Abstract
Objective
This study was conducted to investigate in vitro proangiogenic and anti-inflammatory phenotypes and functions and the in vivo efficacy and safety of quality and quantity (QQ) media-cultured mononuclear cells (MNCs) compared with standard cultured MNCs from the peripheral blood of patients with chronic limb-threatening ischemia (CLTI) with atherosclerotic risk factors.
Methods
Peripheral blood MNCs (PBMNCs) from patients with CLTI were cultured in QQ culture media or standard culture media. Phenotypic analysis of progenitor cells (CD34+CD133+), M2 macrophages (CD206+), and inactivated T regulatory cells (CD4+CD25+CD127+), colony-forming assay, and tube formation assay of QQ media-cultured MNCs (QQMNCs) and PBMNCs, were conducted. Intramuscular transplantation of QQMNCs or PBMNCs was performed in the ischemic hindlimb model. The clinical appearance of ischemic limbs was observed, and blood flow in ischemic limbs was measured using a laser Doppler perfusion imager. Outcomes were compared between the QQMNC and PBMNC groups.
Results
Twenty patients with CLTI were included. The mean percentages of CD34+ cells, CD133+ cells, CD34+CD133+ progenitor cells, CD206+ cells, colony-forming cells, and tube formation were significantly higher in the QQMNCs. The mean percentage of CD4+CD25+CD127+ cells was significantly lower in QQMNC. The colony-forming unit count and Dil-acetylated low-density lipoprotein uptake were significantly greater in QQMNCs. The clinical appearance of post-QQMNC-injected limbs was less severe than the appearance of post-PBMNC-injected limbs. Limb perfusion was significantly better in the QQMNCs.
Conclusions
Proangiogenic and anti-inflammatory phenotypes of MNCs cultured in QQ culture media were reproducible. Intramuscular QQMNC transplantation was safe and resulted in better reperfusion of ischemic hindlimbs compared with PBMNCs.
Keywords: Efficacy, Safety, Quality and quantity media-cultured mononuclear cell transplantation, Ischemic hindlimb mouse model
Clinical Relevance
Intramuscular transplantation of quality and quantity media-cultured mononuclear cells from chronic limb-threatening ischemia patient is safe and effective in the ischemic hindlimb mouse model. The Quality and Quantity media-cultured mononuclear cells might become a novel therapeutic approach in no-option chronic limb-threatening ischemia patients.
Article Highlights.
-
•
Type of Research: In vitro and ischemic mouse model study
-
•
Key Findings: Quality and quantity (QQ) culture media aided proangiogenic, anti-inflammatory, and angiogenesis in vitro. In 40 ischemic limb mouse models, intramuscular transplantation of QQ media-cultured mononuclear cells (MNCs) led to better limb reperfusion than transplantation of MNCs cultured in standard culture media.
-
•
Take Home Message: Intramuscular transplantation of QQ media-cultured MNCs is safe and effective in an ischemic hindlimb mouse model.
Chronic limb-threatening ischemia (CLTI) is the most severe form of peripheral arterial disease,1 and CLTI is a significant predictor of limb morbidity and mortality. Rest pain and ischemic tissue loss are common presentations of CLTI.2 Revascularization, via either open surgery or endovascular technique, is the current treatment of choice for CLTI; however, ≤15% to 20% of CLTI revascularizations fail.3 The term "no option critical limb ischemia” (NOCLI) was coined to characterize this problem. NOCLI also includes conditions that make reperfusion impossible. The causes of NOCLI can be physiological and/or anatomical.4 Therapeutic angiogenesis is an alternative treatment for patients with NOCLI. Cell-based therapy to enhance angiogenesis with bone marrow mononuclear cells (BMMNCs) or peripheral blood MNCs (PBMNCs) was reported to be safe and effective5,6; however, a meta-analysis did not show a significant clinical benefit of BMMNC or PBMNC transplantation in patients with CLTI.7,8 The poor outcomes observed in BMMNC and PBMNC transplantation trials may be due to an insufficient number and/or impaired function of BMMNCs or PBMNCs in patients with CLTI.5,9, 10, 11, 12, 13 The proportion of endothelial progenitor cells (EPCs) is <0.1% in BMMNCs and <0.01% in PBMNCs.5,9,14 In addition, the EPC expansion process requires time and effort.5,9,14, 15, 16 patients with CLTI also have impaired function of progenitor cells.10,17 Diabetes mellitus, which is a well-known risk factor for atherosclerosis, creates an inflammatory tissue environment and impedes vascular regeneration.9,17, 18, 19 A novel alternative method is, therefore, needed to increase the number and function of progenitor cells and/or MNCs harvested from patients with CLTI. The culture of PBMNCs in quality and quantity (QQ) culture media was reported in 2014 by Masuda et al.17 In their study, MNCs from healthy volunteers that were cultured in QQ media (QQMNCs) demonstrated enhanced angiogenesis and anti-inflammatory function, not only in vitro, but also in an ischemic hindlimb animal model.17,18 This finding suggests QQMNCs as a potential option for treating patients with CLTI. Chruewkamlow et al20 studied QQMNCs that were developed from PBMNCs harvested from patients with CLTI in vitro. Their results showed that QQ culture media could increase the amount and enhance the angiogenic phenotype of MNCs from patients with CLTI.
The aim of the present study was to investigate the safety and efficacy of QQMNC transplantation in an ischemic hindlimb mouse model to determine its potential benefit for treating patients with CLTI.
Methods
This pilot cross-sectional experimental study prospectively enrolled patients from the CLTI Clinic of the Division of Vascular Surgery, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, from September 2019 to May 2022. Patients diagnosed with CLTI with an atherosclerotic etiology who underwent failed revascularization and/or were considered NOCLI owing to anatomical and/or physiological problems were eligible for inclusion. The diagnostic criteria used were based on the published guidelines for the management of CLTI.1 Patients were diagnosed as CLTI if they presented with ischemic rest pain and/or tissue loss, and they satisfied the corresponding objective hemodynamic criteria. Patients having one or more of the following were excluded: nonatherosclerotic cause of CLTI, such as thromboangiitis obliterans or vasculitis, and concomitant wound infection and/or clinical sepsis. The Ethical Review Committee of the Siriraj Institutional Review Board approved the study protocol (COA no. Si 365/2019), and all enrolled human subjects provided written informed consent to participate in this study. Animal studies were performed after receiving approval of the Siriraj Laboratory Animal Research and Care Center in Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (approval No. SI-ACUP 005/2562).
