Abstract
The genus Passiflora (Passifloraceae) comprises about 500 species. The Passiflora edulis stands out because of its economic and medicinal importance. It is widely planted in tropical and subtropical regions worldwide, especially in South America, the Caribbean, South Africa, and Asia. The aqueous extract of Passiflora edulis Sims f. edulis (Gulupa) leaves is used in traditional medicine for its soothing and tranquilizing effects on the central nervous system. Therefore, evaluating its safety for human use is a fundamental requirement to continue the development of new therapies within the framework of regulatory, preclinical, and clinical guidelines. Here, the sub-acute toxicity study was conducted following the Organization for Economic Cooperation and Development (OECD) guideline 407 for 28 days in Wistar albino rats. The study showed that 1000 mg/kg/day of the aqueous extract in 10 adult Wistar rats (five males and five females) was well tolerated. The hematological results are at normal levels. However, monocytopenia and eosinopenia were observed with a significant difference (P < 0,05) for both male and female rats treated with the aqueous extract of Passiflora edulis. The results show that liver and kidney function profiles were conserved. However, an increase in ALT is observed with significant differences between male and female rats treated with the extract compared to the controls. Study findings were limited to non-adverse histopathological results of a slightly increased incidence of focal periportal lymphocytic infiltrate in the liver and focal corticomedullary nephrocalcinosis in the kidney compared to control. Therefore, the aqueous extract of Passiflora edulis has a good safety profile in oral administration, was well tolerated, and did not cause any lethality or adverse effects in the sub-acute toxicity study in male and female rats. The NOAEL (no observed adverse effect level) for the 28-day subacute toxicity study was considered to be 1000 mg/kg.
Keywords: Passiflora edulis, Oral toxicity, Herbal drugs, Toxicity assessment
Graphical abstract
Highlights
-
•
Animals subjected to the sub-acute oral test at 1000 mg/kg/day exhibited no adverse effects.
-
•
The NOAEL for sub-acute oral 28-day toxicity was 1000 mg/kg/day in a rat model.
-
•
No deaths/changes were seen after 28-day 1000 mg/kg/day extract administration.
1. Introduction
Traditional and complementary medicine (TCM) is used by at least 80% of the Member States across all WHO (World Health Organization) regions, particularly Eastern Mediterranean, Southeast Asia, and Western Pacific regions reported using TCM [1], [2], [3]. Likewise, the growth in the number of Member States developing regulations and national policies for TCM has been reported [1]. It is also informed that this treatment has been gradually integrated into treating chronic diseases such as diabetes [4] and complex diseases like cancer [5], [6]. The increased use of plants in phytotherapy has meant that new and diverse therapeutic resources have become available, along with conserving the traditional knowledge of the countries where they are used [7].
In general, the population perceives treatments with medicinal plants as safe due to their origin [2], [8]; however, the use of these treatments can affect different organs [9] as well as cause interactions with conventional drugs are possible, leading to changes in the efficacy of treatments or toxic manifestations [10]. In this regard, around 80,000 exposures to plants, and homeopathic supplements were reported for 2020 in the United States [11], [12]. Most of these exposures are of minimal toxicity largely because they involve pediatric ingestions of low quantity [12].
Passiflora edulis Sims f. edulis, known as gulupa in Colombia or purple passion fruit worldwide, is a plant native to South America, specifically Brazil. However, since the 19th century, it has been widely distributed in Asia, the Caribbean, Africa, India, and Australia [13]. Among the different varieties of Passiflora edulis, the yellow passion fruit, Passiflora edulis f. flavicarpa, and the purple passion fruit, Passiflora edulis f. edulis, are the main varieties, and their secondary metabolites are of great interest in the pharmaceutical and food industry [14], [15].
The genus Passiflora spp. has been associated with anti-inflammatory and antioxidant activity due to the presence of flavonoids [16], [17], [18]. Passiflora edulis has been used as a sedative, diuretic, anthelmintic, antidiarrheal, stimulant, and in the treatment of hypertension, menopausal symptoms, infant colic, and for the treatment of symptoms of alcoholism, anxiety, migraine, nervousness, and insomnia [19], [20].
Previous reports on evaluating the oral toxicity of Passiflora edulis extracts did not show toxicity signs in hematological and biochemical parameters [21]. For instance, a sub-acute toxicity study of Passiflora nepalensis (Passifloraceae) in male and female rats at the doses of 40, 80, 160, and 320 mg/kg for fourteen consecutive days did not induce any short-term toxicity [22]. Another sub-acute study in Wistar albino rats was conducted by oral administration of 100, 200, 300, and 400 mg/kg of aqueous leaf extract Passiflora edulis. The subacute study indicated that the extract was safe for bone marrow function and was neither hepatotoxic nor nephrotoxic [23]. In an acute toxicity study of Passiflora alata in mice, deaths were not observed for a single dose up to 4800 mg/kg. However, mice acutely treated with extract 150, 300, and 600 mg/kg presented DNA damage determined by comet assay in peripheral blood cells 3 h after treatment. In another sub-acute study, rats treated with aqueous extract from Passiflora alata at 300 mg/kg dose for 14 days did not present biochemical, hematological, or histopathological significant alterations compared to the control group. However, these rats showed irritability and did not show weight gain [24]. An acute oral toxicity study was performed in mice to determine the safety profile following preclinical requirements to demonstrate the absence of effects or alterations induced by the aqueous extract obtained from Passiflora edulis f. edulis at 2000 mg/kg dose [25]. The results showed no abnormal change in mice behavioral patterns and hematologic parameters, including RBC, Hb, WBC, MCV, MCH, platelets, neutrophils, and lymphocytes. In these studies, regardless of the dosing frequency, it is necessary to determine the safety profile following preclinical requirements [26] to develop new therapeutic alternatives such as polymolecular drugs or isolated molecules.
2. Materials and methods
2.1. Animals and ethical approval
Wistar albino rats of 9 weeks of age were maintained in accordance with recommendations regarding housing, temperature, and humidity in the Guide for the Care and Use of Laboratory Animals at the Unit of Comparative Biology accredited facilities. All experimental procedures were approved by the Pontificia Universidad Javeriana Animal Care and Use Committee. The protocols carried out were endorsed by the Institutional Committee for the Care and Use of Laboratory Animals of the Pontificia Universidad Javeriana (CICUA), FUA No. 057–18. All rats were housed with free access to a standard pellet diet and water ad libitum. They were kept at a constant temperature of 22 ± 1 °C, the relative humidity of 55 ± 5 %, and 12/12 h light/dark cycle.
