Abstract
Kinetic parameters of acetate oxidation were determined for the sulfate reducers Desulforhabdus amnigenus and Desulfobacca acetoxidans. Based on these parameters, both sulfate reducers seem to be able to outcompete Methanosaeta spp. for acetate in acetate-fed anaerobic bioreactors. Mixed-substrate studies showed that D. amnigenus degraded acetate and hydrogen simultaneously but preferred lactate, propionate, and ethanol over acetate.
Acetate is a key intermediate in the breakdown of organic matter in anaerobic bioreactors. In anaerobic reactors treating sulfate-rich wastewaters, such as paper mill and food oil industry wastewaters, sulfate reducers compete for these compounds with methanogens (7). The outcome of the competition for acetate is not yet clear. Generally, acetate is utilized by methanogens (3, 12), but in some reactors it is mainly utilized by sulfate reducers (11). Comparison of the acetate utilization kinetics of methanogens and sulfate reducers can give more insight into the competition for acetate. In most methanogenic bioreactors, Methanosaeta spp. are the dominant acetate-degrading methanogens because of their high affinity and low threshold value for acetate compared to Methanosarcina spp. (7). In sulfate-reducing reactors, acetate-degrading sulfate reducers have to compete with Methanosaeta spp. for the available acetate. Unfortunately, kinetic data for acetate utilization by freshwater sulfate reducers are hardly available. Most researchers have studied acetate oxidation by marine sulfate reducers, as reviewed by Oude Elferink et al. (7). It is unlikely that these marine sulfate reducers play an important role in freshwater anaerobic bioreactors.
The aim of the present study was to investigate the oxidation of acetate by freshwater sulfate reducers. For our study we used Desulfobacca acetoxidans, which oxidizes acetate only (9), and the generalist Desulforhabdus amnigenus, which can use a wide variety of substrates (8). Both sulfate reducers have been isolated from anaerobic granular sludge obtained from reactors in which acetate was mainly converted via sulfate reduction.
D. acetoxidans ASRB2 (DSM 11109) and D. amnigenus ASRB1 (DSM 10338), from our own collection, were cultured anaerobically in bicarbonate-buffered medium at 37°C, as described previously (8).
The Michaelis-Menten kinetic parameters Vmax and Km were estimated from acetate depletion curves obtained with concentrated cell suspensions. The depletion data were fitted to an integrated solution of the Michaelis-Menten equation Vmax · t = S0 − S + Km · ln(S0/S) by nonlinear regression analyses (10). In this equation, S0 is the initial substrate concentration, S is the substrate concentration at time t, Vmax is the maximum consumption rate, and Km is the half-saturation constant.
To obtain concentrated cell suspensions, cells were harvested anaerobically by centrifugation in the late exponential phase of growth. The cells were resuspended and washed twice with the bicarbonate-buffered medium and were then transferred to 120-ml serum vials in an anaerobic glove box and sealed with butyl rubber stoppers and aluminum caps. To eliminate interference with growth, cells of D. acetoxidans and D. amnigenus were concentrated approximately 20-fold and 50-fold, respectively. The vials were preincubated at 37°C for 1 h in the presence of 10 mM sulfate for removal of intracellular acetate. For both bacteria, four independent acetate depletion experiments were carried out, starting with, respectively, 1, 2.5, 4, and 6 mM of acetate as the initial concentration. At the end of each experiment, the protein content of the cell suspensions was estimated by the method of Bradford (1) after disruption of the cells by sonification (five times for 20 s each with an intermittent cooling period of 20 s).
Substrate preferences of D. amnigenus were tested in batch cultures by growing the cells on a single substrate and adding a pulse of a different substrate as soon as the culture reached log phase. The following combinations were tested [starting substrate (mM) plus pulse substrate (mM)]: propionate (17) plus ethanol (11); ethanol (16) plus propionate (17); propionate (17) plus lactate (11); lactate (20) plus propionate (14); acetate (18) plus propionate (17); acetate (22) plus hydrogen (10). For the hydrogen pulse experiments, the cultures were incubated on a rotary shaker (125 rpm). Substrates were measured by gas chromatography and high-performance liquid chromatography (8).
