Abstract
Objectives
Fosmanogepix (APX001), a first-in-class, intravenous (IV) and oral (PO) antifungal prodrug, is being developed to treat invasive fungal diseases (IFDs). Manogepix (APX001A; active moiety) targets fungal glycosylphosphatidylinositol-anchored cell wall transfer protein 1, inhibiting cell wall synthesis causing loss of viability. This open-label, multicentre, Phase 1b study in patients with AML and neutropenia (absolute neutrophil count <500 cells/μL; >10 days) undergoing chemotherapy aimed to assess tolerability, safety and pharmacokinetics (PK) of IV and PO fosmanogepix.
Methods
Of 21 adult AML patients undergoing remission induction chemotherapy, 10 received IV fosmanogepix (600 mg; q24h) and 11 received oral fosmanogepix (500 mg; q24h) over 14 days, with a 28 day follow-up. Patients also received remission induction chemotherapy [sequential high-dose cytarabine and mitoxantrone (S-HAM) or 7 + 3 regimen] for AML and IFD prophylaxis (posaconazole). A two-compartmental PK model from previous studies in healthy volunteers was fitted to manogepix plasma data.
Results
Of 26 fosmanogepix-related adverse events (AEs; IV: 14; PO: 12) in 9 (42.9%) patients [IV: 5 (50%); PO: 4 (36.4%)], none were serious or resulted in fosmanogepix discontinuation. Most frequently occurring fosmanogepix-related AEs were Grade 1/2 nausea [four events in three patients (14.3%)]; vomiting, ALT increase, and delirium [two events; two patients (9.5%) each]. One patient experienced fosmanogepix-related Grade 3 hypertension. Dose-corrected geometric mean ratio of AUC (PO-to-IV) was 95%. Elimination half-lives (∼2 days) were consistent with prior studies in healthy volunteers.
Conclusions
Fosmanogepix was safe and well tolerated in AML patients with neutropenia receiving remission induction chemotherapy. Safety and PK profiles were comparable to healthy volunteers.
Introduction
More than a billion people are affected by fungal disease, with about 1.5 million deaths reported each year.1 Invasive fungal diseases (IFDs) result in significant morbidity and mortality, particularly in those with comorbid conditions.2 Rates of IFDs are generally high in people who are immunocompromised such as persons living with HIV, transplant recipients, people receiving cancer treatment, and in the elderly.3,4
Currently, amphotericin B, azoles and echinocandins constitute the mainstay of treatment for fungal infections.5,6 Despite the availability of these drugs, mortality rates due to IFDs remain high. Echinocandins were introduced in 2002, and isavuconazole was approved in 2015 for the treatment of IFDs. Except for ibrexafungerp and oteseconazole, which were recently approved for the treatment of vulvovaginal candidiasis, no new antifungals have been marketed since then, particularly for the treatment of IFDs.7
Existing treatment options may have limited use in patients with IFDs, due to poor tolerability and toxicity, suboptimal drug exposure at the site of infection, reduced bioavailability of oral formulations while switching dosage forms,8 and pharmacokinetic interactions with other drugs.9 In addition, switching from IV to oral formulations may lead to poor drug exposures and potentially suboptimal efficacy. For example, switching from IV to oral voriconazole led to an 80% decrease in plasma concentrations, although both formulations were administered at the same dose.8 In patients with ALL receiving vincristine chemotherapy, voriconazole and itraconazole coadministration resulted in significant drug–drug interactions leading to adverse drug reactions prohibiting azole use in those patients.10 In addition, rates of resistance are increasing and MDR pathogenic fungi that are resistant to treatment with amphotericin B, azoles and echinocandins have been reported.11
Fosmanogepix (APX001, PF-07842805, E1211), the first member of the ‘gepix’ class of antifungals, is a broad-spectrum antifungal with a novel mechanism of action that inhibits Gwt-1, an essential enzyme for fungal replication and pathogenesis. Inhibition of Gwt-1 leads to the disruption and loss of integrity of the fungal cell wall. Fosmanogepix is metabolized into its active form, manogepix, by systemic alkaline phosphatases.12 Previous Phase 1 studies in healthy volunteers have demonstrated high (>90%) oral bioavailability of manogepix, and maintenance of manogepix plasma drug exposures above estimated antifungal target levels for 7–42 days, even while switching from IV to oral dosing.13,14 Fosmanogepix distribution to sites of infection that are traditionally hard to treat, such as the brain, eyes and abdomen, is observed in animal models.15,16 In addition, the combination of amphotericin B and manogepix has shown varying degrees of synergy against Candida spp. (10% synergy, 90% indifferent). Checkerboard analysis of 18 strains of Aspergillus fumigatus and 4 strains of Aspergillus flavus showed that the effect of the combination of manogepix and amphotericin B was indifferent. Importantly, no antagonism was observed.17 As part of the fosmanogepix clinical development programme, several Phase 1b and Phase 2 clinical trials (NCT04148287, NCT04240886, NCT04240886) have been completed. In addition, several Phase 3 trials are planned and in progress.13,18
Initial results from completed Phase 1 and 2 clinical trials are in line with published preclinical and in vitro susceptibility data and animal models of infection.18–21 The overall goals for the clinical development programme are to determine if fosmanogepix is a safe and effective treatment option for IFDs caused by the major pathogenic fungi (i.e. Candida, Cryptococcus and Aspergillus spp.) including drug-resistant fungi.22,23 The main objective of this Phase 1b study in neutropenic patients with AML undergoing remission induction chemotherapy was to assess the safety, tolerability and pharmacokinetics (PK) of IV and oral fosmanogepix in an immunocompromised population who are at risk of IFDs.
