Abstract
Lacticin 3147 is a broad-spectrum bacteriocin produced by Lactococcus lactis subsp. lactis DPC3147 which is bactericidal against a range of mastitis-causing streptococci and staphylococci. In this study, both lacticin 3147 and the lantibiotic nisin were separately incorporated into an intramammary teat seal product. The seal containing lacticin 3147 exhibited excellent antimicrobial activity and might form the basis of an improved treatment for the prevention of mastitis in dry cows.
Bovine mastitis, one of the most persistent and costly diseases in dairy cows (7), is usually treated or prevented with intramammary antibiotics (10, 11). Although normally very effective, the use of antibiotics to control mastitis has some disadvantages, including the appearance of residues in the milk of treated cows (3). As a result, milk is normally withheld for a period of time following antibiotic administration, with concomitant economic losses. In some instances, broad-spectrum bacteriocins produced by lactic acid bacteria may provide valuable alternatives to antibiotics for the treatment of this disease. These natural inhibitors are particularly suitable for such applications given their use and acceptance as preservatives in foods (4). One such bacteriocin, nisin, which is produced by Lactococcus lactis, is effective against a wide range of gram-positive bacteria, including mastitic pathogens (1). Using nisin as an alternative to antibiotics does not compromise any human applications, and in addition, nisin is readily degraded by digestive enzymes and is, therefore, nontoxic. However, one disadvantage associated with the clinical use of nisin is its poor solubility at physiological pH. Despite this drawback, nisin in combination with lysostaphin (8) has been shown to be an effective treatment for mastitis, with cure rates of 66, 95, and 100% reported for animals infected with Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus uberis, respectively (10, 11). In addition, nisin is now the active ingredient in at least two products currently sold for the prevention of mastitis: Consept (Applied Microbiology, Inc., New York, N.Y.), which is applied as a teat dip, and Wipe-Out (Applied Microbiology), which is used as a teat wipe.
In this study, a novel bacteriocin, lacticin 3147, was evaluated with a view to its possible exploitation for mastitis-related applications. Like nisin, lacticin 3147 exhibits a broad spectrum of inhibition among gram-positive bacteria and is produced by L. lactis (6, 9), a bacterium commonly used as a starter in fermented dairy foods. This specific application involved the incorporation of either nisin or lacticin 3147 into a commercial teat seal. The teat seal is an oil-based formulation which forms a physical barrier against infection in the area of the teat canal and sinus. It consists of a heavy inorganic salt in a paraffin base and is antibiotic free (7). Presently, its use in Ireland is preceded by an antibiotic treatment at the drying-off stage, and the seal is then administered as an added barrier treatment over the antimicrobial protection afforded by the antibiotic. The incorporation of bacteriocin into the teat seal would be advantageous in that the bacteriocin would be localized within the seal in the teat duct and sinus. This study was designed to investigate the efficacy of using a teat seal containing lacticin 3147 to inhibit mastitic pathogens in vitro. Initial experiments focused on the activity of lacticin 3147 against mastitic pathogens from an in-house culture collection.
To test the sensitivity of a strain to lacticin 3147, 20-ml volumes of molten brain heart infusion (BHI) agar were seeded with 50 μl of an overnight culture of each streptococcal strain or with 20 μl of each staphylococcal strain. Sensitivity was determined from triplicate experiments by the agar well diffusion assay (9). The diameter of each zone of inhibition was measured and expressed in millimeters. The concentrated preparation of lacticin 3147 used for sensitivity assays was produced as follows. L. lactis DPC3147 was propagated in TY broth (tryptone at 2.5 g/liter, yeast extract at 5 g/liter, glucose at 10 g/liter, β-glycerophosphate at 19 g/liter, MgSO4 · 7H2O at 0.25 g/liter, MnSO4 · 4H2O at 0.05 g/liter; pH 6.75) overnight at 30°C. The medium had been previously cleared of contaminating proteins by passing it through 500 g of XAD-100 beads (Sigma-Aldrich Company Ltd., Poole, Dorset, England), to which hydrophobic proteins bind. Following incubation, the culture was centrifuged at 10,000 × g to remove the bacteriocin-producing cells. The supernatant was then passed through 50 g of XAD-100 beads at a flow rate of approximately 15 ml/min, thus allowing the bacteriocin to bind. The column was then washed with 40% ethanol, and the bacteriocin was subsequently eluted with 70% propan-2-ol (10 mM acetic acid, pH 2). The propan-2-ol was removed by evaporation with oxygen-free nitrogen gas (BOC Gases, Cork, Ireland), and the resulting bacteriocin-containing solution was bound to a C18 reverse-phase cartridge column (Varian Sample Preparation Products, JVA Analytical Ltd., Dublin, Ireland). This column was washed with 30% ethanol, and the bacteriocin was eluted with 70% propan-2-ol. Again, the propan-2-ol was removed by evaporation with nitrogen gas, and the activity of the resulting bacteriocin solution was determined by the critical dilution assay and expressed as arbitrary units (AU) per milliliter, as described by Ryan et al. (9).
