Abstract
Seasonal changes in the abundance of inducible lysogenic bacteria in a eutrophic estuarine environment were investigated over a 13-month period. Biweekly water samples were collected from Tampa Bay, Fla., and examined for prophage induction by mitomycin C treatment. At the conclusion of the study, we determined that 52.2% of the samples displayed prophage induction, as indicated by significant increases in viral direct counts compared with uninduced controls. Samples that displayed prophage induction occurred during the warmer months (February through October), when surface water temperatures were above 19°C, and no induction was observed in November, December, or January. This study presents clear evidence that there is seasonal variation in the number of inducible lysogenic bacteria in an estuarine environment.
Lysogeny is the process by which a DNA-containing phage maintains a stable symbiosis with its host and is the alternative to lytic replication (1). Lysogeny can be achieved by integration of the viral genome into the host chromosome and transmission of the integrated DNA (termed a prophage) to progeny cells during division. Alternatively, lysogeny can occur by plasmid formation, and the viral DNA is then perpetuated as an extrachromosomal element. A cell carrying a prophage is termed a lysogen (1). The lysogenic state may continue for many generations until the prophage is spontaneously activated or until induction by mutagenic agents, such as UV radiation or mitomycin C, occurs (1). The inducing agents then trigger the host’s DNA repair mechanisms and cause initiation of viral replication and host lysis (1).
Wilcox and Fuhrman (13) and Weinbauer and Suttle (12) reported that the majority of viruses found in the marine environment are lytic and that lysogenic bacteriophages are quantitatively insignificant in coastal waters. Jiang and Paul (7), however, showed that 43% of the bacteria isolated from various marine environments were lysogenic, as determined by prophage induction with mitomycin C. An examination of natural bacterial populations that were inducible by mitomycin C indicated that the proportion of bacteria that were lysogenic ranged from 1.5 to 38% (8). We extended these studies to determine the relative proportion of lysogenic bacteria as a function of season in the estuarine environment of Tampa Bay, Fla., with the intention of relating abundance to various physical, chemical, and biological parameters. We show here that there is a strong seasonal variation in the number of inducible lysogens in this subtropical estuarine environment.
Sampling sites.
Water samples were collected biweekly from Tampa Bay, Fla., at the St. Petersburg city pier for 13 months, from October 1995 to October 1996. The site selected for sampling was on the western shore of the bay and about midway down the bay and was influenced by urban runoff from the city of St. Petersburg.
Induction of lysogenic bacteria by mitomycin C.
Replicate seawater samples (25 ml) either were treated by adding mitomycin C (1 μg ml−1) or were left untreated (controls). Both types of samples were incubated for 24 h at room temperature in the dark and then fixed with 2% glutaraldehyde and stored at 4°C before bacterial direct counts and viral direct counts were determined. The fixed samples used for viral enumeration were concentrated by ultracentrifugation onto 200-mesh Formvar-coated copper grids at 160,070 × g for 60 min at 4°C (2, 3). The grids were then negatively stained with 2% uranyl sulfate (Polysciences, Inc., Warrington, Pa.), rinsed with distilled water, and air dried. Viruslike particles were counted at a magnification of ×50,000 with a Hitachi model 7100 transmission electron microscope. The samples used for bacterial enumeration were stained with 2,4-diamidino-2-phenylindole (DAPI) (Sigma Chemical Co., St. Louis, Mo.) overnight, and 1 ml of each sample was filtered onto an Irgalan black-stained, 25-mm-diameter, 0.2-μm-pore-size Nuclepore filter and counted by epifluorescence microscopy as described elsewhere (9).
Chlorophyll a determination.
Water samples (50 ml) for a chlorophyll a analysis were filtered onto Whatman type GF/F filters and stored frozen until analysis. The filters were extracted in the dark overnight in methanol, and the chlorophyll a content was fluorometrically determined by the method of Holm-Hansen and Riemann (6).
Statistical analysis.