Methods
Ethics
This study was conducted at three sites in Germany (Cologne, Munich and Mainz) and in compliance with the Declaration of Helsinki and the International Council for Harmonization guidelines on Good Clinical Practice (ICH-GCP). Independent ethics committees at each site (Ethics Commission of Cologne University’s Faculty of Medicine; approval # 17-179) approved the study protocol. Written informed consent was obtained from all participants prior to any trial-related investigations or procedures.
Study design and participants
In this open-label, multicentre, Phase 1b trial (NCT03333005; EudraCT No. 2017-000524-10), adults (≥18 years) undergoing remission induction chemotherapy for AML with neutropenia (absolute neutrophil count <500 cells/μL for >10 days) were enrolled. Patients were excluded if they received systemic antifungal therapy for proven or probable IFDs in the 12 months prior to the first administration of study medication, were hepatitis B/C positive or HIV positive, or had QTc interval prolongation of >450 ms. Patients receiving strong inducers of CYP P450 isoenzymes, e.g. rifampicin, rifabutin, ergot alkaloids and carbamazepine, and who could not discontinue these medications at screening were also excluded. Hepatic and renal dysfunction and any different or new cause of neutropenia or immunosuppression were also considered criteria for exclusion.
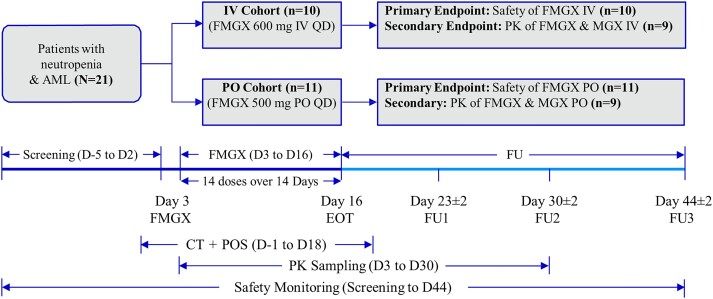
Patients received either IV fosmanogepix, 600 mg once a day (q24h) infused over 3 h (IV cohort) or oral (PO) fosmanogepix, 500 mg q24h (PO cohort). Patients were also receiving remission induction chemotherapy [sequential high-dose cytarabine and mitoxantrone (S-HAM) or 7 + 3 CT regimen] for AML and antifungal prophylaxis (posaconazole) for IFDs per local clinical standards (administered at 300 mg/day in most patients). Fourteen doses of fosmanogepix per participant were administered over 14 consecutive days in both cohorts, from Day 3 to Day 16 [end of treatment (EOT)]. Each participant was in the study for a maximum of 51 days including screening (Days −5 to 2), followed by the start of chemotherapy (Day 1), an overlapping fosmanogepix treatment period (Days 3 to 16 of the chemotherapy cycle) and follow-up for 28 days after EOT (i.e. until Day 44; Figure 1).
Figure 1.
Study design. AML, acute myeloid leukaemia; CT, remission induction chemotherapy; D, day; EOT, end of treatment; FMGX, fosmanogepix; FU, follow-up; IV, intravenous; MGX, manogepix; PK, pharmacokinetics; PO, oral; POS, posaconazole; QD, once a day. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Assessments
The primary endpoint was safety, assessed by frequency of adverse events (AEs), collected from screening to Day 44 (EOT + 28 days follow-up). AEs were reviewed by the investigator, who ascertained whether they were related to fosmanogepix treatment or not. Physical examinations, laboratory tests (including haematology and urinalysis), and 12-lead ECGs were conducted to assess safety. The secondary endpoint was manogepix plasma levels at specified timepoints from Day 3 (start of fosmanogepix treatment) up to Day 30 (EOT + 14 days follow-up). Due to sparse sampling relative to the number of doses over the 14-day fosmanogepix treatment period, two compartmental IV and oral pharmacokinetic (PK) models from previous studies in healthy volunteers (NCT02956499 and NCT02957929) were fitted to the manogepix plasma data.21 Primary PK parameters used to fit the model included clearance [plasma (CL) and intercompartmental (CLD2)] and volumes of distribution [central (V1) and peripheral compartment (V2)]. For the oral model, the clearances and volumes are uncorrected for bioavailability (F), i.e. CL/F etc., and the absorption rate constant, ka, was also estimated. Secondary PK parameters, which were derived from the primary parameters, included rate constants for distribution (α) and elimination (β) and their associated half-lives [distribution (t½α) and elimination (t½β)], volume of distribution—steady state (VSS), maximum concentration at steady state (Cmax) and AUC over the dosing interval at steady state.
