Abstract
OBJECTIVE
Experimental evidence suggests that metabolic syndrome (MetS) is associated with changes in cardiac metabolism. Whether this association occurs in humans is unknown.
RESEARCH DESIGN AND METHODS
821 asymptomatic individuals from the Progression of Early Subclinical Atherosclerosis (PESA) study (50.6 [46.9–53.6] years, 83.7% male) underwent two whole-body 18F-fluorodeoxyglucose positron emission tomography-magnetic resonance (18F-FDG PET-MR) 4.8 ± 0.6 years apart. Presence of myocardial 18F-FDG uptake was evaluated qualitatively and quantitatively. No myocardial uptake was grade 0, while positive uptake was classified in grades 1–3 according to target-to-background ratio tertiles.
RESULTS
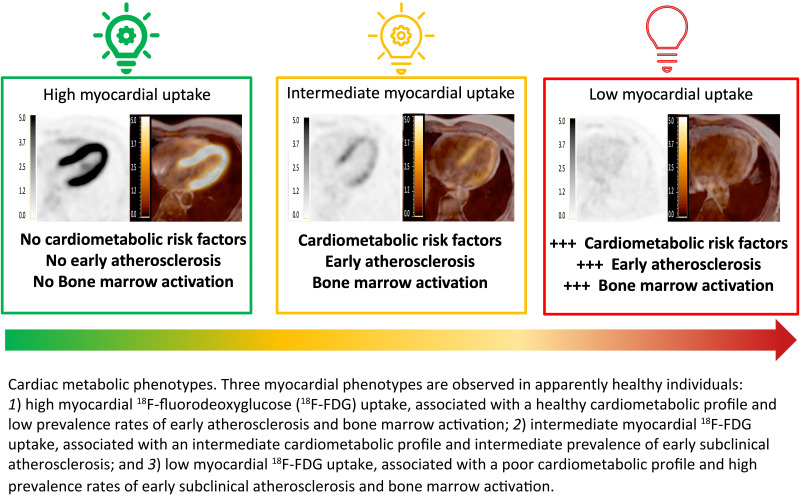
One hundred fifty-six participants (19.0%) showed no myocardial 18F-FDG uptake, and this was significantly associated with higher prevalence of MetS (29.0% vs. 13.9%, P < 0.001), hypertension (29.0% vs. 18.0%, P = 0.002), and diabetes (11.0% vs. 3.2%, P < 0.001), and with higher insulin resistance index (HOMA-IR, 1.64% vs. 1.23%, P < 0.001). Absence of myocardial uptake was associated with higher prevalence of early atherosclerosis (i.e., arterial 18F-FDG uptake, P = 0.004). On follow-up, the associations between myocardial 18F-FDG uptake and risk factors were replicated, and MetS was more frequent in the group without myocardial uptake. The increase in HOMA-IR was associated with a progressive decrease in myocardial uptake (P < 0.001). In 82% of subjects, the categorization according to presence/absence of myocardial 18F-FDG uptake did not change between baseline and follow-up. MetS regression on follow-up was associated with a significant (P < 0.001) increase in myocardial uptake.
CONCLUSIONS
Apparently healthy individuals without cardiac 18F-FDG uptake have higher HOMA-IR and higher prevalence of MetS traits, cardiovascular risk factors, and early atherosclerosis. An improvement in cardiometabolic profile is associated with the recovery of myocardial 18F-FDG uptake at follow-up.
Graphical Abstract
Introduction
The myocardium is an omnivore organ that can generate energy from different substrates: fatty acids, carbohydrates, and ketone bodies (1). The flexibility in substrate utilization is essential, and an optimal cardiac function depends on the myocardium’s ability to combine the intake of the different substrates (2).
Experimental studies suggest that cardiovascular risk factors are associated with changes in cardiac metabolism. Mouse models of metabolic syndrome (MetS) have demonstrated that there is a cardiac metabolic inflexibility associated with MetS, characterized by impaired insulin-induced glucose uptake and oxidation that leads to cardiomyocyte’s mitochondrial energetics impairment (3–5). The hypothesis that MetS is associated with an abnormal cardiac substrate utilization has been recently highlighted in a pig model of MetS, in which a severely reduced availability of glycolytic cycle pathway metabolites has been shown (6). Cardiac metabolism abnormalities have also been observed in mouse models of diabetic cardiomyopathy, in which cardiac uptake and oxidation of fatty acids is increased because of impaired glucose transport (7,8).
In humans, molecular imaging techniques allow the evaluation of cardiac metabolism by means of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) imaging (9). However, most clinical studies testing myocardial 18F-FDG uptake are performed after strict preparation of the patient before the PET imaging (long fasting, high-fat low-carbohydrate diet) that alters cardiac metabolism and precludes its study under physiological conditions (10). Only a few retrospective studies performed in oncologic patients have provided some inconsistent data about myocardial 18F-FDG uptake, which might not be applicable to healthy populations (11,12). Regarding cardiovascular risk factors, diabetes has been associated with a reduced myocardial 18F-FDG uptake, and insulin resistance has been postulated as a potential mechanism (13–18). However, clinical data regarding the associations between MetS and cardiac metabolism are limited.
In this study, we aimed to evaluate whether MetS traits and other cardiovascular risk factors are associated with changes in cardiac metabolism. With that purpose, we assessed myocardial 18F-FDG uptake under physiological conditions in a large cohort of healthy middle-aged adults.
Research Design and Methods
Study Population
The study population consisted of participants from the Progression of Early Subclinical Atherosclerosis (PESA-CNIC-Santander) study (19) who underwent whole-body 18F-FDG PET-magnetic resonance (18F-FDG PET-MR) (20) at baseline and at 5-year follow-up (21).