2.2. Preparation of aqueous extract
Leaves of Passiflora edulis Sims f edulis were collected from the departments of Nariño and Boyacá, Colombia. The taxonomic determination of the species was carried out by the biologist Nestor García in the herbarium of the Pontificia Universidad Javeriana. The leaves were dried in a circulating air oven at a controlled temperature of 35ºC and crushed with a blade mill. The dried and ground material was subjected to an extraction procedure by infusion with boiling water for 10 min, in a ratio (1:10 P/V) of solvent plant material, emulating the traditional use. Subsequently, this extract was subjected to lyophilization. Preparing the aqueous extract of Passiflora edulis under these conditions corresponds to what is done in traditional medicine as an infusion of the leaves. Ultra Performance Liquid Chromatography coupled with Photodiode Array Detection (UPLC-PDA) was in performed Chromatographic profiling. Three batches of the Passiflora edulis extract were prepared. The results of the UPLC-DAD analysis show the chromatographic profile's maintenance between the different batches of the extract. The above allowed us to infer that the chromatographic fingerprints are maintained regardless of the standardized extraction process in each batch. The above allows us to assume that the compounds present in the three batches are conserved in structure and retention times.
2.3. Toxicity assessment
The study was conducted following the recommendations of the Organization for Economic Cooperation and Development (OECD).
2.4. Sub-acute oral toxicity study
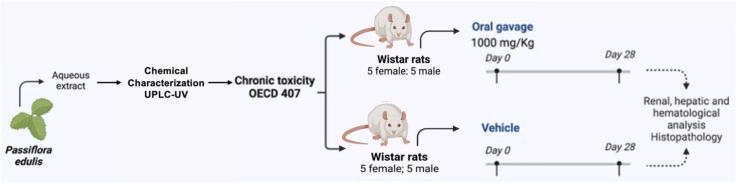
The sub-acute toxicity study was conducted following the Organization for Economic Cooperation and Development (OECD) guideline 407 for 28 days in Wistar albino rats [27]. Twenty Wistar rats of nine weeks old were divided into two groups of ten animals each (five males and five females). One group was treated with a dose of 1000 mg/kg/day with the aqueous extract for 28 days and the other corresponded to the control (vehicle group) received 0.5 ml of physiological saline. As per the OECD guideline, lower dose groups were not included as no effects were expected at 1000 mg/kg/day. The extract or vehicle was administered by oral gavage. The animals were observed daily at least twice a day for 28 days, and weight was recorded twice weekly. Mortality, motor activity, righting reflex, corneal reflex, pineal reflex, the tendency to escape, pallor, piloerection, salivation, and diarrhea, among others, were recorded.
All animals were sacrificed at the end of the study, for which they were anesthetized with isoflurane on day 29 and after fasting overnight. Blood was drawn by intracardiac puncture, and the samples were collected in two tubes (one with EDTA) for the biochemical tests: Urea (BUN), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase, creatinine, total protein, and bilirubin. Another sample was collected with heparin for hematological analysis (erythrocyte count, total and differential leukocyte count, platelet count, hemoglobin, hematocrit). On the other hand, a complete necropsy was conducted on all animals by a veterinary pathologist assisted by a team of trained individuals. The brain, liver, kidneys, heart, adrenals, testes, uterus, ovaries, and spleen were trimmed and weighed immediately. For histopathology, liver and kidney samples were taken and preserved in 10 % buffered formaldehyde. Hematoxylin-eosin (H&E) staining was used for tissue processing.
2.5. Statistical analysis
Data were analyzed using GraphPad Prism v 8.0 (GraphPad Software, La Jolla, California, United States, www.graphpad.com). Data are expressed as mean ± standard deviation of the mean (SD) unless otherwise specified. Blood parameters were processed by factorial analysis of variance (ANOVA). The differences were considered statistically significant when p < 0.05.
3. Results
3.1. Chemical profile of the Passiflora edulis extract
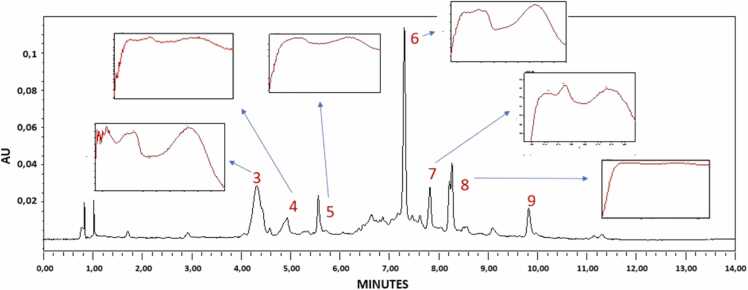
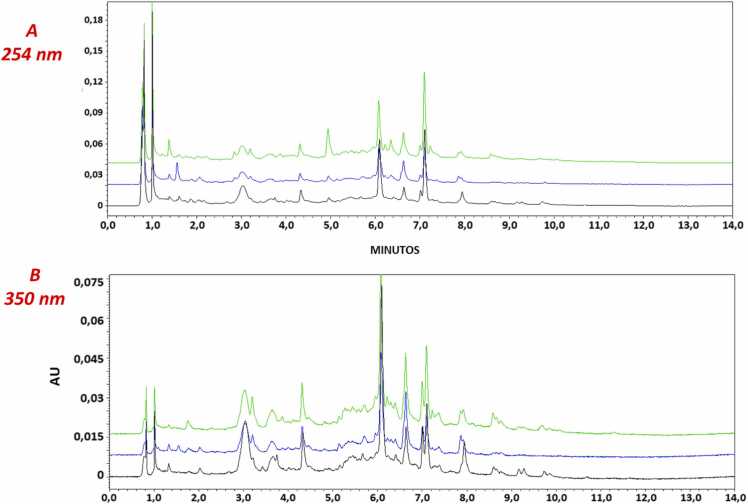
The chromatographic profiles by UPLC-PDA show between minutes 4 and 10 (wavelength 254 nm) peaks corresponding to flavonoids with the typical UV absorption spectra of these molecules (Fig. 1). Among those, peaks 3, 5, 6, 7, and 9 show UV absorption of luteolin derivatives (λmax= 260,270, 350 nm), while peaks 4 and 8 presented UV absorption related to apigenin derivatives (λmax= 270, 340 nm). Some of those flavonoids have already been reported for the genus Passiflora, such as vitexin 2-O-rhamnoside, luteolin 7-O-glucoside, vicenin-2, 6,8-di-C-glucosyl chrysin and spinosyn [20], [24]. The chromatographic profiles of the three batches (L1, L2, and L3) manufactured for the aqueous extract of Passiflora edulis are shown in Fig. 2, where batch-to-batch consistency is observed at 254 and 350 nm, which ensures that the results obtained can be extrapolated to subsequent batches for further preclinical and clinical development.
Fig. 1.
UPLC chromatographic profile and DAD spectra of the aqueous Passiflora edulis extract at 254 nm FE: Phenomenex Kinetex® C18 column (100 ×2.1 mm; 2.6 µm) at temperature: 26 °C. FM: Gradient of acetonitrile and formic acid 0.1%.
Fig. 2.
UPLC chromatographic profile and DAD spectra of the different batches from Passiflora edulis extract: A) 254 nm and B) 350 nm. Black: L1; Blue: L2 and Green: L3. FE: Phenomenex Kinetex® C18 column (100 ×2.1 mm; 2.6 µm) at temperature: 26 °C. FM: Gradient of acetonitrile and formic acid at 0.1%.