Sample size and analysis sets
No statistical tests were performed as the study was not designed for hypothesis testing. A sample size of 20 was considered sufficient to assess safety and PK endpoints. The safety analysis set included participants who received at least one dose of fosmanogepix. These participants were included in the PK analysis set only if adequate plasma concentration data were available for PK analysis.
Results
Of 25 screened participants, 21 received fosmanogepix treatment (IV cohort: 10; PO cohort: 11) and comprised the safety analysis set. Of these, nine patients were enrolled in Cologne, five in Munich, and seven in Mainz. Five participants discontinued the study prematurely (IV cohort: 3; PO cohort: 2) due to an AE (n = 1), death (n = 2) (IV cohort), infection (n = 1) and non-compliance (n = 1) (PO cohort). Of the five participants who discontinued, three (IV cohort: 1; PO cohort: 2) were excluded from the PK analysis set due to lack of data since they received ≤5 doses of fosmanogepix. The PK analysis set thus included 18 patients (n = 9 in each cohort). All 21 participants in the safety analysis set were white, and the majority were female (61.9%). The mean age was 51.9 years (Table 1).
Table 1.
Baseline and demographic characteristics (safety analysis set)
| Parameter | IV cohort (600 mg q24h) n = 10 |
PO cohort (500 mg q24h) n = 11 |
Total N = 21 |
|---|---|---|---|
| Age (years), mean (SD) | 53.1 (13.4) | 50.7 (11.1) | 51.9 (12.0) |
| Gender, n (%) | |||
| Female | 8 (80.0) | 5 (45.5) | 13 (61.9) |
| Male | 2 (20.0) | 6 (54.5) | 8 (38.1) |
| Race, n (%) | |||
| White | 10 (100.0) | 11 (100.0) | 21 (100.0) |
| BMI (kg/m2), mean (SD) | 26.44 (5.73) | 26.24 (5.52) | 26.33 (5.48) |
BMI, body mass index; IV, intravenous; PO, oral; q24h, once a day; SD, standard deviation.
Primary endpoint: safety (AE assessment)
A total of 358 AEs (IV cohort: 227, PO cohort: 131) were reported (Table 2). Overall, in both IV and PO cohorts, AEs were most frequent in the system organ classes (SOCs) of general disorders and administration site conditions (36 and 31 events), gastrointestinal disorders (40 and 25 events) and infections and infestations (20 and 15 events) (Table S1, available as Supplementary data at JAC Online). Pyrexia was the most frequent AE (28 events) affecting 17 (81%) participants (14 events each in IV and PO cohorts).
Table 2.
Safety summary and summary of FMGX-related AEs by SOC and PT (safety analysis set)
| Parameters | IV cohort (600 mg q24h) n = 10 | PO cohort (500 mg q24h) n = 11 | Total N = 21 |
|||
|---|---|---|---|---|---|---|
| Events | n (%) | Events | n (%) | Events | n (%) | |
| AEs | 227 | 10 (100) | 131 | 11 (100) | 358 | 21 (100) |
| SAEs (any grade) | 9 | 5 (50) | 7 | 6 (54.5) | 16 | 11 (52.4) |
| AEs leading to withdrawal/discontinuation | 2 | 2 (20) | 1 | 1 (9.1) | 3 | 3 (14.3) |
| AEs leading to deaths | ||||||
| Related to FMGX treatment | — | 0 | — | 0 | — | 0 |
| Unrelated to FMGX treatment | 3 | 3a (30) | — | 0 | — | 3 (14.3) |
| FMGX-related AEs | 14 | 5 (50.0) | 12 | 4 (36.4) | 26 | 9 (42.9) |
| FMGX-related AEs by SOC and PT | ||||||
| General disorders and administration site conditions | 5 | 2 (20.0) | — | 0 | 5 | 2 (9.5) |
| Fatigue | 1 | 1 (10.0) | — | 0 | 1 | 1 (4.8) |
| Chills | 4 | 1 (10.0) | — | 0 | 4 | 1 (4.8) |
| Gastrointestinal disorders | 4 | 3 (30.0) | 3 | 2 (18.2) | 7 | 5 (23.8) |
| Nausea | 1 | 1 (10.0) | 3 | 2 (18.2) | 4 | 3 (14.3) |
| Diarrhoea | 1 | 1 (10.0) | — | 0 | 1 | 1 (4.8) |
| Vomiting | 2 | 2 (20.0) | — | 0 | 2 | 2 (9.5) |
| Infections and infestations | — | 0 | 2 | 1 (9.1) | 2 | 1 (4.8) |
| Pustular rash | — | 0 | 2 | 1 (9.1) | 2 | 1 (4.8) |
| Skin and subcutaneous tissue disorders | — | 0 | 5 | 2 (18.2) | 5 | 2 (9.5) |
| Rash | — | 0 | 1 | 1 (9.1) | 1 | 1 (4.8) |
| Intertrigo | — | 0 | 4 | 1 (9.1) | 4 | 1 (4.8) |
| Vascular disorders | 2 | 1 (10.0) | 1 | 1 (9.1) | 3 | 2 (9.5) |
| Hypertension | 2 | 1 (10.0) | — | 0 | 2 | 1 (4.8) |
| Vasculitis | — | 0 | 1 | 1 (9.1) | 1 | 1 (4.8) |
| Investigations | 1 | 1 (10.0) | 1 | 1 (9.1) | 2 | 2 (9.5) |
| ALT increased | 1 | 1 (10.0) | 1 | 1 (9.1) | 2 | 2 (9.5) |
| Psychiatric disorders | 2 | 2 (20.0) | — | 0 | 2 | 2 (9.5) |
| Delirium | 2 | 2 (20.0) | — | 0 | 2 | 2 (9.5) |
AEs, adverse events; FMGX, fosmanogepix; IV, intravenous; PO, oral; PT, preferred term; q24h, once a day; SOC, system organ class; SAE, treatment-emergent serious AE.