PESA is an observational, prospective cohort study of 4,184 asymptomatic employees at Santander Bank in Madrid. Exclusion criteria were previous cardiovascular disease, any condition reducing life expectancy or affecting study adherence, morbid obesity (BMI ≥40 kg/m2), or chronic kidney disease (estimated glomerular filtration rate <60 mL/min/m2). The main goal of the PESA study is to characterize atherosclerosis initiation and progression by means of serial multiterritory, multimodality, noninvasive imaging and paired biological sampling (21). At baseline, a subgroup of PESA participants showing atherosclerosis on vascular ultrasound and/or having any coronary artery calcification on computed tomography (CT) were studied by whole-body 18F-FDG-PET-MR (20). Cardiovascular risk factor profiling and biochemical analysis in PESA have been explained in detail elsewhere (19,20,22–24). In this study, MetS was defined when a participant met at least three of the following conditions: central obesity (waist circumference ≥88 cm in women and ≥102 cm in men) (25), elevated fasting plasma triglycerides (≥150 mg/dL), low plasma HDL cholesterol (<40 mg/dL in men or <50 mg/dL in women), elevated fasting plasma glucose (≥100 mg/dL), and high blood pressure (systolic ≥130 mmHg and/or diastolic ≥85 mmHg) (26). The study protocol was approved by the Institutional Review Board at Instituto de Salud Carlos III, Madrid, Spain, and all participants provided written informed consent.
Hybrid PET-MR Acquisition Protocol and Image Analysis
The PET-MR protocol has been published previously (20). In brief, prescan preparation requirements included fasting for 6 h and refraining from exercise in the preceding 24 h. Plasma glucose was measured before the scan. Participants received a target dose of 270–300 MBq 18F-FDG (27) and were asked to remain at rest in a quiet room for 30 min after radiolabel injection. Sequential whole-body PET-MR studies were performed with a Philips Ingenuity TF PET-MR hybrid system (Philips Healthcare, Cleveland, OH). MR was performed with a phased-array 16-channel torso XL coil for aorto-iliac and femoral arteries and a 16-channel neurovascular coil for carotid arteries, using peripheral pulse gating. The imaging protocol and detailed MR parameters have been published previously (20).
Each scan was systematically assessed by two experts (A.D. and R.V.) blinded to the clinical data using dedicated software (IntelliSpace Portal; Philips Healthcare). Fused PET-MR axial images were assessed; the presence of visual myocardial 18F-FDG uptake was defined as positive if an elevated signal was detected compared with blood pool. For semiquantitative assessment, regions of interest encompassing the left ventricular myocardium were drawn in a representative axial image (Supplementary Fig. 1). Maximum standard uptake values (SUVmax) were registered as well as maximum tissue-to-background ratio (TBRmax), where SUVmax was corrected for the mean activity of the blood pool (28). Participants with visual myocardial 18F-FDG uptake were classified in three groups (grades 1–3) according to TBRmax tertiles; absence of uptake was defined as grade 0. Both qualitative and quantitative measurements were highly reproducible: κ index was 1 for both intrareader and interreader reliability on qualitative assessments; intraclass correlation coefficient was 0.98 for intrareader and 0.97 for interreader reliability on quantitative assessments.
Arterial 18F-FDG uptake (a proxy of early atherosclerosis) was assessed in five vascular territories (two carotid arteries, two ilio-femoral arteries, and aorta) (20). Bone marrow 18F-FDG uptake (a proxy of bone marrow activation) was assessed in lumbar vertebrae (L3 and L4) (24).
Statistical Analysis
Normally distributed continuous variables are expressed as mean ± SD, whereas nonnormally distributed variables are expressed as median [Q1–Q3]. Categorical variables are expressed as n (%). Differences between myocardial 18F-FDG uptake and nonuptake groups were assessed as appropriate by the Student t test or Mann-Whitney two-sample statistic for continuous variables and by χ2 or Fisher exact test for categorical variables. Linear trends across groups according to degree of myocardial 18F-FDG uptake were assessed with an extension of the nonparametric Mann-Whitney two-sample statistic. For multivariate analysis, linear regression models were performed. To evaluate the association between MetS, HOMA-insulin resistance (IR), and myocardial 18F-FDG uptake, several models were generated: model 1, adjusting for blood glucose before injection and time from injection to imaging acquisition; and model 2, adjusting for age and sex. For all end points, differences were considered statistically significant at P values < 0.05. Statistical analyses were performed using Stata software version 15 (StataCorp, College Station, TX).
Results
A total of 946 PESA participants underwent whole-body PET-MR at baseline. Reasons for noncompletion of the study were physical intolerance in 21 participants and technical issues with MR attenuation maps in 97 participants; 7 participants were excluded because of limited image quality. Finally, 821 participants constituted the population for the current study (86.8% of the total sample who underwent PET-MR); 706 participants underwent a follow-up PET-MR after a median time of 4.8 ± 0.6 years; 6 participants were excluded because of limited quality imaging; 700 participants (99.2%) constituted the follow-up cohort (Supplementary Fig. 2).
Baseline Characteristics of Participants in Relation to Myocardial 18F-FDG Uptake
The median age of the study population at baseline 18F-FDG PET-MR was 50.6 years [46.9–53.6], and 83.7% of the population were male. Baseline characteristics are presented in Table 1. Within the study population, 156 participants (19.0%) had no myocardial 18F-FDG uptake (grade 0). Myocardial 18F-FDG uptake was present in the remaining 665 participants (81.0%) and was classified in grades 1 to 3 according to TBRmax tertiles (Supplementary Table 1).
Table 1.
