Supplemental Digital Content is available in the text
Keywords: Antiretroviral therapy, Dyslipidemia, Metabolic syndrome, Non-nucleoside reverse transcriptase inhibitor, Nucleoside reverse transcriptase inhibitor, Protease inhibitor
Abstract
Background
Lipid abnormalities are prevalent among people living with human immunodeficiency virus (HIV) (PLWH) and contribute to increasing risk of cardiovascular events. This study aims to investigate the incidence of dyslipidemia and its risk factors in PLWH after receiving different first-line free antiretroviral regimens.
Methods
PLWH who sought care at the Third People's Hospital of Shenzhen from January 2014 to December 2018 were included, and the baseline characteristics and clinical data during the follow-up were collected, including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). The risk factors of dyslipidemia after antiretroviral therapy were analyzed with the generalized estimating equation model.
Results
Among the 7623 PLWH included, the mean levels of TC, HDL-C and LDL-C were 4.23 ± 0.85 mmol/L, 1.27 ± 0.29 mmol/L and 2.54 ± 0.65 mmol/L, respectively, and the median TG was 1.17 (IQR: 0.85−1.68) mmol/L. Compared with that in PLWH receiving tenofovir disoproxil fumarate (TDF) + lamivudine (3TC) + ritonavir-boosted lopinavir (LPV/r), zidovudine (AZT) + 3TC + efavirenz (EFV), and AZT + 3TC + LPV/r, the incidence of dyslipidemia was lower in PLWH receiving TDF + 3TC + EFV. In multivariate analysis, we found that the risks of elevations of TG, TC, and LDL-C were higher with TDF + 3TC + LPV/r (TG: odds ratio [OR] = 2.82, 95% confidence interval [CI]: 2.55−3.11, P < 0.001; TC: OR = 1.24, 95% CI: 1.14−1.35, P < 0.001; LDL: OR = 1.06, 95% CI: 1.00−1.12, P = 0.041), AZT + 3TC + EFV (TG: OR = 1.41, 95% CI: 1.28−1.55, P < 0.001; TC: OR = 1.43, 95% CI: 1.31−1.56, P < 0.001; LDL: OR = 1.18, 95% CI: 1.12−1.25, P < 0.001), and AZT + 3TC + LPV/r (TG: OR = 3.08, 95% CI: 2.65−3.59, P < 0.001; TC: OR = 2.40, 95% CI: 1.96−2.94, P < 0.001; LDL: OR = 1.52, 95% CI: 1.37−1.69, P < 0.001) than with TDF + 3TC + EFV, while treatment with TDF + 3TC + LPV/r was less likely to restore HDL-C levels compared with TDF + 3TC + EFV (OR = 0.95, 95% CI: 0.92−0.97, P < 0.001). In addition to antiretroviral regimens, antiretroviral therapy duration, older age, overweight, obesity and other traditional factors were also important risk factors for dyslipidemia.
Conclusion
The incidence of dyslipidemia varies with different antiretroviral regimens, with TDF + 3TC + EFV having lower risk for dyslipidemia than the other first-line free antiretroviral regimens in China.
Introduction
Antiretroviral therapy (ART) can cause weight gain, dyslipidemia, diabetes mellitus, and metabolic syndrome in people living with human immunodeficiency virus (HIV) (PLWH); however, the risk may vary widely among different combinations of antiretroviral agents, or with the exposure durations to the same antiretroviral regimen.[1–3] Given the findings that PLWH have a higher risk for cardiovascular disease (CVD) than general population,[4,5] development of metabolic complications following long-term antiretroviral therapy may further increase the CVD risk. In addition, the risk of myocardial infarction (MI) also increases with exposure to ART containing abacavir, didanosine, and protease inhibitors (PIs).[6,7]
Since 2003, China has implemented the policy of “Four Frees and One Care” policy, which provided free ART to all PLWH, free voluntary counseling and testing, free services for prevention of mother-to-child transmission (PMTCT), free schooling for children orphaned or otherwise affected by HIV or acquired immune deficiency syndrome (AIDS), and economic assistance to households of PLWH. At present, free antiretroviral agents commonly used in China include: (1) nucleoside reverse transcriptase inhibitors (NRTIs): tenofovir disoproxil fumarate (TDF), zidovudine (AZT), and lamivudine (3TC); (2) non-nucleoside reverse transcriptase inhibitors (NNRTIs): efavirenz (EFV), nevirapine (NVP); and (3) protease inhibitors (PIs): ritonavir-boosted lopinavir (LPV/r). From the second edition of the Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS in 2011[8] to the fourth edition in 2018,[9] it was recommended that the initial ART for adults and adolescents usually included two NRTIs and a third drug. According to the currently available ART in China, 2 NRTIs plus 1 NNRTI or boosted PI are recommended, that is, TDF or AZT + 3TC + EFV, NVP or LPV/r; if not contraindicated, the regimen of TDF + 3TC + EFV is preferred.
In Shenzhen, men who have sex with men (MSM) are the leading risk group for HIV transmission. In our previous study of dyslipidemia among antiretroviral-naïve PLWH, the prevalence of dyslipidemia was as high as 49.0%.[10] The incidence of dyslipidemia after prolonged exposure to the first-line free ART was rarely reported in China. In this study, we aimed to examine the evolution of blood lipids and to explore the risk factors for dyslipidemia in PLWH three years after initiation of different first-line free treatment regimens.
Methods
Ethical approval
This study was approved by the Research Ethics Committee of the Third People's Hospital of Shenzhen (No. 2018-005). Written informed consent was given by all participants. All procedures performed were carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Study design
This study was a retrospective cohort study of PLWH who sought HIV care at the Third People's Hospital of Shenzhen from January 2014 to December 2018. Inclusion criteria were (1) individuals aged 15 years or older who received a confirmed diagnosis of HIV infection by the Centers for Disease Control (CDC) of Shenzhen or other cities or provinces; (2) ART duration ≥3 months; and (3) having baseline and at least one follow-up measurements of blood lipids. Exclusion criteria were (1) presence of tumor or autoimmune diseases at baseline; (2) presence of active syphilis, viral hepatitis, tuberculosis and other active infectious diseases; (3) pregnant or lactating women, or women planning to become pregnant during the study; (4) having no testing for blood lipids within 6 months of ART initiation. The first-line ART included in this study consisted of a combination of 2 NRTIs and 1 NNRTI (EFV or NVP) or a combination of 2 NRTIs and LPV/r. In these regimens, 2 NRTIs included either TDF or AZT in combination with 3TC.