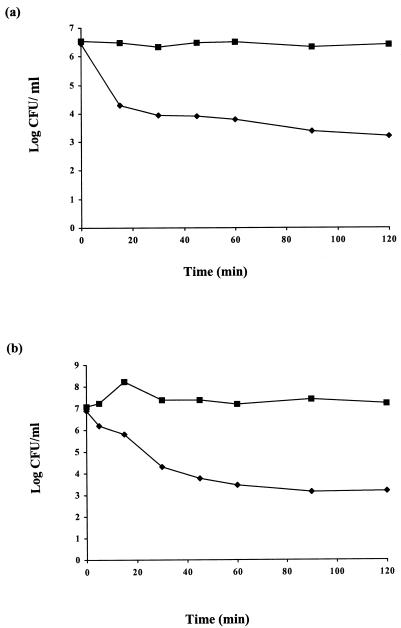
The sensitivities of 24 mastitic streptococcal and staphylococcal strains to lacticin 3147 are shown in Table 1. Lacticin 3147 inhibited all strains tested, although their sensitivities varied considerably, with zone sizes ± standard deviations ranging from 10.1 ± 0.3 mm to 17.0 ± 2.0 mm. In general, however, lacticin 3147 appeared to be more potent against streptococci than staphylococci. Streptococcus dysgalactiae M was found to be the most sensitive of the strains tested, and it was therefore chosen as the indicator strain for subsequent experiments. Lacticin 3147 was shown to cause rapid cell death when added to actively growing cells of sensitive organisms. This bactericidal mode of action of lacticin 3147 was examined using Streptococcus dysgalactiae M (Fig. 1a) and S. aureus 10 (Fig. 1b), which were individually inoculated at 2% (from an overnight culture) in BHI broth and incubated at 37°C for 4 h. The addition of bacteriocin to approximately 106 to 107 of the actively growing cells caused a rapid loss of viability in comparison to control cultures to which no bacteriocin was added. For both S. aureus 10 and Streptococcus dysgalactiae M, >99.9% of cells were killed within 2 h (using 1,280 and 10,240 AU/ml, respectively), demonstrating the potency of this bacteriocin against these mastitic pathogens. Moreover, by adding increasing concentrations of bacteriocin, an increased rate of this killing effect was observed (e.g., only 1 of 106 S. aureus 10 cells survived exposure to 10,240 AU of lacticin 3147/ml for 1 h, thus indicating that the residual populations [as seen in Fig. 1] are not resistant and can be killed by merely increasing the bacteriocin concentration). The frequencies at which these mastitic strains could develop resistance to lacticin 3147 were also investigated. The results demonstrated that the frequency of spontaneous resistance was less than 1 in 1 million cells after 24 h of incubation for Streptococcus dysgalactiae M, while no resistance was observed after 24 h of incubation for S. aureus 10, and these data are in agreement with previous results demonstrating negligible spontaneous resistance to the bacteriocin for the species Listeria monocytogenes (data not shown) and for lactococci (2).
TABLE 1.
