Abstract
Background
Cefiderocol is a novel siderophore cephalosporin with promising activity against most carbapenem-resistant Gram-negative bacteria (CRGNB). However, extensive postmarketing experiences are lacking. This study aimed to analyse the early experience on cefiderocol postmarketing use at three tertiary care hospitals in Italy.
Methods
We retrospectively included patients with infections caused by CRGNB treated with cefiderocol at three Italian tertiary care hospitals from 1 March 2021 to 30 June 2022. A multivariate Cox model was used to identify predictors of 30 day mortality. A propensity score (PS) analysis with inverse probability weighting (IPW) was also performed to compare the treatment effect of cefiderocol monotherapy (CM) versus combination regimens (CCRs).
Results
The cohort included 142 patients (72% male, median age 67 years, with 89 cases of Acinetobacter baumannii infection, 22 cases of Klebsiella pneumoniae, 27 cases of Pseudomonas aeruginosa and 4 of other pathogens). The 30 day all-cause mortality was 37% (52/142). We found no association between bacterial species and mortality. In multivariate analysis, a Charlson Comorbidity Index >3 was an independent predictor of mortality (HR 5.02, 95% CI 2.37–10.66, P < 0.001). In contrast, polymicrobial infection (HR 0.41, 95% CI 0.21–0.82, P < 0.05) was associated with lower mortality. There was no significant difference in mortality between patients receiving CM (n = 70) and those receiving a CCR (n = 72) (33% versus 40%, respectively), even when adjusted for IPW-PS (HR 1.11, 95% CI 0.63–1.96, P = 0.71).
Conclusions
Real-life data confirm that cefiderocol is a promising option against carbapenem-resistant Gram-negative infections, even as monotherapy.
Introduction
The last decades have been characterized by an increasing prevalence of MDR Gram-negative pathogens and related infections. The emergence of numerous drug-resistant pathogens, such as MBL producers, represents an additional challenge in this scenario. Therefore, new antibiotics active against carbapenemase-producing microorganisms are urgently warranted.1
Cefiderocol is a novel siderophore cephalosporin with a unique mechanism of uptake into the bacterial cell; it is also relatively stable to most β-lactamases including serine- and metallo-carbapenemases. In vitro studies have shown excellent activity against carbapenem-resistant Enterobacterales (CRE), carbapenem-resistant Acinetobacter spp. (CRAB) and difficult-to-treat resistant (DTR) Pseudomonas aeruginosa.2 Notably, cefiderocol maintains activity against several strains producing the most prevalent types of MBLs, such as New-Delhi MBL (NDM), imipenemase (IMP) and Verona integron-encoded MBL (VIM).3
However, information on the clinical use of cefiderocol in the treatment of MDR infections is limited so far. The randomized clinical trials leading to US FDA approval of the drug for complicated urinary tract infections (cUTIs) and pneumonias were APEKS-cUTI4 and APEKS-NP,5 respectively. The main limitation of both studies was that they were not focused on MDR pathogens. In Europe, the open-label CREDIBLE-CR study overcome this limitation, showing that cefiderocol had clinical and microbiological efficacy similar to best available therapy against infections caused by carbapenem-resistant Gram-negative bacteria.6
At the time of writing this manuscript, consistent real-life experiences of cefiderocol use are increasing but are still limited.7–10 This study aimed to describe the early experience on cefiderocol postmarketing use at three tertiary care hospitals in Italy, to analyse the predictors of 30 day mortality among patients treated with cefiderocol due to infection with MDR pathogens, and to assess whether the use of cefiderocol-based combinations was associated with different outcomes compared with cefiderocol monotherapy.
Methods
Study population
The study retrospectively involved patients treated with cefiderocol at three Italian tertiary care hospitals [Azienda Ospedaliero-Universitaria Careggi (AOU-C), Firenze; Ospedali Riuniti, Ancona; Ospedale Cotugno, Napoli] from 1 March 2021 to 30 June 2022.
Eligibility criteria for enrollment in the analysis were: (i) age ≥18 years; (ii) microbiologically documented infections caused by Gram-negative MDR and DTR pathogens, including CRE, CRAB and DTR P. aeruginosa; and (iii) treatment with cefiderocol for ≥3 days (either monotherapy or combination therapy).
Exclusion criteria were: (i) death in the first 48 h; and (ii) empirical treatment with cefiderocol without infection with carbapenem-resistant pathogens, including CRE, CRAB and DTR P. aeruginosa, documented by positive cultures.
Cefiderocol treatment and outcome
Cefiderocol was administered to all patients with normal renal function at a dose of 2 g every 8 h; patients with impaired renal function received a dose adjusted according to the manufacturer’s indications.11 We defined ‘combination therapy’ as a regimen containing cefiderocol and at least one other antibiotic active against the involved pathogen or with potential synergistic activity in vivo (e.g. sulbactam or fosfomycin). The combination was considered valid if prolonged for ≥48 h.
Data were collected for the whole hospital stay and all patients were followed up until hospital discharge or death. Survival at Day 30 was assessed for all patients discharged before Day 30 from the beginning of cefiderocol treatment.
The primary outcome was 30 day mortality. Secondary outcomes were: microbiological cure, length of in-hospital stay and presence of major clinical events during the hospitalization [septic shock, acute kidney injury (AKI), acute respiratory distress syndrome (ARDS)]. Microbiological cure was defined as negative culture from the index specimen repeated after ≥72 h from the beginning of cefiderocol.
Data collection and variables
Demographic, clinical, laboratory, microbiological, treatment and outcome information were captured by reviewing medical records in each centre. Data were recorded in a secure electronic sheet and sent to the Coordinating Center for analysis.
Baseline patient’s condition included the most important comorbidities and the Charlson Comorbidity Index (CCI).12 Disease severity at presentation was assessed through APACHE-II score13 and MEWS-2 score.14
Infections were classified into bloodstream infections (BSIs), in the presence of positive blood cultures for a carbapenem-resistant pathogen, and non-BSIs, in patients with one or more positive cultures from specimens other than blood (sputum, bronchoalveolar aspirate or lavage, urine, intra-abdominal fluids, biopsies) and consistent clinical and/or radiological signs of infection.
