Abstract
Background
Diabetes is a very common metabolic condition during pregnancy. The number of cases increases with age and obesity. The prevalence of pre-gestational diabetes and gestational diabetes (GD) differs between different ethnic groups.
Objective
The aim of the study was to analyse the prevalence of pre-gestational diabetes and GD in the health region of Lleida. We also studied the GD risk factors during pregnancy according to the country of origin of the pregnant woman.
Methods
We performed a retrospective observational cohort study among pregnant women between 2012 and 2018 in the health region of Lleida. A multivariate model was performed with the different variables analysed by calculating the regression coefficient and its 95% confidence interval (CI).
Results
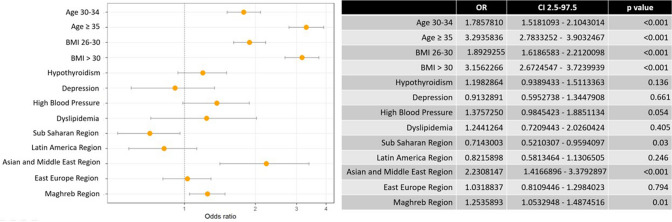
In our sample of 17,177 pregnant women, we observed a prevalence of pre-gestational diabetes and GD of 8.2% and 6.5%, respectively. We found a relationship of gestational diabetes with different factors: age, with 6.8% in 30–34 year-old women and 11.3% in women over 35 (OR 1.78 and 3.29, respectively); overweight, with 8.29% (OR 1.89); and obesity, with 12.9% (OR 3.15). Finally, women from Asia and the Middle East and the Maghreb had a higher risk of diabetes, with 12.2% (OR 2.1) and 9.91% (OR 1.3), respectively, and Sub-Saharan women had a lower risk of it 6.07% (OR 0.71).
Conclusions
GD has different risk factors, such as age, overweight, and obesity. Non-related conditions include hypothyroidism, arterial hypertension, and dyslipidaemia. Finally, pregnant women from the Maghreb, and Asia and the Middle East, are at higher risk of developing diabetes during pregnancy; meanwhile, Sub-Saharan origin is protector factor.
Keywords: Gestational diabetes mellitus, Immigrant women, Obesity, Population-based study
Background
Hyperglycaemia during pregnancy includes pre-gestational (diagnosed before pregnancy), overt diabetes (hyperglycemia first recognised during pregnancy which meets the thresholds of diabetes in non-pregnant adults) and gestational (diagnosed during pregnancy) diabetes. The prevalence of the former among pregnant women is 1–12% [1], while gestational diabetes (GD) is the most common metabolic complication during pregnancy [2] and affects about one in six women (17%) [3]. It usually occurs in the middle of the pregnancy period, approximately between 24 and 28 weeks of gestation [4]. Both pre-gestational and gestational diabetes have been associated with the risk of maternal–foetal complications [5–12]. In particular, GD is associated with an increased risk of perinatal complications and the development of diabetes by the mother and her offspring [13, 14].
The aetiology of diabetes is multifactorial and not fully defined. However, in most cases, hyperglycaemia is the result of impaired glucose tolerance either due to dysfunction of pancreatic β cells, or to defects in the insulin signalling cascade that causes chronic insulin resistance [15, 16]. During pregnancy, high levels of diabetic hormones (prolactin, placental lactogen, progesterone, and cortisol) increase peripheral insulin resistance. In addition, there is an increase in energy demand and insulin to increase the body mass. As a result of insulin resistance, glucose intolerance appears, and the pancreas tries to fight insulin resistance by increasing its production. Finally, GD develops in women with a dysfunction in this cascade [17].
The incidence of type 1 and type 2 diabetes among women in reproductive age is growing globally, together with the incidence of obesity in the population and the average age of pregnant women. Diabetes has been linked to other prevalent conditions during pregnancy, although there are no conclusive studies to date. These conditions include hypothyroidism, which is the second most common medical condition during pregnancy, hypertension, and dyslipidaemia [18–20]. Finally, the country of origin of the pregnant woman is among the factors determining the prevalence of GD. Indeed, women from Maghreb [11, 12, 21] and Asia and the Middle East [22–29] are at higher risk of developing this metabolic disease. Spain has seen significant changes in the percentage of births, fertility, and immigration. The birth rate fell by 24.6% and the fertility rate (children per woman) decreased from 1.4 to 1.3 (1.2 for natives and 1.7 for immigrants) in 2018. The proportion of births among immigrant women has increased. In Catalonia, the percentage of live births was 70.4% from Spanish women and 29.6% from immigrant women [30].
