Abstract
The microbial synthesis of paclitaxel is attractive for its short-cycle, cost-effectiveness, and sustainability. However, low paclitaxel productivity, depleted capacity during subculture and storage, and unclear biosynthesis mechanisms restrain industrial microbial synthesis. Along with the isolation of various paclitaxel-producing microorganisms and the development of versatile molecular tools, tremendous promises for microbial paclitaxel synthesis have become increasingly prominent. In this review, we summarize the progress of microbial synthesis of paclitaxel in recent years, focusing on paclitaxel-producing endophytes and representative engineering microorganism hosts that were used as chassis for paclitaxel precursor synthesis. Numerous wide-type microbes can manufacture paclitaxel, and fermentation process optimization and strain improvement can greatly enhance the productivity. Engineered microbes can efficiently synthesize precursors of paclitaxel by introducing exogenous synthetic pathway. Mining paclitaxel synthetic pathways and genetic manipulation of endophytes will accelerate the construction of microbial cell factories, indefinitely contributing to paclitaxel mass production by microbes. This review emphasizes the potential and provides solutions for efficient microbial paclitaxel mass production.
Keywords: Paclitaxel, Microbial fermentation, Endophytes, Process optimization, Synthetic pathway
Graphical abstract
Highlights
-
•
Paclitaxel-producing microbes have the potential for paclitaxel mass production.
-
•
Process optimization will promote microbial productivity and stability.
-
•
Rewiring synthetic pathway is feasible for paclitaxel heterogeneous production.
-
•
Engineered endophytic microorganisms are promising paclitaxel microbial cell factories.
1. Introduction
As an effective anticancer drug originally extracted from Taxus plants, paclitaxel still cannot meet clinical demand due to the low yield and high cost in production. Compared with chemical synthesis and suspension culture of Taxus cells, microbial paclitaxel production has advantages of short production cycle, higher cost-benefit, and easier manipulation [1]. Generally, improving microbial paclitaxel synthesis needs selecting highly productive and stable paclitaxel-producing microorganisms, constructing optimal microbial chassis, and optimizing fermentation process [2,3].
In recent years, different genera of paclitaxel-producing microorganisms have been reported, which are potential candidate hosts for paclitaxel synthesis [4,5]. However, microbial paclitaxel productivity is generally very low. Screening novel microbes originating from different sources has expanded the diversity of paclitaxel-producing microorganisms, among which some possess yield of paclitaxel up to 1 mg/L [6,7]. Commercial scale-up of microbial paclitaxel requires further evaluation of the productivity stability of these high-yielding microorganisms. Unfortunately, attenuation of the metabolic capacity during subculture and storage has been found in many kinds of paclitaxel-producing microbes, and the machinery of this degeneration is still unclear, resulting a great distance to industrialization [8,9]. Thus, selecting high-yield strains, rewiring the synthetic pathway, and establishing a systematic and high-efficient optimization strategy are necessary to improve microbial paclitaxel synthesis [10,11]. However, integral paclitaxel metabolic pathway is still unknown in endophytes, and only partially elucidated in Taxus plants, limiting the metabolic optimization of paclitaxel synthetic pathway in paclitaxel-producing microbes [12]. De novo synthesis of paclitaxel using microbial chassis is a fascinating approach, despite the entire synthetic pathway refactoring cannot be achieved yet [13]. Model microbial chassis such as Escherichia coli, Saccharomyces cerevisiae, and Bacillus subtilis often have distinct advantages [13,14]. Microbial expression systems for the biosynthesis of prophase intermediates involving in paclitaxel synthesis have been constructed [15]. These heterologous production systems have potential for microbial paclitaxel supplement due to high efficiency, easy-handle and low costs [16]. However, the problem of poor functionality of CYPs genes and low titer of target products has not been fundamentally fixed [14].
In this review, we discuss recent advances in paclitaxel synthesis by paclitaxel-producing endophytes and the precursors of paclitaxel by recombinant microorganisms. On one side, with the isolation and identification of paclitaxel-producing endophytes from various origins, combined with fermentation process optimization and strain improvement, paclitaxel productivity has been considerably strengthened. On the other side, we address how to explore promising industrial microorganisms and endophytes as cell factories to enhance paclitaxel and its precursors production. Depending on tremendous advancements in aforementioned topics, microbial synthesis shows great potential for efficient and sustainable mass production of paclitaxel.
2. Screening wide-type paclitaxel-producing microorganisms
The discovery of the paclitaxel-producing endophytic Taxomyces andreanae in Taxus brevifolia opened the door to explore plant endophytic for paclitaxel synthesis [4]. After that, tremendous studies reported the isolation of paclitaxel-producing microorganisms from versatile niches. Table 1 listed some representative paclitaxel-producing microorganisms, with three highlighted isolates producing more than 1 mg/L of paclitaxel, making them valuable resources for potential paclitaxel mass production.
Table 1.
some representative microorganisms producing paclitaxel.
| Genera | Endophyte | Host | Paclitaxel Yield(μg/L) |
Methods of Optimization | Final yield (μg/L) | Reference |
|---|---|---|---|---|---|---|
| Alternaria | Alternaria alternata MF5 | Taxus | 5700 | - | - | [19] |
| Alternaria brassicicola MVR1 | Terminalia arjuna | 140.8 | [24] | |||
| Alternaria tenuissima TER995 | Taxus arjuna | 37.92 | Bioprocess optimization | 124.32 | [31] | |
| Annulohypoxylon | Annulohypoxylon sp. | Taxus wallichiana Zucc. | 282.05 | – | – | [32] |
| Aspergillus flavipes ATCC 24,487 | Rhizosphere | 185 | Addition of fluconazole (1.0 μg/ml) or P. gracilior leaf (0.5 g) | 320/210 | [33] | |
| Aspergillus | Aspergillus flavus MW485934.1 | jojoba | 88.6 | Bioprocess optimization and γ-irradiation | 375.9 | [34] |
|
Aspergillus fumigatus TXD105 |
Tsxus distichum | 84.41 | Bioprocess optimization | 307.03 | [31] | |
| Aspergillus fumigatus | Taxus sp. | 1590 | - | - | [6] | |
| Aspergillus oryzae | Tarenna asiatica | 95.04 | – | – | [35] | |
| Aspergillus terreus EFB108 | Podocarpus gracilior | 114.2 | Physicochemical optimization and addition of surface-sterilized P. gracilior leaves | 432 | [23] | |
| Cladosporium | Cladosporium cladosporioides MD2 | Taxus media | 800 | – | – | [36] |
| Cladosporium sphaerospermum AUMC 6896 | Clover leaf weevil | 3.732 | Adding ammonium acetate (20 mg/L) | 30.365 | [37] | |
| Epicoccum | E. nigrum TXB502 | Taxus baccata | 61.35 | Bioprocess optimization, γ-irradiation and immobilization | 1364.63 | [38] |
| Fusarium redolens | Taxus brevifolia | 70 | Bioprocess optimization | 198 | [39] | |
| Fusarium. | Fusarium solani Tax-3 | Taxus chinensis | 163.35 | – | – | [18] |
| Grammothele | Grammothele lineata SDL-CO-2015-1 | Corchorus olitorius | 382.2 | – | – | [22] |
| Lasiodiplodia | Lasiodiplodia theobromae SKJM1101 | Piper nigrum | 247 | – | – | [40] |
| Metarhizium | Metarhizium anisopliae H-27 | Taxus chinensis | 846.1 | – | – | [17] |
| Metarizium | Metarizium anisopliae AUMC 5130 | clover leaf weevil | 0.0023 | Adding both ammonium acetate (20 mg/L) and Salicylic acid (90 mg/L) | 116.373 | [37] |
| Nodulisporium | Nodulisporium sylviform HQD33 | Taxus cuspidate | 51.06–125.70 | Strain improving | 516.37 | [41] |
| Penicillium | Penicillium chrysogenum R16 | Glycin max | 170 | Bioprocess optimization | 250 | [26] |
| Penicillium polonicum AUMC14487 | Ginko biloba | 90.53 | Bioprocess optimization and γ-irradiation | 401.2 | [25] | |
| Pestalotiopsis | Pestalotiopsis hainanensis | Ailuropoda melanoleuca | 1466.87 | - | - | [7] |
| Pestalotiopsis | Pestalotiopsis microspora | Taxodium mucronatum | 283.11 | Addition of Salicylic acid(300 μM) | 625.47 | [42] |
| Phoma | Phoma medicaginis | Taxus wallichiana var. mairei | 1215 | – | – | [43] |
2.1. Paclitaxel-producing microorganisms from Taxus
Several endophytic microbes of Taxus have been shown to serve as potential sources of bioactive paclitaxel. Metarhizium anisopliae H-27 and Fusarium solani Tax-3, both originated from Taxus chinensis tissue, produced paclitaxel up to 846.1 μg/L and 163.35 μg/L, respectively [17,18]. Furthermore, another example that Alternaria alternata MF5, derived from the bark of female Taxus yew, can yield paclitaxel up to 5.7 mg/L after 60 h of fermentation, which is the fastest endophyte in terms of paclitaxel accumulation rate [19]. The high yield of up to 1.60 mg/L paclitaxel from Aspergillus fumigatus KU-837249 was reported, albeit inconsistent data [6]. However, no further reports validate its reproducibility and scalability during repeated subculturing. The underlying reason may be its poor purity or inability to stably maintain desired yield [20,21]. Besides, there is not yet distinct molecular mechanisms of paclitaxel biosynthesis in this strain. These limitations are still a nonnegligible hurdle for achieving its industrial availability.