Microbiology
All isolates were identified by MALDI-TOF MS (MALDI-TOF Biotyper; Bruker Daltonics GmbH, Leipzig, Germany). When performed (61/142), susceptibility to cefiderocol was assessed through broth microdilution (BMD) using iron-depleted cation-adjusted Mueller–Hinton broth at AOU-C or disc diffusion (cefiderocol disc at 30 mg; Liofilchem, Roseto degli Abruzzi, Italy) at Cotugno Hospital and Ospedali Riuniti following EUCAST guidelines for Enterobacterales and P. aeruginosa. Breakpoints, when available, were considered according to EUCAST breakpoint tables.15
The presence of carbapenemase genes among Enterobacterales and P. aeruginosa isolates was investigated by immunochromatographic assay (Resist5, Coris Bioconcept, Belgium) or Xpert Carba-R assay (Cepheid, Sunnyvale, CA, USA), according to local standard procedures.
Concerning the definition of susceptibility to cefiderocol of Acinetobacter baumannii we considered resistant all isolates with MIC above 2 mg/L as for Enterobacterales (pharmacokinetic-pharmacodynamic breakpoints).
Statistical analysis
A convenience sample, including all patients meeting eligibility criteria in the three recruiting sites during the study period, was used. Continuous variables are expressed as medians and IQRs; categorical variables are expressed as percentages of the group to which they belong. The Mann–Whitney U test was used to compare non-normally distributed continuous variables; the Kruskal–Wallis test was used in case of comparison of three or more groups. Categorical variables were evaluated by the two-tailed Fisher exact test. The variables emerging from the univariate analysis with P values <0.05 were included in a multivariate Cox model; moreover, all possible confounders were tested with the likelihood ratio, and the final goodness of fit of the model was tested through the Hosmer–Lemeshow test. The Kaplan–Meier estimator was used for survival analysis, and the log-rank test for survival comparison. For each patient, we calculated the propensity score (PS) to receive a combination regimen. The covariates included to create the PS were chosen according to all potential risk factors for negative outcome that emerged from our analysis. A PS weighting was then performed using inverse probability of treatment weighting (IPTW) to estimate the average treatment effect of cefiderocol combination therapy versus cefiderocol monotherapy. The multivariate Cox model was performed on the weighted population to compare the outcome between the two treatment groups, and the HR with 95% CI was reported.
Ethics
Local Ethics Committees (registry number 23248) approved the data collection. Informed consent for medical record consultation was obtained from each patient. The study was conducted in agreement with the ethical principles of the Declaration of Helsinki.
Results
Population characteristics
Patients treated with cefiderocol during the study period in the three sites numbered 189. Among them, 142 adults (72.5% males) with a median age of 66 years (IQR 54–75), met the inclusion criteria (see Figure S1, available as Supplementary data at JAC Online). More than half of the cases received cefiderocol in the ICU (55.6%). Concerning pre-existing comorbidities, 57% of patients had a CCI >3, with a median value of 4 (IQR 2–7).
About one-third of patients (n = 45, 31.7%) had a positive blood culture, associated with cUTI (7%), lower respiratory tract infections (LRTIs, 38%), intra-abdominal infections (IAIs, 4%) or acute bacterial skin and skin structure infections (ABSSSIs, 9%). ABSSSIs (n = 9) included four traumatic wound infections, two cases of cellulitis and three post-surgical wounds. The remaining cases with positive blood culture (42%) were classified as primary bloodstream infections and included 11 who were likely central line related (58%). In the other two-thirds of cases (n = 97, 68%), MDR pathogens were obtained from respiratory specimens (n = 78, 58%), abscess drainage (n = 10, 7%), urine culture (n = 11, 8%) or tissue biopsy (n = 13, 9%). Complete demographic and clinical information is given in Table 1.
Table 1.
Characteristics of survivors and deceased patients treated with cefiderocol
| Variable | SurvivorsCSBARLINE (N = 90) | DeceasedCSBARLINE (N = 52) | P value |
|---|---|---|---|
| Age, median (IQR), years | 65 (51–73) | 68 (59–78) | 0.06 |
| Males, N (%) | 68 (75.6) | 35 (67.3) | 0.33 |
| Ward category, N (%) | |||
| ICU | 43 (47.8) | 36 (69.2) | <0.05 |
| Medical ward | 33 (36.7) | 15 (28.9) | |
| Surgical ward | 14 (15.6) | 1 (1.9) | |