There are few studies that analyse the prevalence of pre-gestational and gestational diabetes in our environment, as well as risk factors associated with the disease during pregnancy. Moreover, the increase in immigration in recent decades in Spain led to a concomitant increase in the number of pregnant women from different populations. In this context, our study had the aim of studying the prevalence of pre-gestational and gestational diabetes in the health region of Lleida and gestational diabetes risk factors, according to the different ethnic groups.
Methods
Study design and data collection
We conducted a retrospective cohort observational study among pregnant women between 2012 and 2018 in the health region of Lleida. We included patients who gave birth at the Arnau University Hospital in Vilanova de Lleida between 1 January 2012 and 31 December 2018. Data were obtained through the CMBD (“Conjunt Mínim de Base de dades”), the eCAP computerised medical history database, and the database of the Catalan Health Service. The latter collects the data of the prescriptions from the Social Security.
Participants
We studied women who have given birth between 1 January 2012 and 31 December 2018. We collected pregnancy data from the date of the last period until the date of delivery. Women who did not belong to the health region of Lleida were excluded. To evaluate the representation of the sample, we calculated the percentage of pregnant women studied (registered at the Arnau University Hospital in Vilanova de Lleida) with respect to the total number of pregnant women in the Lleida health region (registered in the database of the “Institut estadístic de Catalunya”, Idescat) (Table 1).
Table 1.
Number of pregnant women in the health region of Lleida by years, number of pregnant women in the sample, and percentage of the latter with respect to the former
| Year | Part of the sample | Part of Idescat | Idescat/sample (%) |
|---|---|---|---|
| 2012 | 3635 | 3788 | 90 |
| 2013 | 3370 | 3535 | 89 |
| 2014 | 3308 | 3592 | 86 |
| 2015 | 3162 | 3426 | 86 |
| 2016 | 3180 | 3283 | 90 |
| 2017 | 3034 | 3197 | 88 |
| 2018 | 3001 | 3029 | 93 |
The main variable was the presence of pre-gestational or gestational diabetes, and the diagnosis was done with the O'Sullivan test. This test consists in the determination of plasma glycaemia 1 h after the oral administration of 50 g of glucose. Diabetes was diagnosed when blood glucose was above 140 mg/dl (7.8 mmol/l). In this case, the diagnosis was confirmed by Oral Glucose Tolerance Test (OGTT) The OGTT was performed between 24 and 28th gestation weeks with 100 g of glucose and the subsequent determination of blood glucose at the beginning and after the 1st, 2nd, and 3rd hour [17]. The eCAP diabetes registration code corresponds to the CIE-10 code O24.9. Other variables, registered at the early pregnant period, were the age of the pregnant woman; her BMI; hypothyroidism (code E03.9 and E02 of the ICD-10); hypertension (code I10–I16 of the ICD-10); dyslipidaemia (code E78 of the ICD-10); depression (codes F32.0–F32.9, F33.0–F33.3, F33.8, F33.9, F34.1, and F41 0.2 of the ICD-10); and the region of origin (Latin America, Asia and the Middle East, Europe, Eastern Europe, and Maghreb) [31, 32].
Statistical analyses
Numerical variables are described using the mean and the standard deviation, while categorical variables are described using absolute and relative frequencies. Differences between DM groups were assessed using an ANOVA or the Chi-square test, depending on whether the variables were quantitative or categorical, respectively. The risk of developing gestational diabetes according to the different variables was evaluated using a multivariate logistic regression using the diagnosis of DM during pregnancy as the response variables and the rest of the variables as predictors. Odds Ratios with the respective 95% confidence intervals were calculated. Statistical significance was established at p < 0.05. The analysis was carried out with R software, version 4.1.2.
Ethical aspects
This study was approved by the ethics and clinical research committee “Institute for Primary Health Care Research Jordi Gol i Gurina (IDIAPJGol)” under the code 19/194-P. The study was conducted in accordance with the principles of the Declaration of Helsinki. We performed a pseudonymised retrospective descriptive cross-sectional study according to Additional Provision 17.2.d LOPD-GDD for research purposes, without the need to obtain the consent of the data holders. There was a technical and functional separation between the research team and the performer pseudonymisation, and data are only accessible to the research team. Technical measures have been taken to prevent re-identification and access by third parties through the CMBD database (“Conjunt Minim de Base de Dades”) the E-CAP computerised medical history database and the Catalan Health Service database.