2.2. Paclitaxel-producing microorganisms from other sources
Many paclitaxel-producing microbes isolated from Taxus tissues are also presented in other niches, demonstrating that paclitaxel-producing microorganisms may have a wider distribution [22]. Indeed, paclitaxel-producing endophytes isolated from non-Taxus medicinal plants are becoming increasingly common [23,24]. The discovery of paclitaxel-producing endophytes from other plant species have further expanded the biological diversity. Endophytes Grammothele lineata SDL-CO-2015-1 isolated from the herb Jut is the first paclitaxel producer that belongs to the Basidiomycota phylum, revealing that this phylum may harbor other potential candidates for paclitaxel production [22]. Paclitaxel-producing endophyte Penicillium polonicum from non-Taxus sources shows antimicrobial and antiproliferative properties similar to authentic paclitaxel and retains 80 % paclitaxel yield after 5 generations of subculturing [25]. Moreover, some paclitaxel-producing microorganisms from non-plants sources also represent a high potential for these strains to be promising alternatives. For example, the maximum amounts of paclitaxel-producing Penicillium chrysogenum from the rhizosphere region of Glycine max could reach 250 μg/L [26]. In addition, a soil-dwelling saprophyte was identified as Aspergillus flavipes, and no significant paclitaxel productivity decline was found after 10th subculturing [27]. Interestingly, Pestalotiopsis hainanensis isolated from animal provides higher paclitaxel yield than endophytic Pestalotiopsis fungi from other resources [7]. Exploring various paclitaxel-producing endophytic, parasitic and saprophytic microbes may overcome the limitations of traditional host organisms and provide a robust production platform.
The attenuation of paclitaxel-producing potency among endophytes is a common physiological phenomenon. It is reported that the initial paclitaxel yield of C. cladosporioides MD2 isolated from Taxus was 800 μg/L, contrasting to only 5–7 μg/L after 5 years of storage and subculturing [28]. The paclitaxel yield (350 ng/μL) of Periconia sp. isolated from Torreya grandifolia plant decreases to 118 ng/μL after three rounds of subculturing [21]. This decline in productivity implies that endophytes may have unique paclitaxel synthetic machinery that profoundly differs from Taxus [29]. Mining paclitaxel synthesis pathway, maintaining stable producing potency and obtaining higher productivity in endophytic microbe remains prerequisites for the large-scale production of paclitaxel [30]. Searching for potential microbes with stable molecular machinery system is a promising avenue for microbial paclitaxel production. Further research indefinitely needed to do is evaluation of their production stability, elucidate paclitaxel biosynthesis machinery and seek effective strategies to restore the productivity and develop high-effective microbial-based bioprocess for the scale-up of microbial paclitaxel.
3. Strategies to improve paclitaxel production in wide-type endophytes
3.1. Optimizing nutrients supply and fermentation parameters
Optimizing the nutrient composition of the culture medium could improve the growth of endophytes and the biosynthesis of paclitaxel (Fig. 1a). For example, the highest paclitaxel accumulation in A. fumigatus was reached in M1D media, while Alternaria tenuissima gave the highest paclitaxel yield in FBM media [31]. The type and ratio of different nutrients in medium also affect paclitaxel yield. The stimulatory effect of certain carbon and nitrogen sources on the synthesis of paclitaxel was widely recognized in endophytes, e.g., sucrose and ammonium nitrate are the optimal carbon and nitrogen sources for paclitaxel production by A. fumigatus TSD105 and Fusarium redolen [39,44]. Replacing sucrose in M1D medium with xylose as the carbon source resulted in induction of paclitaxel biosynthesis by endophyte Aspergillus terreus [44]. Moreover, adding solutes such as potassium chloride also showed stimulatory effect on paclitaxel accumulation by Paraconiothyrium variabile and Epicoccum nigrum [45].
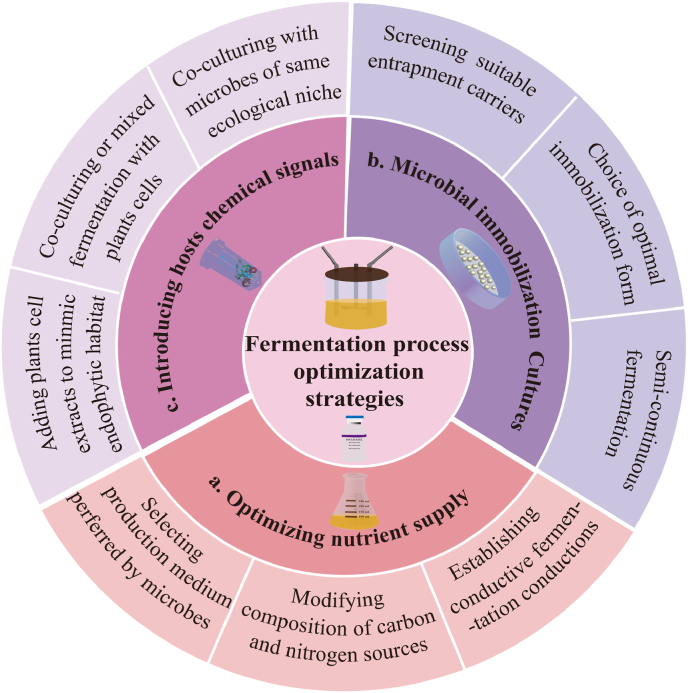
Fig. 1.
Strategies for fermentation optimization during paclitaxel production by endophytes. a. Optimizing nutrient supply to coordinate microbial growth and secondary metabolism. b. Applying microbial immobilization cultures to improve the overall efficiency of the production process. c. Introducing chemical signals from hosts to trigger the expression of genes in paclitaxel biosynthetic pathway.
Same to other secondary metabolites, the accumulation of paclitaxel is influenced by fermentation models and parameters. Cost-effective fermentation process is indispensable for its scaling up. It was found that solid-state fermentation systems gave higher concentrations of paclitaxel than liquid fermentation systems for endophytic Nigrospora sp [46]. Optimizing aeration and/or agitation rate to coordinate microbial growth and paclitaxel synthesis is fundamental in liquid fermentation [26]. The optimal agitation rate required to maximize paclitaxel production varied among microorganisms, with A. fumigatus and A. tenuissima producing the maximum at 120 rpm, whereas 150 rpm was the favorable speed for A. fumigatus TMS-26 [31]. Moreover, applying semi-continuous processing could greatly enhance fermentation productivity. El-Sayed et al. reported considerable yield enhancement after six cycles of semi-continuous fermentation of A. fumigatus TXD105-GM6 and A. tenuissima TER995-GM3 [47]. In all, as indicated in Table 1, the yield of paclitaxel by endophytes can be significantly increased by optimizing nutrients supply and fermentation conditions.
3.2. Immobilizing microbial cells
Microbial immobilization allows physical separation of cell and metabolite, also enables high-density enrichment and reuse of cells. Therefore, it is particularly useful for mass production of growth-inhibiting compounds like paclitaxel. In fact, immobilization technology has been reported to increase paclitaxel output by Taxus cells [48]. Entrapment carriers such as calcium alginate, gelatin, and others have been investigated to immobilize endophytes, achieving high-density enrichment of microbes and increase of paclitaxel output (Fig. 1b) [38,48]. Calcium alginate was considered the most suitable entrapment carrier for immobilizing Aspergillus fumigatus and Alternaria tenuissima mycelia and spores of mutant strains, as remarkable improvement of paclitaxel yields was achieved over free-cell cultures [48].
3.3. Introducing chemical signals from host plants
Unfortunately, recent findings suggested that the expression of paclitaxel biosynthetic gene cluster in endophytes may be dependent on chemical signals from the host plant and that artificial media cannot provide this specific microenvironment [49]. To overcome this limitation, one approach is adding plant extracts to mimic the habitat of endophytes and provide the necessary stimulus to microbes (Fig. 1c). For instance, adding gibberellic acid and cell extracts of Corylus avellana enhances paclitaxel yield by 11.5-fold in E. nigrum strain YEF2 [49]. Likewise, significant increase could be induced by supplementing plant wood or bark extracts for endophytic fungus Paraconiothyrium SSM001 [50]. Another approach is co-culture or mixed fermentation with plant cells. A 136.6-fold increase in paclitaxel output was achieved when co-culturing E. nigrum mycelium with host plants than endophytes fermented alone [51].