PBMNC preparation
Fifteen milliliters of peripheral blood from venipuncture of a forearm superficial vein was collected from study patients with CLTI into a specimen collection tube containing heparin. Density gradient centrifugation of collected PBMNCs was performed using Lymphocyte Separation Solution (Sigma-Aldrich Corporation, St. Louis, MO). Isolated PBMNCs were cultured in either QQ culture media or standard culture media at a concentration of 2 × 106 cells/2 mL in a six-well Primaria dish (BD Biosciences, San Jose, CA) for 7 days.17,21
QQ culture media
QQ culture media is composed of Stem Line II Solution (#S0192; Sigma-Aldrich) and the addition of the following five recombinant human proteins: 100 ng/mL of stem cell factor (cat. no. #300-07; PeproTech, Rocky Hill, NJ), 20 ng/mL of thrombopoietin (#300-18; PeproTech), 100 ng/mL of Flt-3 ligand (#300-19; PeproTech), 50 ng/mL of vascular endothelial growth factor (#100-20; PeproTech), and 20 ng/mL of IL-6 (#200-06; PeproTech).17
Standard culture media
The standard culture media was composed of 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Waltham, MA) and Roswell Park Memorial Institute-1640 Medium (Gibco; Thermo Fisher Scientific).17,21
Phenotypic analysis of progenitor cells, T regulatory cells, and M2 macrophages
The cultured cells were harvested after 7 days. The harvested cells were washed two times with 2% FBS and 0.02% sodium azide phosphate-buffered saline (NaN3 PBS) and then suspended in 2 mmol/L of ethylenediaminetetra-acetic acid/0.2% bovine serum albumin/PBS buffer. After supplementation with 10 μL of FcR blocking reagent (Miltenyi Biotec, Bergisch Gladbach, Germany), the suspended cells were incubated at 4°C for 30 minutes. Staining of incubated cells was then performed using a combination of monoclonal antibodies (mAbs) that are markers of progenitor cells (CD34+CD133+), M2 macrophages (CD206+), and inactivated regulatory T cells (CD4+CD25+CD127+).
Two separate panels were used for phenotypic analysis in this study. The first panel was used to analyze progenitor cells and M2 macrophages. The mAbs used in this panel were CD34-FITC (#343504; BioLegend, San Diego, CA), CD11c-PE (#371504; BioLegend), CD133-APC (#372806; BioLegend), CD3-PE-Cy7 (300,420; BioLegend), CD206-APC-Cy7 (#321119; BioLegend), and CD11b-PerCP Cy5.5 (#101228; BioLegend). The cells were incubated with 5 μL of each mAb at 4°C for 30 minutes and then washed 2 times with 2% FBS and 0.02% NaN3 PBS. The incubated cells were then fixed in PBS with 1% paraformaldehyde (Sigma-Aldrich). Flow cytometry was performed using a BD LSR Fortessa Flow Cytometer (BD Biosciences).17,22
The second panel was used to analyze inactivated regulatory T (CD4+CD25+CD127+) cells. The mAbs used were CD25-PE (#302606; BioLegend), CD127-APC (#351316; BioLegend), CD3-Pe- Cy7 (#300420; BioLegend), and CD4-APC-Cy7 (#300518; BioLegend).17,22 The cells were incubated with 5 μL of each mAb at 4°C for 30 minutes and then washed two times with 2% FBS and 0.02% NaN3 PBS. All experiments were performed a minimum of three times, and the mean values were recorded. The proportions of CD34+CD133+ cells, CD206+ cells, and CD4+CD25+CD127+ cells in PBMNCs and QQMNCs were compared.
EPC colony formation assay
Harvested MNCs at 1 × 105 cells/mL were resuspended with 200 μL of 30% FBS/PBS. The following recombinant human cytokines were then added: human stem cell factor (#300-07; PeproTech) at 66.7 ng/mL; human vascular endothelial growth factor (#100-20; PeproTech) at 33.3 ng/mL; human IL-3 (#200-03; PeproTech) at 13.3 ng/mL; human insulin-like growth factor 1 (#100-11; PeproTech) at 33.3 ng/mL; human fibroblast growth factor basic (#100-18B; PeproTech) at 33.3 ng/mL; and human epidermal growth factor (#100- 15; PeproTech) at 33.3 ng/mL. The cells were resuspended with complete MethoCult media (#04236; STEMCELL Technologies, Inc., Vancouver, British Columbia, Canada) at a final volume of 2 mL. The cells were then cultured at 37°C for 14 days.17
Phase-contrast light microscopy (Eclipse TE300; Nikon Instruments, Tokyo, Japan) was used to assess EPC colony-forming cells (EPC). A colony was defined as the presence of ≥50 cells.23 All experiments were performed at least three times, and the mean values were recorded. The numbers of colonies of PBMNCs and QQMNCs were compared.
Tube formation assay
Labeling of MNCs was performed using 20 μg/mL of acetylated low-density lipoprotein and 1,10-dioctadecyl-3,3,30,30-tetramethyl-indocarbocyanine perchlorate (Dil-Ac-LDL) (Biomedical Technologies, Inc., Stoughton, MA) at 4 × 104 cells/500 μL in a 37°C carbon dioxide (CO2) incubator for 30 minutes. The labeled MNCs were then centrifuged at 400×g for 10 minutes, washed with 2% FBS/PBS, and then suspended in 2% FBS/PBS at 1 × 103 cells/50 μL. Coculture of labeled MNCs and human umbilical vein endothelial cells (HUVECs) was performed at an MNC to HUVEC ratio of 1 × 103 to 1.5 × 104 cells in a total volume of 100 μL. The cell mixture was incubated in a water bath at 37°C. One hundred microliters of the incubated cells was then transferred into a precoated Matrigel (Corning, Inc., Corning, NY) (thin coat method) 50 μL/well in a 96-well plate and incubated in a CO2 incubator at 37°C for 10 hours.
A Nikon Ti-S Intensilight Ri1 NIS-D inverted fluorescence microscope (Nikon Instruments) was used to assess tube formation. All experiments were performed at least three times, and the mean values were recorded. The fluorescence intensity of labeled PBMNCs in HUVECs from PBMNCs and QQMNCs was compared.17
In vivo study
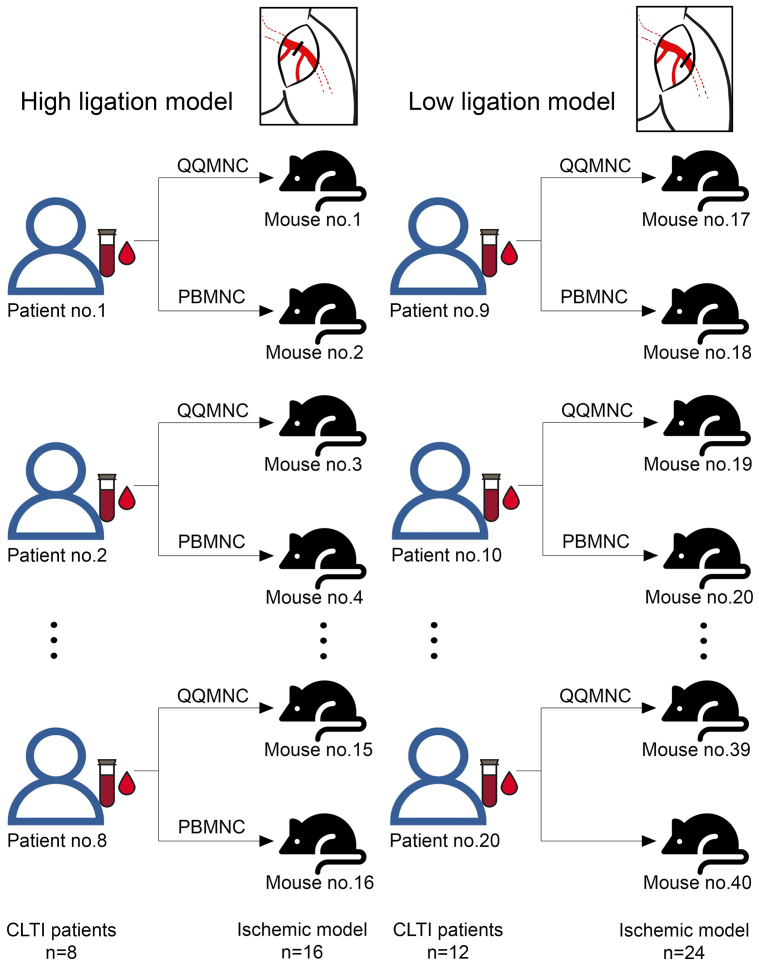
Animal care and the laboratory where animal experiments were conducted in this study are both regulated by the Siriraj Laboratory Animal Research and Care Center. All animal procedures were performed under anesthesia. A postprocedural analgesic ketoprofen dose of 3 mg/kg was given to all study mice. After completion of the experiment, animals were humanely killed in a CO2 chamber under anesthesia. In this study, blood from each human patient with CLTI was divided in half so that 50% could be cultured in QQ culture media, and the other 50% could be cultured in standard culture media. Cultured MNCs from the two different types of media were then transplanted into different mice. Because there were 20 patients, we used 40 mice for the in vivo study (Fig 1).