| Hospital, N (%) | |||
| AOU-C | 38 (42.2) | 19 (36.5) | 0.44 |
| Ospedali Riuniti di Ancona | 43 (47.8) | 24 (46.2) | |
| Ospedale Cotugno | 9 (10) | 9 (17.3) | |
| Underlying condition, N (%) | |||
| Diabetes | 16 (17.8) | 20 (38.5) | <0.05 |
| Heart failure | 10 (11.1) | 14 (26.9) | <0.05 |
| COPD | 15 (16.7) | 11 (21.2) | 0.51 |
| Coronary heart disease | 10 (11.1) | 10 (19.2) | 0.21 |
| Chronic renal failure | 10 (11.1) | 14 (26.9) | <0.05 |
| Cerebrovascular disease | 13 (14.4) | 11 (21.2) | 0.4 |
| Neoplasm | |||
| Localized neoplasm | 8 (8.9) | 9 (17.3) | 0.16 |
| Metastatic neoplasm | 6 (6.7) | 6 (11.5) | |
| Obesity (missing 51) | 11 (19.6) | 9 (25.7) | 0.6 |
| Smoking (missing 64) | |||
| Smoker | 19 (41.3) | 9 (28.1) | 0.53 |
| Former smoker | 13 (28.3) | 11 (34.4) | |
| Charlson Comorbidity Index, median (IQR) | 3 (2–6) | 6 (4–8) | <0.001 |
| MEWS score, median (IQR) | 2 (0–4) | 3 (2–5) | <0.05 |
| APACHE-II, median (IQR) | 13 (8–18) | 19 (14–25) | <0.001 |
| Reason for hospidalization, N (%) | |||
| Infection | 14 (15.6) | 12 (23.1) | 0.12 |
| Trauma | 16 (17.8) | 3 (5.8) | |
| COVID-19 | 21 (23.3) | 9 (17.3) | |
| Respiratory failure | 2 (2.2) | 3 (5.8) | |
| Cardiovascular diseases | 13 (14.4) | 6 (11.5) | |
| Hepatic diseases | 4 (4.4) | 2 (3.9) | |
| Surgical intervention | 14 (15.6) | 7 (13.5) | |
| Others | 6 (6.7) | 10 (19.2) | |
| Type of infection, N (%) | |||
| Bacteraemia | 14 (15.6) | 5 (9.6) | 0.5 |
| UTI | 8 (8.9) | 4 (7.7) | |
| IAI | 9 (10) | 4 (7.7) | |
| Pneumonia | 46 (51.1) | 35 (67.3) | |
| ABSSSI | 6 (6.7) | 3 (5.8) | |
| Others | 7 (7.8) | 1 (1.9) | |
| Positive blood cultures, n (%) | 31 (34.4) | 14 (26.9) | 0.45 |
| Resistance to cefiderocol,a n (%) | 12 (32) | 5 (20) | 0.39 |
| Type of bacterium, n (%) | |||
| A. baumannii | 56 (62.2) | 33 (63.5) | |
| K. pneumoniae | 13 (14.4) | 9 (17.3) | |
| P. aeruginosa | 19 (21.1) | 8 (15.4) | 0.76 |
| Others | 2 (2.2) | 2 (3.9) | |
| Coinfection, N (%) | |||
| Overall | 62 (68.9) | 32 (61.5) | 0.46 |
| Gram-negative | 16 (25.8) | 11 (34.4) | <0.05 |
| Gram-positive | 23 (37.1) | 8 (25) | |
| Mixed | 14 (22.6) | 2 (6.3) | |
| Fungal | 9 (14.5) | 11 (34.4) | |
| Therapy, N (%) | |||
| >10 days of antibiotic treatment before cefiderocolb | 26 (36.6) | 21 (52.5) | 0.11 |
| >10 days of cefiderocol treatment | 49 (54.4) | 22 (42.3) | 0.22 |
| Combination therapy | 43 (47.8) | 29 (55.8) | 0.39 |
| One other active antimicrobial | 36 (43.4) | 27 (54) | 0.28 |
| Two other active antimicrobials | 7 (7.8) | 2 (3.9) | 0.49 |
| Outcomes | |||
| In-hospital stay, days, median (IQR) | 54 (30–81) | 32 (22–50) | <0.001 |
| Microbiological cure,c N (%) | 35 (54.7) | 10 (35.7) | 0.12 |
| Major events,d N (%) | |||
| ARDS | 43 (47.8) | 46 (88.5) | <0.001 |
| AKI | 24 (26.7) | 30 (57.7) | <0.001 |
| Septic shock | 30 (33.3) | 40 (76.9) | <0.001 |
Numbers in bold are statistically significant.
aSusceptibility testing for cefiderocol was performed in only 28 isolates of A. baumannii (31.5%), 16 cases of K. pneumoniae (72.7%) and 16 isolates of P. aeruginosa (59.2%).
bRegardless of the antibiotics used.
cMicrobiological cure available on 92/142 patients who had follow-up cultures available.
dAfter entering the observation period.
The most common pathogen observed was A. baumannii (n = 89, 63%) followed by P. aeruginosa (n = 27, 19%) and Klebsiella pneumoniae (n = 22, 16%). Four cases were caused by Escherichia coli (n = 2), Enterobacter cloacae (n = 1) and Stenotrophomonas maltophilia (n = 1). The presence of carbapenemase genes was detected in all isolates of K. pneumoniae (n = 10 KPC, 9 NDM, 3 VIM) and E. coli (n = 1 NDM, 1 VIM), and in 9/27 of P. aeruginosa (n = 8 VIM, 1 KPC). In two cases, co-expression of VIM and KPC was observed.
In 66% of cases there was a coinfection supported by other Gram-negative (29%), Gram-positive (32%), both Gram-negative and Gram-positive (17%), and fungal (21%) pathogens.
Treatment and outcomes
More than 40% of cases received >10 days of different therapy during their hospital stay before diagnosis of the index infection. The median duration of treatment with cefiderocol was 11 days (IQR 7–14 days). In 69% of cases cefiderocol was started within 4 days from culture sampling, and in 88% of cases within 7 days. About half of the cases were managed with a combination therapy consisting of cefiderocol plus at least one other drug active against the MDR isolate (Table 2). The 30 day mortality, calculated from the initiation of cefiderocol therapy, was 37% (52/142).
Table 2.