Data collection
Baseline data of the included patients were collected, including demographic and clinical characteristics and laboratory results. Baseline was defined as the date when the included PLWH firstly sought care for antiretroviral treatment at this hospital. The demographic and clinical data collected included age, sex, body weight, height, route of transmission, and time of HIV diagnosis. Laboratory tests included hematology, total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), plasma HIV RNA and CD4 cell count. After initiation of ART, the patients were evaluated at three-month intervals with the above-mentioned laboratory testing repeated. The observation duration of this study was a maximum of 36 months.
Diagnostic criteria
The diagnosis of HIV infection was made in accordance with the diagnostic criteria of “Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS (2011 Edition)” issued by the AIDS Group of the Infectious Diseases Branch, Chinese Medical Association and the Chinese CDC.[8] Body mass index (BMI) was calculated by the measured weight in kilogram (kg) divided by the square of the height in meters. BMI ≥28 kg/m2 was defined as being obese, 24.0−27.9 kg/m2 overweight, 18.5−23.9 kg/m2 normal, and <18.5 kg/m2 thin.[11] Dyslipidemia was determined according to the Guidelines on National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III[12] and was defined as TC ≥5.20 mmol/L, HDL-C <1.04 mmol/L, LDL-C ≥3.37 mmol/L, or TG ≥1.70 mmol/L.
Statistical analysis
Statistical analysis was performed using R software version 3.6.1 (R studio, Boston, MA, USA). When the measurement data were normally distributed, mean with standard deviation (SD) was used and the intergroup comparisons were performed with the independent samples t-test or analysis of variance. When the measurement data were skewed, median with interquartile range (IQR) was used, and Wilcoxon rank-sum test or Kruskal-Wallis H test was used for intergroup comparisons. The qualitative data were expressed as counts and percentages (%), and χ2 test or Fisher exact probability method was used for intergroup comparisons. Because of repeat measurements of blood lipids, generalized estimating equation (GEE) model was used to identify the factors associated with dyslipidemia in the multivariate analysis. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 7623 ART-naïve adult PLWH were included in this study; 91.8% (n = 7000) were male and the mean age was 32 ± 10 years. The major route of HIV transmission of the included PLWH was MSM. PLWH with CD4 cell counts between 200 and 349 cells/μL accounted for the highest proportion (40.6%, 3097/7623); 29.3% (2233/7623) were in the World Health Organization (WHO) HIV clinical stages III and IV; and 39.0% (2971/7623) had plasma HIV RNA loads between ≥105 and <106 IU/mL, followed by 28.6% (2180/7623) between ≥104 and <105 IU/mL.
The regimen consisting of TDF + 3TC + EFV was the most commonly selected first-line ART, accounting for 87.6% (6678/7623) of the included PLWH, followed by AZT + 3TC + EFV (5.5%, 417/7623), TDF + 3TC + LPV/r (5.4%, 415/7623), and AZT + 3TC + LPV/r (1.5%, 113/7623) [Table 1]. Overall, 93.0% (7093/7623) of the included PLWH started TDF-containing regimens, while 7.0% (530/7623) started AZT-containing regimens; EFV-based regimens were started in 93.0% (7095/7623) and LPV/r-based regimens in 6.9% (528/7623).
Table 1.
Baseline blood lipid levels of people living with HIV with different demographic and clinical characteristics.
| Characteristics | Number (n) | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) |
| Total | 7623 | 4.23 ± 0.85 | 1.17 (0.85, 1.68) | 1.27 ± 0.29 | 2.54 ± 0.65 |
| Sex | |||||
| Male | 7000 | 4.21 ± 0.84 | 1.16 (0.85, 1.67) | 1.25 ± 0.28 | 2.54 ± 0.65 |
| Female | 623 | 4.50 ± 0.94 | 1.19 (0.85, 1.72) | 1.49 ± 0.34 | 2.54 ± 0.65 |
| t/Z values | 7.43∗ | 0.79† | 17.12∗ | 0.10∗ | |
| P values | <0.001 | 0.431 | <0.001 | 0.920 | |
| Age (years) | |||||
| 15–24 | 1504 | 4.04 ± 0.79 | 1.01 (0.75, 1.39) | 1.28 ± 0.28 | 2.41 ± 0.61 |
| 25–34 | 3549 | 4.20 ± 0.83 | 1.14 (0.84, 1.61) | 1.28 ± 0.30 | 2.52 ± 0.63 |
| 35–44 | 1733 | 4.34 ± 0.90 | 1.33 (0.95, 1.91) | 1.25 ± 0.29 | 2.61 ± 0.69 |
| ≥45 | 837 | 4.50 ± 0.88 | 1.32 (0.95, 1.95) | 1.27 ± 0.29 | 2.71 ± 0.66 |
| F/H values | 66.96‡ | 288.17§ | 3.67‡ | 47.52‡ | |
| P values | <0.001 | <0.001 | 0.012 | <0.001 | |
| Route of transmission | |||||
| Men who have sex with men | 5415 | 4.19 ± 0.82 | 1.14 (0.84, 1.62) | 1.25 ± 0.28 | 2.53 ± 0.64 |