Using the analysis by Time-of-Flight Mass Spectrometry (UHPLC-MS-QTOF), the retention times (RT), molecular ion, and some fragmentation patterns that are presented in Table 1 were obtained. According to the molecular mass of the compounds, the results show the presence of molecules between 300 and 600 g/mol, according to the literature, could be associated with the presence of flavonoid compounds, and characteristically those glycosylated as vitexin, isovitexin, vicenin, chrysin [28], [29], [30], [31], [32], [33].
Table 1.
Analysis by Time-of-Flight Mass Spectrometry (UHPLC-MS-QTOF). The table shows the retention times (RT), molecular ion and some fragmentation patterns can be correlated with could be associated with the presence of flavonoid compounds, and characteristically those glycosylated as vitexin, isovitexin, vicenin, chrysin.
| Retention time | [M-H] | Fragments | Identification | UV max abs. nm |
|---|---|---|---|---|
| 2,45 | 431,19126 | - | Vitexin / isovitexin - Glucoside. | 266,346 |
| 3,13 | 593.15009 | 433.20749 340.10273 298.80642 |
Vicenin (apigenin glucoside) | 265, 346 |
| 5,86 | 723.50054 | 713.47185 575.13924 497.33320 298.80640 |
Chrysin - glucoside | 258, 342 |
| 6,86 | 607.16544 | 561.15963 395.07593 |
Spinosin | 266, 346 |
| 6,97 | 697.41488 | 445,25465 | Cyclopassifloside | |
| 9,00 | 697.41501 | 681.41279 680.40880 679.40.366 639.37441 395.07603 327.21722 |
glycoside |
4. Sub-acute study toxicity for 28 days
4.1. Survival and clinical observations
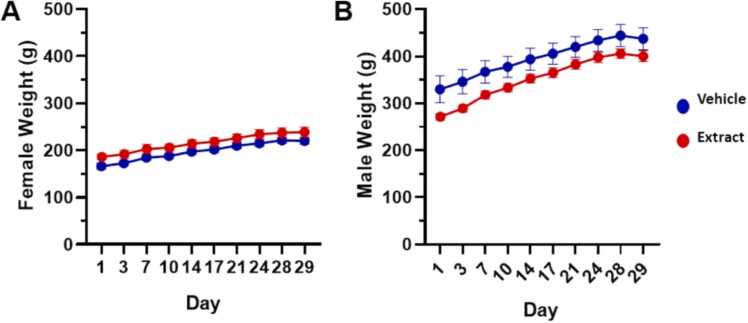
Here, the administration of 1000 mg/Kg/day of the aqueous extract did not generate deaths during the experimental period. There were no clinical findings or observations related to the alteration of the animals' well-being related to the consumption of the extract. As expected, no weight loss was evident in the animals during the 29 days of the study (Fig. 3). However, differences in weight gain were observed between males and females, which can be explained by gender characteristics. There were no treatment-related differences in body weight or body weight gain in male or female rats (Table 2). However, male rats had higher body weights from the start of the study and greater weight gains compared to females. Male rats receiving the extract had lower body weights at the beginning of day 1 of the test and greater body weight gains at the end of the study (32.22 %). However, these differences in body weight compared to control did not reach statistical significance when analyzed using a one-way ANOVA. These differences were interpreted as normal biological variability since these values correspond to the increase in body weight, similar to control males. Furthermore, the weight and body weight gain of female rats receiving the extract were like those of the control group. There were no treatment-related effects on terminal body weights or any organ weights in female and male rats receiving extract compared to control. There were no statistically identified differences in terminal body weights or organ weights in either rat males or females receiving extract compared to controls (Table 3). There were no treatment-related macroscopic pathological or histopathological observations. All pathological observations were interpreted as spontaneous alterations not associated with exposure to the extract.
Fig. 3.
Body weight of male and female rats. Values represent the mean ± SD (n = 10). No significant differences were observed.
Table 2.
Body weight gains summary (g) and weight gains (g) for male and female rats during the study. The table shows weight monitoring on different days and long-term weight gain at 7, 14, 21, and 29 of the subacute study.
| Group | Days on Test |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | Gain | 14 | Gain | 21 | Gain | 29 | Gain | ||
| Male | Vehicle n = 5 |
329.8 ± 63.55 | 366.64 ± 53.22 | 36.84 ± 13.46 | 393.38 ± 51.91 | 63.58 ± 19 | 419.74 ± 49.56 | 89.94 ± 26.03 | 437.30 ± 52.06 | 107.50 ± 26.84 |
| Extract n = 5 |
271.4 ± 15.95 | 318.1 ± 18.81 | 46.70 ± 4.47 | 352.94 ± 20.77 | 81.54 ± 7.27 | 383.10 ± 23.52 | 111.70 ± 11.45 | 400.42 ± 26.39 | 129.02 ± 15.60 | |
| Female | Vehicle n = 5 |
166.4 ± 13.90 | 166.4 ± 13.90 | 18.18 ± 5.05 | 197.42 ± 11.12 | 31.02 ± 8.26 | 210.22 ± 14.39 | 43.82 ± 7.10 | 220.14 ± 15.39 | 53.74 ± 8.39 |
| Extract n = 5 |
185.6 ± 12.66 | 185.6 ± 12.66 | 16.90 ± 11.11 | 213.84 ± 19.20 | 28.24 ± 8.93 | 225.86 ± 19.43 | 40.26 ± 7.83 | 238.54 ± 23.86 | 52.94 ± 11.75 | |
Table 3.
Organ weight summary (g) for male and female rats on the final day of the study. The table shows the weight of the main organs obtained on necropsy.
| Organ | Male |
Female |
||
|---|---|---|---|---|
| Vehicle | Extract | Vehicle | Extract | |
| Liver | 20.05 ± 2.3 | 19.41 ± 2.56 | 10.77 ± 1.14 | 11.34 ± 1.80 |
| Kidney | 3.35 ± 0.47 | 3.01 ± 0.28 | 1.73 ± 0.12 | 1.75 ± 0.21 |
| Lung | 2.46 ± 0.23 | 1.77 ± 0.12 | 1.44 ± 0.12 | 1.24 ± 0.09 |
| Heart | 1.62 ± 0.24 | 1.55 ± 0.14 | 0.88 ± 0.16 | 1.11 ± 0.20 |
| Brain | 1.97 ± 0.11 | 1.86 ± 0.05 | 1.69 ± 0.15 | 1.61 ± 0.04 |
| Testis | 6.40 ± 1.39 | 5.14 ± 0.55 | ||
| Uterus | 1.13 ± 0.24 | 0.65 ± 0.12 | ||
| Pancreas | 1.62 ± 0.24 | 1.55 ± 0.14 | 0.38 ± 0.08 | 0.44 ± 0.14 |
| Spleen | 1.00 ± 0.14 | 0.90 ± 0.13 | 0.57 ± 0.12 | 0.71 ± 0.11 |
| Stomach | 1.85 ± 0.27 | 1.88 ± 0.07 | 1.55 ± 0.20 | 1.35 ± 0.23 |
| Adrenal | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.14 ± 0.01 | 0.08 ± 0.006 |
| Bladder | 0.13 ± 0.01 | 0.12 ± 0.03 | 0.06 ± 0.01 | 0.34 ± 0.39 |
4.2. Hematological parameters
Table 4 shows the hematological parameters of the animals in the study and shows the behavior of the variables analyzed by gender and by treatment. The results do not show important hematological alterations since it is evident that most of these parameters are within the normal ranges reported for Wistar and Sprague Dawley [34], [35]. rats. However, a small monocytopenia is observed with a significant difference (P < 0,05) in monocytes for both male and female rats treated with the aqueous extract of Passiflora edulis. Additionally, eosinopenia was observed with differences in eosinophil counts and percentages between the control and experimental groups for both sexes. No significant changes in total neutrophils were observed. However, no changes associated with loss of animal well-being or weight loss were observed during the study. These results suggest that the aqueous extract of Passiflora edulis is not toxic but induces a slight leukocytopenia with a decrease in monocyte and eosinophil counts.