Fig 1.
The diagram describes how mononuclear cells (MNCs) from each patients with chronic limb-threatening ischemia (CLTI) were transplanted into ischemic model. This study included 20 patients with CLTI. Quality and quantity media-cultured MNCs (QQMNCs) and peripheral blood MNCs (PBMNCs) from each patient with CLTI were transplanted separately into two different mice. QQMNCs and PBMNCs from 8 patients were transplanted into 16 mice with high arterial ligation. QQMNCs and PBMNCs from 12 patients were transplanted into 24 mice with low arterial ligation.
Animal model preparation
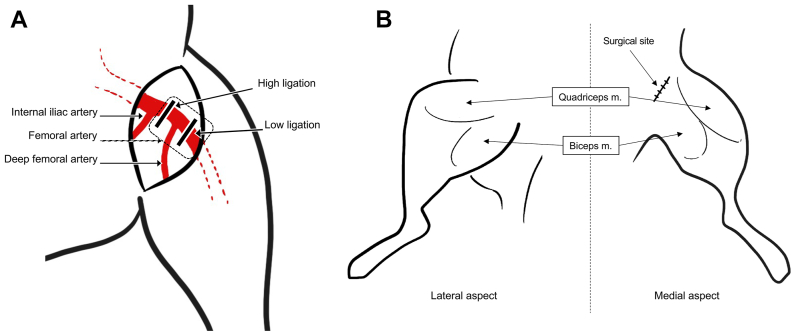
To prevent an immune rejection after human MNC injection, BALB/c nu/nu nude mice (aged 8-10 weeks; bodyweight, 19-22 g) were used in this study. Study mice were obtained from the Mahidol University National Laboratory Animal Center, which is located in Thailand's Nakhon Pathom Province. The left hindlimb was designated to be the ischemic limb in each model. Ischemic model preparation was performed under anesthesia. A cocktail of 100 mg/kg of ketamine and 10 mg/kg of xylazine was given via intraperitoneal route.24 After the study mouse was fully anesthetized, hair at the surgical area was removed and skin was prepared to create a sterile field. With the mouse positioned in the dorsal decubitus position with the hip externally rotated, baseline perfusion was measured using a laser Doppler perfusion imager (Moor Instruments, Axminster, UK) before surgical arterial ligation. The delicate process of preparing the hindlimb ischemia model was then performed using a 10× magnification microscope. A transverse incision at the left groin was made using surgical scissors. Subcutaneous fat and potential progenitor cells around the surgical area were removed using a cotton swab. The femoral artery and its deep branch were identified. There were two types of ischemic models in this study (Fig 2, A). The first was a high arterial ligation model. In this model, we ligated the femoral artery proximal to the origin of the deep femoral artery.25 The second model was a low arterial ligation model with ligation being performed distal to the origin of the deep femoral artery.26, 27, 28 After the target ligation site was identified, blunt-end curved forceps were used to separate the artery from adjacent veins and nerves. Ligation of the intended arterial site was performed using 6-0 polypropylene sutures at the proximal and distal end, and then the artery was divided with surgical scissors. Bleeding was checked for and stopped. The skin was sutured with 6-0 silk. Perfusion measurement using the laser Doppler perfusion imager was obtained after arterial ligation while the mouse was still anesthetized. Mice with hindlimb ischemia were observed for limb gangrene and potential complications for 14 days after MNC transplantation.
Fig 2.
The high and low ligation sites used to induce hindlimb ischemia in mouse model, and the mononuclear cell (MNC) injection sites in this study. (A) High ligation models were created via femoral artery ligation proximal to the origin of the deep femoral artery (labeled as high ligation). Low ligation models were created via femoral artery ligation distal to the origin of the deep femoral artery (labeled as low ligation). (B) Diagram showing the sites of MNC injection at the lateral and medial aspects of the left hindlimb. MNCs were injected intramuscularly at the medial and lateral aspects of the quadriceps muscle (m) and the biceps muscle.
Injection of MNCs
After 1 day of arterial ligation, mice with a viable limb were selected for study of the safety and efficacy of MNC injection. Before transplantation, the PBMNCs and QQMNCs underwent a triple wash procedure with sterile PBS. Subsequently, the supernatant was removed, and the PBMNCs and QQMNCs were resuspended in sterile PBS to achieve a concentration of 12,500 cells/50 μL for each injection site. We also performed an evaluation of endotoxin levels in the cultured supernatants using a Kinetic Turbidimetric Limulus Amebocyte Lysate Assay-USP-NF2022. All cultured cells underwent testing to detect mycoplasma and viral contamination by reverse tancriptase polymerase chain reaction.
The MNC injection procedure was also performed under anesthesia. Intramuscular injection with cultured MNCs, either cells cultured in QQ culture media (QQMNCs) or cells cultured in standard culture media (PBMNCs) was performed. QQMNCs and PBMNCs from each patient with CLTI were transplanted separately into two different mice. QQMNCs and PBMNCs from 8 patients were transplanted into 16 mice with high arterial ligation. QQMNCs and PBMNCs from 12 patients were transplanted into 24 mice with low arterial ligation (Fig 1). MNCs were injected at four sites around the thigh of the ischemic limb, including two sites at the medial and lateral aspects of the quadriceps muscles and two sites at the medial and lateral aspects of the bicep's muscles (Fig 2, B). The injected mice were observed serially for complications for 14 days.
Assessment of limb perfusion
A laser Doppler perfusion imager was used to serially evaluate blood flow in ischemic limb model mice. The area of interest was below the knee to the toes of the mouse model. As previously mentioned, perfusion measurement was performed just before and just after arterial ligation. An additional perfusion measurement was then performed at day 14 after the transplantation of MNCs, and blood flow was compared between QQMNC and PBMNC groups.
Sample size calculation and statistical analysis
To prove the safety of both types of MNCs, we assumed a survival rate of animal models after MNCs transplantation of 95%. Using a 5% alpha error and a 10% allowable error from previous study, the calculated minimum sample size of animal models was 19 mice per group.29,30 The decision was made to increase the sample size to 20 animal models per group. Because we studied 2 types of MNCs, the total number of animal models in this study was 40 mice. Using laser Doppler perfusion imaging to compare the efficacy outcomes between QQMNC and PBMNC injections in both high and low arterial ligation models, a 5% alpha error, and a 10% allowable error, our sample size calculation revealed a minimum of 6 mice per group in our animal models for a minimum total of 24 study mice to demonstrate the efficacy of MNC injection in both high and low arterial ligation models. However, to assess both safety and efficacy outcomes, our study enrolled a total of 40 mice.