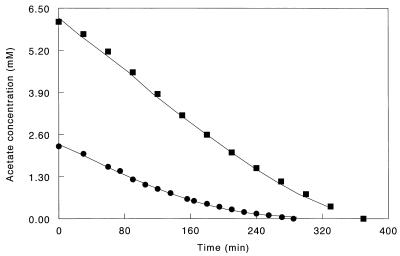
Acetate consumption by concentrated cell suspensions of D. acetoxidans and D. amnigenus followed Michaelis-Menten kinetics (Fig. 1). Thresholds for acetate consumption were not determined, but both strains reached acetate concentrations below the detection limit of our gas chromatographic analysis (15 μM). The theory that methanogens can outcompete sulfate reducers for acetate in anaerobic bioreactors, because of their higher growth rates (13), is clearly not always valid, since D. acetoxidans had a higher growth rate than most Methanosaeta spp. and the growth rate of D. amnigenus was in the same range as that of Methanosaeta soehngenii (Table 1). However, acetate-degrading sulfate reducers from bioreactors seem to have only a slight kinetic advantage over Methanosaeta spp. (Table 1). Therefore, in some reactor studies, acetate-degrading methanogens may have predominated over sulfate reducers to the fact that the duration of the competition study was not long enough to allow sulfate reducers to become dominant (3, 11). Reactor studies by Visser (12) showed, for example, that it can take more than a year before sulfidogens have outcompeted methanogens for acetate.
FIG. 1.
Acetate depletion curve for a concentrated suspension of D. amnigenus cells (▪) and D. acetoxidans cells (•). The markers represent the measured acetate concentrations, while the solid lines are best-fit curves calculated from estimates of Km, Vmax, and the initial acetate concentration (S0) via nonlinear regression analysis.
TABLE 1.
Selected acetate kinetic parameters of D. acetoxidans, D. amnigenus, and the two genera of acetate-degrading methanogens
| Organism | Substrate utilization | μmax (day−1) | Vmax (μmol min−1 g of protein−1) | Km (mM) | Threshold concn (μM) | Reference and/ or source |
|---|---|---|---|---|---|---|
| D. acetoxidans | Specialist | 0.31–0.41 | 43 ± 14a | 0.6 ± 0.4a | <15 | 9; this study |
| D. amnigenus | Generalist | 0.14–0.20 | 28 ± 7a | 0.6 ± 0.4a | <15 | 8; this study |
| Methanosarcina sp. | Generalist | 0.46–0.69 | —b | 3.0 | 190–1,180 | 4, 7 |
| Methanosaeta | ||||||
| M. soehngenii | Specialist | 0.08–0.29 | 76 | 0.4–0.7 | 7–69 | 4, 6, 7 |
| M. concilii | Specialist | 0.21–0.69 | 32 | 0.8–1.2 | — | 6, 7 |
| Strain MTAS | Specialist | 0.37 | 170 | 0.5 | — | 5, 6 |
| Strain MTKO | Specialist | 0.38 | 98 | 1.17 | — | 5, 6 |
Mean of four independent experiments ± standard deviation.
—, not determined.
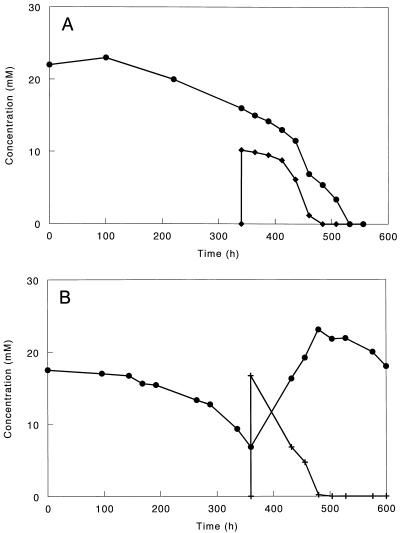
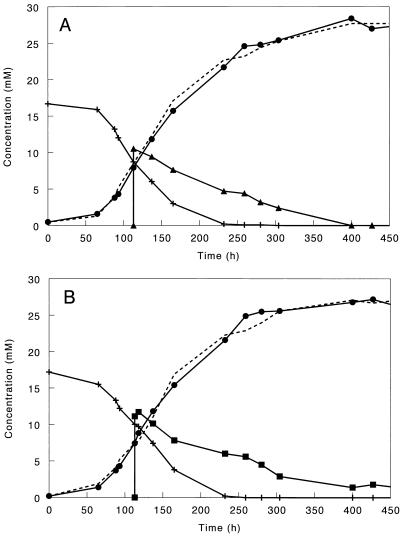
In full-scale anaerobic bioreactors, acetate is not the only organic compound available for microorganisms. From the mixed-substrate studies it is clear that the presence of hydrogen can increase the competitive advantage of D. amnigenus over Methanosaeta spp. because D. amnigenus can use acetate and hydrogen simultaneously (Fig. 2A) while Methanosaeta spp. can use only acetate. How the presence of propionate, lactate, or ethanol influences competition is less clear. The mixed-substrate experiments showed that D. amnigenus was able to degrade propionate and lactate or ethanol simultaneously. However, acetate consumption stopped when these substrates were present in excess. In fact, D. amnigenus even started to produce acetate, because propionate, lactate, and ethanol were incompletely oxidized; i.e., for each mole of propionate, lactate, or ethanol used, 1 mol of acetate was formed (Fig. 2B; Fig. 3). However, it is known that carbon substrates that usually lead to diauxic growth under batch conditions are used simultaneously under limited-carbon conditions (2). Which condition D. amnigenus encounters in sludge is not clear, because substrate availability is related not only to the concentrations in the reactor but also to diffusion of the substrate into the granule and the location of the D. amnigenus cells in the granule. Since D. amnigenus outcompeted the acetate-degrading methanogens in a bioreactor treating complex wastewater (8) and the kinetic properties of this bacterium are similar to those of Methanosaeta spp., one could speculate that the ability to use other substrates besides acetate gives D. amnigenus a competitive advantage over Methanosaeta spp.