Table 4.
Effect of Passiflora edulis extract on hematological parameters of control and experimental groups. *p < 0.05.
| Males - Control (n = 5) | Males - Extract (n = 5) |
Females - Control (n = 5) | Females - Extract (n = 5) | |
|---|---|---|---|---|
| Hematocrit (%) | 52.33 ± 1.55 | 48.30 ± 3.75 | 47.18 ± 3.61 | 46.25 ± 3.66 |
| Hemoglobin (g/dL) | 17.20 ± 0.61 | 16.93 ± 1.21 | 14.74 ± 0.81 | 15.63 ± 1.20 |
| RBCs (IU) | 8.66 ± 0.38 | 8.10 ± 0.46 | 7.48 ± 0.45 | 7.74 ± 0.38 |
| VCM | 60.56 ± 3.79 | 59.60 ± 2.01 | 63.08 ± 3.94 | 59.73 ± 1.90 |
| HCM | 19.88 ± 0.28 | 20.88 ± 0.67 | 19.71 ± 0.43 | 20.20 ± 0.56 |
| MCC | 32.90 ± 1.71 | 35.05 ± 0.35 | 31.36 ± 1.99 | 33.80 ± 0.37 |
| Leukocytes | 8163 ± 666 | 7675 ± 1723 | 6134 ± 1398 | 5025 ± 960 |
| Neutrophils (%) | 20.80 ± 2.36 | 15.00 ± 6.22 | 13.82 ± 3.70 | 11.75 ± 3.40 |
| Eosinophils (%) | 2.09 ± 0.74 * | 0.25 ± 0.50 * | 2.43 ± 0.77 * | 0.25 ± 0.50 * |
| Lymphocytes (%) | 75.76 ± 3.27 | 78.50 ± 9.61 | 82.11 ± 4.52 | 88.00 ± 3.83 |
| Monocytes (%) | 1.50 ± 0.87 * | 0.50 ± 0.57 * | 1.13 ± 0.93 * | 0 * |
| Total Neutrophils (µL) | 1702 ± 277 | 1089 ± 250 | 871 ± 315 | 582 ± 188 |
| Total Eosinophils (µL) | 127 ± 42.45 * | 155 ± 70.29 * | 175.52 ± 34.50 * | 135 ± 27.00 * |
| Total Lymphocytes (µL) | 6175 ± 445 | 6053 ± 1758 | 4995 ± 1025 | 4429 ± 912 |
| Total Monocytes (µL) | 124.7 ± 77.32 * | 77.00 ± 32.37 * | 45.00 ± 52.37 * | 0 * |
| Total Platelets | 949.3 ± 41.88 | 1150 ± 137.4 | 1130 ± 130.1 | 1094 ± 30.18 |
4.3. Biochemical parameters
Regarding the blood biochemistry analysis, Table 5 shows the summary of the liver function profile represented by ALT, GGT, FA, conjugated and unconjugated bilirubin. The data do not show alterations and these parameters are within the normal ranges reported for Wistar and Sprague Dawley rats [34], [35]. However, an increase in ALT is observed with significant differences between male and female rats treated with the extract compared to the control. These data must be correlated with the histological findings to establish whether the extract generates a hepatotoxic effect. Table 6 shows the summary of the renal function profile represented by urea, creatinine, BUN, and plasma proteins. These parameters did not show changes or significant differences between the groups.
Table 5.
Effect of Passiflora edulis extract on liver function profile of control and experimental groups.
| Liver Function Profile | ||||
|---|---|---|---|---|
| Males - Control (n = 5) | Males - Extract (n = 5) | Females -Control (n = 5) | Females - Extract (n = 5) | |
| ALT (U/L) | 50.25 ± 4.19 * | 184.4 ± 271.9 * | 64.17 ± 10.15 * | 328.0 ± 325.0 * |
| GGT (U/L) | 2.88 ± 2.17 | 1.00 ± 1.23 | 2.64 ± 2.16 | 3.20 ± 3.11 |
| AF (IU/L) | 367.6 ± 93.74 | 277.5 ± 10.47 | 246.8 ± 35.31 | 288.7 ± 68.89 |
| Total Bilirubin (mg/dL) | 0.076 ± 0.070 | 0.075 ± 0.095 | 0.022 ± 0.016 | 0 |
| Indirect Bilirubin (mg/dL) | 0.046 ± 0.034 | 0.048 ± 0.055 | 0.012 ± 0.010 | 0 |
| Direct Bilirubin (mg/dL) | 0.074 ± 0.073 | 0.050 ± 0.057 | 0.023 ± 0.016 | 0 |
| Liver Biopsia | Portal triads without changes. | Slight periportal mono-morphonuclear infiltrate | Portal triads without changes. | Slight periportal mono-morphonuclear infiltrate |
Table 6.
Effect of Passiflora edulis extract on kidney function profile of control and experimental groups.