Subgroups of patients treated with cefiderocol monotherapy versus cefiderocol combination therapy
| Variable | Total (N = 142) | Monotherapy (N = 70) | Combination therapy (N = 72) | P value |
|---|---|---|---|---|
| Age, median (IQR), years | 66.5 (54–75) | 66 (57–73) | 67 (50–76) | 0.96 |
| Males, n (%) | 103 (72.5) | 54 (77.1) | 49 (68.1) | 0.26 |
| Ward category, n (%) | ||||
| ICU | 79 (55.6) | 38 (54.3) | 41 (56.9) | 0.88 |
| Medical ward | 48 (33.8) | 25 (35.7) | 23 (31.9) | |
| Surgical ward | 15 (10.6) | 7 (10) | 8 (11.1) | |
| Hospital, n (%) | ||||
| AOU-C | 57 (40.1) | 22 (31.4) | 35 (48.6) | <0.05 |
| Ospedali Riuniti di Ancona | 67 (47.2) | 35 (50) | 32 (44.4) | |
| Ospedale Cotugno | 18 (12.7) | 13 (18.6) | 5 (6.9) | |
| Underlying condition, n (%) | ||||
| Diabetes | 36 (25.3) | 15 (21.4) | 21 (29.2) | 0.34 |
| Heart failure | 24 (16.9) | 10 (14.3) | 14 (19.4) | 0.5 |
| COPD | 26 (18.3) | 9 (12.9) | 17 (23.6) | 0.13 |
| Coronary heart disease | 20 (14.1) | 7 (10) | 13 (18.1) | 0.23 |
| Chronic renal failure | 24 (16.9) | 9 (12.9) | 15 (20.8) | 0.23 |
| Cerebrovascular disease | 24 (16.9) | 12 (17.1) | 12 (16.7) | 1 |
| Neoplasm | ||||
| Localized neoplasm | 17 (12) | 14 (20) | 3 (4.2) | <0.05 |
| Metastatic neoplasm | 12 (8.5) | 6 (8.6) | 6 (8.3) | |
| Obesity (missing 51) | 20 (22) | 7 (14.9) | 13 (29.6) | 0.13 |
| Smoking (missing 64) | ||||
| Smoker | 28 (35.9) | 15 (39.5) | 13 (32.5) | 0.44 |
| Former smoker | 24 (30.8) | 9 (23.7) | 15 (37.5) | |
| Charlson Comorbidity Index, median (IQR) | 4 (2–7) | 4 (3–7) | 4 (2–7) | 0.79 |
| MEWS score, median (IQR) | 2 (1–4) | 2 (1–4) | 5 (2–6) | 0.71 |
| APACHE-II, median (IQR) | 16 (10–20) | 16 (9–20) | 16 (11–20) | 0.92 |
| Reason for hospitalization, n (%) | ||||
| Infection | 26 (18.3) | 16 (22.9) | 10 (14.4) | 0.87 |
| Trauma | 19 (13.4) | 8 (11.4) | 11 (15.3) | |
| COVID-19 | 30 (21.1) | 15 (21.4) | 15 (20.8) | |
| Respiratory failure | 5 (3.5) | 2 (2.9) | 3 (4.2) | |
| Cardiovascular diseases | 19 (13.4) | 8 (11.4) | 11 (15.3) | |
| Hepatic diseases | 6 (4.2) | 3 (4.3) | 3 (4.2) | |
| Surgical intervention | 21 (14.8) | 9 (12.9) | 12 (16.7) | |
| Others | 16 (11.2) | 9 (12.9) | 7 (9.7) | |
| Type of infection, n (%) | ||||
| Bacteraemia | 19 (13.4) | 11 (15.7) | 8 (11.1) | <0.05 |
| UTI | 12 (8.5) | 5 (7.1) | 7 (9.7) | |
| IAI | 13 (9.2) | 7 (10) | 6 (8.3) | |
| LRTI | 81 (57) | 44 (62.9) | 37 (51.4) | |
| ABSSSI | 9 (6.3) | 0 (0) | 9 (12.5) | |
| Others | 3 (2.1) | 3 (4.3) | 5 (6.7) | |
| Positive blood cultures, n (%) | 45 (31.7) | 20 (28.6) | 25 (34.7) | 0.47 |
| Resistance to cefiderocol,a n (%) | 17 (27.9) | 7 (25.9) | 10 (29.4) | 1 |
| Type of bacterium, n (%) | ||||
| A. baumannii | 89 (62.7) | 42 (60) | 47 (65.3) | 0.76 |
| K. pneumoniae | 22 (15.5) | 11 (15.7) | 11 (15.3) | |
| P. aeruginosa | 27 (19) | 14 (20) | 13 (18.1) | |
| Others | 4 (2.8) | 3 (4.3) | 1 (1.4) | |
| Coinfection, n (%) | ||||
| Overall | 94 (66.2) | 45 (64.3) | 49 (68.1) | 0.38 |
| Gram-negative | 27 (28.7) | 12 (26.7) | 15 (30.6) | |
| Gram-positive | 31 (32.3) | 17 (37.8) | 14 (28.6) | |
| Mixed | 16 (17) | 6 (13.3) | 10 (20.4) | |
| Fungal | 20 (21.2) | 10 (22.2) | 10 (20.4) | |
| Therapy, n (%) | ||||
| >10 days of antibiotic treatment before cefiderocolb | 47 (42.3) | 18 (37.5) | 29 (46) | 0.44 |
| >10 days of cefiderocol treatment | 71 (50) | 33 (47.1) | 38 (52.8) | 0.62 |
| Outcome | ||||
| In-hospital stay (IQR), days | 42 (26–65) | 41 (24–64) | 43 (28–70) | 0.3 |
| 30-day mortality, n (%) | 52 (36.6) | 23 (32.9) | 29 (40.3) | 0.39 |
| Microbiological cure,c n (%) | 45 (48.9) | 22 (45.8) | 23 (52.3) | 0.68 |
| Major events,d n (%) | ||||
| ARDS | 89 (62.7) | 42 (60) | 47 (65.3) | 0.6 |
| AKI | 54 (38) | 26 (37.1) | 28 (38.9) | 0.86 |
| Septic shock | 70 (49.3) | 34 (48.6) | 36 (50) | 0.87 |
Numbers in bold are statistically significant.
aSusceptibility testing for cefiderocol was performed in only 61 isolates (43%).
bRegardless of the antibiotics used.
cMicrobiological cure available on 92/142 patients who had follow-up cultures available.
dAfter entering in the observation period.