Additionally, competition for living space and trophic interactions between different endophytes of the same ecological niche trigger paclitaxel biosynthesis [52]. It was reported that co-culturing Taxus plants with only one resident fungus led to a 2.7-fold increase in paclitaxel output, while a 7.8-fold increase achieved by co-culture with two resident fungi [50]. The endophytic B. subtilis is a potent bacterial elicitor, and its intimate interaction with A. flavipes strongly triggered paclitaxel biosynthesis through chromatin remodeling [11]. In fact, mimic the close association among microorganisms by coculturing has been widely applied into natural products research. Coculture endophytic fungal Fusarium tricinctum originated from Eichhornia crassipes with Bacillus subtilis or Streptomyces lividans contributes to the biosynthesis of some novel valuable secondary metabolites, which has not been detected in monoculture [53]. This endophyte-plant or endophyte-endophyte interaction phenomenon is a kind of interspecies communication established for survive and function in their distinct ecological niches during evolution, though no clear understanding of this communication machinery [21,54]. One possible explanation is they serve as signals or transcriptional factors to activate the expression of orphan-gene involved in paclitaxel biosynthesis [55].
3.4. Regulating and maintaining metabolic balance
Regulation of metabolic balance by exogenous growth factors and chemical substances is often important for efficient metabolite accumulation. Indeed, the shortage of precursors is one of the barriers to the massive production of paclitaxel in microorganisms (Fig. 2a). For instance, feeding common precursor isopentenyl pyrophosphate (IPP) and geranylgeranyl diphosphate (GGPP) to endophyte Paraconiothyrium SSM001 could improve paclitaxel yield by 3-fold and 5-fold, respectively [56]. Sodium acetate, an effective precursor in the acetylation reaction of paclitaxel core skeleton, induces the output of paclitaxel in endophytic fungal F. redolens and Aspergillus aculeatinus Tax-6 [39,57]. Like in Taxus cells, phenylalanine may has a positive effect for paclitaxel biosynthesis in endophytes [58].
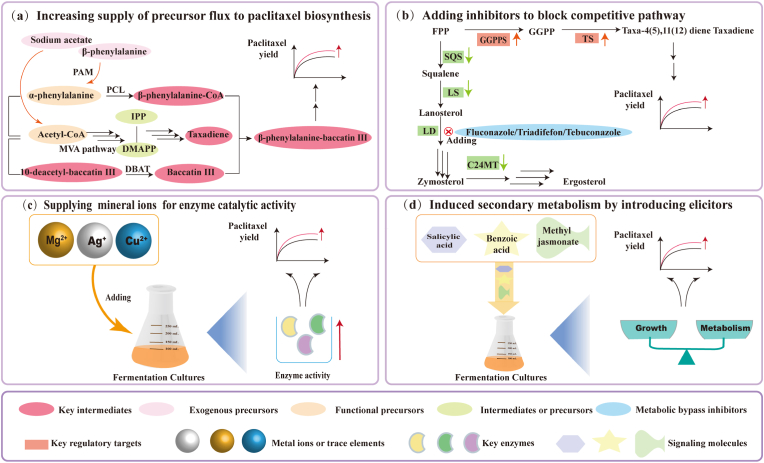
Fig. 2.
Solutions for metabolic regulation during paclitaxel synthesis by endophytes. (a) Increasing supply of precursor to facilitate the move of metabolic fluxes to paclitaxel. PAM, phenylalanine aminomutase; PCL, phenylalanine-CoA ligase; DBAT, 10-deacetybaccatin Ⅲ-10-O-acetytransferase; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate. (b) Adding metabolic bypass inhibitors to block competitive branches. TS, taxadiene synthase; GGPPS, geranylgeranyl diphosphate synthase; SQS, squalene synthase; LS, lanosterol synthase; LD, lanosterol demethylase; C24MT, C-24 methyltransferase. (c) Supplying mineral ions or trace elements to improve enzyme catalytic activity. (d) Introducing elicitors to regulate growth and metabolism balance.
However, high concentrations of exogenous precursors may cause toxicity and some intermediates tend to flow to other metabolic bypasses. Therefore, it is necessary to block the shift of precursors to competing branches and facilitate the conversion of metabolic fluxes to paclitaxel. Adding metabolic inhibitors can be effective in blocking bypass pathways (Fig. 2b). Sterols synthesis inhibitors have negative effect on squalene synthase, thereby promoting accumulation of GGPP towards taxadiene synthesis [2]. For example, adding fluconazole increases the taxadiene synthase (TDS) activity of A. flavipes and A. terreus, and the yield of paclitaxel was increased by 1.8-fold and 1.2-fold, respectively [59]. Moreover, fluconazole induces the expression of transcription factor pbcR, leading to increased paclitaxel production in A. flavipes [33]. However, microbes have unique metabolic backgrounds and growth characteristics, none of desired positive effects was obtained upon fluconazole stimulation in A. terreus EFB108 [23].
It was also demonstrated that suitable concentrations of mineral ions and trace elements is essential for microbial growth and paclitaxel synthesis (Fig. 2c). For example, Ag + can increase paclitaxel output by endophytes A. terreus, and Cu2+ induces the expression and catalytic activity of key enzymes in the paclitaxel synthesis pathway [23,29]. Typically, the yield of paclitaxel by A. aculeatinus Tax-6 has 4-fold increase upon optimization of concentrations of CuSO4 and other inducers [29]. Moreover, low concentrations of Mg2+ could help to stabilize the structure of TS and facilitate the binding of GGPP substrate. Reports on paclitaxel biosynthesis have shown that supplementation with suitable concentration of Mg2+ can increase the titer of paclitaxel by 2.1-fold [2].
Plant defense hormones are believed to trigger biosynthesis of paclitaxel by Taxus plant cells [23]. The yield of paclitaxel by endophytes has also an enhancement upon addition of phytohormones (Fig. 2d). Salicylic acid is responsible for activating GGPP synthase (GGPPS) by increasing reactive oxygen species and fatty acid peroxidation of unsaturated fatty acids, which serves as an induction signal to paclitaxel synthesis [42]. Similarly, adding salicylic acid to cultures of Cladosporium sphaerospermum AUMC 6896 and Metarizium anisopliae AUMC 5130 significantly increased paclitaxel output [37]. Moreover, benzoic acid may have a beneficial role in enhancing paclitaxel production [50]; however, recent radiolabeling studies have negated its direct contribution [23]. In addition, no significant increase in paclitaxel production was observed in several endophytes upon treatment with Methyl jasmonate, another phytohormone that often favors paclitaxel biosynthesis [29].
4. Rewiring industrial microbial hosts for paclitaxel and its precursor production
4.1. Increasing the titer of taxadiene
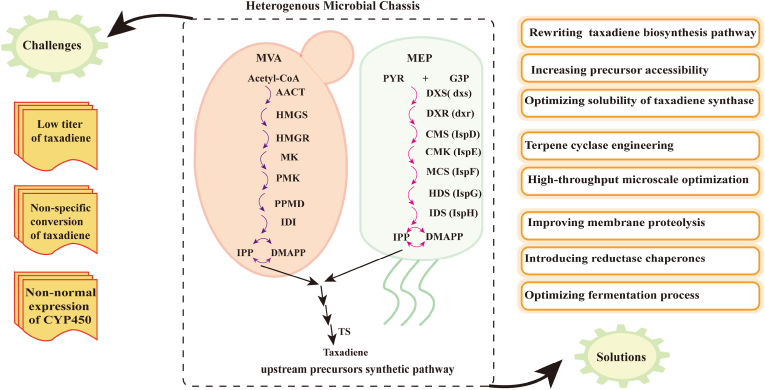
As mature microbial chassis, the fast-growing microbes such as E. coli, S. cerevisiae and B. subtilis can be engineered for potential paclitaxel production by introducing exogenous paclitaxel biosynthetic pathway [9,10]. Although the basic framework of paclitaxel synthesis pathway in plants has been determined, there are still a few genes haven't yet been characterized in plants [60]. These missing enzymes hindered heterogenous expression of complete paclitaxel biosynthetic pathway genes in engineering microbes [61]. At present, low level production of taxadiene, the precursor of paclitaxel was achieved by expressing IPP isomerase, GGPPS and TS [9,62]. Low taxadiene titer might be due to poor accessibility of precursors, high cytotoxicity of intermediates, low activity of key enzymes and presence of competing branches [62]. Current strategies to improve taxadiene titer include three main aspects: rewriting the taxadiene biosynthesis pathway, increasing precursor accessibility and optimizing the solubility of TS (Fig. 3).
Fig. 3.