Characteristics by presence of myocardial 18F-FDG uptake at baseline
| Total population | No uptake (grade 0) | Uptake (grades 1–3) | P value | |
|---|---|---|---|---|
| n | 821 | 156 | 665 | |
| Age, years | 50.6 [46.9–53.6] | 50.8 [48.0–53.5] | 50.5 [46.4–53.6] | 0.196 |
| Male | 684 (83.7) | 120 (77.4) | 564 (85.2) | 0.018 |
| TBRmax | 5.10 [2.75–8.04] | 1.68 [1.36–2.15] | 5.97 [3.91–8.79] | <0.001 |
| Blood glucose before injection, mg/dL | 89 [84–95] | 92 [85–99] | 89 [83–94] | 0.001 |
| Time injection to imaging, min | 132 [124–142] | 134 [124–146] | 131 [124–141] | 0.082 |
| Cardiovascular risk factors | ||||
| Hypertension | 164 (20.1) | 45 (29.0) | 119 (18.0) | 0.002 |
| Dyslipidemia | 479 (58.6) | 86 (55.5) | 393 (59.4) | 0.377 |
| Diabetes | 38 (4.7) | 17 (11.0) | 21 (3.2) | <0.001 |
| Current smoking | 217 (27.1) | 45 (29.4) | 172 (26.5) | 0.466 |
| Family history of CV disease | 169 (20.7) | 36 (23.2) | 133 (20.1) | 0.386 |
| BMI, kg/m2 | 27.2 ± 3.6 | 27.6 ± 4.1 | 27.1 ± 3.5 | 0.096 |
| Body weight, kg | 81.6 ± 13.7 | 81.3 ± 15.3 | 81.6 ± 13.3 | 0.782 |
| Waist circumference, cm | 93.9 ± 11.1 | 94.4 ± 13.3 | 93.8 ± 10.5 | 0.558 |
| Metabolic syndrome and its components | ||||
| Metabolic syndrome | 137 (16.8) | 45 (29.0) | 92 (13.9) | <0.001 |
| Number of components | ||||
| 0 components | 261 (32.0) | 44 (28.4) | 217 (32.8) | 0.286 |
| 1 or 2 components | 418 (51.2) | 66 (42.6) | 352 (53.2) | 0.017 |
| >2 components | 137 (16.8) | 45 (29.0) | 92 (13.9) | <0.001 |
| Central obesity | 214 (26.2) | 50 (32.3) | 164 (24.8) | 0.056 |
| HDL cholesterol, mg/dL | 46.4 ± 11.4 | 46.6 ± 12.9 | 46.3 ± 11.0 | 0.810 |
| Triglycerides, mg/dL | 97 [71–131] | 111 [69–157] | 95 [72–127] | 0.022 |
| Fasting glucose, mg/dL | 91 [85–97] | 94 [87–104] | 90 [84–96] | <0.001 |
| Systolic blood pressure, mmHg | 120.6 ± 12.8 | 120.5 ± 15.2 | 120.7 ± 12.2 | 0.851 |
| Diastolic blood pressure, mmHg | 75.0 ± 9.3 | 75.9 ± 10.6 | 74.8 ± 9.0 | 0.209 |
| Treatment | ||||
| Antihypertensive therapy | 113 (13.8) | 34 (21.8) | 79 (11.9) | 0.001 |
| Lipid-lowering therapy | 120 (14.6) | 26 (16.7) | 94 (14.1) | 0.421 |
| Antidiabetic therapy | 31 (3.8) | 14 (9.0) | 17 (2.6) | <0.001 |
| Biochemistry | ||||
| Total cholesterol, mg/dL | 208.3 ± 34.8 | 204.8 ± 30.1 | 209.1 ± 35.8 | 0.161 |
| LDL cholesterol, mg/dL | 139.7 ± 30.9 | 133.4 ± 27.8 | 141.2 ± 31.4 | 0.004 |
| Hemoglobin A1c, % | 5.5 [5.2–5.7] | 5.6 [5.3–5.8] | 5.4 [5.2–5.7] | 0.001 |
| HOMA-IR, % | 1.27 [0.85–1.98] | 1.64 [0.95–2.67] | 1.23 [0.83–1.88] | <0.001 |
| Insulin, µU/mL | 5.7 [3.9–8.3] | 7.0 [4.3–10.8] | 5.6 [3.8–8.0] | 0.001 |
| Inflammatory markers | ||||
| hs-CRP, mg/dL | 0.11 [0.06–0.20] | 0.11 [0.05–0.20] | 0.10 [0.06–0.19] | 0.467 |
| Ferritin, ng/mL | 122.1 [63.0–202.8] | 107.7 [53.6–199.9] | 123.4 [66.4–202.8] | 0.377 |
| Erythrocyte sedimentation rate (1 h), mm | 5 [4–8] | 6 [4–9] | 5 [4–7] | 0.010 |
| Fibrinogen, mg/dL | 264.7 [236.0–295.3] | 266.1 [231.8–298.1] | 264.4 [236.5–295.1] | 0.990 |
| P-selectin, ng/mL | 135.2 [107.0–165.0] | 136.7 [114.0–169.6] | 134.4 [106.7–164.5] | 0.347 |
| Vascular cell adhesion molecule-1, ng/mL | 644.8 [519.6–820.0] | 640.8 [512.7–814.5] | 646.9 [522.3–822.9] | 0.569 |
| Blood count | ||||
| Red blood cell count, 106 cells/µL | 5.86 [5.01–7.02] | 6.22 [5.28–7.38] | 5.80 [4.96–6.92] | 0.002 |
| Hemoglobin, g/dL | 15.0 [14.3–15.6] | 14.8 [14.1–15.6] | 15.0 [14.3–15.7] | 0.053 |
| Platelet count, 103 cells/µL | 225 [199–256] | 227 [197–263] | 225 [199–255] | 0.500 |
| Leukocytes, 103 cells/µL | 5.86 [5.01–7.02] | 6.22 [5.28–7.38] | 5.80 [4.96–6.92] | 0.002 |
| Segmented neutrophils, 103 cells/µL | 3.33 [2.72–4.21] | 3.50 [2.83–4.51] | 3.30 [2.68–4.13] | 0.022 |
| Lymphocytes, 103 cells/µL | 1.85 [1.56–2.20] | 1.97 [1.66–2.34] | 1.83 [1.54–2.17] | 0.009 |
| Monocytes, 103 cells/µL | 0.42 [0.33–0.52] | 0.42 [0.34–0.53] | 0.41 [0.33–0.52] | 0.542 |
| Eosinophils, 103 cells/µL | 0.13 [0.08–0.20] | 0.13 [0.09–0.21] | 0.12 [0.08–0.20] | 0.180 |
| Basophils, 103 cells/µL | 0.05 [0.03–0.06] | 0.05 [0.03–0.07] | 0.05 [0.03–0.06] | 0.139 |
Data are presented as n (%) or median [Q1–Q3]. CV, cardiovascular; hs-CRP, high-sensitivity C-reactive protein.