| Kindey Function Profile | ||||
|---|---|---|---|---|
| Males - Control (n = 5) | Males - Extract (n = 5) | Females -Control (n = 5) | Females - Extract (n = 5) | |
| Urea (mg/dL) | 58.10 ± 2.71 | 58.12 ± 8.28 | 56.68 ± 5.99 | 69.60 ± 13.79 |
| BUN (mg/dL) | 33.36 ± 11.04 | 27.16 ± 4.08 | 30.57 ± 5.46 | 32.52 ± 6.44 |
| Creatinine (mg/dL) | 0.73 ± 0.05 | 0.60 ± 0.19 | 0.75 ± 0.05 | 0.84 ± 0.18 |
| Total Plasma Proteins (g/dL) | 8.26 ± 0.40 | 6.02 ± 0.51 | 7.56 ± 0.36 | 7.56 ± 0.94 |
| Kidney Biopsia | Corticomedullary tubular nephrocalcifications | |||
4.4. Histopathological examination
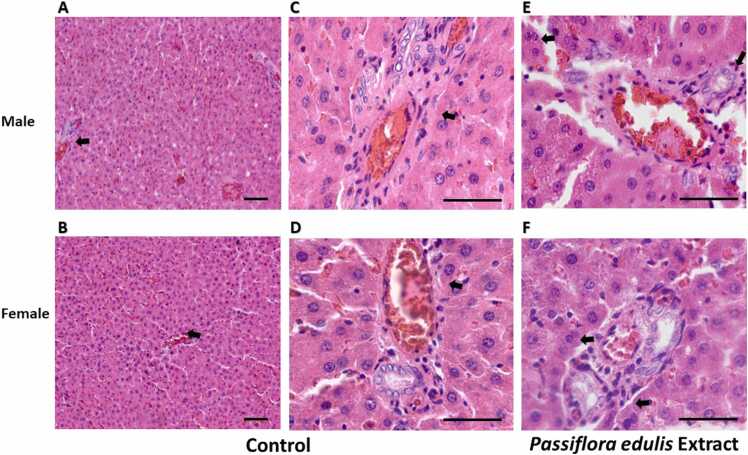
Histopathological examinations of liver sections in rats treated with Passiflora edulis extract are shown in Fig. 4. Microscopic observation of liver sections from the controls and the animals treated with the extract showed healthy liver cells without fatty accumulation, preserved cytoplasm, prominent nucleus, and intact central vein (Fig. 4A-4D). However, the histopathological study revealed the presence of a slight periportal mono-morphonuclear infiltrate (lymphocyte-macrophage) in the liver (Figs. 4E and 4F), with a slight increase in hepatocyte binucleation. The above results suggest that male and female rats administered the extract at 1000 mg/kg dose showed typical liver architecture without necrosis.
Fig. 4.
Hepatic histological study of the animals in sub-acute toxicity. Samples were stained with hematoxylin and eosin (H&E). A. and B. are 10X images, and arrowheads show unchanged portal triads. Bar 10 µm. C. and D. are 40X images of male and female rat samples respectively from the control group. E and F are 40X images of male and female rat samples respectively from the experimental group. Arrowheads show mono-morphonuclear infiltrate and hepatocyte binucleation. Bar 100 µm.
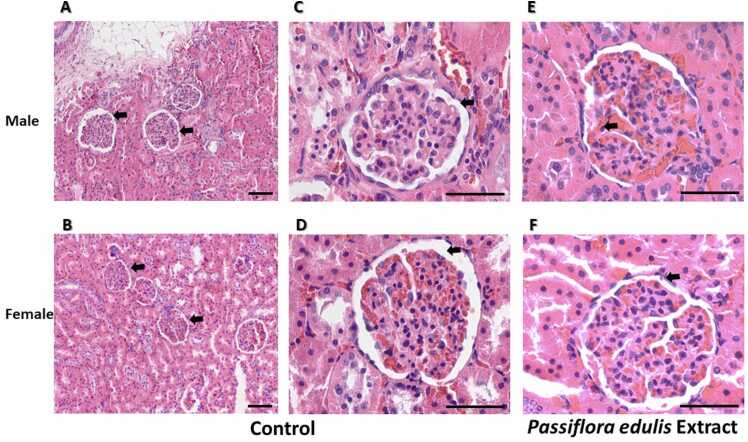
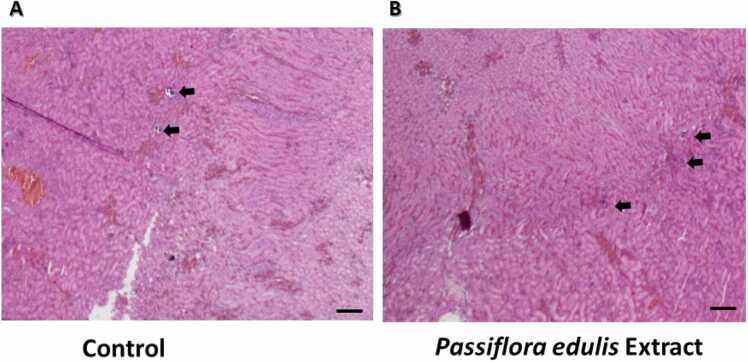
The results of histopathological examination of kidney sections from control animals and those treated with Passiflora edulis extract are shown in Fig. 5. Kidneys from vehicle-administered rats demonstrated intact glomeruli and tubules (Fig. 5A-5D). Rats administered with 1000 mg/kg extract showed normal architectural structures similar to the control group. However, the histological study showed a slight generalized increase of intraglomerular mesangium (Fig. 5E and F) compared with the control, and a slight focal increase of corticomedullary tubular nephron-calcifications was observed in both the control and the experimental groups (Fig. 6A and B).
Fig. 5.
Renal histological study of the animals in sub-acute. Samples were stained with hematoxylin and eosin (H&E). A. and B. are 10X images (Bar 10 µm). C. and D. are 40X images of male and female rat samples respectively from the control group. Arrowheads show unchanged cortico-medullary glomeruli. E and F are 40X images of male and female rat samples respectively from the experimental group. Arrowheads show cortico-medullary glomeruli with increased mesangium. Bar 100 µm.
Fig. 6.
Changes in the renal histological study of the animals in sub-acute. Samples were stained with hematoxylin and eosin (H&E). A. and B. are 10X representative images from female rat samples, respectively, of the control and experimental group. Arrowheads show tubular nephron-calcification (Bar 10 µm). The same finds were observed in the rat male samples.
5. Discussion
The therapeutic properties of Passiflora's infusion (aqueous extract) include traditional medicine such as anxiolytic, sedative, antispasmodic, neuroprotective, anti-inflammatory, and antioxidant [19], [20], [25], [26]. The range of daily dosage varies from 30 mg to 120 mg of total flavonoids expressed as vitexin in an herbal medicine made from Passiflora [36]. In clinical studies that involved the use of Passiflora to treat anxiety 10–100 mg, only one of the studies was conducted with monopreparations of Passiflora. This difficult report doses to use in humans [37]. These preparations can be used acutely or chronically, depending on the pharmacological intention or traditional knowledge. For instance, the therapeutic activity for the central nervous system could involve a more prolonged treatment than its use as an anti-inflammatory. Regardless of the dosing frequency, it is necessary to determine the safety profile following nonclinical requirements [27] to advance in developing new therapeutic alternatives either as polymolecular drugs or as isolated molecules. The oral administration of the aqueous of Passiflora edulis Sims f edulis (300 mg/kg) showed an antidepressant activity [38]. On the other hand, the aqueous extract of Passiflora edulis at 50, 100, and 150 mg/kg showed anxiolytic-like effects in rats' elevated plus-maze and inhibitory avoidance tests. In addition, the extract did not disrupt the rat memory process in habituation to an open-field test [39].