Challenges and solutions for metabolic engineering for taxadiene synthesis by heterogeneous host. The upstream paclitaxel biosynthetic pathway in heterogeneous microbial chassis is shown in dashed black box, which has distinction (the MVA and MEP pathways) in different expression systems. The main challenges (the left) and corresponding solutions (the right) have been listed. AACT, acetoacetyl-CoA thiolase; HMGS, 3-hydroxy-3-methylglutaryl-CoA synthase; HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; MK, mevalonate kinase; PMK, phosphomevalonate kinase; PPMD, mevalonate pyrophosphate decarboxylase; IDI, isopentenyl diphosphate isomerase; DXS, 1-deoxy-d-xylose-5-phosphate racemase; DXR, 1-deoxy-d-xylose-5-phosphate reductoisomerase; CMS, 4-pyrophosphocytidyl-2-C-methyl-D-erythritol synthase; CMK, 4-pyrophosphocytidyl-2-C-methyl-D-erythritol kinase; MCS, 2C-methyl-D-erythritol 2,4-cyclopyriophosphate synthase; HDS, 1-hydroxy-2-methyl-2-(E)-butenyl 4-pyrophosphate synthase; IDS, 1-hydroxy-2-methyl-2-butenyl 4-pyrophosphate reductase.
Rewriting taxadiene synthetic pathway can remove the bottleneck and avoid growth inhibition caused by excessive accumulation of intermediates. Currently, modifying taxadiene biosynthetic pathway via optimizing the expression intensity and copy number of rate-limiting enzymes is a major approach [3]. By applying a systematic multivariate strategy to achieve the ideal expression equilibrium between the intrinsic upstream MEP pathway and the downstream IPP-to-taxadiene synthetic pathway in E. coli, the titer of taxadiene reached 1 g/L [62]. Also, using bidirectional promoters (BDPs) and optimizing expression time interval help a balanced state among different enzymes [15]. For example, the fine-tuning expression of GGPPS and TXS led to a 60-fold increase of taxadiene titer in yeast Komagataella phaffii after introduction of BDPs [63]. Similarly, the final titer of oxygenated taxanes reached 27 mg/L in engineering E. coli cell after metabolic balance [64]. In addition, the supplement of sufficient precursors is important for the scale-up of taxadiene biosynthesis in microbial hosts. The titer of taxadiene by B. subtilis could reach 17.8 mg/L by overexpressing TXS and optimizing the flux of GGPP [65]. Generally, low expression and poor solubility of TS exacerbated the difficulty in engineering taxadiene intermediate within microbial hosts. Researchers have demonstrated that the construction of stable and bioactive fusion protein of TS and GGPPS decreased the physical distance between the substrate GGPP and TS, leading to an elevated titer of taxadiene to 93.5 mg/L [57]. The length of TS truncation, type of promoter and copy number of chromosomal genes also impact microbial paclitaxel biosynthesis [15]. Taxadiene had exceeded 120 mg/L from 20 mg/L via increasing gene copy number and fusing suitable solubility tags in shake flask and bioreactors in S. cerevisiae [66].
4.2. Boosting specific conversion of taxadiene
It was reported that only 10 % of taxadiene was converted to T5α-ol due to the broad product profile of CYP725A4, which is a key obstacle in engineering paclitaxel biosynthesis in heterologous hosts [67,68]. Recent studies demonstrated that the nonspecific product is mainly due to the non-selectivity of the epoxide intermediate rather than to T5αH itself [67]. Previous approaches demonstrate that co-expressing CYP725A with CRP from different sources as a fusion protein resulted in decreased levels of all products [69]. Biggs et al. also found that chimera linkages may damage the functional expression of CYP450 in bacterial systems [70]. Although the yield of byproducts was decreased upon co-expression with Taxus-originated cytochrome B5, an apparent absence of T5α-ol was observed [69]. Moreover, improving the product specificity of the early synthetic pathway may favor alleviating this bottleneck. For example, the conversion of T5α-ol was improved by three approaches, terpene cyclase engineering, P450 engineering, and hydrolase screening [68]. TS engineering was proven to be feasible for improving CYP725A4 selectivity, then the yield of an alternative product taxa-4(20)-11(12)-dien was increased 2.4-fold (Fig. 3) [68]. Besides, the final titer of T5α-ol isomer and T5α-yl-acetate reached 19.2 mg/L and 3.7 mg/L by applying an interdisciplinary approach to optimize the oxidation and acetylation reactions of taxane in S. cerevisiae [71].
4.3. Improving the fitness of CYP450 family protein
CYP450 family enzymes involve in multi-step hydroxylation reactions of paclitaxel synthesis pathway, which are big roadblocks for de novo synthesis of paclitaxel in heterologous microbial cells [15]. The folding and post-translational modifications of these enzymes require multiple chaperones and a functional endosomal system, which are often absent or not fully functional in E. coli [62]. Similarly, in S. cerevisiae, the expression of CYP450 genes can be limited by insufficient supply of heme cofactors [62]. To overcome these limitations, the main solutions to strengthen CYP450 fitness are improving membrane proteolysis properties and introducing reductase chaperones (Fig. 3).
Although a 15000-fold improvement in taxadiene titer can be achieved by applying a multivariate modular metabolism engineering in E. coli, the titer of T5α-ol fell significantly upon the introduction of CYP725A4 [62]. Hence, modifying the hydrophobic N-terminus of membrane proteins and co-expressing of chaperonin are crucial for addressing the poor suitability of CYP450 in E. coli systems [72]. For example, five-fold enhancement of oxygenated diterpene can be achieved by optimizing the expression of CYP450 and CRP (reductase chaperones), and altering reductase partner interaction and N-terminal modifications of P450 and CRP in E. coli [70]. Compared with E. coli, yeast is a more amenable host for CYP450 expression due to its natural endomembrane system [64]. On one hand, function of CYP450 requires the incorporation of CRP for electron transfer [73]. For example, the optimal fusion of CYP725A4 and POR (cognate reductase) is beneficial for the formation of oxygenated taxanes in S. cerevisiae, due to improved activity of CYP725A4 [74]. On the other hand, enhancing the activity of rate-limiting enzymes with stronger promoters is a practicable method. GAL promoter is suitable for the construction of yeast cell factory. The GAL1 promoter drives combined expression of Taxus reductase and THY5a, leading to a 10-fold increase in taxadien-5a-ol in yeast [75]. In addition, riboregulated switchable feedback promoter (rSPF) regulated the expression of CYP725A4 and resulted in a 2.4-fold improvement of oxygenated taxanes in E. coli [76]. A modular co-culture system was also established between E. coli and S. cerevisiae, this mutualistic system combined the merits of different organisms, enabling more efficient and cost-effective production of oxygenated taxanes [77].
5. Engineering paclitaxel-producing endophytes for enhanced biosynthesis
5.1. Genetic manipulation of paclitaxel-producing endophytes
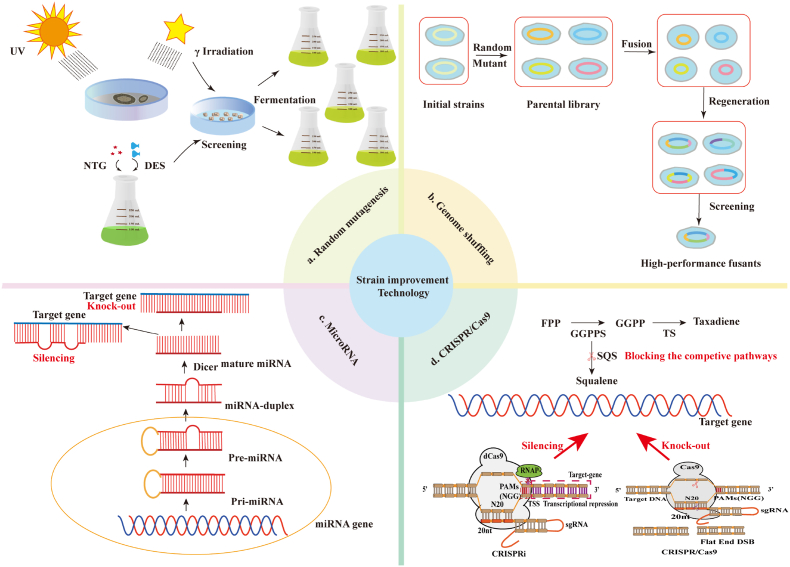
Genetic manipulation of endophytes can be either irrational or rational targeted. Various high-yield mutants have been obtained through random mutagenesis using nitrosoguanidine (NTG), ultraviolet light (UV), diethyl sulfate (DES) and γ irradiation (Fig. 4) [78]. A 10-fold improvement of paclitaxel yield is found in Fusarium maire K178 upon UV irradiation and DES treatment [79]. Also, γ-irradiation results in a 2.42-fold enhancement of paclitaxel production in A. fumigatus TXD105 [80]. However, optimizing mutation conditions to coordinate growth and metabolism is also indispensable. For example, the best mutant dose of γ radiation is 0.75 KGy for A. fumigatus TXD105, but the highest yield of paclitaxel by A. flavus is obtained under 1.0 kGy [34,44]. Furthermore, genome shuffling allows multiple rounds of recursive fusion of protoplasts for rapid recombination in whole genome from multi-parents, making it a practical and effective strain improvement technique for obtaining high-performance fusants [81]. Using genome shuffling technology, Zhao et al. obtained three mutant strains with stable paclitaxel production and showed higher yield than the starting strain [41]. However, success of these irrational designs is largely depended on efficient high-throughput screening.