Absence of myocardial 18F-FDG uptake was associated with higher rates of hypertension (29.0% in participants without uptake versus 18.0% in those with any degree of uptake, P = 0.002), diabetes (11.0% vs. 3.2%, P < 0.001), and MetS (29% vs. 13.9%, P < 0.001) (Table 1). Greater levels of myocardial 18F-FDG uptake were associated with lower rates of MetS (P < 0.001) (Supplementary Table 1). To better evaluate the associations between MetS traits and cardiac metabolism, participants were stratified in three groups according to the number of MetS components: 0 components, 1 or 2 components, or >2 components. Myocardial 18F-FDG uptake assessed by TBRmax significantly decreased with the increase in the number of MetS components (P < 0.001) (Supplementary Fig. 3). The grade 0 myocardial 18F-FDG uptake group also had higher plasma triglycerides (111.0 mg/dL vs. 95.0 mg/dL P = 0.022) (Table 1), higher fasting blood glucose (94 mg/dL vs. 90 mg/dL, P < 0.001), higher hemoglobin A1c (5.6% vs. 5.4%, P = 0.001), a higher insulin resistance index (HOMA-IR, 1.64% vs. 1.23%, P < 0.001), and higher plasma insulin (7.0 µU/mL vs. 5.6 µU/mL, P = 0.001). There was no difference between the no-uptake and uptake groups in family history of cardiovascular disease, smoking, or total and HDL cholesterol.
Participants with no myocardial 18F-FDG uptake had higher counts of leukocytes (6.22 × 103 cells/μL vs. 5.80 × 103, P = 0.002), neutrophils (3.50 × 103 cells/μL vs. 3.30 × 103, P = 0.022), and lymphocytes (1.97 × 103 cells/μL vs. 1.83 × 103 cells/μL, P = 0.009). Absence of myocardial 18F-FDG uptake was associated with higher erythrocyte sedimentation rates at 1 h (6 mm vs. 5 mm, P = 0.01).
When assessed using an exclusively quantitative evaluation (myocardial 18F-FDG uptake divided into quartiles of TBRmax), the same results were observed (Supplementary Table 2). To better evaluate the associations between MetS, insulin resistance, and myocardial 18F-FDG uptake, several models were performed. The associations between MetS and myocardial 18F-FDG uptake (TBRmax) remained significant after adjusting for blood glucose levels before injection and time from injection to imaging (model 1; P = 0.001); these associations were also maintained after adjusting for age and sex (model 2; P < 0.001) (Supplementary Table 3). A similar trend was observed for the association between HOMA-IR and myocardial 18F-FDG uptake when adjusted for model 1 (P = 0.059); the association between HOMA-IR and myocardial 18F-FDG uptake remained significant when adjusted by model 2 (P = 0.003) (Supplementary Table 3).
Associations Between Myocardial 18F-FDG Uptake and Uptake in the Arteries and Bone Marrow
Vascular and bone marrow 18F-FDG uptake patterns in the different myocardial uptake groups are summarized in Supplementary Tables 4 and 5. Participants without myocardial 18F-FDG uptake had a higher prevalence of early atherosclerosis (i.e., arterial 18F-FDG uptake, 58.1% vs. 45.4% in the myocardial 18F-FDG uptake group, P = 0.004), and had more arterial segments with vascular 18F-FDG uptake (1 vs. 0, P = 0.003) and a higher overall arterial SUVmax (1.43 vs. 1.37, P = 0.019). Bone marrow activation was significantly more prevalent in participants without myocardial 18F-FDG uptake (60.3% vs. 47.5%, P = 0.007), and these participants tended to have higher SUVmax values in the bone marrow (1.97 vs. 1.88 in individuals with myocardial 18F-FDG uptake, P = 0.051).
Characteristics at Follow-up in Relation to Myocardial 18F-FDG Uptake
Characteristics of the population at follow-up are summarized in Supplementary Table 6. Seventy participants (10.0%) had no myocardial 18F-FDG uptake (grade 0). Myocardial 18F-FDG uptake in the remaining participants (n = 630, 90.0%) was classified in grades 1–3 according to TBRmax tertiles (Supplementary Table 7).
Most of the associations observed at baseline were replicated at follow-up. Absence of myocardial 18F-FDG uptake was associated with higher rates of diabetes (22.7% vs. 3.9% in those with any degree of uptake, P < 0.001), higher BMI (28.5 kg/m2 vs. 27.1 kg/m2, P = 0.004), and larger waist circumference (98.9 cm vs. 95.6 cm, P = 0.024) and with a trend to higher rates of MetS (24.2% vs. 15.0%, P = 0.051) (Supplementary Table 6). Greater levels of myocardial 18F-FDG uptake were associated with lower rates of MetS (P = 0.001) (Supplementary Table 7), and myocardial 18F-FDG uptake assessed by TBRmax significantly decreased with the increase in the number of MetS components (P = 0.005) (Supplementary Table 8). The grade 0 myocardial 18F-FDG uptake group also had higher plasma triglycerides (126.0 mg/dL vs. 91.0 mg/dL P = 0.001) (Supplementary Table 6), higher fasting blood glucose (96 mg/dL vs. 88 mg/dL, P < 0.001), higher hemoglobin A1c (5.6% vs. 5.4%, P < 0.001), a higher insulin resistance index (HOMA-IR, 2.05% vs. 1.35%, P < 0.001), and higher plasma insulin (8.5 µU/mL vs. 6.3 µU/mL, P < 0.001). The group with no uptake showed lower levels of HDL-cholesterol (47.8 mg/dL vs. 51.4 mg/dL, P = 0.032). There was no difference between the no-uptake and uptake groups in family history of cardiovascular disease, smoking, or total and LDL cholesterol.