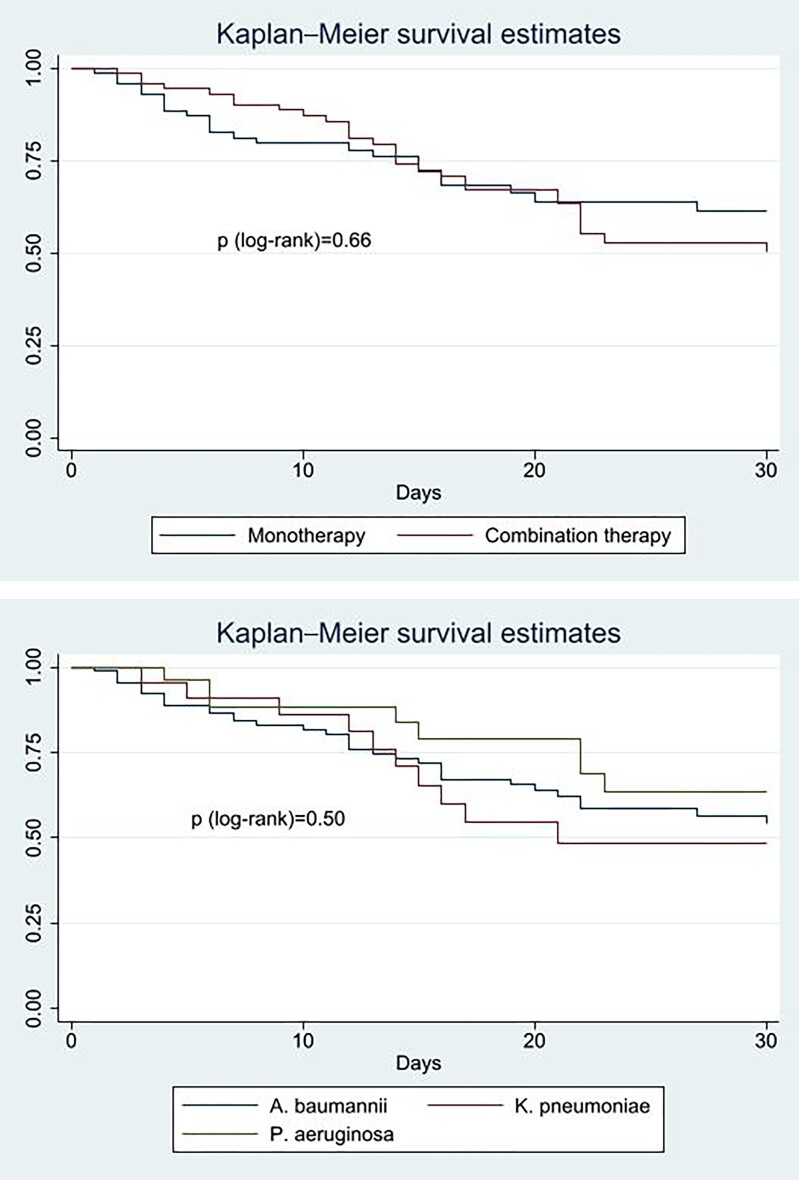
Notably, there were no significant differences in demographic and clinical characteristics between the group treated with cefiderocol monotherapy and those treated with the combination therapy. Patients treated with the combination therapy showed a slightly higher mortality rate (40% versus 33%) without statistical significance. Also, in the subgroup analysis by pathogen type, the outcome of cefiderocol monotherapy was comparable to combination therapy in the treatment of A. baumannii, K. pneumoniae or P. aeruginosa infections (Figure 1).
Figure 1.
Kaplan–Meier estimator of the impact of combination treatment and pathogen type on 30 day mortality. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Microbiological cure rates, calculated among 92 patients with follow-up cultures available, were also comparable between the two groups, as shown in Table 2.
Stratifying by infection type, no statistical association was observed with 30 day mortality. However, a higher number of deaths was observed in the LRTI group (n = 35, 43%), followed by cUTIs (n = 4, 33%), ABSSSIs (n = 3, 33%) and IAIs (n = 4, 31%). BSIs showed the lowest proportion of deaths (n = 5, 26%).
Susceptibility testing for cefiderocol was performed in only 61 of 142 isolates (43%). Of these, 17 (27.9%) were resistant to cefiderocol, including 10 (35.7%) strains of A. baumannii and 7 (43.8%) of K. pneumoniae. Resistance to cefiderocol was not associated with 30 day mortality or a different microbiological cure rate, even if there were no differences in term of monotherapy or combination therapy between cefiderocol-susceptible and -resistant infections (54.5% and 58.8%, respectively, P = 1.00).
Eight patients (6%) had a clinical and microbiological relapse, a median of 11 days (IQR 9–25 days) after cefiderocol discontinuation, and received a second cycle of cefiderocol with a median duration of 12 days (IQR 9–18 days). Susceptibility testing was repeated in only one of these latter episodes, confirming susceptibility to cefiderocol.
Univariate and multivariate analysis for 30 day mortality prediction
Upon univariate analysis of ICU admission, 30 day mortality was associated with pre-existing diabetes, heart failure, chronic renal failure, higher CCI, and severity score at introduction of cefiderocol therapy (APACHE-II and MEWS), and major events occurring during hospital stay, such as AKI (according to KDIGO 2012 criteria16), ARDS (according to 2012 Berlin criteria17) or septic shock (according to Sepsis-3 definition18) (Table 1).
Conversely, no correlation was observed between mortality and pathogen type, source of infection, positive blood culture or coinfection.
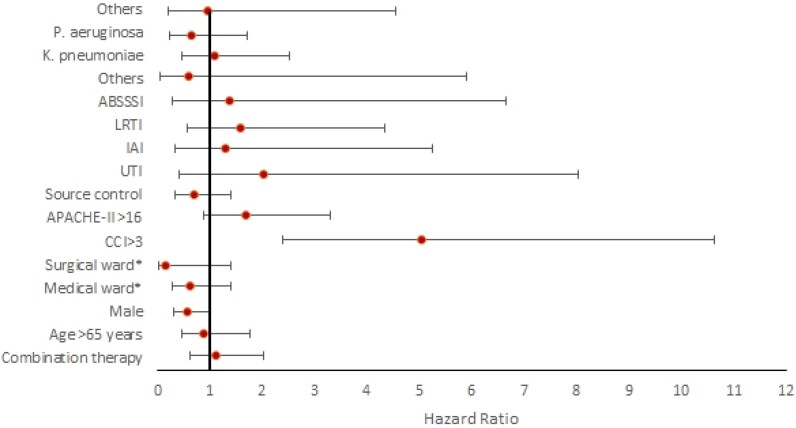
In the multivariate Cox model (Table 3), 30 day mortality was independently associated with a CCI score ≥3 (HR 5.05, 95% CI 2.40–10.62, P < 0.001). At the same time, only the presence of a coinfection (HR 0.46, 95% CI 0.23–0.90, P < 0.05) was associated with lower rate of mortality. The complete results of the multivariable analysis are summarized in Figure 2.