Fig. 4.
Genetic manipulation of paclitaxel-producing endophytes for strain improvement. a. Obtaining fine mutants by introducing chemical and physical mutagen. b. Genome shuffling through protoplast fusion, which introduces multiple mutations in the whole genome from multi-parents. c. Biogenesis of miRNA and gene silencing. d. Gene editing with the CRISPR/Cas9 technology and its application in paclitaxel biosynthesis regulation. The N20 sequence of the sgRNA recognizes the target gene, dCas9-mediated gene expression silencing has no DNA double-strand breaks, and Cas9 protein usually introduces flat-end DNA DSB (double-strand breaks) at the target gene site. NTG, nitrosoguanidine; UV, ultraviolet light; DES, diethyl sulfate; PAMs, protospacer adjacent motif; RNAP, RNA polymerase; FPP, farnesyl pyrophosphate; GGPP, Geranylgeranyl pyrophosphate; GGPPS, geranylgeranyl diphosphate synthase; TS, taxadiene synthase; SQS, squalene synthase.
Further advancements in paclitaxel production in these microorganisms will be more likely obtained by applying synthetic biology technologies such as CRISPR/Cas9-mediated genome editing and RNA interference (RNAi). For example, RNAi and CRISPR/Cas9 technologies can be used to knockdown or knockout the rate-limiting enzyme lanosterol/squalene synthase in the sterol synthesis pathway, facilitate the metabolic flux to paclitaxel [10,82]. However, gene editing is relatively difficult in many paclitaxel-producing endophytes, especially filamentous fungi [82]. Recently, protoplast transformation mediated by electroshock or PEG/CaCl2 has successfully transferred exogenous plasmids containing resistance markers to the paclitaxel-producing endophyte EFY-21 [83]. Agrobacterium-mediated transformation (ATMT) is an effective method for gene transformation in certain endophytes. Bian et al. established an ATMT method for the genus Alternaria and validated the strength of heterologous promoters in A. alternata TPF6 [84]. Successful transformation of paclitaxel-producing fungi has enabled the introduction and optimization of heterologous taxadiene synthesis pathways, which are essential for improved paclitaxel biosynthesis. Moreover, developing high effective genetic manipulation system can indeed be essential. Recently, a feasible CRISPR/Cas9-mediated gene manipulation method is developed, which allows site-specific gene insertion, dual-locus mutations, and long DNA fragment deletions in endophytic fungus Pestalotoiopsis ficiI [85]. Genes involved in paclitaxel biosynthesis are regulated by different promoters and regulatory elements. Therefore, the transcription level of key genes could be optimized by modifying promoter strength, codon usage, and gene copy number. For instance, ectopic expression of GGPPS from Taxus has been proven feasible to enhance the accumulation of paclitaxel in endophytes [56]. In addition, little evidence for paclitaxel biosynthesis pathway was found in microbes, which hindered the directed evolution of paclitaxel pathway genes [5].
5.2. Deep mining of paclitaxel synthesis pathway in endophytes
Genome sequencing technologies and bioinformatics tools provide powerful support for identifying and characterizing paclitaxel biosynthetic mechanisms in endophytes. The key enzymes such as TS and 10-deacetylbacaccatin Ⅲ −10-O-acetyltransferase (DBAT) and C-13 phenylpropanoid side chain-CoA acyltransferase (BAPT) in Taxus have been identified, which provide a reference for mining microbial paclitaxel synthesis pathway and identification of involved candidate genes in endophytes [61]. On one side, the coding sequence can be used as reference to clone and characterize related enzymes in endophytes. For example, dbat gene and WRKY1 transcription factor in endophytes were amplified using primers based on corresponding genes in T. cuspidate [40]. Seven candidate genes in endophytic fungus Penicillium aurantiogriseum NRRL 62431 have also been identified, and the independent evolution of enzymes involved in paclitaxel biosynthesis is elucidated [86]. On the other side, comparative analysis of known paclitaxel biosynthesis genes in Taxus has provided insights into paclitaxel synthetic mechanism and evolution in microbes [12]. Partial potential synthesis pathway from GGPP to 10-DBAT was found in C. cladosporioides MD2, highlighting the diversity of biosynthetic routes in nature [28]. Moreover, comparative transcriptomes analysis of high-yielding mutant strains and starting strains found the expression change of T10βH [87].
However, the industrial utilization of microbial paclitaxel is still hindered by poor productivity, the undesired loss of production. The fundamental bottleneck is the lack of comprehensive understanding of the molecular machinery of paclitaxel biosynthesis, metabolic attenuation and transcript regulation network. One reason is that microenvironmental change may lead to low-level or even silence expression of critical functional genes [87]. Another is that different microbes may have evolved independently paclitaxel biosynthesis pathways, resulting in unannotation or detection of candidate genes due to low sequence identities [28,87]. Moreover, the substantial decrease in productivity during storage or subculture is accompanied by a decrease in the expression of key genes, which limits the in-depth exploration of the paclitaxel synthesis pathway in endophytes [8,28]. The complex attenuation machinery remains an unsolved mystery. A possible speculation is that the isolation of endophytes from hosts lead to disruption of native environmental interaction, resulting in the absence of chemical and biological stimulation, which are often difficult to mimic under in vitro conditions [54,88]. Furthermore, multiple subculturing may alter microbial nutritional requirements, resulted in the metabolic reprogramming and regulatory collapse of secondary and metabolite production in fungal cells [30,88]. Moreover, for the lipophilic nature of paclitaxel and its derivatives, their secretion may lead to cytotoxicity thereby inhibit microbial growth [74]. Overall, paclitaxel biosynthesis machinery in endophytes is still an unfilled gap, which is a prior condition for industrial scale-up of microbial paclitaxel.
6. Concluding remarks
Microbial synthesis provides a promising method for paclitaxel mass production. Isolation and identification of paclitaxel-producing endophytes bring the dawn of microbial synthesis of paclitaxel, and modification of paclitaxel synthesis pathway in microbes promotes the efficiency of microbial cell factories. Optimization of intermediate taxadiene, T5α-ol biosynthesis in heterologous hosts lays the foundation for the reconstitution and heterologous synthesis of the complete paclitaxel synthetic pathway. Future work can be performed from the following aspects: (1) developing high-throughput screening methods for the isolation of more novel, stable, and high-yield paclitaxel-producing microbes; (2) solving the problems of low yield and unstable productivity in microbes with effective strain improvement methods; (3) elucidating the molecular mechanism of paclitaxel synthesis by endophytes based on genomic, transcriptomic, and metabolomic studies; (4) reconstruction of high yield paclitaxel chassis with model microbial hosts or engineering paclitaxel-producing endophytes, using different synthetic biology techniques. Specially, system designing and synthetic engineering paclitaxel biosynthesis pathway in microbes can serve as a promising avenue for the high-level production of paclitaxel or its intermediates, including both industrial strains and paclitaxel-producing wide-type isolates [15]. These include 1) development of more efficient hosts chassis and genetic tools to accelerate construction; 2) characterization of key enzymes and editing of promoters as well as regulators to improve efficiency; and 3) optimization of fermentation bioprocess to ensure productivity.
Authors’ contributions
LC and SZ contributed to the study conception and design. LY, QY, and JM performed literature search and data analysis. LY and QY wrote the first draft of the manuscript. QY and LC critically revised the work. All authors commented on previous versions of the manuscript and approved the final manuscript.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgment
This work was partially supported by a cooperative grant from Henan University of Technology (No. 51100014) and a grant from the Agency of Science and Technology of Henan Province (No. 232102311153, No. 221100110700).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Yanli Qi, Email: yliqi2021@haut.edu.cn.
Chengwei Li, Email: lcw@haut.edu.cn.