Results
Epidemiological data
A sample of 21,375 pregnant women who had given birth at the Arnau University Hospital in Vilanova de Lleida between 2012 and 2018 (both inclusive) was obtained. We excluded 1625 women who did not have a personal identification code (CIP), as well as 2573 women that lacked multiple clinical history data. As a consequence, the final sample was of 17,177 patients (Fig. 1).
Fig. 1.
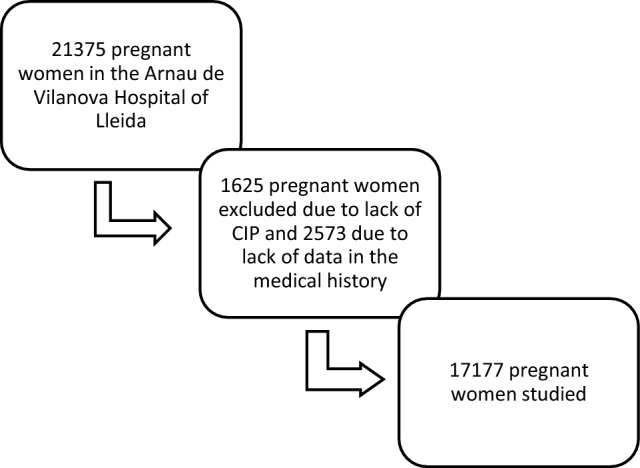
Sample of pregnant women studied
17,177 pregnant women were analysed, the distribution was 12–16% of the sample per year of study. The prevalence of pregnant women with diabetes (pre-gestational diabetes and GD) was 8.2% and GD 6.5%. A percentage of 40.6% of the mothers were < 30 years, and 21.3% equal to or older than 35 years. 24.9% were overweight (BMI 25–30) and 15.0% were obese (BMI ≥ 30). 6.6% of pregnant women had hypothyroidism, 2.3% arterial hypertension, 1.1% dyslipidaemia, and 2.5% depression. Moreover, pregnant women had different ethnic origins: 63.0% were from Western Europe, 14.9% from the Maghreb, 10.2% from Eastern Europe, 5.6% from Sub-Saharan Africa, 4.8% from Latin America, and 1.5% from Asia and the Middle East (Table 2).
Table 2.
Characteristics of the studied population
| N = 17,177 | |
|---|---|
| Year of delivery | 17,177 |
| 2012 | 2740 (15.9%) |
| 2013 | 2525 (14.7%) |
| 2014 | 2491 (14.5%) |
| 2015 | 2419 (14.1%) |
| 2016 | 2418 (14.1%) |
| 2017 | 2317 (13.5%) |
| 2018 | 2224 (12.9%) |
| Age at pregnancy | 17,177 |
| < 30 | 6981 (40.6%) |
| 30–35 | 6543 (38.1%) |
| > 35 | 3653 (21.3%) |
| BMI | 16,803 |
| ≤ 25 kg/m2 | 10,112 (60.2%) |
| 26–30 kg/m2 | 4177 (24.9%) |
| ≥ 30 kg/m2 | 2514(15.0%) |
| Number of pregnancies | 17,177 |
| 1 | 9009 (52.4%) |
| 2 | 5181 (30.2%) |
| 3 | 1870 (10.9%) |
| 4 | 471 (2.74%) |
| > 4 | 471 (2.7%) |
| Preeclampsia | 17,177 |
| No | 17,018 (99.1%) |
| Yes | 159 (0.9%) |
| Multiple pregnancies | 17,177 |
| No | 17,145 (99.8%) |
| Yes | 32 (0.2%) |
| Caesarean section | 17,177 |
| No | 14,201 (82.7%) |
| Yes | 2976 (17.3%) |
| Duration of the pregnancy (qualitative) | 12,962 |
| Abortion | 569 (4.4%) |
| Pre-term | 769 (5.9%) |
| Full-term | 11,296 (87.2%) |
| Prolonged | 328 (2.5%) |
| Pregnancy risk | 15,333 |
| Risk free | 7578 (49.4%) |
| Medium risk | 4527 (29.5%) |
| High risk | 2912 (19.0%) |
| Very high risk | 316 (2.1%) |
| Pre-gestational diabetes mellitus tipus 1 | 17,177 |
| No | 17,124 (98.7%) |
| Yes | 53(0.31%) |
| Pre-gestational diabetes mellitus tipus 2 | 17,177 |
| No | 16,044 (93.4%) |
| Yes | 1123 (6.5%) |
| Overt diabetes mellitus | |
| No | 17,166 (99.94%) |
| Yes | 10 (0.06%) |
| Gestational diabetes | 17,177 |
| No | 16,044 (93.4%) |
| Yes | 1123 (6.5%) |
| Hypothyroidism | 17,177 |
| No | 16,050 (93.4%) |
| Yes | 1127 (6.6%) |
| Arterial hypertension | 17,177 |
| No | 16,778 (97.7%) |
| Yes | 399 (2.3%) |
| Dyslipidaemia | 17,177 |
| No | 16,990 (98.9%) |
| Yes | 187 (1.1%) |
| Depression | 17,177 |
| No | 16,741 (97.5%) |
| Yes | 436 (2.5%) |
| Region of origin | 15,006 |
| Sub-Saharan Africa | 840 (5.6%) |
| Latin America | 717 (4.8%) |
| Asia and the Middle East | 222 (1.5%) |
| Western Europe | 9461 (63.0%) |
| Eastern Europe | 1533 (10.2%) |
| Maghreb | 2233 (14.9%) |
| Newborn weight | 15,133 |
| No Macrosomia | 14,113 (93.3%) |
| Macrosomia | 1020 (6.7%) |
| APGAR test at the first minute | 15,085 |
| ≥ 7 | 14,970 (97.5%) |