Table 3.
Multivariate Cox model for 30 day mortality in hospitalized patients with carbapenem-resistant pathogen infection treated with cefiderocol
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Combination therapya | 1.12 | 0.62–2.02 | 0.71 |
| Age >65 years | 0.89 | 0.46–1.76 | 0.75 |
| Males | 0.57 | 0.30–1.01 | 0.09 |
| Ward category | |||
| ICU | ref. | ||
| Medical ward | 0.63 | 0.28–1.41 | 0.26 |
| Surgical ward | 0.16 | 0.02–1.41 | 0.10 |
| CCI >3 | 5.05 | 2.40–10.62 | <0.001 |
| APACHE-II >16 | 1.70 | 0.88–3.29 | 0.11 |
| Coinfection | 0.46 | 0.23–0.90 | <0.05 |
| Source control | 0.69 | 0.34–1.41 | 0.30 |
| Type of infection | |||
| Bacteraemia | ref | ref | ref |
| UTI | 2.02 | 0.49–8.04 | 0.33 |
| IAI | 1.31 | 0.33–5.24 | 0.70 |
| LRTI | 1.58 | 0.58–4.33 | 0.37 |
| ABSSSI | 1.37 | 0.28–6.64 | 0.70 |
| Others | 0.60 | 0.06–5.9 | 0.66 |
| Type of bacterium | |||
| A. baumannii | ref. | ref. | ref. |
| K. pneumoniae | 1.02 | 0.44–2.34 | 0.95 |
| P. aeruginosa | 0.64 | 0.24–1.71 | 0.38 |
| Others | 0.95 | 0.20–4.56 | 0.95 |
| Propensity score analysis | |||
| Combination regimens (IPTW-adjusted)a | 1.08 | 0.61–1.92 | 0.78 |
Numbers in bold are statistically significant.
aMonotherapy with cefiderocol as reference variable.
Figure 2.
Multivariate Cox model for 30 day mortality in hospitalized patients infected with carbapenem-resistant pathogens treated with cefiderocol. Red spots represent the exact HR value, while horizontal bars represent the confidence interval. Multivariate analysis adjusted for inverse probability of treatment weighting (IPTW) confirmed that combination treatment was not associated with lower 30 day mortality (HR 1.08, 95% CI 0.61–1.92, P = 0.78). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
IPTW using PS balanced the groups well. IPTW-adjusted Cox regression showed that combination treatment was not associated with lower 30 day mortality (HR 1.08, 95% CI 0.61–1.92, P = 0.78).
Discussion
To the best of our knowledge, this is the largest observational cohort study showing real-life postmarketing data on cefiderocol use for MDR Gram-negative infections.
Our study showed an overall 30 day mortality rate of 37%, which was significantly higher than registration trials. APEKS-NP and APEKS-UTI studies were not based on MDR pathogens, preventing a meaningful comparison. The CREDIBLE trial showed an overall 28 day mortality of 25%.6 Because the main prognostic factors were comparable at the time of enrollment (e.g. age, CCI, APACHE-II, ICU), a possible difference could be the distribution of MDR pathogens, which in our cohort was dominated by A. baumannii (63% versus 46% in the CREDIBLE trial).
Moreover, strains resistant to cefiderocol might have increased in the meantime. In our series, data on cefiderocol susceptibility were available for a minority of cases (61/142); however, we observed 28% of resistant isolates. Interestingly, resistance was concentrated in A. baumannii and K. pneumoniae strains, whereas no cases of resistant P. aeruginosa were identified. Epidemiological data from one of the participating centres (AOU-C) focusing on 52 NDM-producing K. pneumoniae isolated between January 2021 and June 2022 revealed that approximately 40% of these strains were resistant to cefiderocol. This outbreak was mostly sustained by clonal expansion of a mutant with the inactivated cirA siderophore receptor gene, which spread independently of cefiderocol exposure.19 A recent comprehensive review of cefiderocol resistance mechanisms highlighted that the NDM enzyme is a proxy for the emergence of cefiderocol resistance through co-expression of additional mechanisms.20,21 Overexpression of the blaNDM gene following increased gene dosage was also reported to be linked to in vivo emergence of cefiderocol resistance under cefiderocol treatment.22,23
Stratifying by type of infection, no significant differences in mortality were observed. However, LRTIs had higher absolute rates of death (43%). Considering that pulmonary penetration of the drug appears to be sufficient, especially in patients with lung inflammation,24–26 this excess mortality may depend on several underlying conditions (e.g. 69% of them were in ICU at the time of treatment with cefiderocol compared with the overall rate of 56%) and confirmed that nosocomial pneumonia and ventilator-associated pneumonia remain challenging entities to manage.
Focusing on A. baumannii, the mortality rate seen in our study was comparable with that observed for polymyxin-based regimens in the CREDIBLE-CR study and in more recent trials based on colistin27 or sulbactam/durlobactam.28 Interestingly, population characteristics were similar when stratified by the three main pathogens, and 30 day mortality for A. baumannii infections was comparable to that of P. aeruginosa and K. pneumoniae, supporting the efficacy of cefiderocol for CRAB. Falcone et al.29 found a 30 day mortality rate of 34% among patients treated with cefiderocol for CRAB infections; in the study by Pascale et al.,30 limited to ICU patients with CRAB infections treated with cefiderocol monotherapy, mortality was higher than in our ICU population (55% versus 46%). Pending further larger randomized trials, the results of real-life observational experiences, including the present study, may suggest reconsidering the role of cefiderocol in the management of CRAB infections with respect to the recommendation by the ESCMID guidelines.31
Limited data are available focusing on the efficacy of cefiderocol for DTR P. aeruginosa. A small study including 17 patients [of whom 14 received cefiderocol combination regimens (CCRs)] reported a 30 day mortality of 24% and a microbiological cure of 77%.32 Bleibtreu et al.33 found 9 out of 12 cases of XDR P. aeruginosa, which were associated with an all-cause mortality rate of 24%. These results were comparable to our experience with P. aeruginosa, as we observed a 30 day mortality of 30% and a microbiological cure rate of 55%. However, due to the small sample size it is not possible to draw a definitive conclusion about the role of cefiderocol against DTR P. aeruginosa.