References
- 1.Liu Y., Zhao F., Wang Q., Zhao Q., Hou G., Meng Q. Current perspectives on paclitaxel: focus on its production, delivery and combination therapy. Mini-Rev Med Chem. 2023 doi: 10.2174/1389557523666230210145150. [DOI] [PubMed] [Google Scholar]
- 2.Shankar Naik B. Developments in taxol production through endophytic fungal biotechnology: a review, Orient. Pharm. Exp. Med. 2018;19(1):1–13. doi: 10.1007/s13596-018-0352-8. [DOI] [Google Scholar]
- 3.Nazhand A., Durazzo A., Lucarini M., Mobilia M.A., Omri B., Santini A. Rewiring cellular metabolism for heterologous biosynthesis of Taxol. Nat Prod Res. 2020;34(1):110–121. doi: 10.1080/14786419.2019.1630122. [DOI] [PubMed] [Google Scholar]
- 4.Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260(5105):214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayed A.S.A., El-Sayed M.T., Rady A., Zein N., Enan G., Shindia A., et al. Exploiting the biosynthetic potency of taxol from fungal endophytes of conifers plants; genome mining and metabolic manipulation. Molecules. 2020;25(13) doi: 10.3390/molecules25133000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar P., Singh B., Thakur V., Thakur A., Thakur N., Pandey D., et al. Hyper-production of taxol from Aspergillus fumigatus, an endophytic fungus isolated from Taxus sp. of the Northern Himalayan region. Biotechnol. Rep. 2019;24 doi: 10.1016/j.btre.2019.e00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Y., Wang Y., Ma X., Wang C., Yue G., Zhang Y., et al. Greater taxol yield of fungus Pestalotiopsis hainanensis from dermatitic scurf of the giant panda (Ailuropoda melanoleuca) Appl Biochem Biotechnol. 2015;175(1):155–165. doi: 10.1007/s12010-014-1254-y. [DOI] [PubMed] [Google Scholar]
- 8.El-Sayed A.S.A., Mohamed N.Z., Safan S., Yassin M.A., Shaban L., Shindia A.A., et al. Restoring the Taxol biosynthetic machinery of Aspergillus terreus by Podocarpus gracilior Pilger microbiome, with retrieving the ribosome biogenesis proteins of WD40 superfamily. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-47816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W.C., Gong T., Zhu P. Advances in exploring alternative Taxol sources. RSC Adv. 2016;6(54):48800–48809. doi: 10.1039/c6ra06640b. [DOI] [Google Scholar]
- 10.Sabzehzari M., Zeinali M., Naghavi M.R. Alternative sources and metabolic engineering of Taxol: advances and future perspectives. Biotechnol Adv. 2020;43 doi: 10.1016/j.biotechadv.2020.107569. [DOI] [PubMed] [Google Scholar]
- 11.El-Sayed A.S.A., Shindia A.A., AbouZeid A., Koura A., Hassanein S.E., Ahmed R.M. Triggering the biosynthetic machinery of Taxol by Aspergillus flavipes via cocultivation with Bacillus subtilis: proteomic analyses emphasize the chromatin remodeling upon fungal-bacterial interaction. Environ Sci Pollut Res. 2021;28(29):39866–39881. doi: 10.1007/s11356-021-13533-1. [DOI] [PubMed] [Google Scholar]
- 12.Xiong X., Gou J., Liao Q., Li Y., Zhou Q., Bi G., et al. The Taxus genome provides insights into paclitaxel biosynthesis. Nat Plants. 2021;7(8):1026–1036. doi: 10.1038/s41477-021-00963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A., Bhatia S.K., Banyal A., Chanana I., Kumar A., Chand D., et al. An overview on Taxol production technology and its applications as anticancer agent. Biotechnol Bioproc Eng. 2022;27(5):706–728. doi: 10.1007/s12257-022-0063-3. [DOI] [Google Scholar]
- 14.Li J., Mutanda I., Wang K., Yang L., Wang J., Wang Y. Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana. Nat Commun. 2019;10(1):4850. doi: 10.1038/s41467-019-12879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutanda I., Li J., Xu F., Wang Y. Recent advances in metabolic engineering, protein engineering, and transcriptome-guided insights toward synthetic production of Taxol. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.632269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M., Xie W., Luo Z., Li C.-X., Hua Q., Xu J.-H. Improving solubility and copy number of taxadiene synthase to enhance the titer of taxadiene in Yarrowia lipolytica. Synth Syst Biotechnol. 2023;8(2):331–338. doi: 10.1016/j.synbio.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K., Ding X., Deng B., Chen W. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J Ind Microbiol Biotechnol. 2009;36(9):1171–1177. doi: 10.1007/s10295-009-0598-8. [DOI] [PubMed] [Google Scholar]
- 18.Deng B.W., Liu K.H., Chen W.Q., Ding X.W., Xie X.C. Fusarium solani, Tax-3, a new endophytic taxol-producing fungus from Taxus chinensis. World J Microbiol Biotechnol. 2008;25(1):139–143. doi: 10.1007/s11274-008-9876-2. [DOI] [Google Scholar]
- 19.Yang N., Pan X., Chen G., Sarsaiya S., Yu J., Fan X., et al. Fermentation engineering for enhanced paclitaxel production by Taxus media endophytic fungus MF-5 (Alternaria sp) J Biobased Mater Bioenergy. 2018;12(6):545–550. doi: 10.1166/jbmb.2018.1806. [DOI] [Google Scholar]
- 20.Christensen S.B. Drugs that changed society: microtubule-targeting agents belonging to taxanoids, macrolides and non-ribosomal peptides. Molecules. 2022;27(17) doi: 10.3390/molecules27175648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra S., Sahu P.K., Agarwal V., Singh N. Exploiting endophytic microbes as micro-factories for plant secondary metabolite production. Appl Microbiol Biotechnol. 2021;105(18):6579–6596. doi: 10.1007/s00253-021-11527-0. [DOI] [PubMed] [Google Scholar]
- 22.Das A., Rahman M.I., Ferdous A.S., Amin A., Rahman M.M., Nahar N., et al. An endophytic Basidiomycete, Grammothele lineata, isolated from Corchorus olitorius, produces paclitaxel that shows cytotoxicity. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sayed A.S.A., Safan S., Mohamed N.Z., Shaban L., Ali G.S., Sitohy M.Z. Induction of Taxol biosynthesis by Aspergillus terreus, endophyte of Podocarpus gracilior Pilger, upon intimate interaction with the plant endogenous microbes. Process Biochem. 2018;71:31–40. doi: 10.1016/j.procbio.2018.04.020. [DOI] [Google Scholar]
- 24.Gill H., Vasundhara M. Isolation of taxol producing endophytic fungus Alternaria brassicicola from non-Taxus medicinal plant Terminalia arjuna. World J Microbiol Biotechnol. 2019;35(5):74. doi: 10.1007/s11274-019-2651-8. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Fatah S.S., El-Batal A.I., El-Sherbiny G.M., Khalaf M.A., El-Sayed A.S. Production, bioprocess optimization and gamma-irradiation of Penicillium polonicum, as a new Taxol producing endophyte from Ginko biloba. Biotechnol. Rep. 2021;30 doi: 10.1016/j.btre.2021.e00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Sayed A., Enan G., Al-Mohammadi A.R., A H.M., El-Gazzar N. Detection, purification and elucidation of chemical structure and antiproliferative activity of taxol produced by Penicillium chrysogenum. Molecules. 2020;25(20) doi: 10.3390/molecules25204822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi J., Yuan J., Jiao P., Yang Y., Zhu X. A new taxol-producing fungus (Pestalotiopsis malicola) and evidence for taxol as a transient product in the culture. Afr J Biotechnol. 2013;10(34):6647–6654. doi: 10.1186/1471-2164-12-359. [DOI] [Google Scholar]
- 28.Miao L.Y., Mo X.C., Xi X.Y., Zhou L., De G., Ke Y.S., et al. Transcriptome analysis of a taxol-producing endophytic fungus Cladosporium cladosporioides MD2. Amb Express. 2018;8(1):41. doi: 10.1186/s13568-018-0567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao W., Ling F., Yu L., Huang Y., Wang T. Enhancing taxol production in a novel endophytic fungus, Aspergillus aculeatinus Tax-6, isolated from Taxus chinensis var. mairei. Fungal Biol. 2017;121(12):1037–1044. doi: 10.1016/j.funbio.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Kusari S., Singh S., Jayabaskaran C. Rethinking production of Taxol(R) (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014;32(6):304–311. doi: 10.1016/j.tibtech.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Ismaiel A.A., Ahmed A.S., Hassan I.A., El-Sayed E.R., Karam El-Din A.A. Production of paclitaxel with anticancer activity by two local fungal endophytes, Aspergillus fumigatus and Alternaria tenuissima. Appl Microbiol Biotechnol. 2017;101(14):5831–5846. doi: 10.1007/s00253-017-8354-x. [DOI] [PubMed] [Google Scholar]