Regarding the chemical profile of the Passiflora edulis extract, the mass spectrometry data (Table 1) suggest the presence of a group of compounds of the glycosylated flavonoid type with masses between 400 and 600 g/mol. According to the literature, they may correspond to 4 flavonoids: vitexin/iso-vitexin ([M-H]-: 431.19129), vicenin ([M-H]-: 593.15009), chrysin ([M-H]-: 723.50054) and spinosyn ([M-H]-: 607.16544). These molecules of flavonoids can present cores of apigenin, chrysin, and swertisin, respectively. Results previously reported by Farag et al. [32] identified flavone glycoside conjugates as the major class of compounds in Passiflora spp., using mass spectrophotometry and liquid chromatography with diode array detection with UV maxima 270 and 325–350 nm. Additionally, as suggested by Urrego et al. [33] we also propose the presence of the cyclopassifloside compound ([M-H]-: 697.41501) in Passiflora edulis f edulis sims. Other authors have reported the absence of absorption of some compounds at wavelengths > 205 nm and detection with UHPLC-DAD-ELSD of the presence of terpene compounds without chromophore groups, possibly saponins and cyclopasifloic acid glycoside terpenoid structures in species of Passiflora spp [31], [32], [33], [40].
A previous acute oral toxicity study was performed in our lab in mice to determine the safety profile and the absence of effects or alterations induced by the Passiflora edulis aqueous extract at 2000 mg/kg single dose. All animals survived to the end of the test and were observed for 14 days. Our data suggest that the Passiflora edulis extract is well tolerated for mice. For this reason, we select the 1000 mg/kg dose for the sub-acute study.
This study determined the sub-acute toxicity for 28 days of an aqueous Passiflora edulis f edulis extract. This preparation, in which batch-to-batch consistency was confirmed, was based on traditional Colombian knowledge. The NOAEL for sub-acute oral 28-day toxicity was 1000 mg/kg/day in a rat model. This result suggests low toxicity seen in acute and 7-day toxicity studies with an aqueous Passiflora edulis f edulis extract at oral doses of up to 100, 200, 300, and 400 mg/kg body weight in a rat model [21]. Considering the components reported for aqueous Passiflora edulis f edulis extract [20], [21], [22], [23], [24], [25], [26], [27], the literature reports that extracts with glycosylated flavonoids have low toxicity in oral administration [41].
Determining weight gain or relative organ weight is essential to identify possible damage from exposure to toxic substances in the extract. The weight of the damaged organ would be altered depending on the degree of toxicity and the proportion of the body weight. In this subacute toxicity study, the extract administered up to 1000 mg/kg did not cause any change in clinical signs, mortality, or morbidity. In addition, weight gain was observed in the animals throughout the 29 days of the study. These results suggest that the extract did not cause any detrimental effect on the relative weight of the organs compared to the control animals at the end of the study.
The liver is the main organ metabolizing and detoxifying drugs and environmental chemicals. For this reason, evaluating liver function is essential to determine the toxicity of drugs and plant extracts. AST, ALT, ALP, total protein, and total bilirubin concentration are liver disease's most common clinical biomarkers [27]. Serum ALT activity has been used as an essential biomarker of liver injury in preclinical studies, as damaged hepatocytes release ALT into the extracellular space with increased blood [27]. In this study, serum ALT increased values compared to the control groups in male and female rats. The ALT levels of 184 U/L and 328.0 U/L suggest the diagnostic criteria of liver damage, compared to the control groups of 50.25 U/L and 64.17 U/L in male and female rats, respectively. However, histopathological examination of the liver does not correlate with alterations in cellular structure, inflammation, or any other unfavorable effect.
In the present study, the control and extract-treated groups observed no significant alterations in the serum levels of GGT, FA, total protein, and total bilirubin. The present study found that administration of Passiflora edulis extract at a dose of 1000 mg/kg did not adversely affect the liver. Likewise, histological examination of the liver showed small changes in the tissue and induced a statistically significant increase in ALT. However, the results suggest that administration of the extract is relatively safe for the liver. These results can be correlated with several works that have reported that Passiflora edulis showed hepatoprotection effect in rats [42], [43], [44], [45]. In addition, in ethanol-induced hepatic injury, the daily treatment with Fruit juice protected the liver and decreased AST and ALT activity, inflammation, and oxidative stress [44].
The results obtained from the histopathological study show in the liver the presence of a slight mono-morphonuclear infiltrate (lymphocyte-macrophage), periportal, and a slight increase in hepatocyte binucleation. The above may suggest a mild chronic proinflammatory hepatic parenchymal proinflammatory effect with associated mild intrahepatic cholangitis. This effect could be unrelated to the direct cytopathic effect of administering the aqueous extract at a dose of 1000 mg/kg/day for 28 days, given that the hepatic and cholangiositic profile evidenced between the control and experimental groups did not show any statistical difference in the hepatic biochemical biomarkers. Toxicological studies in repeated doses at 28 days [46], [47] after oral administration of 1000 mg/kg/day of a glycosylated flavonoid similar to those reported for the extract of Passiflora, have also found a minimal focal and multifocal infiltrate in the liver, where the authors suggest that such histopathological liver finding may not be related to toxicological significance.
The kidneys are considered frequent targets of toxicity. Monitoring biochemical markers and histological changes is radical for determining kidney health. The biochemical results did not show significant differences in the urea, creatinine, and BUN levels. These results suggest that Passiflora extract does not affect the normal renal function following sub-acute exposure compared to the control group. Similarly, histological analysis showed no differences in the tissue architecture of the organ between the treatment and control groups.
The renal analysis showed histopathological results with a slight focal increase of corticomedullary tubular nephron-calcifications; a related toxicological change may not justify that because the appearance of spontaneous nephrocalcinosis has been reported for the experimental animal model [48] together with the fact that no signs of loss of animal welfare were evidenced. These corticomedullary tubular nephron-calcifications have been related to different factors, such as diet changes [49], which can significantly reduce urinary pH [50]. The renal histopathological study showed a slight generalized increase of the intraglomerular mesangium; it should be noted that this change did not modify the renal filtration function since the creatinine result between the control and experimental groups with the administration of 1000 mg/Kg/day of the aqueous extract was similar.
The hematopoietic system is very vulnerable to toxic substances; therefore, it is essential to determine the physiological and pathological states. In this study, subacute administration of Passiflora edulis extract caused significant changes in the leukocyte profile of rats treated with the extract compared to the control.
However, it should be noted that the histopathological scenarios reported do not express direct cytopathological findings, or at least in a toxicological magnitude associated with the experiment, which explains the results of the hematological and biochemical analyses (Table 2, Table 3). However, hematology does show a significant difference in the decrease in monocyte and eosinophil counts, which the high variability of the data can explain. On the other hand, the significant variability of the reference values reported for the species in the literature and the experimental ones is given by the high standard deviations reported for the evaluated parameters, on average around 50% (Table 1).
Finally, the aqueous extract of Passiflora edulis f edulis had a good safety profile in oral administration for the examined parameters, was well tolerated, and did not cause any lethality or adverse effects in the sub-acute toxicity study in male and female rats. The present study provides data on the chemical profile and sub-acute toxicity of the aqueous extract Passiflora edulis, commonly used in traditional medicine. However, other studies should be performed to evaluate the extract's safety, such as acute, subacute, and chronic toxicity, genotoxicity, and carcinogenicity, to understand better the plant's safety profile and its potential use in traditional medicine.
