinit Kullavanijaya, Email: pinitkul@hotmail.com.
Rungsun Rerknimitr, Email: ercp@live.com.
Funding
This work was supported by the Royal College of Physicians of Thailand (grant number RES_64_190_30_049) and Pancreas Research Unit, Faculty of Medicine, Chulalongkorn University.
Author Contributions
P. Kongkam: concept, design, data collection, analysis, drafting, and final approval of the manuscript; K.T.: data collection, analysis, drafting, and final approval of the manuscript; D.W.S.: concept, design, and final approval of the manuscript; T.L.: concept, design, final approval of the manuscript; V.S.: concept, design, and final approval of the manuscript; C.N.: concept, design, and final approval of the manuscript; T.J.: concept, design, and final approval of the manuscript; A.C.: data collection and final approval of the manuscript; S.J.K.: concept, design, biostatistician, and final approval of the manuscript; P.A.: concept, design, and final approval of the manuscript; W.R.: concept, design, and final approval of the manuscript; P. Kullavanijaya: concept, design, and final approval of the manuscript; R.R.: concept, design, and final approval of the manuscript.
Conflict of Interest
Pradermchai Kongkam and Dong Wan Seo are editorial board members of the journal. This article was subject to the journal's standard procedures, with peer review handled independently of the editors and their research groups.
References
- 1.Kongkam P Benjasupattananun P Taytawat P, et al. Pancreatic cancer in an Asian population. Endosc Ultrasound 2015;4(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strobel O, Büchler MW. Clinical practice guidelines—what is the evidence? Nat Rev Clin Oncol 2016;13(10):593–594. [DOI] [PubMed] [Google Scholar]
- 3.Sohal DPS Kennedy EB Khorana A, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36(24):2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saccomandi P, Lapergola A, Longo F, Schena E, Quero G. Thermal ablation of pancreatic cancer: a systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia 2018;35(1):398–418. [DOI] [PubMed] [Google Scholar]
- 5.Oken MM Creech RH Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5(6):649–655. [PubMed] [Google Scholar]
- 6.Gaidhane M Smith I Ellen K, et al. Endoscopic ultrasound–guided radiofrequency ablation (EUS-RFA) of the pancreas in a porcine model [Internet]. Gastroenterol Res Pract 2012;2012:431451. doi: 10.1155/2012/431451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotton PB Eisen GM Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010;71(3):446–454. [DOI] [PubMed] [Google Scholar]
- 8.Song TJ Seo DW Lakhtakia S, et al. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc 2016;83(2):440–443. [DOI] [PubMed] [Google Scholar]
- 9.Crinò SF D'Onofrio M Bernardoni L, et al. EUS-guided radiofrequency ablation (EUS-RFA) of solid pancreatic neoplasm using an 18-gauge needle electrode: feasibility, safety, and technical success. J Gastrointestin Liver Dis 2018;27(1):67–72. [DOI] [PubMed] [Google Scholar]
- 10.Scopelliti F Pea A Conigliaro R, et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc 2018;32(9):4022–4028. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P Arbuck SG Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 12.Giardino A Girelli R Frigerio I, et al. Triple approach strategy for patients with locally advanced pancreatic carcinoma [Internet]. HPB (Oxford) 2013;15(8):623–627. doi: 10.1111/hpb.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang JY, Sutton B, Hawes RH, Varadarajulu S. EUS-guided celiac ganglion radiofrequency ablation versus celiac plexus neurolysis for palliation of pain in pancreatic cancer: a randomized controlled trial (with videos). Gastrointest Endosc 2019;89(1):58–66.e3. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc 1999;50(3):392–401. [DOI] [PubMed] [Google Scholar]
- 15.Date RS, Biggins J, Paterson I, Denton J, McMahon RF, Siriwardena AK. Development and validation of an experimental model for the assessment of radiofrequency ablation of pancreatic parenchyma. Pancreas 2005;30(3):266–271. [DOI] [PubMed] [Google Scholar]
- 16.Date RS, McMahon RF, Siriwardena AK. Radiofrequency ablation of the pancreas. I: definition of optimal thermal kinetic parameters and the effect of simulated portal venous circulation in an ex-vivo porcine model. JOP 2005;6(6):581–587. [PubMed] [Google Scholar]
- 17.Kim HJ Seo D-W Hassanuddin A, et al. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc 2012;76(5):1039–1043. [DOI] [PubMed] [Google Scholar]