All statistical analyses were performed using SPSS Statistics version 18 (SPSS, Inc., Chicago, IL) and Prism 6 software (GraphPad Software, Inc., San Diego, CA). Categorical variables are given as number and percentage. Continuous variables are reported as mean plus/minus standard deviation or median (minimum and maximum) depending on the distribution of the continuous data. Categorical data were analyzed using the χ2 test or Fisher's exact test depending on the size of the sample. Continuous data were compared using the Student t test and Mann-Whitney U test for normally and non-normally distributed continuous data, respectively. Univariate logistic regression was performed to identify significant clinical risk factors associated with the abnormal clinical appearance of the ischemic model after MNC transplantation. Factors with a P value of <.10 from that analysis were included in the subsequent multivariate logistic regression analysis to identify independent predictors of an abnormal clinical appearance after MNC transplantation. The results of those two analyses are shown as odds ratio (OR) and adjusted OR (aOR) and their corresponding 95% confidence intervals (95% CIs), respectively. All P values were two tailed, and values of <.05 were considered to reflect statistical significance.
Results
Baseline patient with CLTI characteristics
This study included 20 patients with CLTI with a mean age of 65.9 ± 8.56 years; 17 patients (85%) were male. Diabetes mellitus, hypertension, current smoker status, and chronic kidney disease was found in 12 (60%), 17 (85%), 11 (55%), and 6 (30%) patients, respectively. The mean toe pressure was 20.4 ± 19.8 mm Hg. The clinical presentation of rest pain, gangrene, and nonhealing ulcer was found in 2 (10%), 12 (60%), and 6 (30%) patients, respectively. The baseline demographic and clinical characteristics of the 20 enrolled patients with CLTI are summarized in Table I.
Table I.
Baseline demographic and clinical characteristics of patients with chronic limb-threatening ischemia (CLTI) (n = 20)
| Characteristics | Value |
|---|---|
| Age, years | 65.9 ± 8.56 |
| Male gender | 17 (85) |
| Ankle pressure, mm Hg | 122.5 ± 96.0 |
| Ankle-brachial index | 0.8 ± 0.58 |
| Toe pressure, mm Hg | 20.4 ± 19.8 |
| Clinical presentation | |
| Rest pain | 2 (10) |
| Gangrene | 12 (60) |
| Nonhealing ulcer | 6 (30) |
| Diabetes mellitus | 12 (60) |
| Chronic kidney disease | 6 (30) |
| Hypertension | 17 (85) |
| Dyslipidemia | 16 (80) |
| Current smoker status | 11 (55) |
Values are mean ± standard deviation or number (%).
Phenotypic analysis of progenitor cells, T regulatory cells, and M2 macrophages
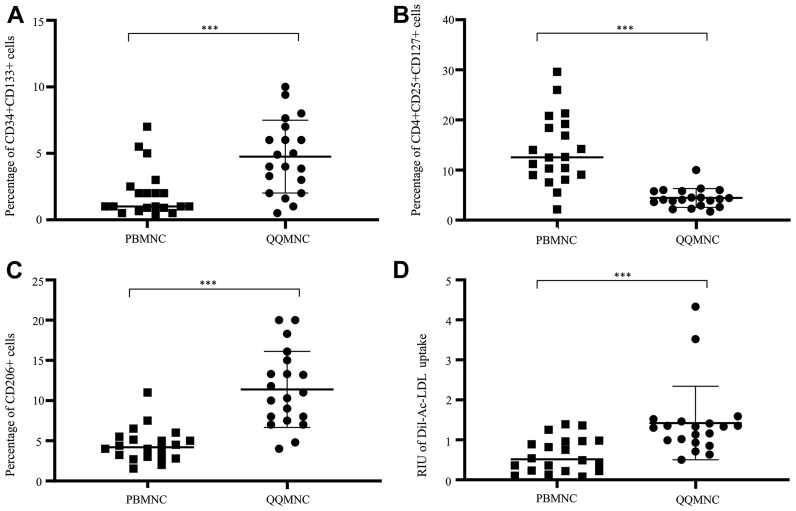
The outcomes of in vitro studies compared between the QQMNC and PBMNC groups are shown in Table II. The mean percentage of CD34+ cells was significantly greater in QQMNCs than in PBMNCs (14.58 ± 4.6% vs 7.2 ± 4.47%, respectively; P < .001). The mean percentage of CD133+ cells was significantly greater in QQMNCs than in PBMNCs (12.39 ± 5.83% vs 4.45 ± 2.78%, respectively; P < .001). The mean percentage of CD34+CD133+ cells was significantly greater in QQMNCs than in PBMNCs (4.72 ± 2.62% vs 2.08 ± 1.93%, respectively; P < .001). The mean percentage of CD206+ cells was significantly greater in QQMNCs than in PBMNCs (11.79 ± 4.98% vs 4.55 ± 2.33%, respectively; P < .001). The mean percentage of CD4+CD25+CD127+ cells was significantly greater in PBMNCs than in QQMNCs (4.56 ± 1.97% vs 13.61 ± 6.85%, respectively; P < .001).
Table II.
Outcomes of in vitro study compared between the quality and quantity media-cultured mononuclear cell (QQMNC) and peripheral blood mononuclear cell (PBMNC) groups (n = 20)
| Evaluated parameters | QQMNC group | PBMNC group | P value |
|---|---|---|---|
| CD34+ cells | 14.58 ± 4.6% | 7.2 ± 4.47% | <.001 |
| CD133+ cells | 12.39 ± 5.83% | 4.45 ± 2.78% | <.001 |
| CD34+CD133+ cells | 4.72 ± 2.62% | 2.08 ± 1.93% | <.001 |
| CD206+ cells | 11.79 ± 4.98% | 4.55 ± 2.33% | <.001 |
| CD4+CD25+CD127+ cells | 4.56 ± 1.97% | 13.61 ± 6.85% | <.001 |
| CFU count (2 × 105 cells/dish) | 7.3 ± 4.6 | 1.6 ± 0.8 | <.001 |
| Dil-Ac-LDL uptake (RIU) | 1.47 ± 0.98 | 0.58 ± 0.43 | <.001 |
CD, Cluster of differentiation; CFU, colony-forming unit; CLTI, chronic limb-threatening ischemia; Dil-Ac-LDL, Dil-acetylated low-density lipoprotein; RIU, reference intensity unit.
Data presented as mean ± standard deviation.
Boldface entries indicate statistical significance.
EPC-CFA, tube formation assay, and culture cell endotoxin and contamination
The mean colony-forming unit count was significantly greater in QQMNCs than in PBMNCs (7.3 ± 4.6 vs 1.6 ± 0.8, respectively; P < .001). The mean reference intensity units of Dil-Ac-LDL uptake in coculture was significantly greater in QQMNCs than in PBMNCs (1.47 ± 0.98 vs 0.58 ± 0.43, respectively; P < .001). Selected in vitro angiogenic phenotypes, anti-inflammatory phenotypes, and the function of PBMNCs and QQMNCs are shown in Fig.3.
Fig 3.