Total of four patients died during the study; one patient died before receiving any study drug and was considered a screen failure.
A total of 26 AEs (IV cohort: 14, PO cohort: 12), observed in 9 (42.9%) participants [IV cohort: 5 (50%), PO cohort: 4 (36.4%)], were considered related to fosmanogepix treatment by the investigator; none of these were serious and none resulted in study drug discontinuation (Table 2). Except for one participant with Grade 3 hypertension (IV cohort), all other fosmanogepix-related AEs were Grade 1 (mild) or Grade 2 (moderate); none were Grade 4 or Grade 5. The most frequently occurring fosmanogepix-related AE was nausea [four events in three patients (14.3%)], followed by vomiting, an increase in ALT, and delirium [two events in two patients (9.5%) each]. Vomiting and delirium occurred only in the IV cohort (Table 2). Of the two events of ALT increase (Grade 2, reported in one patient in each cohort), one event occurred 3 days after fosmanogepix administration and preceded an episode of febrile neutropenia and sepsis. The second event occurred 5 days after the first dose of fosmanogepix, following a Klebsiella pneumoniae infection and pyrexia. In both patients, no increases in other markers of drug-induced liver injury were detected and both events were reported as recovered/resolved. Overall, the majority of events reported to be fosmanogepix related recovered or were recovering at the last assessment timepoint. Interruption of fosmanogepix treatment occurred in a single participant (IV cohort) who experienced three events of chills, possibly related to fosmanogepix.
All serious AEs, clinically significant laboratory findings, vital signs, ECG and physical examination findings observed in this study were considered unrelated to fosmanogepix treatment. A total of 16 AEs (IV cohort: 9, PO cohort: 7) were reported in 11 (52.4%) participants [IV cohort: 5 (50%), PO cohort: 6 (54.5%)] and considered serious; none of those were found to be fosmanogepix related by the investigator (Table 2). Additionally, the AEs that were reported in Phase 1 studies in healthy volunteers (headache and dizziness) were not reported.23 Chemotherapy-induced nausea and vomiting (CINV) occurred occasionally and was attributed to remission induction chemotherapy, with no apparent augmentation due to the administration of fosmanogepix.
No confirmed cases of invasive fungal infections were reported during the trial. One patient experienced a suspected case of abdominal candidiasis during the follow-up period (approximately 18 days after fosmanogepix administration was completed), which was treated with IV caspofungin. This case did not meet the criteria of a fosmanogepix breakthrough of infection since a fungal infection was suspected (not confirmed) and occurred several days after fosmanogepix administration was completed. A total of three patients discontinued study treatment, two due to AEs [Grade 3 acute kidney injury (IV cohort) and life-threatening Grade 4 pneumonia (PO cohort); both fosmanogepix unrelated (assessed by investigator)] and one due to withdrawal of consent. Overall, four deaths were reported; one participant died due to disease progression before receiving any study drug (screen failure), and three participants died of neutropenic colitis, sepsis and bronchopulmonary haemorrhage (one participant each, IV cohort), respectively. None of these deaths was considered related to fosmanogepix treatment or study procedures (Table 2).
All clinically significant laboratory findings observed in this study were attributed to the underlying condition of AML and remission induction chemotherapy by the investigator. Expected abnormalities in haematology occurred in all patients. Approximately 50% of all abnormal values were rated as clinically significant. Most abnormal serum chemistry values were not clinically significant. No unusual patterns in vital signs were observed, with blood pressure and heart rate mostly within normal ranges. QTc intervals were also mostly within normal ranges.