FIG. 2.
Substrate consumption by D. amnigenus growing in batch cultures with acetate (•) and pulsed with hydrogen (⧫) (A) or propionate (+) (B).
FIG. 3.
Acetate (•) production by D. amnigenus growing in batch cultures with propionate (+) and pulsed with ethanol (▴) (A) or lactate (▪) (B). The broken lines represent calculated acetate concentrations, assuming incomplete oxidation of propionate, lactate, and ethanol.
Acknowledgments
This work was supported by a grant from Senter-IOP Environmental Technology (IOP 90209) and by Paques Environmental Technology, Balk, The Netherlands.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Egli T, Lendenmann U, Snozzi M. Kinetics of microbial growth with mixtures of carbon sources. Antonie van Leeuwenhoek. 1993;63:289–298. doi: 10.1007/BF00871224. [DOI] [PubMed] [Google Scholar]
- 3.Isa Z, Grusenmeyer S, Verstraete W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: technical aspects. Appl Environ Microbiol. 1986;51:572–579. doi: 10.1128/aem.51.3.572-579.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jetten M S M, Stams A J M, Zehnder A J B. Acetate threshold values and acetate activating enzymes in methanogenic bacteria. FEMS Microbiol Ecol. 1990;73:339–344. [Google Scholar]
- 5.Ohtsubo S, Miyahara H, Demizu K, Kohno S, Miura I. Isolation and characterization of new Methanothrix strains. Int J Syst Bacteriol. 1991;41:358–362. [Google Scholar]
- 6.Ohtsubo S, Demizu K, Kohno S, Miura I, Ogawa T, Fukuda H. Comparison of acetate utilization among strains of an aceticlastic methanogen, Methanothrix soehngenii. Appl Environ Microbiol. 1992;58:703–705. doi: 10.1128/aem.58.2.703-705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oude Elferink S J W H, Visser A, Hulshoff Pol L W, Stams A J M. Sulfate reduction in methanogenic bioreactors. FEMS Microbiol Rev. 1994;15:119–136. [Google Scholar]
- 8.Oude Elferink S J W H, Maas R N, Harmsen H J M, Stams A J M. Desulforhabdus amnigenus gen. nov. sp. nov., a sulfate reducer isolated from anaerobic granular sludge. Arch Microbiol. 1995;164:119–124. [PubMed] [Google Scholar]
- 9.Oude Elferink, S. J. W. H., W. M. Akkermans-van Vliet, J. J. Bogte, and A. J. M. Stams. Desulfobacca acetoxidans gen. nov. sp. nov., a novel acetate-degrading sulfate reducer isolated from sulfidogenic granular sludge. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 10.Robinson J A, Characklis W G. Simultaneous estimation of Vmax, Km, and the rate of endogenous substrate production (R) from substrate depletion data. Microb Ecol. 1984;10:165–178. doi: 10.1007/BF02011423. [DOI] [PubMed] [Google Scholar]
- 11.Visser A, Alphenaar P A, Gao Y, van Rossem G, Lettinga G. Granulation and immobilisation of methanogenic and sulfate reducing bacteria in high rate anaerobic reactors. Appl Microbiol Biotechnol. 1993;40:575–581. [Google Scholar]
- 12.Visser A. The anaerobic treatment of sulfate containing wastewater. Ph.D. thesis. Wageningen, The Netherlands: Wageningen Agricultural University; 1995. [Google Scholar]
- 13.Yoda M, Kitagawa M, Miyaji Y. Long term competition between sulfate-reducing and methane-producing bacteria for acetate in an anaerobic biofilm. Water Res. 1987;21:1547–1556. [Google Scholar]