Dot density frequency plot of selected in vitro phenotypes and functions of peripheral blood mononuclear cells (PBMNCs) and quality and quantity media-cultured MNC (QQMNCs). (A–C) Results of flow cytometry analyses of PBMNCs and QQMNCs. (A) Analysis of hematopoietic stem cells (CD34+CD133+). The percentage of CD34+CD133+ cells was significantly higher in QQMNCs than in PBMNCs. (B) Analysis of inactive T regulatory cells (CD4+CD25+CD127+) after CD3+ cells and CD4+ cells were gated. The percentage of inactivated regulatory T cells (CD4+CD25+CD127+) was significantly higher in PBMNCs than in QQMNCs. (C) Analysis of M2 macrophages (CD206+) after CD3 cells were gated. The percentage of CD206+ cells was significantly higher in QQMNCs than in PBMNCs. (D) Analysis of Dil-acetylated low-density lipoprotein (Dil-Ac-LDL) uptake of tube formation assay results compared between MNCs cultured in standard media and MNCs cultured in QQ media. Dil-Ac-LDL uptake was significantly higher in QQMNCs than in PBMNCs. ∗∗∗P < .001. CD, cluster of differentiation.
The results for all cultured cells indicated endotoxin levels of <0.100 EU/mL and the absence of mycoplasma and viral contamination.
Clinical outcomes and assessment of limb perfusion of in vivo study
No complications (other than cyanosis or gangrene) or mortality were observed during the 14-day observation period after MNC transplantation in any study mouse. In vivo clinical outcomes and limb perfusion of ischemic hindlimb mouse model compared between the QQMNC and PBMNC groups are summarized in Table III. Of the 20 hindlimbs injected with QQMNCs, 16 (80%) appeared normal at 14 days after MNC injection. The other four hindlimbs (20%) in the QQMNC group appeared cyanotic. None of the hindlimbs in this group developed limb gangrene.
Table III.
In vivo clinical outcomes and limb perfusion of ischemic hindlimb mouse model compared between the quality and quantity media-cultured mononuclear cell (QQMNC) and peripheral blood mononuclear cell (PBMNC) groups
| Evaluated parameters | PBMNC group | QQMNC group | P value |
|---|---|---|---|
| Overall (n = 40) | (n = 20) | (n = 20) | |
| Limb appearance | .096 | ||
| Normal appearing limb | 10 (50) | 16 (80) | |
| Limb cyanosis | 6 (30) | 4 (20) | |
| Limb gangrene | 4 (20) | 0 (0) | |
| Complication | 0 (0) | 0 (0) | |
| Laser Doppler perfusion imaging (aPU) | |||
| After ligation | 334.8 ± 126.0 | 283.0 ± 119.0 | .301 |
| 14-days post-MNC transplantation | 434.3 ± 230.7 | 641.9 ± 232.2 | .020 |
| High arterial ligation model (n = 16) | (n = 8) | (n = 8) | |
| Limb appearance | .041 | ||
| Normal appearing limb | 1 (12.5) | 6 (75.0) | |
| Limb cyanosis | 4 (50.0) | 2 (25.0) | |
| Limb gangrene | 3 (37.5) | 0 (0.0) | |
| Laser Doppler perfusion imaging (aPU) | |||
| After ligation | 213.4 ± 132.4 | 168.1 ± 103.4 | .574 |
| 14-days post-MNC transplantation | 258.3 ± 154.5 | 468.9 ± 225.3 | .038 |
| Low arterial ligation model (n = 24) | (n = 12) | (n = 12) | |
| Limb appearance | .96 | ||
| Normal appearing limb | 9 (75.0) | 10 (83.3) | |
| Limb cyanosis | 2 (16.7) | 2 (16.7) | |
| Limb gangrene | 1 (8.3) | 0 (0.0) | |
| Laser Doppler perfusion imaging (aPU) | |||
| After ligation | 386.2 ± 90.5 | 343.8 ± 88.6 | .291 |
| 14-days post-MNC transplantation | 501.2 ± 219.7 | 702.0 ± 242.4 | .114 |
aPU, Arbitrary perfusion unit; MNC, mononuclear cell.
Values are number (%) or mean ± standard deviation.
Boldface entries indicate statistical significance.
Of the 20 hindlimbs injected with PBMNCs, 10 limbs (50%) appeared normal, 6 (30%) limbs were cyanotic, and 4 (20%) limbs developed gangrene. The difference in clinical appearance (ie, normal appearance vs abnormal appearance, including cyanosis and gangrene) was not statistically significantly different between groups (P = .096). Limb perfusion as measured by laser Doppler perfusion imager at baseline (before MNC injection) was not significantly different between the two transplantation groups (P = .301). Limb perfusion measured on day 14 after MNC injection was significantly higher in mice injected with QQMNCs than in mice injected with PBMNCs (P = .020).
High arterial ligation model
In vivo clinical outcomes and limb perfusion of high arterial ligation ischemic hindlimb mouse models compared between the QQMNC and PBMNC groups are summarized in Table III. The number of abnormal limbs among the hindlimbs injected with PBMNCs and the hindlimbs injected with QQMNCs was seven (87.5%) and two (25%) limbs, respectively (P = .041). Limb perfusion measured at baseline (before MNC injection) was not significantly different between groups (P = .574). However, limb perfusion measured at day 14 after MNC injection was significantly higher in limbs injected with QQMNCs (P = .038).
Low arterial ligation model
In vivo clinical outcomes and limb perfusion in the low arterial ligation ischemic hindlimb mouse model compared between the QQMNC and PBMNC groups are given in Table III. Nine limbs (75%) in the PBMNC group and 10 limbs (83.3%) in the QQMNC group appeared normal at 14 days after MNC transplantation. There was no significant difference in limb perfusion between groups after ligation (P = .291) or at day 14 after MNC transplantation (P = .114) in the low arterial ligation model.
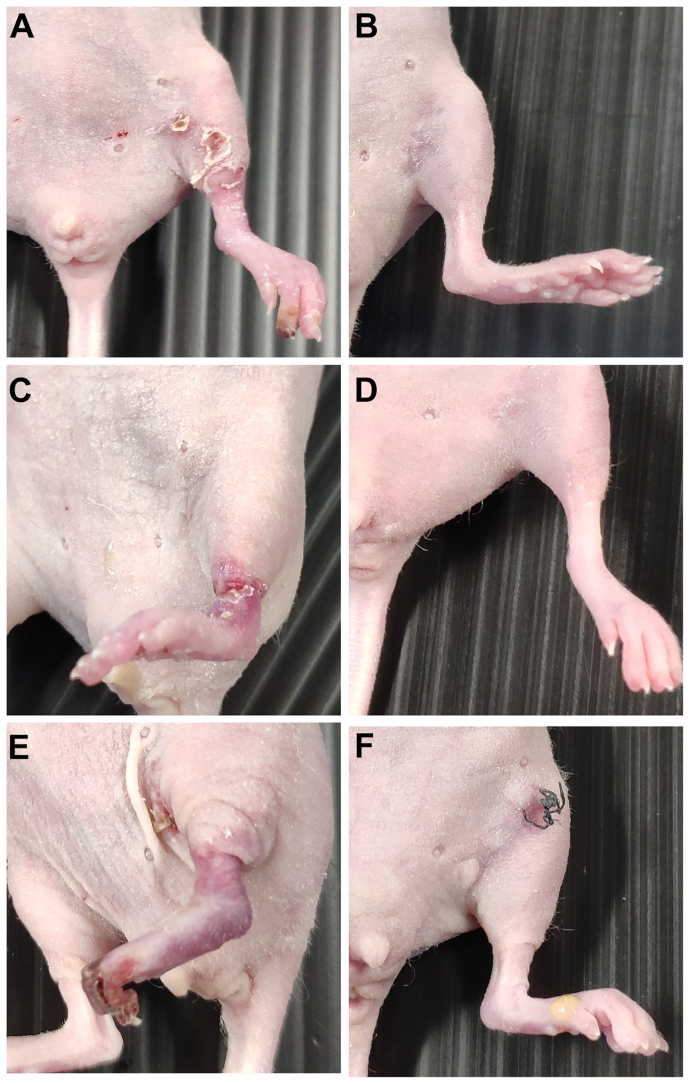
Examples of the clinical appearance of mice hindlimbs transplanted with MNCs from the same patient with CLTI are shown in Fig 4. At 14 days after transplantation, the clinical appearance of limbs transplanted with QQMNCs was better than the clinical appearance of the limbs transplanted with PBMNCs. This trend could be observed in both the low and high arterial ligation models.