Studies addressing the role of cefiderocol against MBL-producing Enterobacterales in a real-life setting are still lacking. In our experience we had 22 cases of MBL-producing K. pneumoniae, 2 cases of E. coli and 1 case of E. cloacae. Among them, 30 day mortality rate and microbiological cure were 44% and 47%, respectively.
Resistance to cefiderocol was not associated with poor outcome, suggesting that data obtained in vitro could disagree with in vivo performance. However, given the EUCAST warnings about cefiderocol susceptibility testing and the heterogeneity in methods between the sites, the interpretation of these results remains challenging.34
According to our results, there was no significant difference in 30 day mortality between groups receiving CCR and monotherapy. This finding contrasts with the recommendations included in the recent guidelines issued by ESCMID and the IDSA, which suggest a combination therapy including two in vitro active antibiotics for patients with moderate to severe and high-risk CRAB infections.31,35 In particular, IDSA guidelines recommend the use of cefiderocol for CRAB only as part of a combination scheme.35 Consistent with our findings, a small case series of ICU patients (n = 10) with A. baumannii infections showed good efficacy of monotherapy (30 day mortality 10%).36 Another small study (n = 18) comparing monotherapy with combination therapy showed comparable results in patients treated with cefiderocol alone (30 day mortality was 29% in the combination therapy and 25% in the monotherapy group).37
The apparent protective role of the coinfection observed in the multivariate model may reflect a possible predominant role of a second, less virulent and ‘easier-to-treat’ pathogen.
Concerning microbiological cure, our results were in line with the CREDIBLE trial (48.9% versus 48%), but markedly different from other real-life studies, where microbiological eradication ranged from 28% to 82.6%.29,30
The main limitation of our study is related to the retrospective observational design. A control group comprising MDR infections treated with non-cefiderocol regimens was not provided; however, a comparison with different regimens against MDR was beyond the scope of this study. Moreover, most of the isolates were not tested for cefiderocol susceptibility, due to the well-known challenges in susceptibility testing during the first few months after cefiderocol is marketed.
Conclusions
Despite its limitations, cefiderocol has proven to be an important option for addressing emerging MDR pathogens, possibly even when the drug is used alone. The potential use in monotherapy deserves attention considering both the toxicity profile of common companion drugs (e.g. colistin), and the purpose of antimicrobial management. Randomized studies are urgently needed to reconsider the role of cefiderocol against A. baumannii infections and to compare its performance with aztreonam-based regimens against MBL Enterobacterales.
Supplementary Material
Contributor Information
Matteo Piccica, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Michele Spinicci, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Infectious and Tropical Diseases Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy.
Annarita Botta, Department of Infectious Disease and Infectious Emergencies, AORN dei Colli, Cotugno Hospital, Naples, Italy.
Vincenzo Bianco, Department of Infectious Disease and Infectious Emergencies, AORN dei Colli, Cotugno Hospital, Naples, Italy.
Filippo Lagi, Infectious and Tropical Diseases Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy.
Lucia Graziani, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Alessandro Faragona, Department of Biomedical Sciences and Public Health, Università Politecnica delle Marche, Ancona, Italy.
Roberto Parrella, Department of Infectious Disease and Infectious Emergencies, AORN dei Colli, Cotugno Hospital, Naples, Italy.
Tommaso Giani, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Clinical Microbiology and Virology Unit, Florence Careggi University Hospital, Florence, Italy.
Andrea Bartolini, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Gianluca Morroni, Clinic of Infectious Diseases, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy.
Mariano Bernardo, Microbiology Unit, AORN Ospedali dei Colli-Monaldi Hospital, Naples, Italy.
Gian Maria Rossolini, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Clinical Microbiology and Virology Unit, Florence Careggi University Hospital, Florence, Italy.
Marcello Tavio, Unit of Emerging and Immunosuppressed Infectious Diseases, Department of Gastroenterology and Transplantation, Azienda Ospedaliero-Universitaria ‘Ospedali Riuniti’, Ancona, Italy.
Andrea Giacometti, Department of Biomedical Sciences and Public Health, Università Politecnica delle Marche, Ancona, Italy; Clinic of Infectious Diseases, Azienda Ospedaliero-Universitaria delle Marche, Ancona, Italy.
Alessandro Bartoloni, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Infectious and Tropical Diseases Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy.
Funding
This work was supported by Shionogi & Co, which funded InformaPRO for the publication process by covering the language editing review and the article processing charge.
Transparency declarations
The authors have no conflicts of interest to declare. The lead author (M.P.) affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Author contributions
Study conceptualization: M.P., M.S., A. Botta, A. Bartoloni. Data collection: M.P., A. Botta, A.F., V.B., L.G. Data elaboration and interpretation: M.P., M.S., F.L., G.M.R., T.G., A. Bartolini, M.B., G.M. Manuscript writing: M.P., M.S. Patient management and manuscript reviewing: M.P., M.S., G.M.R., T.G., F.L. Project supervision: A. Bartoloni, G.M.R., M.T., A.G., R.P.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online.