- 32.Gauchan D.P., Velez H., Acharya A., Ostman J.R., Lunden K., Elfstrand M., et al. Annulohypoxylon sp. strain MUS1, an endophytic fungus isolated from Taxus wallichiana Zucc., produces taxol and other bioactive metabolites. 3 Biotech. 2021;11(3):152. doi: 10.1007/s13205-021-02693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Sayed A.S.A., Ali D.M.I., Yassin M.A., Zayed R.A., Ali G.S. Sterol inhibitor “Fluconazole” enhance the Taxol yield and molecular expression of its encoding genes cluster from Aspergillus flavipes. Process Biochem. 2019;76:55–67. doi: 10.1016/j.procbio.2018.10.008. [DOI] [Google Scholar]
- 34.Abdel-Fatah S.S., El-Sherbiny G.M., Khalaf M., Baz A.F.E., El-Sayed A.S.A., El-Batal A.I. Boosting the anticancer activity of Aspergillus flavus "endophyte of jojoba" taxol via conjugation with gold nanoparticles mediated by gamma-irradiation. Appl Biochem Biotechnol. 2022;194(8):3558–3581. doi: 10.1007/s12010-022-03906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suresh G., Kokila D., Suresh T.C., Kumaran S., Velmurugan P., Vedhanayakisri K.A., et al. Mycosynthesis of anticancer drug taxol by Aspergillus oryzae, an endophyte of Tarenna asiatica, characterization, and its activity against a human lung cancer cell line. Biocatal Agric Biotechnol. 2020;24 doi: 10.1016/j.bcab.2020.101525. [DOI] [Google Scholar]
- 36.Zhang P., Zhou P.P., Yu L.J. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr Microbiol. 2009;59(3):227–232. doi: 10.1007/s00284-008-9270-1. [DOI] [PubMed] [Google Scholar]
- 37.El-Maali N.A., Mohrram A.M., El-Kashef H., Gamal K. Novel resources of Taxol from endophytic and entomopathogenic fungi: isolation, characterization and LC-Triple mass spectrometric quantification. Talanta. 2018;190:466–474. doi: 10.1016/j.talanta.2018.07.089. [DOI] [PubMed] [Google Scholar]
- 38.El-Sayed E.R., Zaki A.G., Ahmed A.S., Ismaiel A.A. Production of the anticancer drug taxol by the endophytic fungus Epicoccum nigrum TXB502: enhanced production by gamma irradiation mutagenesis and immobilization technique. Appl Microbiol Biotechnol. 2020;104(16):6991–7003. doi: 10.1007/s00253-020-10712-x. [DOI] [PubMed] [Google Scholar]
- 39.Garyali S., Kumar A., Reddy M.S. Enhancement of taxol production from endophytic fungus Fusarium redolens. Biotechnol Bioproc Eng. 2014;19(5):908–915. doi: 10.1007/s12257-014-0160-z. [DOI] [Google Scholar]
- 40.Sah B., Subban K., Chelliah J. Cloning and sequence analysis of 10-deacetylbaccatin III-10-O-acetyl transferase gene and WRKY1 transcription factor from taxol-producing endophytic fungus Lasiodiplodia theobromea. FEMS Microbiol Lett. 2017;364(24) doi: 10.1093/femsle/fnx253. [DOI] [PubMed] [Google Scholar]
- 41.Zhao K., Ping W., Zhang L., Liu J., Lin Y., Jin T., et al. Screening and breeding of high taxol producing fungi by genome shuffling. Sci China Ser C Life Sci. 2008;51(3):222–231. doi: 10.1007/s11427-008-0037-5. [DOI] [PubMed] [Google Scholar]
- 42.Subban K., Subramani R., Srinivasan V.P.M., Johnpaul M., Chelliah J. Salicylic acid as an effective elicitor for improved taxol production in endophytic fungus Pestalotiopsis microspora. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaiyou J., Li M., Xiqiao H. An endophytic fungus efficiently producing paclitaxel isolated from Taxus wallichiana var. mairei. Medicine. 2017;96(27) doi: 10.1097/MD.0000000000007406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Sayed E.-S.R., Ismaiel A.A., Ahmed A.S., Hassan I.A., Karam El-Din A.-Z.A. Bioprocess optimization using response surface methodology for production of the anticancer drug paclitaxel by Aspergillus fumigatus and Alternaria tenuissima: enhanced production by ultraviolet and gamma irradiation. Biocatal Agric Biotechnol. 2019;18 doi: 10.1016/j.bcab.2019.01.034. [DOI] [Google Scholar]
- 45.Somjaipeng S., Medina A., Magan N. Environmental stress and elicitors enhance taxol production by endophytic strains of Paraconiothyrium variabile and Epicoccum nigrum. Enzym Microb Technol. 2016;90:69–75. doi: 10.1016/j.enzmictec.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Sanchez J., Flores-Bustamante Z.R., Dendooven L., Favela-Torres E., Soca-Chafre G., Galindez-Mayer J., et al. A comparative study of Taxol production in liquid and solid-state fermentation with Nigrospora sp. a fungus isolated from Taxus globosa. J Appl Microbiol. 2010;109(6):2144–2150. doi: 10.1111/j.1365-2672.2010.04846.x. [DOI] [PubMed] [Google Scholar]
- 47.El-Sayed E.R., Ahmed A.S., Hassan I.A., Ismaiel A.A., Karam El-Din A.A. Semi-continuous production of the anticancer drug taxol by Aspergillus fumigatus and Alternaria tenuissima immobilized in calcium alginate beads. Bioproc Biosyst Eng. 2020;43(6):997–1008. doi: 10.1007/s00449-020-02295-8. [DOI] [PubMed] [Google Scholar]
- 48.El-Sayed E.R., Ahmed A.S., Hassan I.A., Ismaiel A.A., Karam El-Din A.A. Strain improvement and immobilization technique for enhanced production of the anticancer drug paclitaxel by Aspergillus fumigatus and Alternaria tenuissima. Appl Microbiol Biotechnol. 2019;103(21–22):8923–8935. doi: 10.1007/s00253-019-10129-1. [DOI] [PubMed] [Google Scholar]
- 49.Salehi M., Moieni A., Safaie N. Elicitors derived from Hazel (Corylus avellana L.) cell suspension culture enhance growth and paclitaxel production of Epicoccum nigrum. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-29762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman S.S., Raizada M.N. Interactions between Co-Habitating fungi elicit synthesis of Taxol from an endophytic Fungus in host Taxus plants. Front Microbiol. 2013;4:3. doi: 10.3389/fmicb.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salehi M., Moieni A., Safaie N., Farhadi S. New synergistic co-culture of Corylus avellana cells and Epicoccum nigrum for paclitaxel production. J Ind Microbiol Biotechnol. 2019;46(5):613–623. doi: 10.1007/s10295-019-02148-8. [DOI] [PubMed] [Google Scholar]
- 52.Chagas F.O., Dias L.G., Pupo M.T. A mixed culture of endophytic fungi increases production of antifungal polyketides. J Chem Ecol. 2013;39(10):1335–1342. doi: 10.1007/s10886-013-0351-7. [DOI] [PubMed] [Google Scholar]
- 53.Ebrahim W., El-Neketi M., Lewald L.-I., Orfali R.S., Lin W., Rehberg N., et al. Metabolites from the fungal endophyte Aspergillus austroafricanus in axenic culture and in fungal–bacterial mixed cultures. J Nat Prod. 2016;79(4):914–922. doi: 10.1021/acs.jnatprod.5b00975. [DOI] [PubMed] [Google Scholar]
- 54.Wang W.-X., Kusari S., Sezgin S., Lamshöft M., Kusari P., Kayser O., et al. Hexacyclopeptides secreted by an endophytic fungus Fusarium solani N06 act as crosstalk molecules in Narcissus tazetta. Appl Microbiol Biotechnol. 2015;99(18):7651–7662. doi: 10.1007/s00253-015-6653-7. [DOI] [PubMed] [Google Scholar]
- 55.El-Sayed A.S.A., George N.M., Abou-Elnour A., El-Mekkawy R.M., El-Demerdash M.M. Production and bioprocessing of camptothecin from Aspergillus terreus, an endophyte of Cestrum parqui, restoring their biosynthetic potency by Citrus limonum peel extracts. Microb Cell Factories. 2023;22(1) doi: 10.1186/s12934-022-02012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soliman S.S.M., Mosa K.A., El-Keblawy A.A., Husseiny M.I. Exogenous and endogenous increase in fungal GGPP increased fungal Taxol production. Appl Microbiol Biotechnol. 2017;101(20):7523–7533. doi: 10.1007/s00253-017-8509-9. [DOI] [PubMed] [Google Scholar]
- 57.Wang J.-Y., Huang Z.-Y., Wu Q.-Y., Pan J., Li C.-X., Xu J.-H. Facile biosynthesis of taxadiene by a newly constructed Escherichia coli strain fusing enzymes taxadiene synthase and geranylgeranyl pyrophosphate synthase. Process Biochem. 2022;122:129–136. doi: 10.1016/j.procbio.2022.09.005. [DOI] [Google Scholar]