| < 7 | 379 (2.5%) |
| APGAR test at the fifth minute | 15,087 |
| ≥ 7 | 14,970 (99.2%) |
| < 7 | 117 (0.8%) |
Risk factors for diabetes in pregnancy
Pre-gestational and gestational diabetes were more common among older women: 14.2% of pregnant women over the age of 35 had diabetes, in comparison to 4.4% under the age of 30. This difference was statistically significant. Another significantly different variable was the BMI: 16.1% of pregnant women with obesity presented diabetes, in comparison to 5.3% with normal weight. Moreover, 11.1% of patients with hypothyroidism had diabetes, in comparison to 7.9% of patients without hypothyroidism; 19.3% of hypertensive patients were also diabetics, in comparison to 7.9% of non-hypertensive patients; and 15.0% of patients with dyslipidemia had diabetes, in comparison to 8% of patients without dyslipidemia. In the analysis of the region of origin, statistically significant differences were also evident, with a higher proportion of gestational diabetes among women from Asia and the Middle East (12.2%), and from the Maghreb (9.9%) (Table 3).
Table 3.
Relationship of diabetes with different variables
| No diabetes | Pre-gestational diabetes | Gestational diabetes | p | |
|---|---|---|---|---|
| N = 15,773 | N = 271 | N = 1123 | ||
| Age at pregnancy | < 0.001 | |||
| < 30 | 6675 (95.6%) | 42 (0.6%) | 262 (3.75%) | |
| 30–34 | 5965 (91.2%) | 124 (1.9%) | 448 (6.85%) | |
| ≥ 35 | 3133 (85.8%) | 105 (2.88%) | 413 (11.3%) | |
| BMI | < 0.001 | |||
| ≤ 25 kg/m2 | 9574 (94.7%) | 103 (1.02%) | 433 (4.28%) | |
| 26–30 kg/m2 | 3748 (89.8%) | 79 (1.89%) | 346 (8.29%) | |
| > 30 kg/m2 | 2105 (83.9%) | 80 (3.19%) | 325 (12.9%) | |
| Number of pregnancies | < 0.001 | |||
| 1 | 8353 (92.8%) | 75 (0.83%) | 576 (6.40%) | |
| 2 | 4743(91.6%) | 114 (2.20%) | 322 (6.22%) | |
| 3 | 1682 (90.0%) | 48 (2.57%) | 138 (7.39%) | |
| 4 | 573 (88.8%) | 20 (3.10%) | 52 (8.06%) | |
| > 4 | 422 (89.6%) | 14 (2.97%) | 35 (7.43%) | |
| Hypothyroidism | < 0.001 | |||
| No | 14,773 (92.1%) | 241 (1.50%) | 1028 (6.41%) | |
| Yes | 1000 (88.9%) | 30 (2.67%) | 995 (8.44%) | |
| Arterial hypertension | < 0.001 | |||
| No | 15,452 (92.1%) | 247(1.47%) | 1070 (6.38%) | |
| Yes | 321 (80.7%) | 24(6.03%) | 53 (13.3%) | |
| Dyslipidaemia | < 0.001 | |||
| No | 15,614 (92.0%) | 262(1.54%) | 1104 (6.50%) | |
| Yes | 159 (85.0%) | 9(4.81%) | 19 (10.2%) | |
| Depression | 1.000 | |||
| No | 15,373 (91.9%) | 263(1.57%) | 1095 (6.54%) | |
| Yes | 400 (91.7%) | 8(1.83%) | 28 (6.42%) | |
| Region of origin | < 0.001 | |||
| Sub-Saharan Africa | 768 (91.4%) | 21(2.50%) | 51 (6.07%) | |
| Latin America | 673 (93.9%) | 3(0.42%) | 41 (5.72%) | |
| Asia and the Middle East | 190 (85.6%) | 5(2.25%) | 27 (12.2%) | |
| Europe | 8683 (91.8%) | 142(1.50%) | 632 (6.68%) | |
| Eastern Europe | 1431 (93.5%) | 11(0.72%) | 89 (5.81%) | |
| Maghreb | 1971 (88.4%) | 38(1.70%) | 221 (9.91%) |
Finally, in Fig. 2, a multivariate analysis showed statistically significant differences, with a higher risk of gestational diabetes linked to: age of 30 years or older; BMI greater than or equal to 25; and origin from the Maghreb or Asia and the Middle East. Being of sub-Saharan origin has a protective factor for developing the GD. Other variables, such as hypothyroidism, high blood pressure, dyslipidaemia, and depression, were not significantly different between diabetics and non-diabetics.