Secondary endpoint: PK of manogepix
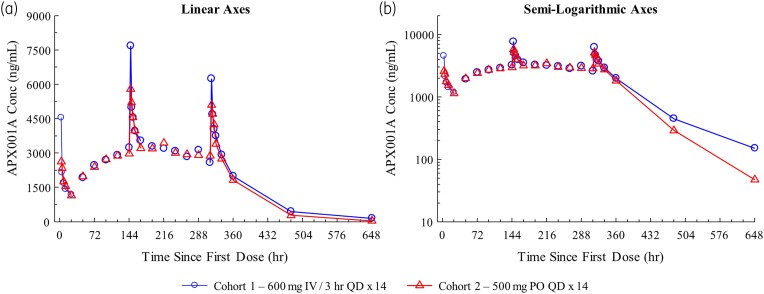
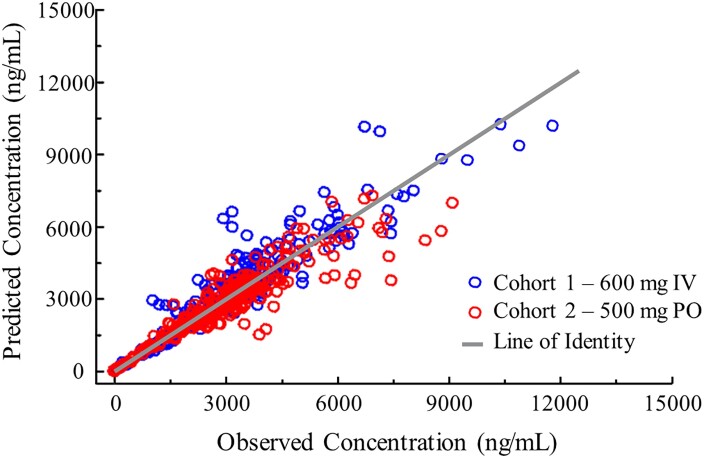
The geometric mean observed manogepix plasma concentrations for IV and PO administration are illustrated in Figure 2. The model-predicted versus observed individual participant concentrations were randomly distributed along a line of identity, indicating that the models were consistent with the data (Figure 3). The geometric mean observed Cmax on Days 7 and 14 were comparable between cohorts and consistent with the geometric mean model-predicted Cmax, further demonstrating the fit of the data to the models (Table 3). Observed Cmax on Day 14 was assessed in only eight participants since one participant (IV cohort) received only 10 doses (instead of 14 doses).
Figure 2.
Plasma concentrations (geometric mean) of MGX after IV or PO administration. Geometric mean plasma concentrations after IV or PO administration. (a) Linear representation. (b) Semi-log representation. The observed Cmax of FMGX was comparable between the IV and PO cohorts. FMGX, fosmanogepix; hr, hour; IV, intravenous; MGX, manogepix; PO, oral; QD, once a day. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Relationship between observed and model-predicted plasma FMGX concentrations after IV and PO administration. Model-predicted plasma concentrations were concordant with observed plasma concentrations, with the majority of observations lying along a line of identity and demonstrating that the IV and PO models were consistent with the data. FMGX, fosmanogepix; IV, intravenous; PO, oral. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 3.
Summary of PK parameters of MGX (PK analysis set)
| Parameter, geometric mean (% CV) | IV cohort (600 mg q24h) n = 9 |
PO cohort (500 mg q24h) n = 9 |
|---|---|---|
| Primary | ||
| First-order absorption rate constant (1/h) | — | 1.49 (141.3) |
| Clearance (mL/h) | 3696 (29.7) | 3879 (21.5) |
| Central compartment volume of distribution (L) | 61.4 (43.3) | 110 (66.3) |
| Intercompartmental clearance (mL/h) | 16 203 (200) | 5130 (322) |
| Peripheral compartment volume of distribution (L) | 188 (79.0) | 78.9 (113) |
| Secondary | ||
| Cmax (ng/mL) | 8033 (24.9) | 5610 (37.5) |
| Primary | ||
| AUC (ng·h/mL) | 124 217 (29.7) | 98 634 (21.5) |
| Distribution half-life (h) | 1.52 (112) | 4.43 (163) |
| Elimination half-life (h) | 60.9 (43.6) | 47.4 (52.2) |
| Volume of distribution, steady state (L) | 270 (43.7) | — |
| Observed | ||
| Cmax Day 7 (ng/mL) | 7876 (23.9) | 6319 (29.6) |
| Cmax Day 14 (ng/mL) | 6254 (38.9)a | 5616 (35.3) |
The CL and V values are CL/F and V/F for PO treatment where F = bioavailability. Primary and secondary parameters are from fitting the compartmental model to the data. Primary parameters were used to fit the model, and secondary parameters were calculated from primary parameters. Observed parameters were taken from observed data. AUC, area under the curve over a dosing interval at steady-state; Cmax, maximum plasma concentration; CV, coefficient of variation; h, hour; IV, intravenous; MGX, manogepix; PK, pharmacokinetic; PO, oral; q24h, once a day
n = 8 [1 participant (IV cohort) received only 10 doses; Cmax Day 14 could not be calculated for that participant].
PK parameters between IV and PO cohorts were in agreement as assessed by geometric mean values of CL and CL/F and V1 and V1/F. The dose-corrected geometric mean ratio of AUC (PO to IV) was 95%, demonstrating the high oral bioavailability of fosmanogepix. Elimination half-lives after IV (60.9 h) and PO (47.4 h; Table 3) administration were consistent with prior studies in healthy volunteers.24,25
Haematocrit (HCT) values were found to affect the plasma concentration of manogepix. Measured plasma concentrations of manogepix were found to be about 27% lower in blood with low HCT values [∼21.5% (in patients with AML)] compared with blood with normal HCT values (∼41.5%). Assuming similar blood volumes in both AML patients and healthy volunteers, results were comparable since overall systemic exposure to manogepix was similar in both populations and consistent with a drug that favours the plasma compartment.