- 58.Wang Z.-J., Zhang W., Zhang J.-W., Guo M.-J., Zhuang Y.-p. Optimization of a broth conductivity controlling strategy directed by an online viable biomass sensor for enhancing Taxus cell growth rate and Taxol productivity. RSC Adv. 2016;6(47):40631–40640. doi: 10.1039/c5ra26540a. [DOI] [Google Scholar]
- 59.El-Sayed A.S.A., Fathalla M., Yassin M.A., Zein N., Morsy S., Sitohy M., et al. Conjugation of Aspergillus flavipes Taxol with porphyrin increases the anticancer activity of taxol and ameliorates its cytotoxic effects. Molecules. 2020;25(2) doi: 10.3390/molecules25020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuang X., Sun S., Wei J., Li Y., Sun C. Iso-Seq analysis of the Taxus cuspidata transcriptome reveals the complexity of Taxol biosynthesis. BMC Plant Biol. 2019;19(1):210. doi: 10.1186/s12870-019-1809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kasaei A., Mobini-Dehkordi M., Mahjoubi F., Saffar B. Isolation of taxol-producing endophytic fungi from Iranian Yew through novel molecular approach and their effects on human breast cancer cell line. Curr Microbiol. 2017;74(6):702–709. doi: 10.1007/s00284-017-1231-0. [DOI] [PubMed] [Google Scholar]
- 62.Ajikumar P.K., Xiao W.H., Tyo K.E., Wang Y., Simeon F., Leonard E., et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. 6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogl T., Kickenweiz T., Pitzer J., Sturmberger L., Weninger A., Biggs B.W., et al. Engineered bidirectional promoters enable rapid multi-gene co-expression optimization. Nat Commun. 2018;9(1):3589. doi: 10.1038/s41467-018-05915-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Q.-Y., Huang Z.-Y., Wang J.-Y., Yu H.-L., Xu J.-H. Construction of an Escherichia coli cell factory to synthesize taxadien-5α-ol, the key precursor of anti-cancer drug paclitaxel, Bioresour. Bioprocess. 2022;9(1) doi: 10.1186/s40643-022-00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdallah, Pramastya H., van Merkerk R., Sukrasno W.J. Quax. Metabolic engineering of Bacillus subtilis toward Taxadiene biosynthesis as the first committed step for Taxol production. Front Microbiol. 2019;10:218. doi: 10.3389/fmicb.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nowrouzi B., Li R.A., Walls L.E., d'Espaux L., Malci K., Liang L., et al. Enhanced production of taxadiene in Saccharomyces cerevisiae. Microb Cell Factories. 2020;19(1):200. doi: 10.1186/s12934-020-01458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edgar S., Zhou K., Qiao K., King J.R., Simpson J.H., Stephanopoulos G. Mechanistic insights into taxadiene epoxidation by taxadiene-5alpha-hydroxylase. ACS Chem Biol. 2016;11(2):460–469. doi: 10.1021/acschembio.5b00767. [DOI] [PubMed] [Google Scholar]
- 68.Edgar S., Li F.S., Qiao K., Weng J.K., Stephanopoulos G. Engineering of Taxadiene synthase for improved selectivity and yield of a key Taxol biosynthetic intermediate. ACS Synth Biol. 2017;6(2):201–205. doi: 10.1021/acssynbio.6b00206. [DOI] [PubMed] [Google Scholar]
- 69.Sagwan-Barkdoll L., Anterola A.M. Taxadiene-5alpha-ol is a minor product of CYP725A4 when expressed in Escherichia coli. Biotechnol Appl Biochem. 2018;65(3):294–305. doi: 10.1002/bab.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biggs B.W., Lim C.G., Sagliani K., Shankar S., Stephanopoulos G., De Mey M., et al. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2016;113(12):3209–3214. doi: 10.1073/pnas.1515826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walls L.E., Malci K., Nowrouzi B., Li R.A., d'Espaux L., Wong J., et al. Optimizing the biosynthesis of oxygenated and acetylated Taxol precursors in Saccharomyces cerevisiae using advanced bioprocessing strategies. Biotechnol Bioeng. 2021;118(1):279–293. doi: 10.1002/bit.27569. [DOI] [PubMed] [Google Scholar]
- 72.Rouck J.E., Biggs B.W., Kambalyal A., Arnold W.R., De Mey M., Ajikumar P.K., et al. Heterologous expression and characterization of plant Taxadiene-5alpha-Hydroxylase (CYP725A4) in Escherichia coli. Protein Expr Purif. 2017;132:60–67. doi: 10.1016/j.pep.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang L., Huang L., Cai J., Xu Z., Lian J. Functional expression of eukaryotic cytochrome P450s in yeast. Biotechnol Bioeng. 2021;118(3):1050–1065. doi: 10.1002/bit.27630. [DOI] [PubMed] [Google Scholar]
- 74.Nowrouzi B., Lungang L., Rios-Solis L. Exploring optimal Taxol(R) CYP725A4 activity in Saccharomyces cerevisiae. Microb Cell Factories. 2022;21(1):197. doi: 10.1186/s12934-022-01922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dejong J.M., Liu Y., Bollon A.P., Long R.M., Jennewein S., Williams D., et al. Genetic engineering of Taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol Bioeng. 2006;93(2):212–224. doi: 10.1002/bit.20694. [DOI] [PubMed] [Google Scholar]
- 76.Glasscock C.J., Biggs B.W., Lazar J.T., Arnold J.H., Burdette L.A., Valdes A., et al. Dynamic control of gene expression with riboregulated switchable feedback promoters. ACS Synth Biol. 2021;10(5):1199–1213. doi: 10.1021/acssynbio.1c00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou K., Qiao K., Edgar S., Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol. 2015;33(4):377–383. doi: 10.1038/nbt.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu F., Wang S., Li Y., Zheng M., Xi X., Cao H., et al. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol Lett. 2018;40(5):797–807. doi: 10.1007/s10529-018-2531-6. [DOI] [PubMed] [Google Scholar]
- 79.Xu F., Tao W., Cheng L., Guo L. Strain improvement and optimization of the media of taxol-producing fungus Fusarium maire. Biochem Eng J. 2006;31(1):67–73. [Google Scholar]
- 80.El-Sayed E.R., Ahmed A.S., Al-Hagar O.E.A. Agro-industrial wastes for production of paclitaxel by irradiated Aspergillus fumigatus under solid-state fermentation. J Appl Microbiol. 2020;128(5):1427–1439. doi: 10.1111/jam.14574. [DOI] [PubMed] [Google Scholar]
- 81.Magocha T.A., Zabed H., Yang M., Yun J., Zhang H., Qi X. Improvement of industrially important microbial strains by genome shuffling: current status and future prospects. Bioresour Technol. 2018;257:281–289. doi: 10.1016/j.biortech.2018.02.118. [DOI] [PubMed] [Google Scholar]
- 82.El-Sayed A.S.A., Abdel-Ghany S.E., Ali G.S. Genome editing approaches: manipulating of lovastatin and taxol synthesis of filamentous fungi by CRISPR/Cas9 system. Appl Microbiol Biotechnol. 2017;101(10):3953–3976. doi: 10.1007/s00253-017-8263-z. [DOI] [PubMed] [Google Scholar]
- 83.Wei Y.M., Zhou X.W., Liu L., Lu J., Wang Z.N., Yu G., et al. An efficient transformation system of taxol-producing endophytic fungus EFY-21 (Ozonium sp) Afr J Biotechnol. 2010;9(12):1726–1733. doi: 10.5897/AJB2010.000-3019. [DOI] [Google Scholar]
- 84.Bian G., Yuan Y., Tao H., Shi X., Zhong X., Han Y., et al. Production of taxadiene by engineering of mevalonate pathway in Escherichia coli and endophytic fungus Alternaria alternata TPF6. Biotechnol J. 2017;12(4) doi: 10.1002/biot.201600697. [DOI] [PubMed] [Google Scholar]
- 85.Xu X., Huang R., Yin W.B. An optimized and efficient CRISPR/Cas9 system for the endophytic fungus Pestalotiopsis fici. J Fungi. 2021;7(10) doi: 10.3390/jof7100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Y., Zhao H., Barrero R.A., Zhang B., Sun G., Wilson I.W., et al. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genom. 2014;15(1):69. doi: 10.1186/1471-2164-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiao W., Tang T., Ling F. Comparative transcriptome analysis of a taxol-producing endophytic fungus, Aspergillus aculeatinus Tax-6, and its mutant strain. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-67614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohamed N.Z., Shaban L., Safan S., El-Sayed A.S.A. Physiological and metabolic traits of Taxol biosynthesis of endophytic fungi inhabiting plants: plant-microbial crosstalk, and epigenetic regulators. Microbiol Res. 2023;272 doi: 10.1016/j.micres.2023.127385. [DOI] [PubMed] [Google Scholar]