Sensitivities of various mastitic streptococcal and staphylococcal strains to concentrated lacticin 3147a
| Strain | Inhibition zoneb (mm) |
|---|---|
| Streptococcus agalactiae H | 12.4 ± 0.4 |
| Streptococcus dysgalactiae M | 17.0 ± 2.0 |
| Streptococcus uberis 1 | 13.0 ± 0.1 |
| Streptococcus uberis 2 | 13.5 ± 0.3 |
| Streptococcus uberis 3 | 14.1 ± 0.7 |
| Streptococcus uberis 4 | 13.6 ± 0.3 |
| Streptococcus uberis 5 | 12.1 ± 0.4 |
| Staphylococcus aureus V | 12.3 ± 2.2 |
| Staphylococcus aureus 1 | 11.7 ± 1.0 |
| Staphylococcus aureus 2 | 11.5 ± 0.5 |
| Staphylococcus aureus 5 | 11.6 ± 1.1 |
| Staphylococcus aureus 10 | 12.5 ± 1.4 |
| Staphylococcus aureus 12 | 13.2 ± 1.1 |
| Staphylococcus aureus 13 | 11.6 ± 1.1 |
| Staphylococcus aureus 22 | 14.1 ± 1.8 |
| Staphylococcus aureus 27 | 10.1 ± 0.3 |
| Staphylococcus aureus 36 | 11.5 ± 0.5 |
| Staphylococcus aureus 423 | 12.2 ± 0.9 |
| Staphylococcus aureus 425 | 11.2 ± 0.4 |
| Staphylococcus aureus 431 | 12.7 ± 1.4 |
| Staphylococcus aureus 426 | 14.7 ± 2.8 |
| Staphylococcus aureus 427 | 13.5 ± 0.6 |
| Staphylococcus aureus 429 | 10.8 ± 1.2 |
| Staphylococcus aureus 430 | 11.6 ± 2.0 |
All staphylococci and streptococci were aerobically incubated in BHI at 37°C. All strains were obtained from Dairy Products Research Centre, Moorepark, Fermoy, County Cork, Ireland, except S. aureus V, which was obtained from the American Type Culture Collection, Rockville, Md.
Results of triplicate assays.
FIG. 1.
Bactericidal effect of lacticin 3147 on Streptococcus dysgalactiae M (a) and S. aureus 10 (b). Lacticin 3147 was added to Streptococcus dysgalactiae M cultures at a final concentration of 10,240 AU/ml and to S. aureus 10 cultures at a final concentration of 1,280 AU/ml. ⧫, bacteriocin added; ▪, no bacteriocin added (controls).
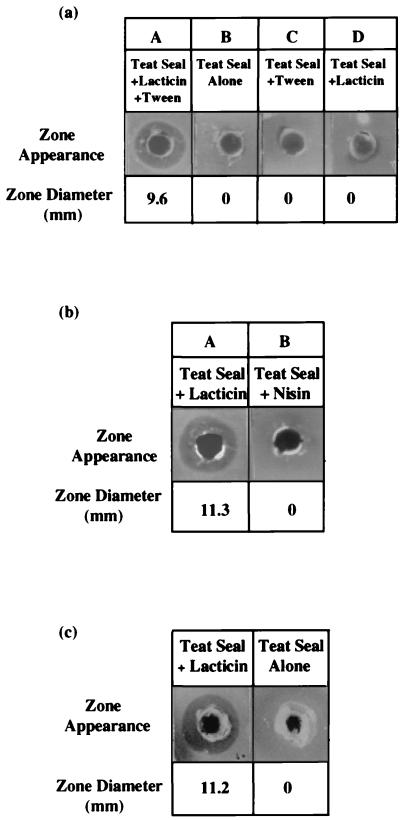
Given that lacticin 3147 was effective at inhibiting various mastitic pathogens, the potential for incorporating the bacteriocin into a teat seal was investigated, with a view to formulating an improved dry-cow product. Concentrated lacticin 3147 was added to a 5-g teat seal preparation, and the bacteriocin-supplemented preparation was mixed to form an emulsion. Bacteriocin activity in the teat seal was assayed by the agar well diffusion method. Molten Mueller-Hinton agar (Merck, Darmstadt, Germany) was seeded with 200 μl of Streptococcus dysgalactiae M, and wells (4.6 mm in diameter) were bored in the agar. Teat seal preparations were carefully dispensed into the wells so that the seals were in contact with the walls of the wells. Very little bacteriocin could be detected in the teat seal preparations containing bacteriocin alone. This may have been due to partitioning of the bacteriocin into the oil-based seal, given that the bacteriocin is hydrophobic (unpublished data). However, the release of the bacteriocin could be improved significantly by addition of Tween 80 (at a final concentration of 2%) to the bacteriocin prior to mixing it with the teat seal. While teat seals containing both lacticin 3147 and Tween 80 produced clear zones of inhibition against Streptococcus dysgalactiae M (Fig. 2a, panel A), no inhibitory activity was shown with seals containing either Tween 80 (Fig. 2a, panel C) or lacticin 3147 (Fig. 2a, panel D) alone.