| Heterosexual | 2087 | 4.35 ± 0.92 | 1.24 (0.89, 1.81) | 1.32 ± 0.32 | 2.57 ± 0.68 |
| Others | 121 | 4.27 ± 0.80 | 1.37 (1.02, 2.06) | 1.23 ± 0.31 | 2.49 ± 0.67 |
| F/H values | 66.96‡ | 50.33§ | 2.89‡ | 40.75‡ | |
| P values | <0.001 | <0.001 | 0.055 | <0.001 | |
| BMI | |||||
| Thin | 676 | 3.98 ± 0.86 | 1.02 (0.76, 1.37) | 1.30 ± 0.33 | 2.35 ± 0.62 |
| Normal | 4296 | 4.22 ± 0.85 | 1.10 (0.82, 1.56) | 1.29 ± 0.30 | 2.52 ± 0.64 |
| Overweight | 1845 | 4.31 ± 0.82 | 1.28 (0.92, 1.84) | 1.25 ± 0.27 | 2.61 ± 0.65 |
| Obese | 806 | 4.36 ± 0.86 | 1.51 (1.06, 2.20) | 1.19 ± 0.26 | 2.64 ± 0.68 |
| F/H values | 31.84‡ | 324.15§ | 33.59‡ | 34.23‡ | |
| P values | <0.001 | <0.001 | <0.001 | <0.001 | |
| CD4 cell count (cells/μL) | |||||
| <200 | 2064 | 4.11 ± 0.88 | 1.22 (0.88, 1.74) | 1.22 ± 0.30 | 2.45 ± 0.64 |
| 200−349 | 3097 | 4.24 ± 0.82 | 1.10 (0.83, 1.57) | 1.29 ± 0.29 | 2.55 ± 0.64 |
| ≥350 | 2462 | 4.33 ± 0.85 | 1.21 (0.86, 1.77) | 1.30 ± 0.29 | 2.60 ± 0.66 |
| F/H values | 37.25‡ | 48.67§ | 52.82‡ | 32.62‡ | |
| P values | <0.001 | <0.001 | <0.001 | <0.001 | |
| WHO HIV clinical stage | |||||
| I−II | 5390 | 4.27 ± 0.83 | 1.14 (0.84, 1.63) | 1.29 ± 0.28 | 2.57 ± 0.65 |
| III−IV | 2233 | 4.14 ± 0.90 | 1.24 (0.89, 1.76) | 1.22 ± 0.31 | 2.46 ± 0.65 |
| t/Z values | 5.98∗ | 6.75† | 8.86∗ | 6.89∗ | |
| P values | <0.001 | <0.001 | <0.001 | <0.001 | |
| HIV RNA load (IU/ml) | |||||
| <104 | 1646 | 4.37 ± 0.93 | 1.31 (0.91, 1.98) | 1.34 ± 0.32 | 2.53 ± 0.67 |
| 104−9.9 × 104 | 2180 | 4.31 ± 0.81 | 1.10 (0.81, 1.55) | 1.30 ± 0.28 | 2.58 ± 0.65 |
| 105−9.9 × 105 | 2971 | 4.18 ± 0.83 | 1.14 (0.85, 1.59) | 1.24 ± 0.28 | 2.53 ± 0.64 |
| ≥106 | 826 | 3.98 ± 0.83 | 1.23 (0.91, 1.76) | 1.17 ± 0.28 | 2.47 ± 0.64 |
| F/H values | 48.79‡ | 111.04§ | 78.21‡ | 6.01‡ | |
| P values | <0.001 | 0.051 | <0.001 | <0.001 | |
| Antiretroviral regimen | |||||
| TDF+3TC+EFV | 6678 | 4.20 ± 0.82 | 1.13 (0.84, 1.61) | 1.27 ± 0.28 | 2.54 ± 0.64 |
| TDF+3TC+LPV/r | 415 | 4.34 ± 1.02 | 1.70 (1.12, 2.54) | 1.29 ± 0.38 | 2.49 ± 0.70 |
| AZT+3TC+EFV | 417 | 4.47 ± 0.94 | 1.24 (0.90, 1.74) | 1.28 ± 0.31 | 2.65 ± 0.65 |
| AZT+3TC+LPV/r | 113 | 4.80 ± 1.24 | 1.68 (1.16, 2.29) | 1.44 ± 0.46 | 2.74 ± 0.85 |
| F/H values | 32.74‡ | 210.09§ | 13.26‡ | 8.28‡ | |
| P values | <0.001 | <0.001 | <0.001 | <0.001 | |
Data were presented as mean ± SD or median (IQR). 3TC: Lamivudine; AZT: Zidovudine; BMI: Body mass index; EFV: Efavirenz; HDL-C: High-density lipoprotein cholesterol; HIV: Human immunodeficiency virus; IQR: Interquartile range; LDL-C: Low-density lipoprotein cholesterol; LPV/r: Ritonavir-boosted lopinavir; SD: Standard deviation; TC: Total cholesterol; TDF: Tenofovir disoproxil fumarate; TG: Triglyceride; WHO: World Health Organization. ∗t value. †Z value. ‡F value. §H value.
Blood lipid levels in PLWH with different demographic and clinical characteristics
The mean levels and comparisons of different blood lipid parameters in all included PLWH of different demographic and clinical characteristics are shown in Table 1. At baseline, the mean levels of TC, HDL-C and LDL-C were 4.23 ± 0.85 mmol/L, 1.27 ± 0.29 mmol/L, and 2.54 ± 0.65 mmol/L, respectively; and the median TG was 1.17 (IQR: 0.85−1.68) mmol/L. The levels of TC, TG and LDL-C were higher in the elderly (except that TG was lower in the age group of ≥45 years than that in the age group of 35−44 years), and overweight and obese patients. TC levels were significantly higher in women (t = 7.43, P < 0.001) and those with the heterosexual transmission (F = 66.96, P < 0.001), higher CD4 cell count (F = 37.25, P < 0.001), earlier WHO HIV clinical stages (t = 5.98, P < 0.001) and lower HIV RNA load (F = 48.79, P < 0.001). TG levels were higher in those with higher BMI (H = 324.15, P < 0.001), in the older patients (H = 288.17, P < 0.001), those with the heterosexual transmission (F = 50.33, P < 0.001), and those in the WHO HIV clinical stages III-IV (Z = 6.75, P < 0.001). LDL-C levels were also significantly higher in heterosexuals (F = 40.75, P < 0.001) and those with a higher BMI (F = 34.23, P < 0.001), higher CD4 cell count (F = 32.62, P < 0.001), and earlier WHO HIV clinical stages (t = 6.89, P < 0.001). In contrast, HDL-C levels were significantly lower in men (t = 17.12, P < 0.001), and those with overweight and obesity (F = 33.59, P < 0.001), lower CD4 cell count (F = 52.82, P < 0.001), later WHO HIV clinical stages (t = 8.86, P < 0.001), and higher HIV RNA load (F = 78.21, P < 0.001) [Table 1].