Fig 4.
Clinical appearance of mouse left hindlimbs at 14 days after mononuclear cell (MNC) injection. (A, B) Low arterial ligation ischemic model transplanted with MNCs from mouse number 14. (A) The limb appeared cyanotic at 14 days after peripheral blood MNC (PBMNC) injection. (B) The limb appeared normal at 14 days after quality and quantity media-cultured MNC (QQMNC) injection. (C, D) High arterial ligation ischemic model transplanted with MNCs from mouse number 7. (C) The limb appeared cyanotic at 14 days after PBMNC injection. (D) The limb appeared normal at 14 days after QQMNC injection. (E, F) High arterial ligation ischemic model transplanted with MNCs from mouse number 4. Toe gangrene was observed at 14 days after PBMNC injection. (F) The limb appeared normal at 14 days after QQMNC injection.
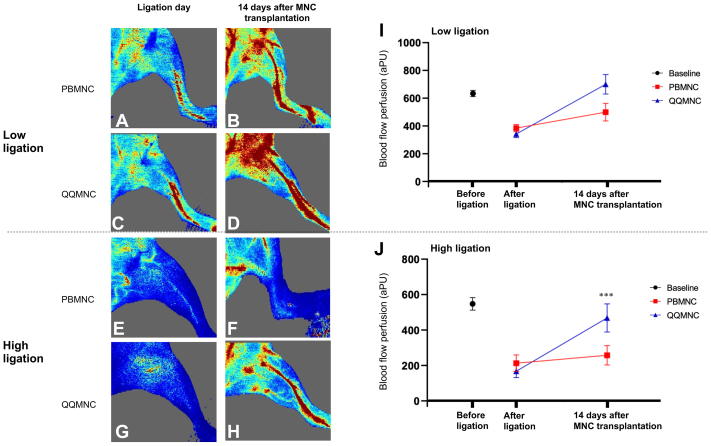
The results of blood flow perfusion measurement before and after MNC transplantation are shown in Fig 5. After ligation, blood flow decreased from baseline (before ligation). However, there was no significant difference in measured flow between groups after ligation in either the low or high ligation model. At 14 days after MNC transplantation, perfusion in hindlimbs transplanted with QQMNCs was higher than perfusion in hindlimbs transplanted with PBMNCs; however, that difference between groups was only statistically significant in the high arterial ligation model (P = .038).
Fig 5.
Results of blood flow perfusion measurement. (A–H) Examples of laser Doppler perfusion images of ischemic mouse hindlimb models. (A–D) Two low ligation models transplanted with mononuclear cell (MNCs) from a patient with chronic limb-threatening ischemia (CLTI). (A and B) Perfusion images of a peripheral blood MNC (PBMNC)-transplanted model after ligation and at 14-days post-MNC transplantation. (C, D) Perfusion images of a quality and quantity media-cultured MCS (QQMNC)-transplanted model after ligation and at 14-days after MNC transplantation. (E–H) Two high ligation models transplanted with MNCs from a different patient with CLTI. (E, F) Perfusion images of a PBMNC-transplanted model after ligation and at 14 days after MNC transplantation. (G, H) Perfusion images of a QQMNC-transplanted model after ligation and at 14 days after MNC transplantation. (I, J) Flow difference in the low (I) and high (J) ligation models compared between the PBMNC and QQMNC groups at baseline (before ligation), after ligation, and at 14 days after MNC transplantation (which was 15 days after ligation). Each line graph represents a mean ± standard error. After ligation, flow perfusion decreased in all models. However, flow perfusion was not significantly different between the PBMNC and QQMNC groups in both the low and high ligation models after ligation. At 14 days after MNC transplantation, flow perfusion in both the high and low ligation models transplanted with QQMNCs was higher than in the PBMNC transplantation models, but a significant difference between groups was only observed in the high ligation model at 14 days after MNC transplantation (∗∗∗P < .05). PBMNC represents the model transplanted with PBMNCs, and QQMNC represents the model transplanted with QQMNCs. aPU, arbitrary perfusion unit.
Univariate and multivariate analysis of factors associated with abnormal clinical limb appearance
The results of univariate analysis to identify factors significantly associated with abnormal clinical limb appearance at 14 days after MNC injection are summarized in Table IV. The results of multivariate analysis to identify factors independently associated with abnormal clinical limb appearance at 14 days after MNC injection are shown in Table V. High ligation model (OR, 4.878; 95% CI, 1.211-19.608; P = .041) and QQMNC injection (OR, 0.250; 95% CI, 0.061-1.017; P = .096) were the only two factors with a P value or <.10 on univariate analysis, so they were both included in the multivariate analysis. Both of those factors survived multivariate analysis, as follows: high arterial ligation was found to be independently associated with higher odds of abnormal clinical appearance at day 14 after MNC transplantation (aOR, 6.21; 95% CI, 1.304-29.412; P = .022), and QQMNC injection was found to be independently associated with lower odds of abnormal clinical appearance at 14 days after MNC injection (aOR, 0.192; 95% CI, 0.039-0.937; P = .041). In addition, paired analysis per patient showed that the outcomes of QQMNCs were better than the outcomes of PBMNCs in every patient.
Table IV.
Univariate analysis to identify factors significantly associated with abnormal clinical limb appearance at 14 days after mononuclear cell (MNC) injection (n = 40)
| Factors | Limb appearance |
P value | |
|---|---|---|---|
| Abnormal limb (n = 14) | Normal limb (n = 26) | ||
| Age, years | 66.8 ± 10.6 | 65.3 ± 7.7 | .326 |
| Current smoker status | 8 (57.1) | 14 (53.8) | 1 |
| Male gender | 12 (85.7) | 22 (84.6) | 1 |
| Hypertension | 13 (92.9) | 21 (80.8) | .399 |
| Diabetes mellitus | 10 (71.4) | 14 (53.8) | .329 |
| Dyslipidemia | 13 (92.9) | 19 (73.1) | .222 |
| Chronic kidney disease | 4 (28.6) | 8 (30.8) | .127 |
| High ligation model | 9 (64.3) | 7 (26.9) | .041 |
| QQMNC injection | 4 (28.6) | 16 (61.5) | .096 |
| PBMNC injection | 10 (71.4) | 10 (38.5) | |
PBMNC, Peripheral blood mononuclear cell; QQMNC, quality and quantity media-cultured mononuclear cell.