When assessed using an exclusively quantitative evaluation (myocardial 18F-FDG uptake divided into quartiles of TBRmax), the same results were observed (Supplementary Table 9). The associations between MetS and myocardial 18F-FDG uptake were maintained after adjusting by model 1 (P = 0.050) and model 2 (P = 0.012), and so were the associations between HOMA-IR and myocardial 18F-FDG uptake (model 1: P = 0.001; model 2: P < 0.001) (Supplementary Table 3).
Longitudinal Assessment of Myocardial FDG Uptake
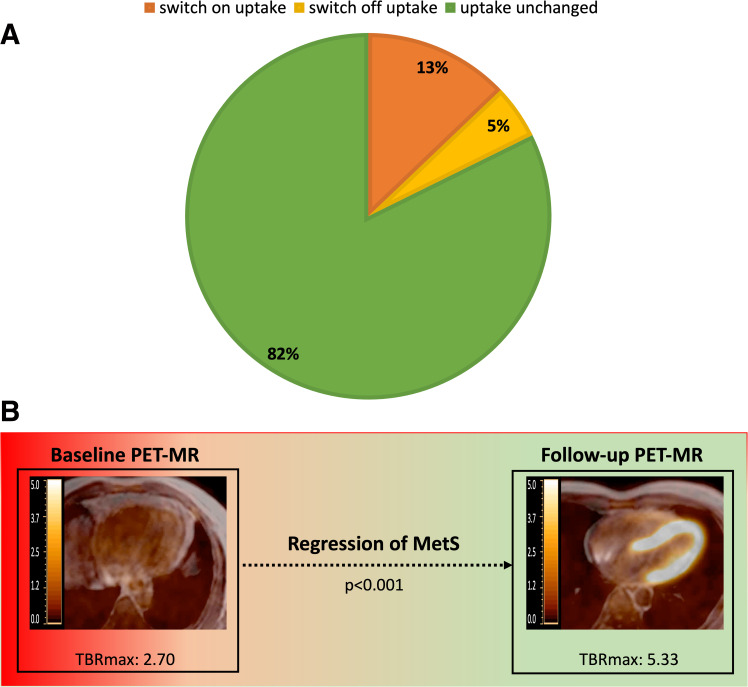
PET-MR was available at both baseline and follow-up in 612 participants (87.4% of 700 PET-MR at follow-up). At follow-up, presence of myocardial 18F-FDG uptake did not change in 82.0% of participants (n = 502); in 81 participants (13.2%) who did not have uptake at baseline, myocardial 18F-FDG uptake was present at follow-up; finally, myocardial 18F-FDG uptake disappeared in 29 participants (4.7%) at follow-up (Fig. 1A). Forty-six participants (7.9%) met MetS criteria both at baseline and follow-up; MetS had regressed in 45 participants (7.7%) and appeared de novo in 50 (8.6%) participants at follow-up (Supplementary Table 10). MetS regression at follow-up was associated with an increase in myocardial 18F-FDG uptake (TBRmax: 2.70 at baseline vs. 5.33 at follow-up, P < 0.001), while myocardial 18F-FDG uptake did not significantly change when MetS was present at both baseline and follow-up (Fig. 1B and Supplementary Table 10). The presence of MetS at baseline was associated with less myocardial 18F-FDG uptake at follow-up (P = 0.015), and this association was maintained after adjusting by model 1 (P = 0.05) and model 2 (P = 0.012) (Supplementary Table 3).
Figure 1.
Progression of myocardial 18F-FDG uptake progression according to changes in cardiometabolic profile. A: Progression of myocardial 18F-FDG uptake from baseline to follow-up. B: Regression of MetS is associated with an increase in myocardial 18F-FDG uptake.
Conclusions
The current study evaluated cardiac metabolism by means of myocardial 18F-FDG uptake in a population of apparently healthy individuals. Absence of myocardial 18F-FDG uptake was associated with the presence of MetS and cardiovascular risk factors, as well as with bone marrow activation and early atherosclerosis. The same associations were observed when evaluated at 5-year follow-up, which allowed confirmation of the findings. At follow-up, presence of myocardial uptake was unchanged in the great majority of participants; MetS regression at follow-up was associated with an increase in myocardial 18F-FDG uptake.
The study of cardiac metabolism in humans is possible by means of myocardial 18F-FDG uptake evaluation (9). Some studies using cardiac 18F-FDG PET have been performed in oncology patients; however, the results are heterogenous and might not be extrapolated to healthy populations (11,12). Most cardiac 18F-FDG PET studies are performed to evaluate different cardiac conditions, particularly inflammatory diseases, for which an aggressive preparation is required in order to annul myocardial physiological glucose uptake, and thus do not provide information on cardiac basal metabolism. In contrast, our study was performed in apparently healthy individuals with no modification of metabolic conditions before the study, thus reflecting homeostatic cardiac status.