Evolution of dyslipidemia in PLWH receiving different ART regimens
The trends of dyslipidemia varied among PLWH receiving different ART regimens, as shown in Figure 1. The number of patients in the cohort at various follow-up points stratified by regimen was presented in Supplementary Table 1, http://links.lww.com/CM9/A417. Among them, the rate of hypertriglyceridemia (TG ≥1.70 mmol/L) at baseline was lower in patients receiving TDF + 3TC + EFV (1545/6904, 22.4%) and AZT + 3TC + EFV (118/442, 26.7%), and higher in those receiving TDF + 3TC + LPV/r (221/434, 50.9%) and AZT + 3TC + LPV/r (60/124, 48.4%). Three months after ART initiation, the TG levels of the included PLWH receiving either one of the four regimens increased significantly, and the rates of hypertriglyceridemia in those receiving TDF + 3TC + EFV, TDF + 3TC + LPV/r, and AZT + 3TC + LPV/r showed decreasing trends during the 3 years of follow-up; in contrast, that in those receiving AZT + 3TC + EFV showed a gradually increasing trend.
Figure 1.
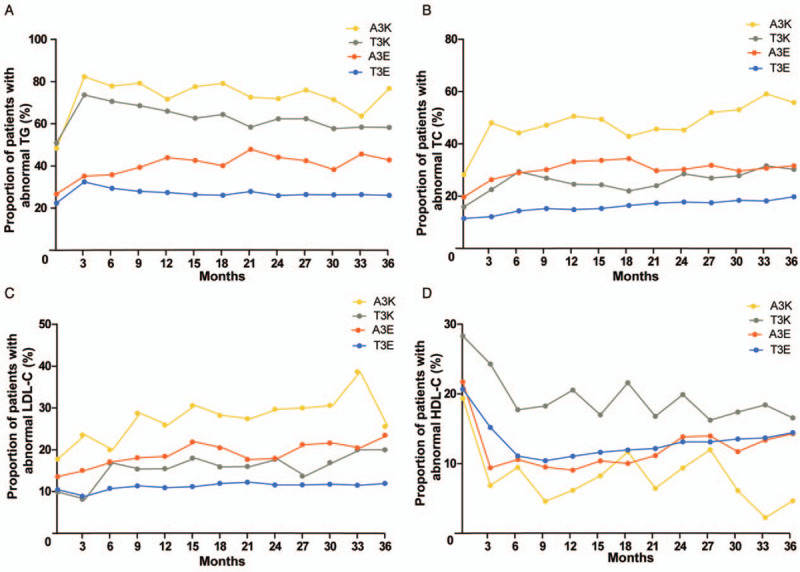
Evolution of dyslipidemia in people living with HIV receiving different ART regimens during 36 months of follow-up. Dynamic changes in proportions of patients with abnormal TG (A), TC (B), LDL-C (C), and HDL-C (D). A3K: Zidovudine (AZT) + lamivudine (3TC) + ritonavir-boosted lopinavir (LPV/r); ART: Antiretroviral therapy; HDL-C: High-density lipoprotein cholesterol; HIV: Human immunodeficiency virus; LDL-C: Low-density lipoprotein cholesterol; TG: Triglyceride; TC: Total cholesterol; T3K: Tenofovir disoproxil fumarate (TDF) + 3TC + LPV/r; A3E: AZT + 3TC + efavirenz (EFV); T3E: TDF + 3TC + EFV.
The highest rate of hypercholesterolemia (TC ≥5.20 mmol/L) at baseline was found in PLWH treated with AZT + 3TC + LPV/r (35/124, 28.2%), followed by AZT + 3TC + EFV (87/442, 19.7%) and TDF + 3TC + EFV (790/6904, 11.4%). After ART initiation, there were increasing trends of hypercholesterolemia in all of the four groups.
At baseline, the highest rate of patients with LDL-C ≥3.37 mmol/L was found in PLWH receiving AZT + 3TC + LPV/r (22/124, 17.7%), followed by AZT + 3TC + EFV (60/442, 13.6%), but was lower in those receiving TDF + 3TC + EFV and TDF + 3TC + LPV/r. After ART initiation, the rates of elevated LDL-C level in PLWH receiving AZT + 3TC + LPV/r, AZT + 3TC + EFV and TDF + 3TC + LPV/r showed gradually increasing trends, but that in those receiving TDF + 3TC + EFV remained low, with no significant increase during the follow-up.
The rate of HDL-C <1.04 mmol/L at baseline was 28.3% (123/434) in PLWH receiving TDF + 3TC + LPV/r, which was higher than those of the other three regimens. Three months after ART initiation, the rates of decreased HDL-C level in PLWH receiving TDF + 3TC + LPV/r and TDF + 3TC + EFV decreased significantly and continued to decrease until 6 months after ART initiation. Subsequently, the rate of decreased HDL-C level in PLWH receiving TDF + 3TC + LPV/r fluctuated and showed a decreasing trend, while that in PLWH receiving TDF + 3TC + EFV showed a gradually increasing trend from the 9th month onward. Three months after ART initiation, the rate of HDL-C <1.04 mmol/L decreased sharply in PLWH receiving any regimen, but that in PLWH receiving AZT + 3TC + EFV fluctuated and showed an increasing trend 12 months after initiation of ART, while, that in PLWH receiving AZT + 3TC + LPV/r fluctuated and showed a gradually decreasing trend.
Analysis of risk factors of dyslipidemia after ART
Multivariate analysis showed that the risk of elevations of TG, TC, and LDL-C levels and a decreased HDL-C level varied with different antiretroviral regimens. Compared with PLWH receiving TDF + 3TC + EFV, PLWH receiving TDF + 3TC + LPV/r, AZT + 3TC + EFV, and AZT + 3TC + LPV/r were more likely to have elevated TG, TC, and LDL-C levels [Table 2]; PLWH receiving TDF + 3TC + LPV/r were more likely to have a decreased HDL-C level, while PLWH receiving the other two regimens were less likely to have a decreased HDL-C level compared with those receiving TDF + 3TC + EFV.