Multisample regression and comparison by analysis of variance and two-sample comparison with a t test were performed by using Statgraphics software (Manugistics Inc., Rockville, Md.). Statistical comparisons of viral direct count and bacterial direct count data were performed by using the averages of the values from four grids per replicate slide per sample.
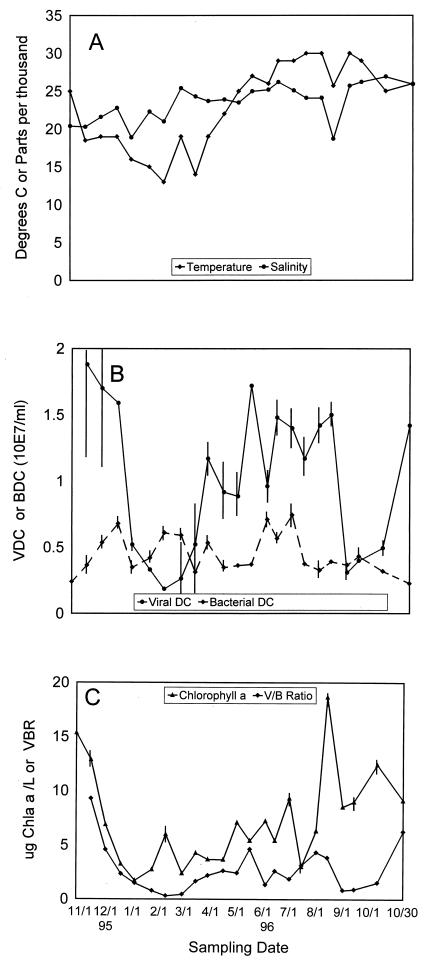
Figure 1A shows the seasonal variations in salinity and temperature in Tampa Bay. The water temperature dropped 7°C between the first two sampling dates and continued to drop to an unseasonably low 13°C during February. In general, the water temperatures were below 20°C for the winter season and began to increase in April. Salinity followed rainfall, which was quite erratic during the winter and spring of 1996 (data not shown). The peaks in salinity during the first half of the study were associated with months in which there was very little rainfall. The summer rains, which normally decrease salinity during the summer, were not as great as in previous years (the 1995 rainfall for June through October was 46.37 in., and the 1996 rainfall for June through October was 27.33 in. [National Climatic Data Center, Asheville, N.C.]).
FIG. 1.
(A) Seasonal variation in salinity and temperature. Both parameters were measured at the time of sample collection. (B) Total viral direct counts (VDC) and total bacterial direct counts (BDC) measured during the 13-month seasonal study. Viral direct counts were determined by negative staining and transmission electron microscopy. Bacterial direct counts were determined by DAPI staining and epifluorescence microscopy. DC, direct counts. (C) Chlorophyll a (Chla) concentrations and VBR (V/B Ratio) in Tampa Bay during the 13-month seasonal study.
A seasonal pattern in viral and bacterial abundance was observed (Fig. 1B), and the virus concentrations were lowest in the winter and highest in late fall and summer. There was a decrease in viral abundance (from 1.59 × 107 to 0.52 × 107 viruses ml−1) between December and January, which corresponded with a decrease in water temperature. This trend continued until the first February sample, which contained the lowest concentration of viruses (0.186 × 107 viruses ml−1) and had the lowest recorded water temperature (13°C). An increase in bacterial direct counts coincided with a decrease in viral direct counts during the winter, with the bacterial numbers increasing from 2.4 × 106 to 6.79 × 106 cells ml−1 and the viral numbers decreasing from 1.88 × 107 to 0.52 × 107 viruses ml−1. The increase in bacterial abundance was preceded by increases in the water temperature from 13 to 27°C by the end of May. The average bacterial direct count in this study was 4.42 × 106 ± 1.5 × 106 cells ml−1.
The chlorophyll a values were lowest during the winter months (3.85 ± 1.67 μg liter−1) and highest during the summer months (9.27 ± 4.25 μg liter−1) (Fig. 1C). A chlorophyll a peak (6 ± 0.54 μg liter−1) was observed with the first February sample, which coincided with the coldest water temperature recorded (13°C) and the lowest viral direct count (0.186 × 107 viruses ml−1).