Values are number (%) or mean ± standard deviation.
Boldface entries indicate statistical significance.
Factors with a P-value<.10 were included in subsequent multivariate analysis.
Table V.
Multivariate analysis to identify factors independently associated abnormal clinical limb appearance at 14 days after mononuclear cell (MNC) injection
| Factors | Crude OR (95% CI) | P value | aOR (95% CI) | P value |
|---|---|---|---|---|
| High ligation model | 4.878 (1.211-19.608) | .041 | 6.21 (1.304-29.412) | .022 |
| QQMNC injection | 0.250 (0.061-1.017) | .096 | 0.192 (0.039-0.937) | .041 |
aOR, Adjusted odds ratio; CI, confidence interval; OR, odds ratio; QQMNC, quality and quantity media-cultured mononuclear cell.
Boldface entries indicate statistical significance.
Factors with a P value of <.10 from univariate analysis were included in this multivariate analysis.
Discussion
Therapeutic angiogenesis was reported to be an alternative treatment for patients with CLTI, especially in patients with NOCLI1; however, a meta-analysis study of intramuscular BMMNC or PBMNC injection did not show a clear clinical benefit of these treatments in patients with CLTI.7,8 The aforementioned finding may be due to the fact that patients with CLTI normally have multiple comorbidities, including problems associating with aging, diabetes mellitus, hypertension, dyslipidemia, and/or chronic kidney disease, which individually and collectively impair the number and function of MNCs.5,9,14,16 In this study, we investigated the efficacy and safety of intramuscular transplantation of QQMNCs, which is a novel cell-based therapy for angiogenesis, in an ischemic hindlimb mouse model to evaluate the therapeutic potential of QQMNCs in patients with CLTI.17 The results of this study demonstrated that QQMNC transplantation improves limb clinical outcomes and enhances tissue perfusion in the ischemic hindlimb of mice when compared with the outcomes of PBMNC transplantation. No complication other than the anticipated potential adverse outcomes (ie, cyanosis or gangrene) was found and there was no mortality during the 14-day observation period in either group. There was also no evidence of any abnormal tissue growth in either group. These findings suggest QQMNC injection to be safe for therapeutic angiogenesis in patients with CLTI; however, a clinical trial is needed to evaluate the benefit of autologous QQMNC transplantation in patients with CLTI.
All of the patients included in this study had CLTI, and 90% of them presented with ischemic gangrene or nonhealing ulcer, which are the most severe manifestations of CLTI.1 Multiple factors could have influenced the disappointing clinical outcomes of the prior generation of therapeutic neovascularization in patients with CLTI, including BMMNC and PBMNC injection.7,8 In contrast, there is some compelling evidence that supports the benefit of QQMNC injection compared with PBMNC injection. First, the QQMNCs in our study contained a higher number of proangiogenic progenitor cells (CD34+CD133+) compared with the number found in PBMNCs. This factor could have influenced better outcomes in the ischemic hindlimb mouse model transplanted with QQMNCs in the present study. Second, we found that QQ culture media not only promotes MNC expansion and EPC differentiation, but also enhances anti-inflammatory cell phenotypes.17 QQMNCs had a higher number of anti-inflammatory M2 macrophages (CD206+) and a lower number of inactivated regulatory T cells (CD4+CD25+CD127+ cells).20 A previous study reported that a higher percentage of inactivated regulatory T cells may suggest or indicate a more pronounced proinflammatory state.20 Consequently, the lower percentage of inactivated T cells in QQMNCs may suggest a lower inflammatory profile compared with PBMNCs. Common comorbidities of patients with CLTI, including aging and diabetes, cause proinflammatory status,11,12,31 and increased inflammation can decrease the regenerative capability of the vascular system.17,19,31 Interestingly, in vivo studies that harvested MNCs from diabetes patients and then transplanted the QQMNCs into diabetes-induced mice resulted in better outcomes compared with non-QQMNC transplantation.17,32 Therefore, the increased number of progenitor cells and anti-inflammatory phenotype of QQMNCs may have influenced the favorable outcomes of the in vivo experiments in our study.
The two different levels of artery ligation (high ligation and low ligation) designed into this study were aimed to compare with different levels of severity of ischemia found in real-world clinical practice. The high arterial ligation model was designed to represent a higher level of arterial occlusion disease.25, 26, 27 Importantly, our multivariate analysis identified the high ligation model as an independent risk factor for increased risk of limb cyanosis and/or gangrene in our animal model. The outcomes in our study, including the rate of normal limb appearance and limb perfusion measured by laser Doppler perfusion imager, were better in the QQMNC group than in the PBMNC group. A similar result was found in the study by Masuda et al17 showed significantly lower rates of autoamputation and higher blood flow measured by laser Doppler perfusion imager in the QQMNC transplanted group compared with the PMNMC transplanted group. Tanaka et al32 reported enhanced efficacy of QQMNCs vs non-QQ in the treatment of diabetic wounds. Moreover, the magnitude of outcome improvement was more pronounced in the high ligation model in our study. This finding may suggest the enhanced therapeutic benefit of QQMNCs compared with PBMNCs, even in more severe disease.
We found transplantation of QQMNCs from patients with CLTI to be safe in animal model. There were no animal deaths in our study during the 14-day observation period. The potential early adverse effects of therapeutic angiogenesis, infection, and hemorrhage also did not occur. Other in vivo studies also demonstrated the safety of QQMNC transplantation. Masuda et al17 studied QQMNCs from healthy volunteers transplanted into ischemic hindlimb animal model. No adverse event or animal death during the observation period was reported in their study.17 The aforementioned study by Tanaka et al32 evaluated the effect of QQMNCs from diabetic patients in a wound healing model of diabetes-induced mice and they also reported no animal death or adverse event after QQMNC transplantation.
Limitations
This study has some mentionable limitations. First, we used mice aged 8 to 10 weeks, which may not reflect the pathophysiology of a normally older patient with CLTI; however, mice of this age were previously reported to be appropriate for the study of neovascularization.25,28 Both high and low ligation models were used in several studies of limb ischemia.25, 26, 27 However, other comorbidities, such as diabetes, were not accounted for in our model. The use of an atherosclerotic and/or diabetic mouse model should be further evaluated.33 Second, our study compared safety and efficacy between two different types of MNCs (QQMNCs vs PBMNCs). There was no ischemic model without MNCs transplantation as a negative control group because the evidence suggests that results of PBMNCs transplantation were comparable with culture media injection in ischemic limb model.17 Third, a histologic analysis was not performed in our study, so we could not determine what components of neovascularization (ie, angiogenesis and/or arteriogenesis), or what proportions of those components were responsible for causing a higher rate of perfusion in the QQMNC group. However, the study by Masuda et al17 showed improved angiogenesis and arteriogenesis in QQMNC transplanted models. Their group also reported anti-inflammatory and antifibrotic effects of QQMNCs. They also reported the transplanted cell biodistribution, which revealed that transplanted QQMNCs exhibited a higher degree of differentiation into endothelial cells compared with PBMNCs, which contributed to the formation of vascular structures.17 This topic should be investigated further in different ischemic models to improve our understanding of therapeutic neovascularization and inflammation in ischemic limb. Fourth, the 14-day observation period in this study might be too short to demonstrate tumor formation and growth.34 Other studies using QQMNCs did not report any teratogenic adverse events during a 14-day period of observation.17,18 There is currently no standard tumorigenicity test for QQMNCs35; however, clinicians and researchers should remain vigilant in their observation for abnormal tissue growth. Furthermore, we did not compare PBMNCs at the time of isolation with PBMNCs after one week of culture. This should be further studied to investigate any additional proangiogenic, proarteriogenic, and anti-inflammatory properties of PBMNCs.