Funding
The authors would like to thank Ministerio de Ciencia, tecnología e Innovación, al Ministerio de Educación Nacional, al Ministerio de Industria, Comercio y Turismo e ICETEX, 2ª Convocatoria Ecosistema científico - Colombia Científica 792–2017, Programa “Generación de alternativas terapéuticas en cáncer a partir de plantas a través de procesos de investigación y desarrollo traslacional, articulados en sistemas de valor sostenibles ambiental y económicamente” (Contrato No. FP44842–221-2018), y Vicerrectoría de investigación, Pontificia Universidad Javeriana, Grant IDs 006354, 006365 y 007163.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the Colombian Environmental Ministry for allowing the use of genetic resources and products derived (Contract number 212/2018; Resolution 210/2020). The authors would also like to thank María Lucía Correal, DVM, from the Animal care facility of Pontificia Universidad Javeriana.
Handling Editor:Prof. L.H. Lash
Data availability
Data will be made available on request.
References
- 1.Organization WH . World Health Organization,; 2019. WHO Global Report on Traditional and Complementary Medicine 2019. [Google Scholar]
- 2.Falzon C.C., Balabanova A. Phytotherapy: an introduction to herbal medicine. Prim. Care Clin. Pract. 2017;44:217–227. doi: 10.1016/j.pop.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Riddle J.M. Historical data as an aid in pharmaceutical prospecting and drug safety determination. J. Alter. Complement. Med. 1998;5:195–201. doi: 10.1089/acm.1999.5.195. [DOI] [PubMed] [Google Scholar]
- 4.Governa P., Baini G., Borgonetti V., et al. Phytotherapy in the management of diabetes: a review. Molecules. 2018;23:105. doi: 10.3390/molecules23010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P., McClees S.F., Afaq F. Pomegranate for prevention and treatment of cancer: an update. Molecules. 2017;22:177. doi: 10.3390/molecules22010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliga M.S., Dsouza J.J. Amla (Emblica officinalis Gaertn), a wonder berry in the treatment and prevention of cancer. Eur. J. Cancer Prev. 2011;20:225–239. doi: 10.1097/CEJ.0b013e32834473f4. [DOI] [PubMed] [Google Scholar]
- 7.Antonio G.D., Tesser C.D., Moretti-Pires R.O. Fitoterapia na atenção primária à saúde. Rev. Saude Publica. 2014;48:541–553. doi: 10.1590/S0102-311X2007000600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuzzolin L., Zaffani S., Benoni G. Safety implications regarding use of phytomedicines. Eur. J. Clin. Pharmacol. 2006;62:37–42. doi: 10.1007/s00228-005-0050-6. [DOI] [PubMed] [Google Scholar]
- 9.Capasso R., Izzo A.A., Pinto L., et al. Phytotherapy and quality of herbal medicines. Fitoterapia. 2000;71:S58–S65. doi: 10.1016/s0367-326x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 10.Williamson E.M. Drug interactions between herbal and prescription medicines. Drug Saf. 2003;26:1075–1092. doi: 10.2165/00002018-200326150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gummin D.D., Mowry J.B., Beuhler M.C., et al. 2020 Annual report of the American association of poison control centers’ national poison data system (NPDS): 38th annual report. Clin. Toxicol. 2021;59:1282–1501. doi: 10.1080/15563650.2021.1989785. [DOI] [PubMed] [Google Scholar]
- 12.Froberg B., Ibrahim D., Furbee R.B. Plant poisoning. Emerg. Med. Clin. North Am. 2007;25:375–433. doi: 10.1016/j.emc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Faleiro F.G., Morera M.P., Costa A.M., et al 2020. Pasifloras: especies cultivadas en el mundo.
- 14.He X., Luan F., Yang Y., et al. Passiflora edulis: An insight into current researches on phytochemistry and pharmacology. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadioli I.L., da Cunha M., de S.B., de Carvalho M.V.O., et al. A systematic review on phenolic compounds in Passiflora plants: exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2018;58:785–807. doi: 10.1080/10408398.2016.1224805. [DOI] [PubMed] [Google Scholar]
- 16.De Santana F.C., Shinagawa F.B., Araujo E. da S., et al. Chemical composition and antioxidant capacity of Brazilian Passiflora seed oils. J. Food Sci. 2015;80:C2647–C2654. doi: 10.1111/1750-3841.13102. [DOI] [PubMed] [Google Scholar]
- 17.Argentieri M.P., Levi M., Guzzo F., Avato P. Phytochemical analysis of Passiflora loefgrenii Vitta, a rich source of luteolin-derived flavonoids with antioxidant properties. J. Pharm. Pharmacol. 2015;67:1603–1612. doi: 10.1111/jphp.12454. [DOI] [PubMed] [Google Scholar]
- 18.Ingale S.P., Kasture S.B. Antioxidant and antiparkinsonian activity of Passiflora incarnata leaves. Orient Pharm. Exp. Med. 2014;14:231–236. [Google Scholar]
- 19.Taïwe G.S., Kuete V. Medicinal Spices and Vegetables from Africa. Elsevier,; 2017. Passiflora edulis; pp. 513–526. [Google Scholar]
- 20.Urrego N., Sepúlveda P., Aragón M., et al. Flavonoids and saponins from Passiflora edulis f. edulis leaves (purple passion fruit) and its potential anti-inflammatory activity. J. Pharm. Pharmacol. 2021;73:1530–1538. doi: 10.1093/jpp/rgab117. [DOI] [PubMed] [Google Scholar]
- 21.Devaki K., Beulah U., Akila G., Gopalakrishnan V.K. Effect of aqueous extract of Passiflora edulis on biochemical and hematological parameters of Wistar albino rats. Toxicol. Int. 2012;19:63. doi: 10.4103/0971-6580.94508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S.S., Verma S., Nayak G., Singhai A.K., Ganesh N. Acute and sub-acute toxicity studies of Passiflora nepalensis in rats. Rev. Bras. De. Farmacogn. 2011;21:730–736. [Google Scholar]
- 23.Devaki K., Beulah U., Akila G., Gopalakrishnan V.K. Effect of aqueous extract of passiflora edulis on biochemical and hematological parameters of Wistar albino rats. Toxicol. Int. 2012;19:63–67. doi: 10.4103/0971-6580.94508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeira J.M., Fenner R., Betti A.H., Provensi G., Lacerda Le.A., Barbosa P.R., et al. Toxicity and genotoxicity evaluation of Passiflora alata Curtis (Passifloraceae) J. Ethnopharmacol. 2010;128:526–532. doi: 10.1016/j.jep.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 25.Anurangi C.R., Shamina S. Preliminary phytochemical screening and acute & subacutetoxicity study on different concentrations of unripen fruit peel flour of Passiflora edulis in male albino rats. World J. Pharm. Pharm. Sci. 2018;7:828–834. doi: 10.20959/wjpps20183-11113. [DOI] [Google Scholar]
- 26.2022. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. OECD.
- 27.2008. Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents. OECD.