The virus-to-bacterium ratio (VBR) (5, 14) is used to characterize the relationship between bacterial and viral communities. In the seasonal study, the VBR ranged from 0.305 to 9.29, with the highest values obtained at the two October sampling times (Fig. 1C). The seasonal pattern of the VBR most closely resembled the seasonal distribution of the viral direct counts (Fig. 1B), with the highest VBR coinciding with the highest viral counts and the lowest VBR coinciding with the lowest temperature recorded (13°C) and the lowest viral direct counts (0.186 × 107 viruses ml−1).
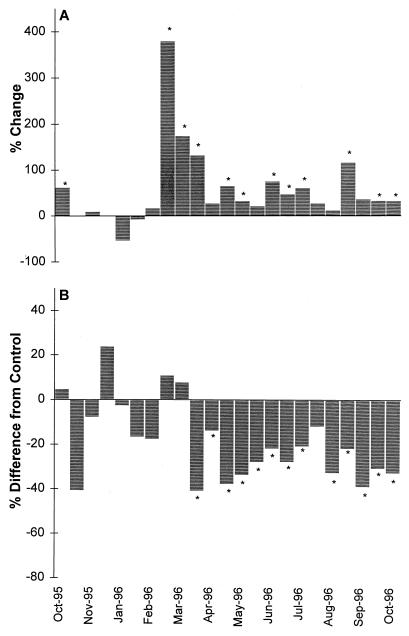
Figure 2 and Table 1 show the results of induction by mitomycin C of indigenous lysogenic bacteria from the Tampa Bay sampling site. Based on our previous criterion for prophage induction in environmental samples, whereby an increase in phage counts must be accompanied by a decrease in bacterial counts (8), 39% of the samples exhibited prophage induction. However, we feel that small changes in bacterial direct counts caused by prophage induction might not be detected by the methods currently used for bacterial enumeration. Therefore, we assumed that positive induction occurred when there was a significant increase in viral direct counts compared with the control. On the basis of this criterion, 52.2% of the samples resulted in prophage induction. If all decreases in bacterial concentration in response to mitomycin C were considered prophage-induced lysis, then 0 to 41% of the bacterial population contained inducible prophage (Table 1).
FIG. 2.
(A) Percent change in viral abundance during the seasonal study. The percent change was calculated by dividing the mitomycin C-treated sample value by the control sample value and subtracting 100 to determine the percent increase or decrease in relation to the control sample. (B) Percent difference in bacterial direct counts from the control sample value. The percent decrease was calculated by subtracting the ratio of mitomycin C-treated bacterial direct counts to control bacterial direct counts from 100. Statistically significant increases or decreases (95% confidence interval) are indicated by asterisks.
TABLE 1.
Induction of indigenous lysogenic bacteria present in seasonal samples from the St. Petersburg city pier, Tampa Bay, Fla.a
| Date (mo/day/yr) | Sample | Bacterial direct counts (n = 4)
|
Viral direct counts (n = 4)
|
% Lysogenic bacteria calculated by:
|
|||
|---|---|---|---|---|---|---|---|
| Cells/ml (106) | % of control | Viruses/ml (107) | % of control | Mortality | Avg burst size | ||