Fig. 2.
Multivariate analysis of the association of maternal risk factors with gestational diabetes
Discussion
In our sample of 17,177 pregnant women, we observed a prevalence of pre-gestational diabetes and GD of 8.2% and 6.5%, respectively. We found a relationship of gestational diabetes with different factors: age, with 6.8% in 30–34 year-old women and 11.3% in women over 35 (OR 1.78 and 3.29, respectively); overweight, with 8.29% (OR 1.89); and obesity, with 12.9% (OR 3.15). Finally, women from Asia and the Middle East and the Maghreb had a higher risk of diabetes, with 12.2% (OR 2.1) and 9.91% (OR 1.3), respectively, and Sub-Saharan women had a lower risk of it 6.07% (OR 0.71).
The latest studies show values of 8–25% of pregnancies with GD, with oscillations depending on the diagnostic criteria of the region and the population [32–34]. In our study, the prevalence was lower than in these ones (6.6%) and similar to others, such as the one conducted in the US with more than 12 million cases. Here, the age-adjusted GD ratio increased significantly from 47.6 to 63.5 per 1000 live births from 2011 to 2019.
Our multivariate analysis showed age and BMI as factors associated with diabetes, consistent with the study by Li et al. [11]. Moreover, Ferreira et al. identified gestational age and obesity as the two most important independent factors contributing to GD [35–38]. These along with family history of diabetes and multiple pregnancies are considered well-catalogued risk factors for GD. Finally, an Australian study estimated that 8.6%, 15.6%, and 19.5% of GDs could have been prevented by eliminating overweight, obesity, and maternal morbid obesity, respectively [39].
In our study, we found that 8.86% of diabetic pregnant women have hypertension, in comparison to 4.72% of non-diabetic ones, being these differences non-significative. Against with our results, Daly et al. found that women diagnosed with GD are twice as likely to develop hypertension [46].
With regard to dyslipidaemia, we did not observe any association with GD. On the contrary, Baumfeld et al. [47] found an increased risk of preeclampsia and/or GD in women with low HDL cholesterol and high pre-conception levels of triglycerides. O’Malley et al. concluded that the epidemiological association between GD and dyslipidaemia is mediated by maternal obesity, as women exclusively with obesity or GD did not have a high risk of dyslipidaemia in comparison to the rest [48]. A recent systematic review with meta-analysis considered the elevation of blood triglycerides to be the most important risk factor for GD. Similarly, high levels of total cholesterol, LDL, and VLDL; high triglycerides/HDL ratio; and low levels of HDL were found to be more common in the GD group [49]. In addition, the study by Diboun et al. provides data on metabolic impairment in women during the second trimester, identifying significant associations between the number of different metabolites (glutamate, branched amino acids, phosphatidylcholines and some triglycerides) [50] and diabetes mellitus 2 and GD.
Different articles associated depression with diabetes. Numerous studies either examined if diabetes was a risk factor for developing depression or if depression was a risk factor for diabetes during pregnancy; however, there is no clear consensus [51]. In particular, in our study, we did not observe this association. However, a systematic review and meta-analysis of recent cohort studies [52] concluded that women with pre-gestational and gestational diabetes had a statistically significant risk of prepartum depression, in comparison to women without diabetes.