Discussion
Results from this Phase 1b study of fosmanogepix in patients with neutropenia and AML receiving remission induction chemotherapy and posaconazole prophylaxis indicate that fosmanogepix was safe and well tolerated and that the safety and PK profile of fosmanogepix is comparable with previously published healthy volunteer data. Most AEs were unrelated to fosmanogepix treatment. One participant experienced suspected abdominal candidiasis. This case did not meet the criteria of a fosmanogepix breakthrough infection since a fungal infection was not confirmed and occurred several days after fosmanogepix administration was completed. No fosmanogepix-related instances of headache or dizziness were reported, and the frequency and intensity of the observed incidence of nausea and vomiting were similar to what is expected with remission induction chemotherapy,26 with no apparent augmentation due to fosmanogepix administration. Although fosmanogepix treatment was interrupted by mild AEs (chills) in a single patient, none of the discontinuations, serious AEs or deaths reported during the study were related to fosmanogepix treatment. No unexpected side effects or toxicities were observed, and no PK interactions between fosmanogepix and either induction chemotherapy or posaconazole antifungal prophylaxis were observed. In addition, no increases in manogepix levels were observed in this study due to coadministration of posaconazole. Previous studies on azoles have shown that drug interactions with CYP P450-sensitive substrates (e.g. immunosuppressants, chemotherapy, antivirals, statins) may lead to high exposures of these drugs and may necessitate regular therapeutic drug monitoring to ensure that target plasma exposures are maintained.27,28 The lack of such drug–drug interaction toxicities with fosmanogepix observed in the current study may indicate that the risk of drug–drug interactions caused by the coadministration of fosmanogepix and drugs that are metabolized by CYP P450 enzyme is low.
After the administration of a 14 day course of IV (600 mg q24h) or oral (500 mg q24h) fosmanogepix, PK exposures were similar, with a dose-corrected geometric mean AUC ratio (PO to IV) of 95%. This is in line with previous data from healthy volunteers that demonstrated oral bioavailability of >90% and indicated complete conversion of fosmanogepix to manogepix.25 Findings from this study suggest that transitioning from IV to oral fosmanogepix due to changes in care settings or patient tolerance is feasible.13 Geometric mean half-lives were 60.9 h after IV administration and 47.4 h after oral administration. In healthy volunteers, a half-life of approximately 60 h was reported for both dose forms.24,25
After administration, fosmanogepix is rapidly converted to manogepix, with fosmanogepix concentrations lower than the limit of quantification within 8 h of starting the IV infusion. In previous Phase 1 studies of oral and IV fosmanogepix, manogepix plasma exposures were linear and interparticipant variability was low.24,25 After IV administration of 600 mg fosmanogepix over a 3 h infusion period, the observed geometric manogepix Cmax on Day 7 was 7876 ng/mL. Similarly, the observed geometric manogepix Cmax on Day 7 was 6319 ng/mL for the oral cohort. At the EOT on Day 14, maximum plasma concentrations were 6254 ng/mL for the IV cohort and 5616 ng/mL for the oral cohort. Observed exposures were slightly lower than those in healthy volunteers, which may be due to differences in HCT between the two populations.
Overall, this study assessed the safety and PK of fosmanogepix in a small group of immunocompromised patients at high risk of fungal infections. However, confounding of AE relatedness for expected fosmanogepix-related events due to concomitant administration of remission induction chemotherapy may have occurred. In conclusion, fosmanogepix was safe and well tolerated in AML patients with neutropenia receiving induction chemotherapy and demonstrated a safety and PK profile that was comparable to that observed in healthy volunteers. No clinically relevant drug interactions with induction chemotherapy or posaconazole were observed. No new safety signals or toxicities were identified, and no adverse drug interactions were reported. Further investigations of fosmanogepix in patients with fungal infections are ongoing.
Supplementary Material
Acknowledgements
We thank all investigators, staff and volunteers for participating in study-related activities and procedures. Wes acknowledge Kripa Madnani (PhD, CMPP™; employee of Pfizer Inc.) for providing medical writing assistance under the guidance of the authors. Varkha Agrawal (PhD, CMPP™) and Kanchan Bhati, both employees of Pfizer Inc., provided editorial assistance.
Contributor Information
Oliver A Cornely, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Cologne, Germany; University Hospital of Cologne, Department of Internal Medicine, Medical Faculty, University Hospital of Cologne, Cologne, Germany.
Helmut Ostermann, Department of Hematology/Oncology, Ludwig Maximilian University of Munich, Munich, Germany.
Philipp Koehler, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Cologne, Germany; University Hospital of Cologne, Department of Internal Medicine, Medical Faculty, University Hospital of Cologne, Cologne, Germany.
Daniel Teschner, University Medical Center of the Johannes Gutenberg University Mainz, Department of Hematology, and Medical Oncology, Mainz, Germany; Department of Internal Medicine II, University Hospital Würzburg, Würzburg, Germany.
Endrik Limburg, Clinical Trials Centre Cologne (CTCC), University of Cologne, Cologne, Germany.
William G Kramer, Kramer Consulting LLC, North Potomac, MD, USA.
Sara H Barbat, Amplyx Pharmaceuticals Inc., San Diego, CA, USA.