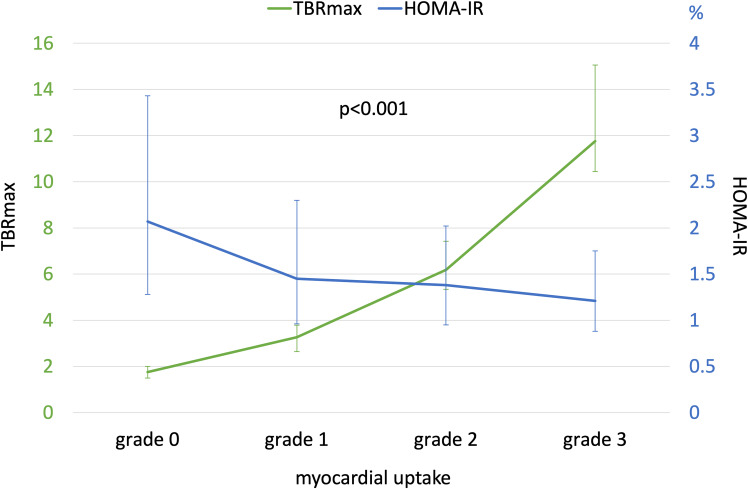
Several preclinical studies have postulated that there is an association between MetS and an abnormal myocardial metabolism. The proposed mechanisms are impaired myocardial sensitivity to insulin, altered myocardial substrate utilization, and abnormal cardiac mitochondrial function (3,4,29). Experimental models of MetS have shown that the heart defends against MetS by severely altering glycolysis and activating alternative pathways for energy production (5,6). Clinical data regarding the associations between MetS and cardiac metabolism are limited; one small study using 18F-FDG PET observed that, among subjects with type 2 diabetes, those with MetS showed a reduction in myocardial glucose metabolic rate as compared with those without MetS (30). The role of insulin resistance in altered myocardial metabolism has been previously suggested in clinical studies; indeed, one study using endomyocardial biopsies showed that myocardial mitochondrial oxidative capacity for different substrates was lower in a group with impaired glucose tolerance among subjects without apparent heart failure (31,32). Myocardial insulin resistance might also explain why studies in diabetes have shown a decrease in myocardial 18F-FDG uptake in patients with diabetes compared with subjects without diabetes (13–18). Our results support these observations, as they demonstrate that variations in myocardial 18F-FDG uptake are associated with MetS traits and diabetes in the PESA cohort (apparently healthy individuals). As postulated by experimental models, MetS might alter cardiac metabolism and increase the utilization of alternative energy substrates other than glucose by the myocardium, which might explain our findings (i.e., lower myocardial 18F-FDG uptake in MetS). The contributory role of the different components of the MetS is well reflected by a progressive reduction in myocardial 18F-FDG uptake as the number of components of the MetS increases (Supplementary Fig. 3). In our study, insulin resistance (assessed by HOMA-IR) also showed an association with cardiac metabolism; the increase in HOMA-IR was associated with a progressive decrease in myocardial 18F-FDG uptake (Fig. 2).
Figure 2.
Myocardial 18F-FDG uptake and insulin resistance.
In the present work, the study population comprised apparently healthy individuals with no history of heart failure or cardiomyopathy. The same population underwent a cardiac MRI study at a previous time point of the study (23), and no overt structural or functional cardiac abnormality was observed. Lower insulin-stimulated myocardial glucose metabolic rate evaluated by myocardial 18F-FDG uptake has been associated with reduced cardiac mechano-energetic efficiency, which represents the capability of the myocardium to convert energy into mechanical work (33). A reduced myocardial energetic efficiency has, in turn, been associated with incident heart failure (34) and cardiovascular events (35). A reduction in myocardial 18F-FDG uptake may indicate a very early stage of myocardial energy disruption, although further studies are warranted to understand these mechanisms.
Moreover, myocardial 18F-FDG uptake was associated with lower prevalence of bone marrow activation (indexed by elevated 18F-FDG uptake in the bone marrow [24]) as well as with lower prevalence of early atherosclerosis, as indexed by elevated arterial metabolic activity (arterial 18F-FDG uptake). Bone marrow activation is triggered by cardiovascular risk factors and metabolic syndrome, it is implicated in the atherosclerotic process long before the appearance of acute cardiovascular events, and it is considered an early phenomenon in the atherosclerosis development (24). As bone marrow activation progresses, it is accompanied by an increase in hematopoietic progenitor cells and inflammatory markers that leads to the very early stages of atherosclerosis (increased arterial 18F-FDG uptake [20]) and, ultimately, to overt atherosclerotic plaque (24). Overall, our data suggest that greater 18F-FDG uptake in the heart (as opposed to absence of uptake) in steady-state conditions is a marker of a healthy cardiovascular state.
In this study, we were able to replicate the results in a very large population at 5-year follow-up under similar conditions, which allowed the evaluation of cardiac metabolism at its physiological state at two time points. Since MetS and insulin resistance increase with age and male sex (36), we evaluated the associations between MetS, HOMA-IR, and myocardial 18F-FDG uptake after adjusting by age and sex, and we confirmed that they were maintained. As previous studies have reported (37), some intraindividual variability in myocardial 18F-FDG uptake was observed between the baseline and follow-up scans (18%); however, these changes were associated with a change in the cardiometabolic risk profile. In the study population, blood glucose measured just before PET-MR was higher in the group with no myocardial 18F-FDG uptake than in participants with any degree of uptake. Moreover, the associations between MetS, HOMA-IR, and myocardial 18F-FDG uptake were maintained after adjusting by blood glucose before injection. Myocardial 18F-FDG uptake is therefore not explained by elevated blood glucose before the procedure, which is in agreement with previous studies (38).
These results suggest that, as the cardiovascular risk profile worsens, myocardial 18F-FDG uptake declines, being lost as the risk profile deteriorates further. Based on both qualitative and quantitative measurements of myocardial 18F-FDG uptake, we identified three phenotypes. A healthy cardiometabolic risk profile is associated with low prevalence of early atherosclerosis and bone marrow activation and greater myocardial 18F-FDG uptake (i.e., normal myocardial substrate utilization). At the other extreme is the phenotype characterized by no cardiac 18F-FDG uptake (i.e., abnormal glucose consumption by the myocardium), which coincides with the poorest cardiometabolic profile and the highest prevalence rates of early atherosclerosis and bone marrow activation. Between these extremes is an intermediate phenotype characterized by intermediate grades of myocardial 18F-FDG uptake (Graphical Abstract).