| 10/27/95 | Control | 1.31 ± 0.3 | 2.37 ± 0.58 | ||||
| Mitomycin C | 105 (I)a | 162 (S) | NAb | 37.3 | |||
| 11/13/95 | Control | 3.63 ± 1.15 | 3.39 ± 0.38 | ||||
| Mitomycin C | 59 (I) | 101 (I) | NA | NA | |||
| 11/30/95 | Control | 4.58 ± 0.79 | 2.45 ± 0.52 | ||||
| Mitomycin C | 92 (I) | 110 (I) | NA | NA | |||
| 12/18/95 | Control | 4.78 ± 0.70 | 3.50 ± 0.69 | ||||
| Mitomycin C | 124 (I) | 100 (I) | NA | NA | |||
| 1/13/96 | Control | 3.24 ± 0.81 | 1.44 ± 0.62 | ||||
| Mitomycin C | 97 (I) | 46 (I) | NA | NA | |||
| 1/23/96 | Control | 4.32 ± 1.0 | 1.20 ± 0.5 | ||||
| Mitomycin C | 83 (I) | 92 (I) | NA | NA | |||
| 2/8/96 | Control | 4.64 ± 0.87 | 2.2 ± 0.4 | ||||
| Mitomycin C | 82 (I) | 117 (I) | NA | NA | |||
| 2/27/96 | Control | 3.34 ± 0.63 | 0.387 ± 0.058 | ||||
| Mitomycin C | 111 (I) | 479 (S) | NA | 14.6 | |||
| 3/14/96 | Control | 2.16 ± 0.29 | 1.31 ± 0.73 | ||||
| Mitomycin C | 108 (I) | 274 (S) | NA | 35.2 | |||
| 3/28/96 | Control | 2.77 ± 0.40 | 1.32 ± 0.67 | ||||
| Mitomycin C | 59 (S) | 232 (S) | 41 | 21.1 | |||
| 4/15/96 | Control | 3.16 ± 0.11 | 2.20 ± 0.49 | ||||
| Mitomycin C | 85 (S) | 127 (I) | NA | NA | |||
| 5/1/96 | Control | 2.85 ± 0.36 | 1.29 ± 0.59 | ||||
| Mitomycin C | 62 (S) | 165 (S) | 38 | 9.7 | |||
| 5/16/96 | Control | 3.26 ± 0.63 | 1.84 ± 0.19 | ||||
| Mitomycin C | 66 (S) | 132 (S) | 34 | 6.03 | |||
| 6/3/96 | Control | 6.48 ± 0.73 | 0.968 ± 0.17 | ||||
| Mitomycin C | 72 (S) | 121 (I) | NA | NA | |||
| 6/14/96 | Control | 3.52 ± 0.26 | 1.48 ± 0.16 | ||||
| Mitomycin C | 78 (S) | 175 (S) | 22 | 10.5 | |||
| 7/1/96 | Control | 7.64 ± 1.0 | 1.43 ± 0.39 | ||||
| Mitomycin C | 72 (S) | 147 (S) | 28 | 2.9 | |||
| 7/15/96 | Control | 4.66 ± 0.53 | 1.40 ± 0.25 | ||||
| Mitomycin C | 79 (S) | 161 (S) | 21 | 6.1 | |||
| 8/1/96 | Control | 2.79 ± 0.22 | 2.61 ± 0.73 | ||||
| Mitomycin C | 88 (I) | 127 (I) | NA | NA | |||
| 8/14/96 | Control | 2.2 ± 0.20 | 1.79 ± 0.34 | ||||
| Mitomycin C | 67 (S) | 112 (I) | NA | NA | |||
| 9/1/96 | Control | 2.48 ± 0.05 | 0.382 ± 0.20 | ||||
| Mitomycin C | 78 (S) | 216 (S) | 22 | 5.9 | |||
| 9/14/96 | Control | 4.26 ± 0.46 | 0.80 ± 0.30 | ||||
| Mitomycin C | 61 (S) | 136 (I) | NA | NA | |||
| 10/11/96 | Control | 3.28 ± 0.42 | 0.634 ± 0.14 | ||||
| Mitomycin C | 69 (S) | 133 (S) | 31 | 2.1 | |||
| 10/30/96 | Control | 1.79 ± 0.41 | 1.31 ± 0.28 | ||||
| Mitomycin C | 67 (S) | 132 (S) | 33 | 7.8 | |||
I, not statistically significant at a 95% confidence interval; S, statistically significant at a 95% confidence interval.
NA, not applicable.