Table 2.
Analysis of associated factors for dyslipidemia in people living with HIV who initiated free first-line antiretroviral therapy.
| TG | TC | HDL-C | LDL-C | |||||
| Risk factors | Exp (B) | P values | Exp (B) | P values | Exp (B) | P values | Exp (B) | P values |
| Sex | ||||||||
| Male | 1 | 1 | 1 | 1 | ||||
| Female | 0.84 (0.77–0.91) | <0.001 | 1.20 (1.12–1.27) | <0.001 | 1.24 (1.21–1.27) | <0.001 | 0.99 (0.95–1.04) | 0.701 |
| Age (years) | ||||||||
| 15−24 | 1 | 1 | 1 | 1 | ||||
| 25−34 | 1.23 (1.16–1.30) | <0.001 | 1.23 (1.18–1.29) | <0.001 | 1.00 (0.99–1.01) | 0.997 | 1.17 (1.13–1.21) | <0.001 |
| 35−44 | 1.44 (1.34–1.54) | <0.001 | 1.41 (1.34–1.48) | <0.001 | 1.00 (0.98–1.02) | 0.907 | 1.27 (1.22–1.32) | <0.001 |
| ≥45 | 1.39 (1.28–1.51) | <0.001 | 1.64 (1.54–1.75) | <0.001 | 1.01 (0.99–1.03) | 0.237 | 1.39 (1.32–1.46) | <0.001 |
| BMI | ||||||||
| Thin | 0.83 (0.77–0.90) | <0.001 | 0.82 (0.78–0.87) | <0.001 | 1.03 (1.01–1.05) | 0.007 | 0.83 (0.79–0.87) | <0.001 |
| Normal | 1 | 1 | 1 | 1 | ||||
| Overweight | 1.35 (1.28–1.43) | <0.001 | 1.18 (1.13–1.23) | <0.001 | 0.97 (0.96–0.99) | <0.001 | 1.16 (1.13–1.20) | <0.001 |
| Obese | 1.79 (1.67–1.93) | <0.001 | 1.28 (1.20–1.35) | <0.001 | 0.93 (0.91–0.94) | <0.001 | 1.25 (1.20–1.31) | <0.001 |
| CD4 count (cells/μl) | ||||||||
| <200 | 1 | 1 | 1 | 1 | ||||
| 200−349 | 0.90 (0.79–1.03) | 0.116 | 1.06 (0.95–1.18) | 0.327 | 1.05 (1.01–1.08) | 0.006 | 1.04 (0.96–1.12) | 0.360 |
| ≥350 | 0.96 (0.84–1.09) | 0.523 | 1.07 (0.96–1.20) | 0.237 | 1.03 (0.99–1.06) | 0.128 | 1.05 (0.97–1.13) | 0.222 |
| Treatment regimen | ||||||||
| TDF+3TC+EFV | 1 | 1 | 1 | 1 | ||||
| TDF+3TC+LPV/r | 2.82 (2.55–3.11) | <0.001 | 1.24 (1.14–1.35) | <0.001 | 0.95 (0.92–0.97) | <0.001 | 1.06 (1.00–1.12) | 0.041 |
| AZT+3TC+EFV | 1.41 (1.28–1.55) | <0.001 | 1.43 (1.31–1.56) | <0.001 | 1.04 (1.01–1.07) | 0.006 | 1.18 (1.12–1.25) | <0.001 |
| AZT+3TC+LPV/r | 3.08 (2.65–3.59) | <0.001 | 2.40 (1.96–2.94) | <0.001 | 1.07 (1.01–1.13) | 0.013 | 1.52 (1.37–1.69) | <0.001 |
| Treatment duration | ||||||||
| 0 month | 1 | 1 | 1 | 1 | ||||
| 6 months | 1.26 (1.23–1.30) | <0.001 | 1.17 (1.15–1.19) | <0.001 | 1.11 (1.10–1.11) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| 12 months | 1.20 (1.17–1.24) | <0.001 | 1.21 (1.19–1.23) | <0.001 | 1.10 (1.09–1.10) | <0.001 | 1.05 (1.04–1.06) | <0.001 |
| 18 months | 1.16 (1.13–1.20) | <0.001 | 1.27 (1.25–1.30) | <0.001 | 1.08 (1.07–1.09) | <0.001 | 1.08 (1.07–1.09) | <0.001 |
| 24 months | 1.13 (1.09–1.17) | <0.001 | 1.30 (1.27–1.32) | <0.001 | 1.06 (1.05–1.06) | <0.001 | 1.06 (1.05–1.07) | <0.001 |
| 30 months | 1.11 (1.07–1.14) | <0.001 | 1.36 (1.33–1.39) | <0.001 | 1.04 (1.03–1.05) | <0.001 | 1.07 (1.06–1.09) | <0.001 |
| 36 months | 1.10 (1.06–1.14) | <0.001 | 1.38 (1.35–1.41) | <0.001 | 1.03 (1.02–1.03) | <0.001 | 1.07 (1.05–1.08) | <0.001 |
| WHO HIV clinical stage | ||||||||
| I–II | 1 | 1 | 1 | 1 | ||||
| III–VI | 0.96 (0.85–1.09) | 0.515 | 0.98 (0.89–1.09) | 0.767 | 0.99 (0.92–1.07) | 0.804 | 1.00 (0.97–1.04) | 0.813 |
| HIV RNA load (IU/mL) | ||||||||
| <104 | 1 | 1 | 1 | 1 | ||||
| 104–9.9 × 104 | 0.90 (0.84–0.96) | 0.001 | 0.99 (0.95–1.04) | 0.759 | 0.99 (0.97–1.01) | 0.185 | 1.01 (0.97–1.04) | 0.810 |
| 105–9.9 × 105 | 0.94 (0.89–1.00) | 0.055 | 0.99 (0.95–1.04) | 0.716 | 0.97 (0.95–0.98) | <0.001 | 1.02 (0.98–1.05) | 0.335 |
| ≥106 | 1.07 (0.99–1.17) | 0.092 | 1.03 (0.96–1.10) | 0.390 | 0.94 (0.92–0.96) | <0.001 | 1.05 (1.00–1.10) | 0.052 |
3TC: Lamivudine; AZT: Zidovudine; BMI: Body mass index; EFV: Efavirenz; HDL-C: High-density lipoprotein cholesterol; HIV: Human immunodeficiency virus; LDL-C: Low-density lipoprotein cholesterol; LPV/r: Ritonavir-boosted lopinavir; TC: Total cholesterol; TDF: Tenofovir disoproxil fumarate; TG: Triglyceride; WHO: World Health Organization.