Studies of the PESA cohort have confirmed that cardiovascular risk factors impact metabolism in distinct organs, establishing associations between a poor cardiovascular risk profile and vascular inflammation (20), global brain hypometabolism (39), and bone marrow reactive activation (24). The current study extends these findings, demonstrating the associations between cardiometabolic risk factors and the metabolism of the heart. Interestingly, the association between cardiometabolic profile and organ 18F-FDG uptake does not always go in the same direction; our results show that risk factors are associated with increased bone marrow 18F-FDG uptake but with decreased myocardial uptake.
Study Limitations
The selection of participants from the PESA cohort to undergo whole-body 18F-FDG PET-MR was based on the presence of subclinical atherosclerosis on baseline vascular ultrasound. Around 20% of participants invited to participate declined enrollment or had MR contraindications, comparable to similar studies (40). As previously reported (20), the first 97 PET studies could not be used because of inaccuracies in MR-based attenuation and reconstruction; however, feasibility was almost 100% thereafter. This is, to our knowledge, the largest study to date of myocardial 18F-FDG-PET-MR performed in healthy individuals. Unlike previous studies performed in cancer or cardiology patients, the current study provides information on an apparently healthy middle-aged population, without aggressive pre-exam preparation, thus allowing the study of cardiac metabolism under physiological conditions.
In conclusion, in apparently healthy individuals, absence of myocardial 18F-FDG uptake is associated with a poorer cardiometabolic risk profile (mainly metabolic syndrome and insulin resistance). Absence of myocardial 18F-FDG uptake was further associated with higher prevalence of early atherosclerosis and bone marrow activation. Changes in myocardial 18F-FDG uptake are associated with changes in the cardiometabolic risk profile.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24059127.
Article Information
Acknowledgments. Simon Bartlett (Centro Nacional de Investigaciones Cardiovasculares) provided English editing.
Funding. The PESA study is funded by the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and Santander Bank. B.I. is supported by the European Commission (grant numbers 819775 and 945118), by the Spanish Ministry of Science and Innovation (PID2019-110369RB-I00), and by the Red Madrileña de Nanomedicina en Imagen Molecular-Comunidad de Madrid (S2017/BMD-3867 RENIM-CM). A.D. is an Alfonso Martin Escudero fellow and is scientifically supported by La Caixa Foundation. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación (MCIN), and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033).
Duality of Interest. J.S.-G. is a Philips employee. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.D. researched data, contributed to discussion, and wrote the first draft of the manuscript. V.F., R.V., I.G.-L., B.O., S.E., A.G.-A., M.G.T., J.S.-G., and B.I. researched data, contributed to discussion, and reviewed and edited the manuscript. A.M.-A., J.S., C.P.-H., H.B., E.L.-P., V.M.d.V., L.F.-F., A.F.-O., and X.R. reviewed and edited the manuscript. All authors approved the final version of the manuscript. B.I. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
The PESA study is funded by the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and Santander Bank. B.I. is supported by the European Commission (grant numbers 819775 and 945118), by the Spanish Ministry of Science and Innovation (PID2019-110369RB-I00), and by the Red Madrileña de Nanomedicina en Imagen Molecular-Comunidad de Madrid (S2017/BMD-3867 RENIM-CM). A.D. is an Alfonso Martin Escudero fellow and is scientifically supported by La Caixa Foundation. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación (MCIN), and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033).
Footnotes
Clinical trial reg. no. NCT01410318, clinicaltrials.gov
References
- 1. Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol 1994;19:59–113 [DOI] [PubMed] [Google Scholar]
- 2. Taegtmeyer H, Lubrano G. Rethinking cardiac metabolism: metabolic cycles to refuel and rebuild the failing heart. F1000Prime Rep 2014;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ilkun O, Wilde N, Tuinei J, et al. Antioxidant treatment normalizes mitochondrial energetics and myocardial insulin sensitivity independently of changes in systemic metabolic homeostasis in a mouse model of the metabolic syndrome. J Mol Cell Cardiol 2015;85:104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Landa-Galvan HV, Rios-Castro E, Romero-Garcia T, Rueda A, Olivares-Reyes JA. Metabolic syndrome diminishes insulin-induced Akt activation and causes a redistribution of Akt-interacting proteins in cardiomyocytes. PLoS One 2020;15:e0228115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karimi M, Petkova V, Asara JM, et al. Metabolomics and the pig model reveal aberrant cardiac energy metabolism in metabolic syndrome. Sci Rep 2020;10:3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ménard SL, Croteau E, Sarrhini O, et al. Abnormal in vivo myocardial energy substrate uptake in diet-induced type 2 diabetic cardiomyopathy in rats. Am J Physiol Endocrinol Metab 2010;298:E1049–E1057 [DOI] [PubMed] [Google Scholar]
- 8. Ouwens DM, Diamant M, Fodor M, et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 2007;50:1938–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peterson LR, Gropler RJ. Radionuclide imaging of myocardial metabolism. Circ Cardiovasc Imaging 2010;3:211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu Y, Grant C, Xie K, Sweiss NJ. Suppression of myocardial 18F-FDG uptake through prolonged high-fat, high-protein, and very-low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. Clin Nucl Med 2017;42:88–94 [DOI] [PubMed] [Google Scholar]
- 11. Inglese E, Leva L, Matheoud R, et al. Spatial and temporal heterogeneity of regional myocardial uptake in patients without heart disease under fasting conditions on repeated whole-body 18F-FDG PET/CT. J Nucl Med 2007;48:1662–1669 [DOI] [PubMed] [Google Scholar]