- 28.Ayres A.S., de Araújo L.L.S., Soares T.C., et al. Comparative central effects of the aqueous leaf extract of two populations of Passiflora edulis. Rev. Bras. Farm. 2015;25:499–505. [Google Scholar]
- 29.Coleta M., Batista M.T., Campos M.G., et al. Neuropharmacological evaluation of the putative anxiolytic effects of Passiflora edulis Sims, its sub‐fractions and flavonoid constituents. Phyther. Res. Int. J. Devoted Pharm. Toxicol. Eval. Nat. Prod. Deriv. 2006;20:1067–1073. doi: 10.1002/ptr.1997. [DOI] [PubMed] [Google Scholar]
- 30.Pineli L., de L., de O., Rodrigues J. da S.Q., Costa A.M., et al. Antioxidants and sensory properties of the infusions of wild passiflora from Brazilian savannah: potential as functional beverages. J. Sci. Food Agric. 2015;95:1500–1506. doi: 10.1002/jsfa.6852. [DOI] [PubMed] [Google Scholar]
- 31.Costa G., Gazola A., Zucolotto S., Castellanos L., Ramos F., Reginatto F., et al. Chemical profiles of traditional preparations of four South American Passiflora species by chromatographic and capillary electrophoretic techniques. Revista Brasileira de. Rev. Bras. Farmacogn. 2016;26:4. [Google Scholar]
- 32.Farag M.A., Otify A., Porzel A., Michel C.G., Elsayed A., Wessjohann L.A. Comparative metabolite profiling and fingerprinting of genus Passiflora leaves using a multiplex approach of UPLC-MS and NMR analyzed by chemometric tools. Anal. Bioanal. Chem. 2016;408:3125–3143. doi: 10.1007/s00216-016-9376-4. [DOI] [PubMed] [Google Scholar]
- 33.Urrego N., Sepúlveda P., Aragón M., Ramos F.A., Costa G.M., Ospina L.F., et al. Flavonoids and saponins from Passiflora edulis f. edulis leaves (purple passion fruit) and its potential anti-inflammatory activity. J. Pharm. Pharmacol. 2021;73(11):1530–1538. doi: 10.1093/jpp/rgab117. [DOI] [PubMed] [Google Scholar]
- 34.Wolford S.T., Schroer R.A., Gohs F.X., et al. Reference range database for serum chemistry and hematology values in laboratory animals. J. Toxicol. Health. 1986;18:161–188. doi: 10.1080/15287398609530859. [DOI] [PubMed] [Google Scholar]
- 35.Lillie L.E., Temple N.J., Florence L.Z., et al. Reference values for young normal Sprague-Dawley rats: weight gain, hematology and clinical chemistry. Hum. Exp. Toxicol. 1993;15:612–616. doi: 10.1177/096032719601500802. [DOI] [PubMed] [Google Scholar]
- 36.da Fonseca Lyca R., Rodrigues Rafaele de A., Ramos Aline de S., da Cruz Jefferson D., Ferreira José Luiz P., Silva Jefferson Rocha de A., Amaral Ana Claudia F. Herbal medicinal products from passiflora for anxiety: an unexploited potential. Sci. World J. 2020 doi: 10.1155/2020/6598434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobrek Lukasz, Głowacka Krystyna. Depression and its phytopharmacotherapy – a narrative review. Int J. Mol. Sci. 2023;24:4772. doi: 10.3390/ijms24054772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayres A.S.F.S.J., Santos W.B., Junqueira-Ayres D.D., Costa G.M., Ramos F.A., Castellanos L., et al. Monoaminergic neurotransmission is mediating the antidepressant-like effects of Passiflora edulis Sims fo. Edulis. Neurosci. Lett. 2017;660:79–85. doi: 10.1016/j.neulet.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 39.He Xirui, Luan Fei, Yang Yan, Wang Ze, Zhao Zefeng, Fang Jiacheng, Wang Min, Zuo Manhua, Li Yongsheng. Passiflora edulis: an insight into current researches on phytochemistry and pharmacology. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zucolotto S.M., Fagundes C., Reginatto F.H., Ramos F.A., Castellanos L., Duque C., et al. Analysis of C-glycosyl flavonoids from South American Passiflora species by HPLC-DAD and HPLC-MS. Phytochem. Anal. 2012;23:232–239. doi: 10.1002/pca.1348. [DOI] [PubMed] [Google Scholar]
- 41.Ritskes-Hoitinga J., Beynen A.C. Nephrocalcinosis in the rat: a literature review. Prog. Food Nutr. Sci. 1992;16(1):85–124. [PubMed] [Google Scholar]
- 42.Zibadia S., Faridc R., Moriguchid S., Lue Y.R., Fooe L.Y., Tehranic P.M., et al. Oral administration of purple passion fruit peel extract attenuates blood pressure in female spontaneously hypertensive rats and humans. Nutr. Res. 2007;27:408–416. doi: 10.1016/j.nutres.2007.05.004. [DOI] [Google Scholar]
- 43.Kavitha R.V., Kumar J.R., Balasubramanian S., Swamy S.N., Keerthini D., Shambhavi N., et al. Comparative evaluation of hepatoprotective effects of exotic fruits and common vegetables extracts on CCl4 induced hepatotoxicity: an in vitro study. Int. J. Pharm. Sci. Res. 2016;7:3388–3393. doi: 10.13040/IJPSR.0975-8232. [DOI] [Google Scholar]
- 44.Zhang Y.J., Zhou T., Wang F., Zhou Y., Li Y., Zhang J.J., et al. The effects of syzygium samarangense, passiflora edulis and solanum muricatum on alcohol-induced liver injury. Int. J. Mol. Sci. 2016;17:1616. doi: 10.3390/ijms17101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shanmugam S., Sivaraj D., Dos Santos Lima B., Dos Passos Menezes P., de Carvalho Y.M.B.G., de Souza Araújo A.A., Narain N., Serafini M.R., Quintans Júnior L.J., Scotti L., Scotti M.T., Parimelazhagan T. Polyphenols rich Passiflora leschenaultii leaves modulating Farnesoid X Receptor and Pregnane X Receptor against paracetamol-induced hepatotoxicity in rats. Biomed. Pharmacother. 2017;88:1114–1121. doi: 10.1016/j.biopha.2017.01.156. [DOI] [PubMed] [Google Scholar]
- 46.Mukinda J.T. , 2005. Acute and chronic toxicity of the flavonoid-containing plant, Artemisia afra in rodents. [DOI] [PubMed]
- 47.Li Y., Kandhare A.D., Mukherjee A.A., Bodhankar S.L. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2019;105:77–85. doi: 10.1016/j.yrtph.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Dimitrov S., Lange T., Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010;184:503–511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- 49.Ritskes-Hoitinga J., Beynen A.C. Nephrocalcinosis in the rat: a literature review. Prog. Food Nutr. Sci. 1992;16:85–124. [PubMed] [Google Scholar]
- 50.Mohamed H., Alhaidary A., Beynen A. Nephrocalcinosis and urinary mineral concentrations in rats fed diets containing various concentrations of magnesium. J. Anim. Vet. Adv. 2010;9:2405–2408. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.