References
- 1. WHO . Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed [Google Scholar]
- 2. Tamma PD, Hsu AJ. Defining the role of novel β-lactam agents that target carbapenem-resistant Gram-negative organisms. J Pediatric Infect Dis Soc 2019; 8: 251–60. 10.1093/jpids/piz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ito A, Sato T, Ota M et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 2018; 62: e01454-17. 10.1128/AAC.01454-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Portsmouth S, van Veenhuyzen D, Echols R et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2018; 18: 1319–28. 10.1016/S1473-3099(18)30554-1 [DOI] [PubMed] [Google Scholar]
- 5. Wunderink RG, Matsunaga Y, Ariyasu M et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21: 213–25. 10.1016/S1473-3099(20)30731-3 [DOI] [PubMed] [Google Scholar]
- 6. Bassetti M, Echols R, Matsunaga Y et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. 10.1016/S1473-3099(20)30796-9 [DOI] [PubMed] [Google Scholar]
- 7. Palermo G, Medaglia AA, Pipitò L et al. Cefiderocol efficacy in a real-life setting: single-centre retrospective study. Antibiotics (Basel) 2023; 12: 746. 10.3390/antibiotics12040746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sajib MI, Monteforte M, Go R. Clinical outcome of cefiderocol for infections with carbapenem-resistant organisms. Antibiotics (Basel) 2023; 12: 936. 10.3390/antibiotics12050936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satlin MJ, Simner PJ, Slover CM et al. Cefiderocol treatment for patients with multidrug- and carbapenem-resistant Pseudomonas aeruginosa infections in the compassionate use program. Antimicrob Agents Chemother 2023; 67: e0019423. 10.1128/aac.00194-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rando E, Cutuli SL, Sangiorgi F et al. Cefiderocol-containing regimens for the treatment of carbapenem-resistant A. baumannii ventilator-associated pneumonia: a propensity-weighted cohort study. JAC Antimicrob Resist 2023; 5: dlad085. 10.1093/jacamr/dlad085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shionogi Inc. FETROJA (Cefiderocol) for injection, for intravenous use: US prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209445s002lbl.pdf
- 12. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 13. Knaus WA, Draper EA, Wagner DP et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 14. Subbe CB, Kruger M, Rutherford P et al. Validation of a modified Early Warning Score in medical admissions. QJM 2001; 94: 521–6. 10.1093/qjmed/94.10.521 [DOI] [PubMed] [Google Scholar]
- 15. European Committee on Antimicrobial Susceptibility Testing (EUCAST) . Breakpoint tables for interpretation of MICs and zone diameters. Version 02.01.2023. https://www.eucast.org/clinical_breakpoints
- 16. Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–30. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 17. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 18. Singer M, Deutschman CS, Seymour CW et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coppi M, Antonelli A, Niccolai C et al. Nosocomial outbreak by NDM-1-producing Klebsiella pneumoniae highly resistant to cefiderocol, Florence, Italy, August 2021 to June 2022. Euro Surveill 2022; 27: 2200795. 10.2807/1560-7917.ES.2022.27.43.2200795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nurjadi D, Kocer K, Chanthalangsy Q et al. New Delhi metallo-beta-lactamase facilitates the emergence of cefiderocol resistance in Enterobacter cloacae. Antimicrob Agents Chemother 2022; 66: e0201121. 10.1128/aac.02011-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karakonstantis S, Rousaki M, Kritsotakis EI. Cefiderocol: systematic review of mechanisms of resistance, heteroresistance and in vivo emergence of resistance. Antibiotics (Basel) 2022; 11: 723. 10.3390/antibiotics11060723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simner PJ, Patel R. Cefiderocol antimicrobial susceptibility testing considerations: the Achilles’ heel of the Trojan horse? J Clin Microbiol 2021; 59: e00951-20. 10.1128/JCM.00951-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choby JE, Ozturk T, Satola SW et al. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect Dis 2021; 21: 597–8. 10.1016/S1473-3099(21)00194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drwiega EN, Rodvold KA. Penetration of antibacterial agents into pulmonary epithelial lining fluid: an update. Clin Pharmacokinet 2021; 61: 17–46. 10.1007/s40262-021-01061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katsube T, Saisho Y, Shimada J et al. Intrapulmonary pharmacokinetics of cefiderocol, a novel siderophore cephalosporin, in healthy adult subjects. J Antimicrob Chemother 2019; 74: 1971–4. 10.1093/jac/dkz123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawaguchi N, Katsube T, Echols R et al. Intrapulmonary pharmacokinetic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, in patients with pneumonia and healthy subjects. J Clin Pharmacol 2022; 62: 670–80. 10.1002/jcph.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaye KS, Marchaim D, Thamlikitkul V et al. Colistin monotherapy versus combination therapy for carbapenem-resistant organisms. NEJM Evid 2023; 2: evidoa2200131. 10.1056/evidoa2200131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watkins RR, Du B, Isaacs R et al. Pathogen-targeted clinical development to address unmet medical need: design, safety, and efficacy of the ATTACK trial. Clin Infect Dis 2023; 76: S210–4. 10.1093/cid/ciad097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falcone M, Tiseo G, Leonildi A et al. Cefiderocol- compared to colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2022; 66: e0214221. 10.1128/aac.02142-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pascale R, Pasquini Z, Bartoletti M et al. Cefiderocol treatment for carbapenem-resistant Acinetobacter baumannii infection in the ICU during the COVID-19 pandemic: a multicentre cohort study. JAC Antimicrob Resist 2021; 3: dlab174. 10.1093/jacamr/dlab174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paul M, Carrara E, Retamar P et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect 2022; 28: 521–47. 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 32. Meschiari M, Volpi S, Faltoni M et al. Real-life experience with compassionate use of cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa (DTR-P) infections. JAC Antimicrob Resist 2021; 3: dlab188. 10.1093/jacamr/dlab188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bleibtreu A, Dortet L, Bonnin RA et al. Susceptibility testing is key for the success of cefiderocol treatment: a retrospective cohort study. Microorganisms 2021; 9: 282–8. 10.3390/microorganisms9020282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.EUCAST. EUCAST warnings concerning antimicrobial susceptibility testing products or procedures. https://www.eucast.org/ast-of-bacteria/warnings [Google Scholar]
- 35. Tamma PD, Aitken SL, Bonomo RA et al. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 2022; 74: 2089–114. 10.1093/cid/ciab1013 [DOI] [PubMed] [Google Scholar]
- 36. Falcone M, Tiseo G, Nicastro M et al. Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant Gram-negative infections in intensive care unit patients. Clin Infect Dis 2021; 72: 2021–4. 10.1093/cid/ciaa1410 [DOI] [PubMed] [Google Scholar]
- 37. Corcione S, De Benedetto I, Pinna SM et al. Cefiderocol use in Gram negative infections with limited therapeutic options: is combination therapy the key? J Infect Public Health 2022; 15: 975–9. 10.1016/j.jiph.2022.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.