- 12. Maurer AH, Burshteyn M, Adler LP, Gaughan JP, Steiner RM. Variable cardiac 18FDG patterns seen in oncologic positron emission tomography computed tomography: importance for differentiating normal physiology from cardiac and paracardiac disease. J Thorac Imaging 2012;27:263–268 [DOI] [PubMed] [Google Scholar]
- 13. Ozguven MA, Karacalioglu AO, Ince S, Emer MO. Altered biodistribution of FDG in patients with type-2 diabetes mellitus. Ann Nucl Med 2014;28:505–511 [DOI] [PubMed] [Google Scholar]
- 14. Kim G, Jo K, Kim KJ, et al. Visceral adiposity is associated with altered myocardial glucose uptake measured by 18FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc Diabetol 2015;14:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yokoyama I, Yonekura K, Ohtake T, et al. Role of insulin resistance in heart and skeletal muscle F-18 fluorodeoxyglucose uptake in patients with non-insulin-dependent diabetes mellitus. J Nucl Cardiol 2000;7:242–248 [DOI] [PubMed] [Google Scholar]
- 16. Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes 2002;51:3020–3024 [DOI] [PubMed] [Google Scholar]
- 17. Hu L, Qiu C, Wang X, Xu M, Shao X, Wang Y. The association between diabetes mellitus and reduction in myocardial glucose uptake: a population-based 18F-FDG PET/CT study. BMC Cardiovasc Disord 2018;18:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen R, Jorsal A, Iversen P, et al. Heart failure patients with prediabetes and newly diagnosed diabetes display abnormalities in myocardial metabolism. J Nucl Cardiol 2018;25:169–176 [DOI] [PubMed] [Google Scholar]
- 19. Fernández-Ortiz A, Jiménez-Borreguero LJ, Peñalvo JL, et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J 2013;166:990–998 [DOI] [PubMed] [Google Scholar]
- 20. Fernández-Friera L, Fuster V, López-Melgar B, et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol 2019;73:1371–1382 [DOI] [PubMed] [Google Scholar]
- 21. Ibanez B, Fernández-Ortiz A, Fernández-Friera L, García-Lunar I, Andrés V, Fuster V. Progression of Early Subclinical Atherosclerosis (PESA) study: JACC Focus Seminar 7/8. J Am Coll Cardiol 2021;78:156–179 [DOI] [PubMed] [Google Scholar]
- 22. Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation 2015;131:2104–2113 [DOI] [PubMed] [Google Scholar]
- 23. de la Chica JA, Gómez-Talavera S, García-Ruiz JM, et al. Association between left ventricular noncompaction and vigorous physical activity. J Am Coll Cardiol 2020;76:1723–1733 [DOI] [PubMed] [Google Scholar]
- 24. Devesa A, Lobo-González M, Martínez-Milla J, et al. Bone marrow activation in response to metabolic syndrome and early atherosclerosis. Eur Heart J 2022;43:1809–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva, World Health Org., 2000. (Tech Rep. Ser., no. 894) [PubMed] [Google Scholar]
- 26. Swarup S, Goyal A, Grigorova Y, Zeltser R. Metabolic syndrome. Accessed 2 May 2023. Available from Accessed 2 May 2023. https://www.ncbi.nlm.nih.gov/books/NBK459248/
- 27. Bucerius J, Mani V, Moncrieff C, et al. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: the impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. Eur J Nucl Med Mol Imaging 2013;41:369–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maier A, Liao SL, Lescure T, et al. Pulmonary artery 18F-fluorodeoxyglucose uptake by PET/CMR as a marker of pulmonary hypertension in sarcoidosis. JACC Cardiovasc Imaging 2022;15:108–120 [DOI] [PubMed] [Google Scholar]
- 29. Ilkun O, Boudina S. Cardiac dysfunction and oxidative stress in the metabolic syndrome: an update on antioxidant therapies. Curr Pharm Des 2013;19:4806–4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Succurro E, Vizza P, Papa A, et al. Metabolic syndrome is associated with impaired insulin-stimulated myocardial glucose metabolic rate in individuals with type 2 diabetes: a cardiac dynamic 18F-FDG-PET study. Front Cardiovasc Med 2022;9:924787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zweck E, Scheiber D, Schultheiss HP, et al. Impaired myocardial mitochondrial respiration in humans with prediabetes: a footprint of prediabetic cardiomyopathy. Circulation 2022;146:1189–1191 [DOI] [PubMed] [Google Scholar]
- 32. Zweck E, Scheiber D, Jelenik T, et al. Exposure to type 2 diabetes provokes mitochondrial impairment in apparently healthy human hearts. Diabetes Care 2021;44:e82–e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Succurro E, Cicone F, Papa A, et al. Impaired insulin-stimulated myocardial glucose metabolic rate is associated with reduced estimated myocardial energetic efficiency in subjects with different degrees of glucose tolerance. Cardiovasc Diabetol 2023;22:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Losi MA, Izzo R, Mancusi C, et al. Depressed myocardial energetic efficiency increases risk of incident heart failure: The Strong Heart Study. J Clin Med 2019;8:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Simone G, Izzo R, Losi MA, et al. Depressed myocardial energetic efficiency is associated with increased cardiovascular risk in hypertensive left ventricular hypertrophy. J Hypertens 2016;34:1846–1853 [DOI] [PubMed] [Google Scholar]
- 36. Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care 2010;33:2457–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thut DP, Ahmed R, Kane M, Djekidel M. Variability in myocardial metabolism on serial tumor (18)F-FDG PET/CT scans. Am J Nucl Med Mol Imaging 2014;4:346–353 [PMC free article] [PubMed] [Google Scholar]
- 38. de Groot M, Meeuwis APW, Kok PJM, Corstens FHM, Oyen WJG. Influence of blood glucose level, age and fasting period on non-pathological FDG uptake in heart and gut. Eur J Nucl Med Mol Imaging 2005;32:98–101 [DOI] [PubMed] [Google Scholar]
- 39. Cortes-Canteli M, Gispert JD, Salvadó G, et al. Subclinical atherosclerosis and brain metabolism in middle-aged individuals: The PESA study. J Am Coll Cardiol 2021;77:888–898 [DOI] [PubMed] [Google Scholar]
- 40. Fayad ZA, Mani V, Woodward M, et al.; dal-PLAQUE Investigators . Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011;378:1547–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]