In addition to antiretroviral therapy, female, older age, overweight and obesity were independent risk factors for high TC; male, older age, overweight and obesity were independent risk factors for high TG; overweight and obesity were risk factors for low HDL-C; and older age, overweight, and obesity were risk factors for low LDL-C [Table 2]. ART duration was also an independent factor for dyslipidemia. Compared with baseline, PLWH had a higher risk of elevated TG, TC, and LDL levels at 6, 12, 18, 24, 30, and 36 months after ART initiation; however, HDL-C recovered at different time points of ART from baseline [Table 2].
Discussion
Immune activation and persistent inflammation after HIV infection, and exposure to antiretroviral agents can lead to dyslipidemia in PLWH and increase the risk of cardiovascular morbidity and mortality.[4,5,13] This study analyzed the evolution of dyslipidemia and its risk factors among PLWH in Shenzhen who received the four most commonly used, first-line free antiretroviral regimens. We found that, within 3 years of ART initiation, the rates of elevated TG and LDL-C levels in PLWH treated with TDF + 3TC + EFV were maintained at a low level, while the rates of dyslipidemia in PLWH treated with the other three regimens increased from baseline to varying degrees. Compared with TDF + 3TC + EFV, the risks of elevated TG, TC and LDL-C levels were higher in PLWH receiving TDF + 3TC + LPV/r, AZT + 3TC + EFV, and AZT + 3TC + LPV/r, and treatment with TDF + 3TC + LPV/r was a risk factor for a decreased HDL-C level. In addition to ART, older age, overweight, obesity, and ART duration were also independent risk factors of dyslipidemia.
Hypertriglyceridemia is commonly seen in PLWH.[14] A meta-analysis of 14 trials involving 21,023 people in sub-Saharan Africa between 2003 and 2014 showed that ART was associated with an increased risk of hypertriglyceridemia (relative risk, 2.05, 95% confidence interval: 1.51–2.77).[15] Mild to moderate hypertriglyceridemia was associated with an increased risk of CVD.[16] Each 1-mmol/L increase in plasma TG level can lead to a 1.4- to 1.8-fold increase in the risk of CVD.[17–18] In this study, we found that the rate of hypertriglyceridemia in PLWH receiving TDF + 3TC + EFV remained stable from baseline throughout the 3 years of follow-up. However, the rate of hypertriglyceridemia in other regimens, especially those containing LPV/r (TDF + 3TC + LPV/r and AZT + 3TC + LPV/r), increased to varying degrees from baseline, suggesting that PIs may be more likely to cause hypertriglyceridemia than NNRTIs, which was similar to the findings of Périard et al[19] and Ucciferri et al.[20] The possible mechanism for hypertriglyceridemia was that PIs interfere with lipid metabolism-related proteins and cholesterol metabolism-related proteins, and inhibit proteasome-mediated apolipoprotein cleavage, resulting in the over-production and over-secretion of triglyceride-rich lipoproteins.[21,22] Other studies have found that PI-associated hypertriglyceridemia and low HDL cholesterolemia were associated with APO gene polymorphisms, and apolipoprotein APOA5–1131TC gene polymorphisms are associated with PI-related hyperlipidemia.[23,24] It was suggested that TDF + 3TC + EFV is preferred in patients with dyslipidemia without special contraindications, and the combination of LPV/r-containing regimen should be avoided if alternative ART is available; and blood lipids should be monitored after ART initiation. In addition, it has been shown that PLWH with hypertriglyceridemia consumed higher amounts of total fat, saturated fat, trans fat and cholesterol than HIV-negative individuals.[25] The increased intake of saturated fat is closely related to TG levels, suggesting that PLWH should be counseled for limiting intake of saturated fat.
Increased LDL-C levels are a major risk factor for the development of atherosclerosis[26] and a major target for lipid-lowering therapy[12]. In our study, we found that the blood lipids at baseline and the rate of increased LDL-C level after ART initiation remained at a low level with TDF + 3TC + EFV, while the rates of increased LDL-C levels with the other regimens showed gradually increasing trends from baseline, suggesting that the TDF + 3TC + EFV had less effect on LDL-C among the first-line antiretroviral regimens in China. The rate of hypercholesterolemia was similar to that of increased LDL-C. After ART initiation, the rates of hypercholesterolemia showed gradually increasing trends in all four groups, but the increase in the rate of hypercholesterolemia was the smallest in PLWH receiving TDF + 3TC + EFV. The mechanism may be that TDF exerts a lipid-lowering effect, similar to statins,[27,28] and switch of other NRTIs to TDF or initiation of TDF-containing regimens may improve blood lipids in PLWH with hypercholesterolemia.[29–31] These results suggest that TDF + 3TC + EFV with less effect on LDL-C is preferred in the absence of contraindications.