Finally, regarding the country of origin, we observed that 14.4% of Asian pregnant women developed diabetes. This value coincides with the one found in other studies, such as the one conducted by Hod et al., in which they observed that 13% of women in China developed GD [53]. Another meta-analysis showed that the prevalence of GD in Asia was 20.9% [27–29]. In general, the prevalence of GD is increasing globally, with different studies predicting that 90% of new GD cases will occur in Asia [22–24]. An increase in the prevalence of GD among Asian and Moroccan women is indeed shown in several other studies, such as the one by Shah et al. [54, 55]. They studied the non-Hispanic Asian population/Pacific islands in the US (which included India, China, the Philippines, Japan, Korea, and Vietnam), and observed that the GD ratio significantly increased from 69.9 to 102.7 per 1000 live births per year from 2011 to 2019 [56]. There are some studies that demonstrate a higher risk of GD in Sub-Saharan woman; however, in our study, we observed a lower risk to develop it [57].
Our study has some limitations. First, the analysis was extracted from a database for clinical and administrative purposes. However, the National Health System is public, so the entire population has free access to Primary Care, Obstetrics services, and complementary tests, and all pregnant women can be screened. Second, our database does not include covariates such as history of diabetes in the family, diabetes history, history of previous impaired fasting plasma glucose, impaired glucose tolerance, macrosomia, thyroid autoimmunity and socioeconomic status and lifestyle (diet and physical activity), which are also risk factors for diabetes. Nevertheless, our multivariate analysis included all types of diabetes during pregnancy, involving heterogeneous subgroups.
Conclusions
In this study, we found an association of diabetes during pregnancy with age and obesity, and a higher prevalence in the immigrant population from Asia and the Maghreb. The origin from Sub-Saharan Region had a protective effect to develop GD.
The high prevalence of diabetes during pregnancy poses a major challenge to prevent sequelae for both the mother and the baby, so it should be considered a public health priority. It is essential to prioritise preventive health care for women both before conception and during pregnancy by reducing risk factors.
We consider that more studies are needed to provide information on diabetes control, associated risk factors, and most susceptible populations, to finally provide better medical, family, and social intervention.
Acknowledgements
The authors would like to thank Dr. Miquel Butí for her valuable contribution and support in the design and creation of the database; Laura Azlor for her contribution to statistical analysis; and the Gol i Gurina Foundation for the formalisation of the study and revision of the draft manuscript. All the authors read and approved the final manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. There has been no funding in the article.
Availability of data and materials
Unidentified survey data will be provided by sending an email request to miriam_oros@hotmail.com.
Declarations
Conflict of interest
The authors state that there are no conflicts of interest.
Ethical statement
This study has been approved by the Comitè d'Ètica i Investigació Clínica “Institut d'Investigació en Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol)” with code 19/194-P. It was carried out in accordance with the principles of the Helsinki Declaration. We performed a pseudonymised retrospective descriptive cross-sectional study according to Additional Provision 17.2.d LOPD-GDD for research purposes, without the need to obtain the consent of the data holders. There is a technical and functional separation between the research team and the pseudonymisation of the interpreter, and that the data are only accessible to the research team. Technical measures have been taken to prevent the re-identification and access to data by third parties through the CMBD database (“Conjunt Mínim de Base de Dades”), the eCAP computerised medical history database, and the database of the Servei Català de la Salut.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Diabetes mellitus y embarazo guia de práctica clínica actualizada (2020) Grupo Español de Diabetes y Embarazo Sociedad Española de Diabetes y Sociedad Española de Ginecología y Obstetricia [Internet]. SED. Available from: https://www.sediabetes.org/grupos_de_trabajo/diabetes-y-embarazo/. Accessed 30 Jan 2022