Margaret Tawadrous, Pfizer Inc., 445 Eastern Point Road, Groton, CT 06340, USA.
Michael R Hodges, Amplyx Pharmaceuticals Inc., San Diego, CA, USA.
Funding
Financial support was provided by Amplyx Pharmaceuticals, Inc., now a subsidiary of Pfizer Inc., for conducting these studies and preparing the manuscript.
Transparency declarations
M.R.H. was an employee of Amplyx (now a Pfizer Inc. subsidiary). M.R.H. was previously an employee of Pfizer and holds Pfizer stock and is currently a consultant for Pfizer. O.A.C. reports: grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer and Scynexis; consulting fees from AbbVie, Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, IQVIA, Janssen, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, Pardes, Pfizer, PSI, Scynexis and Seres; honoraria for lectures from Abbott, AbbVie, Al-Jazeera Pharmaceuticals, Astellas, Gilead, Grupo Biotoscana/United Medical/Knight, Hikma, Medscape, med update, Merck/MSD, Mylan, Noscendo, Pfizer and Shionogi; payment for expert testimony from Cidara; participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, MedPace, Paratek, PSI, Pulmocide, Shionogi and The Prime Meridian Group; a patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7); stocks from Core Consulting; and honoraria from Novartis, AstraZeneca, Cerus, Vifor and Vertex. H.O. reports: consulting fees from MSD, Novartis, AstraZeneca, Grifols, SOBI, argenx, UCB, Servier and Pfizer; and was part of an independent data monitoring committee (IDMC) at Johnson & Johnson. D.T. reports: research grants from Deutsche Krebshilfe, Gilead, JOGU Mainz and Wilhelm-Sander-Stifung; honoraria from AAEF Rheinland-Pfalz, ACI Clinical, Akademie für Infektionsmedizin, AstraZeneca, BioNTech, Gilead, F2G, iQone, LAEK Hessen, MSD, Noscendo, Octapharma, Pfizer, Takeda and Tillotts; has been a consultant to ACI Clinical, BioNTech, German Society for Haematology and Medical Oncology (DGHO), Gilead, iQone, MSD, NCO, Noscendo, Octapharma, Pfizer, Sanofi and Takeda; and travel grants from AbbVie, Astellas, Cellgene, DGHO, German Society for Internal Medicine (DGIM), Gilead, Jazz, Medac, Paul Ehrlich Society (PEG) and Tillotts. P.K. reports: grants or contracts from German Federal Ministry of Research and Education (BMBF) Bundesweites Forschungsnetz Angewandte Surveillance und Testung (B-FAST) and Nationales Pandemie Kohorten Netz (NAPKON), German National Pandemic Cohort Network) of the Network University Medicine (NUM) and the State of North Rhine-Westphalia; consulting fees from Ambu GmbH, Gilead Sciences, Mundipharma Research Limited, Noxxon N.V. and Pfizer Pharma; honoraria for lectures from Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma, Bio-Rad Laboratories Inc., European Confederation of Medical Mycology, Gilead Sciences, GPR Academy Ruesselsheim, HELIOS Kliniken GmbH, med update GmbH, MedMedia, MSD Sharp & Dohme GmbH, Pfizer Pharma GmbH, Scilink Comunicación Científica SC and University Hospital and LMU Munich; participation on an Advisory Board for Ambu GmbH, Gilead Sciences, Mundipharma Research Limited and Pfizer Pharma; a pending patent currently being reviewed at the German Patent and Trade Mark Office; and other non-financial interests from Elsevier, Wiley and Taylor & Francis Online outside the submitted work. M.T. is a Pfizer employee and holds Pfizer stock. W.G.K. is an employee of Kramer Consulting LLC. E.L. and S.B. have no conflicts of interest to declare.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. Bongomin F, Gago S, Oladele RO et al. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017; 3: 57. 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rayens E, Norris KA. Prevalence and healthcare burden of fungal infections in the United States, 2018. Open Forum Infect Dis 2022; 9: ofab593. 10.1093/ofid/ofab593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravikumar S, Win MS, Chai LY. Optimizing outcomes in immunocompromised hosts: understanding the role of immunotherapy in invasive fungal diseases. Front Microbiol 2015; 6: 1322. 10.3389/fmicb.2015.01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barchiesi F, Orsetti E, Mazzanti S et al. Candidemia in the elderly: what does it change? PLoS One 2017; 12: e0176576. 10.1371/journal.pone.0176576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen SC, Perfect J, Colombo AL et al. Global guideline for the diagnosis and management of rare yeast infections: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect Dis 2021; 21: e375–e86. 10.1016/S1473-3099(21)00203-6 [DOI] [PubMed] [Google Scholar]
- 6. Hoenigl M, Salmanton-Garcia J, Walsh TJ et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 2021; 21: e246–e57. 10.1016/S1473-3099(20)30784-2 [DOI] [PubMed] [Google Scholar]
- 7. Fisher MC, Alastruey-Izquierdo A, Berman J et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 2022; 20: 557–71. 10.1038/s41579-022-00720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harada S, Niwa T, Hoshino Y et al. Influence of switching from intravenous to oral administration on serum voriconazole concentration. J Clin Pharm Ther 2021; 46: 780–5. 10.1111/jcpt.13352 [DOI] [PubMed] [Google Scholar]