FIG. 2.
(a) Tween 80 dependency. Tween 80 is needed for release of lacticin 3147 from the oil-based teat seal (A); there is no antimicrobial activity associated with teat seal alone (B), from seals containing Tween 80 alone (C), or from seals containing lacticin 3147 alone (D). (b) Comparison of antimicrobial activities of lacticin 3147 (A) and nisin (B) when incorporated into teat seals under identical conditions. (c) Residual lacticin 3147 activity in recovered teat seal. The control, containing no lacticin, gives no zone of inhibition.
The effect of this lacticin 3147-containing teat seal is twofold; first, the seal itself will provide an effective physical barrier against infection at the teat orifice, and second, the bacteriocin will inhibit any infectious gram-positive microorganisms which have the potential to evade the teat seal plug. Interestingly, in parallel experiments using concentrated nisin (prepared as described for lacticin 3147, using L. lactis NCDO496 as the nisin-producing strain), no inhibition of Streptococcus dysgalactiae M was observed (Fig. 2b). Nisin has been shown to be inhibitory to Streptococcus dysgalactiae M in previous experiments (results not shown). The final activity of both lacticin 3147 and nisin solutions, using L. lactis HP as the indicator strain, was 80,920 AU/ml. Both bacteriocins were diluted 10-fold in sodium phosphate buffer (100 mM; pH 7). The final pH values were 6.84 and 6.86 for lacticin 3147 and nisin, respectively. The two diluted bacteriocin solutions were then assayed against L. lactis HP to determine if their activities were affected by the rise in pH. The nisin activity had decreased to 2,560 AU/ml, while the lacticin 3147 activity was 10,240 AU/ml. Thus (allowing for the 10-fold dilution with buffer), nisin had lost approximately 75% of its activity while lacticin 3147 did not appear to have lost any activity. Finally, 400 μl of each bacteriocin solution was incorporated separately into two teat seals at final concentrations of 2,560 AU/g of teat seal for lacticin 3147 and 640 AU/g of teat seal for nisin. The surfactant Tween 80 was added to both bacteriocin solutions at a concentration of 2% prior to their addition to the teat seal (nisin activity is enhanced by the addition of surfactants such as Tween 80 [5]). The teat seals were then assayed against Streptococcus dysgalactiae M to check for inhibition. As expected, the seal containing lacticin 3147 exhibited inhibition against the indicator strain, whereas the seal containing nisin gave negligible inhibition. Hence, although nisin is inhibitory to many mastitic strains, it appears that its insolubility at physiological pH makes it unavailable as an inhibitory agent. Lacticin 3147, therefore, may be a more suitable candidate for such applications.
The teat seal containing lacticin 3147 and Tween 80 was subsequently introduced into an infection-free teat of an adult cow to test for irritancy in vivo. After 6 h, the seal was recovered and residual bacteriocin activity was assayed against Streptococcus dysgalactiae M by the well diffusion method described earlier. The recovered seal was initially heated for 10 min at 80°C, and 0.1 g was inserted into a well. Residual activity was observed as a clear zone of inhibition (Fig. 2c). For the next 4 days, the somatic cell counts were monitored during the morning and evening milkings to ensure that the bacteriocin-containing teat seal was nonirritating. No notable increase in somatic cell count over that of the untreated controls was observed, suggesting that this bacteriocin is a nonirritating agent (data not shown). Hence, the teat seal appears to provide a safe yet effective vehicle for localization of the bacteriocin in the teat canal. Moreover, the resultant seal retains its bacteriocin activity even after being infused in the teat of an animal.