HDL can transport cholesterol from peripheral tissues such as blood vessel wall to the liver for catabolism and has an anti-atherosclerotic effect. In addition, HDL also has anti-inflammatory and antioxidant effects, thus protecting vascular intima. HIV may increase cholesterol transfer to apolipoprotein B (ApoB)-containing lipoproteins by affecting HDL metabolism and the ability of reverse cholesterol transport,[32] resulting in a decrease in HDL. The HDL-C level decreases significantly with the progression of HIV infection.[33] PLWH who did not receive ART had a more significant decrease in HDL-C than those receiving ART.[34] Our study found that the rates of dyslipidemia in terms of decreased HDL-C levels declined more rapidly within the first 3 months of treatment with the four antiretroviral regimens investigated in this study, but the rates in PLWHs receiving TDF + 3TC + EFV and AZT + 3TC + EFV subsequently fluctuated and showed a long-term increasing trend, while those in PLWH receiving TDF + 3TC + LPV/r and AZT + 3TC + LPV/r fluctuated and showed a gradually decreasing trend. Previous studies have shown that EFV-containing regimens might increase HDL-C levels.[3,35] In contrast, our study revealed that the rates of decreased HDL-C levels stabilized and gradually increased with the two EFV-containing regimens (AZT + 3TC + EFV and TDF + 3TC + EFV) 1 year after initiation of ART. The discrepancy between our findings and those of previous studies could be explained by the different study populations and designs. Our study was a retrospective study including PLWH of ethnic Chinese and losses to follow-up of blood lipids were not uncommon during the study. Prospective studies with regular follow-up of blood lipids are warranted to confirm our findings.
Dyslipidemia is an independent risk factor for CVD.[12] Early identification and screening of risk factors affecting dyslipidemia are of great importance in the long-term successful management of HIV infection. In our study, we found that the risks of elevated TG, TC, and LDL-C levels were higher with TDF + 3TC + LPV/r, AZT + 3TC + EFV, and AZT + 3TC + LPV/r using TDF + 3TC + EFV as a reference; while TDF + 3TC + LPV/r was a risk factor for a decreased HDL-C level. Duration of exposure to ART was also an independent factor affecting blood lipids. In addition to treatment factors, we found that traditional factors such as older age, overweight and obesity were also important risk factors for dyslipidemia in PLWH. However, no associations between CD4, plasma HIV RNA, WHO HIV disease stage at baseline and post-ART blood lipids were found in this study.
Due to the cost of antiretroviral drugs and the adverse effects after long-term exposure to antiretroviral drugs, we have to make a comprehensive consideration of various factors in selecting the most suitable treatment regimen for PLWH. The baseline condition of PLWH should be evaluated before ART initiation; and, for PLWH with obvious increases in blood lipids, fatty liver, and presence of other cardiovascular risks at baseline, LPV/r-containing regimens should be selected carefully. If there are no special contraindications, the regimen of TDF + 3TC + EFV should be considered for PLWH in China with obesity, high BMI, and hypercholesterolemia and hypertriglyceridemia before ART initiation. Other interventions are needed to quit smoking, promote physical activity and control weight. Although it is not yet clear whether treatment with statins is sufficient to significantly reduce the risk of CVD in PLWH, statins can simultaneously treat dyslipidemia and reduce inflammation, thereby potentially reducing this risk of CVD.
The limitations of this study include: the sample sizes of PLWH receiving regimens other than TDF + 3TC + EFV were small. Not all PLWH included in this study had blood testing regularly and losses to follow-up occurred at different time points; moreover, we did not collect data on the adherence of the included PLWH to the antiretroviral regimens prescribed during the follow-up nor did we have information on diet, exercise, smoking or treatment with lipid-modifying agents. The majority of PLWH in this study were young and middle-aged men, and our findings may not be generalizable to other PLWH in different age groups. Our study included only the PLWH who were receiving first-line free ART in China. The findings cannot be generalized to those who received ART containing integrase strand transfer inhibitors (InSTIs), or combination regimens consisting of NNRTI, PI or InSTI that were administered because of intolerance or virologic failure.
In conclusion, the rates of dyslipidemia are different among different antiretroviral regimens. TDF + 3TC + EFV has a lower rate of dyslipidemia than the other first-line free antiretroviral regimens available to PLWH in China. Blood lipids should be monitored regularly when ART is initiated and continued and counseling and management of modifiable risk factors for dyslipidemia should be pursued.
Funding
This work was supported by the grants from the Sanming Project of Medicine in Shenzhen [Nos. SZSM201612014 and SZSM201512029], Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515011197), and Science and Technology Innovation Committee of Shenzhen Municipality (No. JCYJ20190809115617365).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Sun LQ, Liu JY, He Y, Zhou Y, Xu LM, Zhang LK, Zhao F, Liu XN, Song Y, Cao TZ, Tian YM, Rao M, Wang H. Evolution of blood lipids and risk factors of dyslipidemia among people living with human immunodeficiency virus who had received first-line antiretroviral regimens for 3 years in Shenzhen. Chin Med J 2020;133:2808–2815. doi: 10.1097/CM9.0000000000001245
Li-Qin Sun and Jia-Ye Liu contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.cmj.org).