- 2.Saravanan P, Diabetes in Pregnancy Working Group, Maternal Medicine Clinical Study Group, Royal College of Obstetricians and Gynaecologists, UK Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8(9):793–800. doi: 10.1016/S2213-8587(20)30161-3. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation (2022) 9th edition. IDF Diabetes Atlas [Internet]. https://diabetesatlas.org/atlas/ninth-edition/. Accessed 13 Mar 2022
- 4.American Diabetes Association Summary of revisions: standards of medical care in diabetes—2021. Diabetes Care. 2020;44(Supplement_1):S4–6. doi: 10.2337/dc21-Srev. [DOI] [PubMed] [Google Scholar]
- 5.Padmanabhan S, Zen M, Lee V, Cheung NW. Pre-existing diabetes in pregnancy. Minerva Endocrinol. 2016;41(1):122–137. [PubMed] [Google Scholar]
- 6.Abell SK, Boyle JA, de Courten B, Soldatos G, Wallace EM, Zoungas S, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57(3):308–314. doi: 10.1111/ajo.12521. [DOI] [PubMed] [Google Scholar]
- 7.Klingensmith GJ, Pyle L, Nadeau KJ, Barbour LA, Goland RS, Willi SM, et al. Pregnancy outcomes in youth with type 2 diabetes: the TODAY study experience. Diabetes Care. 2016;39(1):122–129. doi: 10.2337/dc15-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon LE, O’Malley EG, Reynolds CME, Turner MJ. The impact of revised diagnostic criteria on hospital trends in gestational diabetes mellitus rates in a high income country. BMC Health Serv Res. 2020;20(1):795. doi: 10.1186/s12913-020-05655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewandowska M. The role of maternal weight in the hierarchy of macrosomia predictors; overall effect of analysis of three prediction indicators. Nutrients. 2021;13(3):801. doi: 10.3390/nu13030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ro A, Goldberg RE, Kane JB. Racial and ethnic patterning of low birth weight, normal birth weight, and macrosomia. Prev Med. 2019;118:196–204. doi: 10.1016/j.ypmed.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Wei T, Ni W, Zhang A, Zhang J, Xing Y, et al. Incidence and risk factors of gestational diabetes mellitus: a prospective cohort study in Qingdao, China. Front Endocrinol. 2020 doi: 10.3389/fendo.2020.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amiri FN, Faramarzi M, Bakhtiari A, Omidvar S. Risk factors for gestational diabetes mellitus: a case-control study. Am J Lifestyle Med. 2021;15(2):184–190. doi: 10.1177/1559827618791980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coustan DR. Gestational diabetes mellitus. Clin Chem. 2013;59(9):1310–1321. doi: 10.1373/clinchem.2013.203331. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 15.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalano PM. Trying to understand gestational diabetes. Diabetes Med. 2014;31(3):273–281. doi: 10.1111/dme.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ACOG ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 18.Gong L-L, Liu H, Liu L-H. Relationship between hypothyroidism and the incidence of gestational diabetes: a meta-analysis. Taiwan J Obstet Gynecol. 2016;55(2):171–175. doi: 10.1016/j.tjog.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Guzmán-Juárez W, Avila-Esparza M, Contreras-Solís RE, Levario-Carrillo M. Factors associated with gestational hypertension and preeclampsia. Ginecol Obstet Mex. 2012;80(7):461–466. [PubMed] [Google Scholar]
- 20.Chodick G, Tenne Y, Barer Y, Shalev V, Elchalal U. Gestational diabetes and long-term risk for dyslipidemia: a population-based historical cohort study. BMJ Open Diabetes Res Care. 2020;8(1):e000870. doi: 10.1136/bmjdrc-2019-000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe A, Giveon S, Rubin C, Novikov I, Ziv A, Kalter-Leibovici O. Gestational diabetes risk in a multi-ethnic population. Acta Diabetol. 2020;57(3):263–269. doi: 10.1007/s00592-019-01404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overview. Diabetes in pregnancy: management from preconception to the postnatal period | Guidance|NICE [Internet]. NICE. (2022) https://www.nice.org.uk/guidance/ng3. Accessed 30 Jan 2022
- 23.World Health Organization . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy [internet] World Health Organization; 2022. [PubMed] [Google Scholar]
- 24.American Diabetes Association 14. Management of diabetes in pregnancy: standards of medical care in diabetes 2020. Diabetes Care. 2020;43(Suppl 1):S183–S192. doi: 10.2337/dc20-S014. [DOI] [PubMed] [Google Scholar]