All of the samples that displayed positive induction were obtained during the summer and fall of the seasonal study, which suggests that temperature or some other biological factor controlled by temperature may control prophage induction. The estimated burst sizes (based on changes in viral direct counts divided by changes in bacterial direct counts) for these samples ranged from 2.1 to 15.5. A second approach used to determine the percentage of lysogenic bacteria in the marine environment is to assume an average burst size (ca. 30) and calculate the number of lysogens by dividing the increased virus numbers by this burst size (8). The percentage of lysogenic bacteria calculated from the average burst size ranged from 2.1 to 37.3%. The results of both methods used to determine the percentage of lysogenic bacteria are presented in Table 1.
The multiple regression analysis of the seasonal study data showed that the percent increase in virus abundance was positively correlated with salinity (r = 0.52), with positive induction occurring at salinities above 23 ppt and only one sample induced below this level of salinity. Salinity was also positively correlated with temperature (r = 0.65). These results imply that the variation in viral abundance could be explained by the variation in salinity and temperature and are consistent with previous reports for this marine environment (7). The induced viral direct counts were positively correlated with the control viral counts (r = 0.73) and the total viral direct counts (r = 0.57), which implies that a large viral population favors lysogeny. This relationship has also been observed in offshore studies on the occurrence of lysogeny (8).
In order to determine the significance of lysogeny in the marine environment, a seasonal study was performed to measure the abundance of lysogens in a eutrophic estuary, Tampa Bay, Fla. Previous studies on lysogeny in the marine environment brought into question the significance of this process for bacterial mortality or phage production (8, 12). Weinbauer and Suttle (12) reported that 0.07 to 4.4% of the total bacterial community was lysogenic, whereas Jiang and Paul (8) showed that up to 38% of the bacterial population was lysogenized in various estuarine environments. In this study, we determined that 0 to 37.3% (average, 6.9%) of the indigenous bacteria present in the Tampa Bay estuarine environment were lysogenic, as calculated by using an average burst size of 30 (8). The percentage ranged from 0 to 41% (average, 11.7%) when bacterial mortality was used to determine the lysogenic fraction (this method assumed that the increase in viral numbers and the decrease in bacterial numbers in the induced samples were caused solely by lysogenic induction).
Seasonal studies on viral abundance in various lake and marine environments have indicated that the greatest viral concentrations occur during the spring and summer, suggesting that there is a possible correlation with water temperature (2, 4, 7, 11). This phenomenon was also observed in our seasonal study. The virus numbers were greatest during the summer months (average, 1.32 × 107 viruses ml−1) and smallest during the winter (0.57 × 107 viruses ml−1). With the onset of warmer water temperatures during the spring and summer, positive induction was observed. When the water temperature was 19°C or greater, the percent increase in virus numbers was significantly greater than the increase in the control, which allowed detection of prophage induction. Decreases in bacterial numbers compared with the control also corresponded with these episodes of induction. With the onset of winter and cooler water temperatures (the water temperature decreased from 25 to 13°C), no induction was observed. Similar observations were made for Lake Superior (10), suggesting that bacterial activity controlled by temperature may be a significant factor in prophage induction. Once initial warming occurred in February (the water temperature increased from 13 to 19°C), a chlorophyll a peak was observed, and then 2 weeks later (when the next sample was obtained), a “lysogeny bloom” was observed and there was the greatest measured percent increase in induced virus numbers compared with the control (a 379% increase). However, a significant increase in inducible prophage was observed only in the viral counts. A concomitant decrease in bacterial numbers was not observed. If the initial bacterial counts and not the control counts at the end of the experiment were used, a significant decrease (P < 0.05) in the bacterial counts was observed.
Our data indicate that there is a strong interaction between the presence of inducible lysogens and the total viral direct counts. In fact, the greatest percent increase in inducible prophage (second February 1996 sample) corresponded with the initiation of the seasonal increase in viral direct counts. This could be interpreted as a seasonal regulatory switch, when lysogens go from a dormant state to inducibility. In cultures, induction does not occur readily in starved, slowly growing, or nonexponentially growing cells (1). The dormant state, which we hypothesize is related to low activity, is caused by cooler water temperatures or lack of dissolved organic carbon or both. In fact, the increase in viral direct counts observed seasonally may be in part a result of prophage induction and the attack and lysis of sensitive hosts by induced prophages. For example, if on average 30% of the population is lysogenized and a portion of the lysogenized cells (say one-third) are induced by some environmental event, then this could result in 1.2 × 107 viruses produced per ml, which is close to the total viral counts measured. If only 8% of the cells are lysogenized and one-half of these cells are induced, there would be 4.8 × 106 viruses produced per ml, still a significant proportion of the viral population.