In conclusion, the novel broad-host-range bacteriocin lacticin 3147 has considerable potential for the prevention of mastitis. Given the problems associated with antibiotics, their use in prophylactic preventative mastitis treatments may be restricted in the future, and for this reason there is a growing need for effective natural alternatives. Previous studies have shown that teat seals alone provide an effective physical barrier against infection in dry cows, and preliminary experiments in animals, recently performed in this laboratory, have indicated that a teat seal in combination with lacticin 3147 protects against Streptococcus dysgalactiae in artificial-infection studies.
Acknowledgments
This work was supported by both European Union structural funds and by Dairy Levy funds. M.P.R. was supported by the Teagasc Walsh Fellowship Programme.
We gratefully acknowledge Jim Morgan and Vincent McNally (Cross Vetpharm Group Ltd., Tallaght, Dublin 24, Ireland) for helpful discussions and for providing teat seal.
REFERENCES
- 1.Broadbent J R, Chou C, Gillies K, Kondo J K. Nisin inhibits several gram-positive, mastitis-causing pathogens. J Dairy Sci. 1989;72:3342–3345. doi: 10.3168/jds.S0022-0302(89)79496-0. [DOI] [PubMed] [Google Scholar]
- 2.Coakley M, Fitzgerald G F, Ross R P. Application and evaluation of the phage resistance- and bacteriocin-encoding plasmid pMRC01 for the improvement of dairy starter cultures. Appl Environ Microbiol. 1997;63:1434–1440. doi: 10.1128/aem.63.4.1434-1440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craven N. Efficacy and financial value of antibiotic treatment of bovine clinical mastitis during lactation—a review. Br Vet J. 1987;143:412–422. doi: 10.1016/0007-1935(87)90018-2. [DOI] [PubMed] [Google Scholar]
- 4.Delves-Broughton J. Nisin and its uses as a food preservative. Food Technol. 1990;44:100–112. [Google Scholar]
- 5.Jung D S, Bodyfelt F W, Daeschel M A. Influence of fat and emulsifiers on the efficacy of nisin in inhibiting Listeria monocytogenes in fluid milk. J Dairy Sci. 1990;75:387–393. doi: 10.3168/jds.S0022-0302(92)77773-X. [DOI] [PubMed] [Google Scholar]
- 6.McAuliffe O, Ryan M P, Ross R P, Hill C, Breeuwer P, Abee T. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl Environ Microbiol. 1998;64:439–445. doi: 10.1128/aem.64.2.439-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meaney W J. Effect of dry period teat seal on bovine udder infection. Ir J Agric Res. 1977;16:293–299. [Google Scholar]
- 8.Oldham E R, Daley M J. Lysostaphin: use of a recombinant bactericidal enzyme as a mastitis therapeutic. J Dairy Sci. 1991;74:4175–4182. doi: 10.3168/jds.S0022-0302(91)78612-8. [DOI] [PubMed] [Google Scholar]
- 9.Ryan M P, Rea M C, Hill C, Ross R P. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl Environ Microbiol. 1996;62:612–619. doi: 10.1128/aem.62.2.612-619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sears P, Peele J, Lassauzet M, Blackburn P. Proceedings of the 3rd International Mastitis Seminar. 1995. Use of antimicrobial proteins in the treatment of bovine mastitis; pp. 17–18. [Google Scholar]
- 11.Sears P M, Wilson D J, Gonzalez R N. Proceedings of the XVIIth World Buiatrics Congress and the XXVth American Association of Bovine Practitioners Conference. Vol. 2. 1992. The potential role of antimicrobial proteins in the treatment of bovine mastitis; pp. 138–142. [Google Scholar]