- 9. Waterer G. Advances in anti-fungal therapies. Mycopathologia 2021; 186: 665–72. 10.1007/s11046-021-00560-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang L, Yu L, Chen X et al. Clinical analysis of adverse drug reactions between vincristine and triazoles in children with acute lymphoblastic leukemia. Med Sci Monit 2015; 21: 1656–61. 10.12659/MSM.893142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arastehfar A, Gabaldon T, Garcia-Rubio R et al. Drug-resistant fungi: an emerging challenge threatening our limited antifungal armamentarium. Antibiotics (Basel) 2020; 9: 877. 10.3390/antibiotics9120877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoenigl M, Sprute R, Egger M et al. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 2021; 81: 1703–29. 10.1007/s40265-021-01611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaw KJ, Ibrahim AS. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi (Basel) 2020; 6: 239. 10.3390/jof6040239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy SE, Bicanic T. Drug resistance and novel therapeutic approaches in invasive candidiasis. Front Cell Infect Microbiol 2021; 11: 759408. 10.3389/fcimb.2021.759408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee A, Wang N, Carter CL et al. Therapeutic potential of fosmanogepix (APX001) for intra-abdominal candidiasis: from lesion penetration to efficacy in a mouse model. Antimicrob Agents Chemother 2021; 65: e02476-20. 10.1128/AAC.02476-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petraitiene R, Petraitis V, Maung BBW et al. Efficacy and pharmacokinetics of fosmanogepix (APX001) in the treatment of Candida endophthalmitis and hematogenous meningoencephalitis in nonneutropenic rabbits. Antimicrob Agents Chemother 2021; 65: e01795-20. 10.1128/AAC.01795-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watanabe NA, Miyazaki M, Hata K. In Vitro Activity of E1210 and In Vivo Activity of E1211, a Water-Soluble Prodrug of E1210, in Combination with Other Antifungals . 52nd ICAAC, San Francisco, USA, 2014. Abstract number 1378.
- 18. Vazquez JA, Pappas PG, Boffard K et al. Clinical efficacy and safety of a novel antifungal, fosmanogepix, in patients with candidemia caused by Candida auris: results from a Phase 2 trial. Antimicrob Agents Chemother 2023; 67: e0141922. 10.1128/aac.01419-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badali H, Patterson HP, Sanders CJ et al. Manogepix, the active moiety of the investigational agent fosmanogepix, demonstrates in vitro activity against members of the Fusarium oxysporum and Fusarium solani species complexes. Antimicrob Agents Chemother 2021; 65: e02343-20. 10.1128/AAC.02343-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiederhold NP, Najvar LK, Shaw KJ et al. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother 2019; 63: e01120-19. 10.1128/AAC.01120-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodges MR, Ople E, Wedel P et al. Safety and pharmacokinetics of intravenous and oral fosmanogepix, a first-in-class antifungal agent, in healthy volunteers. Antimicrob Agents Chemother 2023; 67: e0162322. 10.1128/aac.01623-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giacobbe DR, Magnasco L, Sepulcri C et al. Recent advances and future perspectives in the pharmacological treatment of Candida auris infections. Expert Rev Clin Pharmacol 2021; 14: 1205–20. 10.1080/17512433.2021.1949285 [DOI] [PubMed] [Google Scholar]
- 23. Hoenigl M, Sprute R, Arastehfar A et al. Invasive candidiasis: investigational drugs in the clinical development pipeline and mechanisms of action. Expert Opin Investig Drugs 2022; 31: 795–812. 10.1080/13543784.2022.2086120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges MR, Ople E, Shaw KJ et al. First-in-human study to assess safety, tolerability and pharmacokinetics of APX001 administered by intravenous infusion to healthy subjects. Open Forum Infect Dis 2017; 4 Suppl 1: S526. 10.1093/ofid/ofx163.1370 [DOI] [Google Scholar]
- 25. Hodges MR, Ople E, Shaw KJ et al. Phase 1 study to assess safety, tolerability and pharmacokinetics of single and multiple oral doses of APX001 and to investigate the effect of food on APX001 bioavailability. Open Forum Infect Dis 2017; 4 Suppl 1: S534. 10.1093/ofid/ofx163.1390 [DOI] [Google Scholar]
- 26. Gupta K, Walton R, Kataria SP. Chemotherapy-induced nausea and vomiting: pathogenesis, recommendations, and new trends. Cancer Treat Res Commun 2021; 26: 100278. 10.1016/j.ctarc.2020.100278 [DOI] [PubMed] [Google Scholar]
- 27. Brüggemann RJM, Alffenaar J-WC, Blijlevens NMA et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 2009; 48: 1441–58. 10.1086/598327 [DOI] [PubMed] [Google Scholar]
- 28. Nivoix Y, Ledoux MP, Herbrecht R. Antifungal therapy: new and evolving therapies. Semin Respir Crit Care Med 2020; 41: 158–74. 10.1055/s-0039-3400291 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.