References
- 1.Waters DD, Hsue PY. Lipid abnormalities in persons living with HIV infection. Can J Cardiol 2019; 35:249–259. doi: 10.1016/j.cjca.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Non LR, Escota GV, Powderly WG. HIV and its relationship to insulin resistance and lipid abnormalities. Transl Res 2017; 183:41–56. doi: 10.1016/j.trsl.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Fontas E, van Leth F, Sabin CA, Friis-Møller N, Rickenbach M, d’Arminio Monforte A, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different drugs associated with different lipid profiles? J Infect Dis 2004; 189:1056–1074. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 4.Shah A, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsue PY, Waters DD. Time to recognize HIV infection as a major cardiovascular risk factor. Circulation 2018; 138:1113–1115. doi: 10.1161/CIRCULATIONAHA.118.036211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 7.Study Group DAD, Friis-Møller N, Reiss P, Sabin CA, Weber R, Ad M, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 8.Group AIDS. Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the Diagnosis and Treatment of HIV/AIDS (2011 Edition). Chin J Infect Dis 2011; 29:629–640. doi: 10.3760/cma.j.issn.1000-6680.2011.10.018. [Google Scholar]
- 9.AIDS and Hepatitis C Professional Group. Society of Infectious Diseases, Chinese Medical Association; Chinese Center for Disease Control and Prevention. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018) (in Chinese). Chin J Intern Med 2018; 57:867–884. doi: 10.3760/cma.j.issn.0578-1426.2018.12.002. [Google Scholar]
- 10.Sun LQ, Liu JY, Wang H, Xu L, Zhou Y, Zhang L, et al. Prevalence and influencing factors of dyslipidemia among treatment-naive HIV-infected individuals in Shenzhen in 2018. Infect Dis Info 2020; 33:122–128. doi:10.3969/j.issn.1007-8134.2020.02.006. [Google Scholar]
- 11.Cooperative Meta-analysis Group of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin J Epidemiol 2002; 23:5–10. doi: 10.1046/j.1440-6047.11.s8.9.x. [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. doi: 10.1001/jama.285.19.2486. [PubMed] [Google Scholar]
- 13.Ye P. Key points and interpretation of Chinese expert consensus on hypertriglyceridemia and its cardiovascular risk management. Chin Circul J 2017; 32:42–44. [Google Scholar]
- 14.Hsue PY, Waters DD. Bonow RO, Mann DL, Tomaselli GF, Bhatt D. Cardiovascular abnormalities in HIV-infected individuals. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine, 2-Volume Set 11th Edition.Amsterdam, Netherlands: Elsevier; 2019. 1651–1661. [Google Scholar]
- 15.Ekoru K, Young EH, Dillon DG, Gurdasani D, Stehouwer N, Faurholt-Jepsen D, et al. HIV treatment is associated with a two-fold higher probability of raised triglycerides: Pooled Analyses in 21 023 individuals in sub-Saharan Africa. Glob Health Epidemiol Genom 2018; 3:e7.doi: 10.1017/gheg.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ 2007; 176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011; 32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2:655–666. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Périard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation 1999; 100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 20.Ucciferri C, Falasca K, Vignale F, Di Nicola M, Pizzigallo E, Vecchiet J. Improved metabolic profile after switch to darunavir/ritonavir in HIV positive patients previously on protease inhibitor therapy. J Med Virol 2013; 85:755–759. doi: 10.1002/jmv.23543. [DOI] [PubMed] [Google Scholar]
- 21.Liang JS, Distler O, Cooper DA, Jamil H, Deckelbaum RJ, Ginsberg HN, et al. HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med 2001; 7:1327–1331. doi: 10.1038/nm1201-1327. [DOI] [PubMed] [Google Scholar]
- 22.Hui DY. Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res 2003; 42:81–92. doi: 10.1016/s0163-7827(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 23.Fauvel J, Bonnet E, Ruidavets JB, Ferrières J, Toffoletti A, Massip P, et al. An interaction between apo C-III variants and protease inhibitors contributes to high triglyceride/low HDL levels in treated HIV patients. AIDS 2001; 15:2397–2406. doi: 10.1097/00002030-200112070-00007. [DOI] [PubMed] [Google Scholar]
- 24.Guardiola M, Ferré R, Salazar J, Alonso-Villaverde C, Coll B, Parra S, et al. Protease inhibitor-associated dyslipidemia in HIV-infected patients is strongly influenced by the APOA5-1131T->C gene variation. Clin Chem 2006; 52:1914–1919. doi: 10.1373/clinchem.2006.069583. [DOI] [PubMed] [Google Scholar]
- 25.Joy T, Keogh HM, Hadigan C, Lee H, Dolan SE, Fitch K, et al. Dietary fat intake and relationship to serum lipid levels in HIV-infected patients with metabolic abnormalities in the HAART era. AIDS 2007; 21:1591–1600. doi: 10.1097/QAD.0b013e32823644ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone NJ, Bilek S, Rosenbaum S. Recent national cholesterol education program adult treatment panel III update: adjustments and options. Am J Cardiol 2005; 96:53E–59E. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Crane HM, Grunfeld C, Willig JH, Mugavero MJ, Van Rompaey S, Moore R, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS 2011; 25:185–195. doi: 10.1097/QAD.0b013e328341f925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamara DA, Smith C, Ryom L, Reiss P, Rickenbach M, Phillips A, et al. Longitudinal analysis of the associations between antiretroviral therapy, viraemia and immunosuppression with lipid levels: the D:A:D study. Antivir Ther 2016; 21:495–506. doi: 10.3851/IMP3051. [DOI] [PubMed] [Google Scholar]
- 29.Hemkens LG, Ewald H, Santini-Oliveira M, Bühler JE, Vuichard D, Schandelmaier S, et al. Comparative effectiveness of tenofovir in treatment-naïve HIV-infected patients: systematic review and meta-analysis. HIV Clin Trials 2015; 16:178–189. doi: 10.1179/1945577115Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 30.Valantin MA, Bittar R, de Truchis P, Bollens D, Slama L, Giral P, et al. Switching the nucleoside reverse transcriptase inhibitor backbone to tenofovir disoproxil fumarate + emtricitabine promptly improves triglycerides and low-density lipoprotein cholesterol in dyslipidaemic patients. J Antimicrob Chemother 2010; 65:556–561. doi: 10.1093/jac/dkp462. [DOI] [PubMed] [Google Scholar]
- 31.Moyle GJ, Orkin C, Fisher M, Dhar J, Anderson J, Wilkins E, et al. A randomized comparative trial of continued abacavir/lamivudine plus efavirenz or replacement with efavirenz/emtricitabine/tenofovir DF in hypercholesterolemic HIV-1 infected individuals. PLoS One 2015; 10:e0116297.doi: 10.1371/journal.pone.0116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose H, Hoy J, Woolley I, Tchoua U, Bukrinsky M, Dart A, et al. HIV infection and high density lipoprotein metabolism. Atherosclerosis 2008; 199:79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong C, Liu E, Okuma J, Spiegelman D, Guerino C, Njelekela M, et al. Dyslipidemia in an HIV-positive antiretroviral treatment-naive population in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 2011; 57:141–145. doi: 10.1097/QAI.0b013e318219a3d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonfanti P, De Soeio GV, Ricci E, Antinori A, Martinelli C, Vichi F, et al. The feature of metabolic syndrome in HIV naive patients is not the same of those treated: results from a prospective study. Biomed Pharmacother 2012; 66:348–353. doi: 10.1016/j.biopha.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Negredo E, Ribalta J, Ferré R, Salazar J, Rey-Joly C, Sirera G, et al. Efavirenz induces a striking and generalized increase of HDL-cholesterol in HIV-infected patients. AIDS 2004; 18:819–821. doi: 10.1097/00002030-200403260-00017. [DOI] [PubMed] [Google Scholar]