We do not know what environmental conditions might lead to large-scale induction of natural populations. Ackermann and DuBow (1) list a variety of treatments shown to cause prophage induction in bacterial cultures, including a range of DNA-damaging agents, radiation, heat, and pressure, as well as other phages and plasmids. We have demonstrated that induction of natural populations by aromatic hydrocarbon pollutants and elevated temperatures occurs (8). It is possible that naturally occurring processes and anthropogenic activities cause large-scale prophage induction and that these events can contribute significantly to the total viral direct counts and the seasonal variability in such counts. In fact, the widespread observation of extremely high viral direct counts in estuarine samples may be the result of induction of prophages by anthropogenic or natural causes. Estuaries are often stressed by pollutants, and a range of environmental pollutants have been shown to result in prophage induction (8). Naphthalene, phenanthrene, and pyrene all cause prophage induction in natural populations of marine bacteria. It is interesting that the greatest induction (percent increase in prophage) occurred during the second seasonal induction in February. In the summer, a smaller increase in phage was observed, suggesting that a proportion of the indigenous lysogens might be induced by anthropogenic and naturally occurring agents found in the estuary studied.
Acknowledgments
This research was supported in part by grants OCE 9502775, OCE 9214614, and BIR-9512122 from the National Science Foundation.
We are grateful to Sunny Jiang for valuable suggestions and comments during the course of this study.
REFERENCES
- 1.Ackermann H W, DuBow M S. Viruses of prokaryotes. 1. General properties of bacteriophages. Boca Raton, Fla: CRC Press; 1987. [Google Scholar]
- 2.Bergh Ø, Børsheim K, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 3.Børsheim K, Bratbak G, Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990;56:352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demuth J, Neve H, Witzel K. Direct electron microscopic study on the morphological diversity of bacteriophage populations in Lake Plußsee. Appl Environ Microbiol. 1993;59:3378–3384. doi: 10.1128/aem.59.10.3378-3384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara S, Terauchi K, Korike I. Abundance of viruses in marine waters: assessment by epifluorescence and transmission electron microscopy. Appl Environ Microbiol. 1991;57:2731–2734. doi: 10.1128/aem.57.9.2731-2734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm-Hansen O, Riemann B. Chlorophyll a determination: improvements in methodology. Oikos. 1978;30:438–448. [Google Scholar]
- 7.Jiang S C, Paul J H. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar Ecol Prog Ser. 1994;104:163–172. [Google Scholar]
- 8.Jiang S C, Paul J H. Occurrence of lysogenic bacteria in marine microbial communities as determined by prophage induction. Mar Ecol Prog Ser. 1996;142:27–38. [Google Scholar]
- 9.Paul J H. Use of Hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982;43:939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapper, M. A., and R. E. Hicks. Morphology and abundance of free and temperate viruses in Lake Superior. Limnol. Oceanogr., in press.
- 11.Waterbury J B, Valois F W. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol. 1993;59:3393–3399. doi: 10.1128/aem.59.10.3393-3399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinbauer M G, Suttle C A. Potential significance of lysogeny to bacteriophage production and bacterial mortality in coastal waters of the Gulf of Mexico. Appl Environ Microbiol. 1996;62:4374–4380. doi: 10.1128/aem.62.12.4374-4380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox R M, Fuhrman J A. Bacterial viruses in coastal seawater: lytic rather than lysogenic production. Mar Ecol Prog Ser. 1994;114:35–45. [Google Scholar]
- 14.Wommack K E, Hill T T, Kessel M, Tussek-Cohen E, Colwell R R. Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol. 1992;58:2965–2970. doi: 10.1128/aem.58.9.2965-2970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]