Author Contributions
Conception and design: WC, NC, NS, KV, CW, CR
Analysis and interpretation: WC, NC, NS
Data collection: WC, NC, NS, SJ
Writing the article: WC, NS
Critical revision of the article: WC, NC, NS, KV, SJ, CW, CR
Final approval of the article: WC, NC, NS, KV, SJ, CW, CR
Statistical analysis: WC
Obtained funding: NS
Overall responsibility: NS
Disclosures
None.
Acknowledgments
The authors gratefully acknowledge the patients that generously agreed to participate in this study, and Mr Suthipol Udompunturak for his kind and competent assistance with statistical analyses.
Footnotes
This study was funded by a grant from the Health Systems Research Institute (HSRI), Nonthaburi, Thailand (grant no. 62-031). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Conte M.S., Bradbury A.W., Kolh P., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Abdul Wahid S.F., Ismail N.A., Wan Jamaludin W.F., et al. Autologous cells derived from different sources and administered using different regimens for 'no-option' critical lower limb ischaemia patients. Cochrane Database Syst Rev. 2018;8 doi: 10.1002/14651858.CD010747.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutirangura P., Ruangsetakit C., Wongwanit C., Sermsathanasawadi N., Chinsakchai K. Pedal bypass with deep venous arterialization: the therapeutic option in critical limb ischemia and unreconstructable distal arteries. Vascular. 2011;19:313–319. doi: 10.1258/vasc.2010.oa0278. [DOI] [PubMed] [Google Scholar]
- 5.Lawall H., Bramlage P., Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–453. doi: 10.1016/j.jvs.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 6.Tateishi-Yuyama E., Matsubara H., Murohara T., et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 7.Rigato M., Monami M., Fadini G.P. Autologous cell therapy for peripheral arterial disease: systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 2017;120:1326–1340. doi: 10.1161/CIRCRESAHA.116.309045. [DOI] [PubMed] [Google Scholar]
- 8.Sermsathanasawadi N., Pruekprasert K., Chruewkamlow N., et al. Peripheral blood mononuclear cell transplantation to treat no-option critical limb ischaemia: effectiveness and safety. J Wound Care. 2021;30:562–567. doi: 10.12968/jowc.2021.30.7.562. [DOI] [PubMed] [Google Scholar]
- 9.Raval Z., Losordo D.W. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res. 2013;112:1288–1302. doi: 10.1161/CIRCRESAHA.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burt R.K., Testori A., Oyama Y., et al. Autologous peripheral blood CD133+ cell implantation for limb salvage in patients with critical limb ischemia. Bone Marrow Transplant. 2010;45:111–116. doi: 10.1038/bmt.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rea I.M., Gibson D.S., McGilligan V., McNerlan S.E., Alexander H.D., Ross O.A. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fessler J., Ficjan A., Duftner C., Dejaco C. The impact of aging on regulatory T-cells. Front Immunol. 2013;4:231. doi: 10.3389/fimmu.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasa M., Fichtlscherer S., Aicher A., et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 14.Ishida A., Ohya Y., Sakuda H., et al. Autologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemia. Circ J. 2005;69:1260–1265. doi: 10.1253/circj.69.1260. [DOI] [PubMed] [Google Scholar]
- 15.Huang P., Li S., Han M., Xiao Z., Yang R., Han Z.C. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–2160. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 16.Frangogiannis N.G. Cell therapy for peripheral artery disease. Curr Opin Pharmacol. 2018;39:27–34. doi: 10.1016/j.coph.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda H., Tanaka R., Fujimura S., et al. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka R., Masuda H., Fujimura S., et al. Quality-quantity control culture enhances vasculogenesis and wound healing efficacy of human diabetic peripheral blood CD34+ cells. Stem Cells Transl Med. 2018;7:428–438. doi: 10.1002/sctm.17-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Royen N., Schirmer S.H., Atasever B., et al. START Trial: a pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation. 2005;112:1040–1046. doi: 10.1161/CIRCULATIONAHA.104.529552. [DOI] [PubMed] [Google Scholar]
- 20.Chruewkamlow N., Pruekprasert K., Phutthakunphithak P., et al. Novel culture media enhances mononuclear cells from patients with chronic limb-threatening ischemia to increase vasculogenesis and anti-inflammatory effect. Stem Cell Res Ther. 2021;12:520. doi: 10.1186/s13287-021-02592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honge B.L., Petersen M.S., Olesen R., Moller B.K., Erikstrup C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 24.Greco A., Ragucci M., Liuzzi R., et al. Repeatability, reproducibility and standardisation of a laser Doppler imaging technique for the evaluation of normal mouse hindlimb perfusion. Sensors. 2012;13:500–515. doi: 10.3390/s130100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellingman A.A., Bastiaansen A.J., de Vries M.R., et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. 2010;40:796–803. doi: 10.1016/j.ejvs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Limbourg A., Korff T., Napp L.C., Schaper W., Drexler H., Limbourg F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 27.Scholz D., Ziegelhoeffer T., Helisch A., et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 28.Aref Z., de Vries M.R., Quax P.H.A. Variations in surgical procedures for inducing hind limb ischemia in mice and the impact of these Variations on neovascularization assessment. Int J Mol Sci. 2019;20:3704. doi: 10.3390/ijms20153704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enderlein G. Daniel, Wayne W.: Biostatistics — A Foundations for Analysis in the Health Sciences. Wiley & Sons, New York—Chichester—Brisbane—Toronto—Singapore, 6th ed. 1995, 780 S., £58.—, ISBN 0–471–58852-0 (cloth) Biom J. 1995;37:744. [Google Scholar]
- 30.Friede T., Kieser M. Sample size recalculation in internal pilot study designs: a review. Biom J. 2006;48:537–555. doi: 10.1002/bimj.200510238. [DOI] [PubMed] [Google Scholar]
- 31.Asahara T., Murohara T., Sullivan A., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka R., Ito-Hirano R., Fujimura S., et al. Ex vivo conditioning of peripheral blood mononuclear cells of diabetic patients promotes vasculogenic wound healing. Stem Cells Transl Med. 2021;10:895–909. doi: 10.1002/sctm.20-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilyas I., Little P.J., Liu Z., et al. Mouse models of atherosclerosis in translational research. Trends Pharmacol Sci. 2022;43:920–939. doi: 10.1016/j.tips.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Ylä-Herttuala S., Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- 35.Kawamata S., Kanemura H., Sakai N., Takahashi M., Go M.J. Design of a tumorigenicity test for induced pluripotent stem cell (iPSC)-derived cell products. J Clin Med. 2015;4:159–171. doi: 10.3390/jcm4010159. [DOI] [PMC free article] [PubMed] [Google Scholar]