- 25.Gupta LS, Wu CC, Young S, Perlman SE. Prevalence of diabetes in New York City, 2002–2008: comparing foreign-born South Asians and other Asians with US born whites, blacks, and Hispanics. Diabetes Care. 2011;34(8):1791–1793. doi: 10.2337/dc11-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35(7):1492–1498. doi: 10.2337/dc11-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, Dong L, Zhang CP, Li B, Wen J, Gao W, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabetes Med. 2011;28(6):652–657. doi: 10.1111/j.1464-5491.2010.03205.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Chen L, Xiao K, Horswell R, Besse J, Johnson J, et al. Increasing incidence of gestational diabetes mellitus in Louisiana, 1997–2009. J Womens Health (Larchmt) 2012;21(3):319–325. doi: 10.1089/jwh.2011.2838. [DOI] [PubMed] [Google Scholar]
- 29.Rajab KE, Issa AA, Hasan ZA, Rajab E, Jaradat AA. Incidence of gestational diabetes mellitus in Bahrain from 2002 to 2010. Int J Gynaecol Obstet. 2012;117(1):74–77. doi: 10.1016/j.ijgo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Instituto Nacional de Estadística (2022) Cifras de Población (CP) a 1 de julio de 2018. Estadística de Migraciones (EM). Primer semestre de 2018. INE. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177000&menu=ultiDatos&idp=1254735573002. Accessed 13 Mar 2022
- 31.Cruz I, Serna C, Rué M, Real J, Soler-Gonzalez J, Galván L. Duration and compliance with antidepressant treatment in immigrant and native-born populations in Spain: a four year follow-up descriptive study. BMC Public Health. 2012;11(12):256. doi: 10.1186/1471-2458-12-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alejandro EU, Mamerto TP, Chung G, Villavieja A, Gaus NL, Morgan E, et al. Gestational diabetes mellitus: a harbinger of the vicious cycle of diabetes. Int J Mol Sci. 2020;21(14):E5003. doi: 10.3390/ijms21145003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mecacci F, Lisi F, Vannuccini S, Ottanelli S, Rambaldi MP, Serena C, et al. Different gestational diabetes phenotypes: which insulin regimen fits better? Front Endocrinol (Lausanne) 2021;12:630903. doi: 10.3389/fendo.2021.630903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behboudi-Gandevani S, Parajuli R, Vaismoradi M. A systematic review of the prevalence of gestational diabetes in Norway. Int J Environ Res Public Health. 2021;18(4):1423. doi: 10.3390/ijerph18041423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–1083. doi: 10.1001/jama.1997.03550130052036. [DOI] [PubMed] [Google Scholar]
- 36.de Ferreira LAP, de Piccinato CA, Cordioli E, Zlotnik E. Pregestational body mass index, weight gain during pregnancy and perinatal outcome: a retrospective descriptive study. Einstein (Sao Paulo) 2020;18:eAO4851. doi: 10.31744/einstein_journal/2020AO4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36(1):56–62. doi: 10.2337/dc12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang Y, Li D-T, Chen M-X, Gong Y-H, Zhang X, Yang W-Y, et al. Associations of pre-pregnancy body mass index and gestational weight gain with gestational diabetes mellitus: a cohort study in Southwest China. Sichuan Da Xue Xue Bao Yi Xue Ban. 2019;50(1):83–87. [PubMed] [Google Scholar]
- 39.Mnatzaganian G, Woodward M, McIntyre HD, Ma L, Yuen N, He F, et al. Trends in percentages of gestational diabetes mellitus attributable to overweight, obesity, and morbid obesity in regional Victoria: an eight-year population-based panel study. BMC Pregnancy Childbirth. 2022;22(1):95. doi: 10.1186/s12884-022-04420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia M, Wu Y, Lin B, Shi Y, Zhang Q, Lin Y, et al. Meta-analysis of the association between maternal subclinical hypothyroidism and gestational diabetes mellitus. Int J Gynaecol Obstet. 2019;144(3):239–247. doi: 10.1002/ijgo.12751. [DOI] [PubMed] [Google Scholar]
- 41.Tirosh D, Benshalom-Tirosh N, Novack L, Press F, Beer-Weisel R, Wiznitzer A, et al. Hypothyroidism and diabetes mellitus - a risky dual gestational endocrinopathy. PeerJ. 2013;1:e52. doi: 10.7717/peerj.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawal S, Tsai MY, Hinkle SN, Zhu Y, Bao W, Lin Y, et al. A longitudinal study of thyroid markers across pregnancy and the risk of gestational diabetes. J Clin Endocrinol Metab. 2018;103(7):2447–2456. doi: 10.1210/jc.2017-02442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L-M, Du W-J, Dai J, Zhang Q, Si G-X, Yang H, et al. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a Chinese population. PLoS ONE. 2014;9(10):e109364. doi: 10.1371/journal.pone.0109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oguz A, Tuzun D, Sahin M, Usluogullari AC, Usluogullari B, Celik A, et al. Frequency of isolated maternal hypothyroxinemia in women with gestational diabetes mellitus in a moderately iodine-deficient area. Gynecol Endocrinol. 2015;31(10):792–795. doi: 10.3109/09513590.2015.1054801. [DOI] [PubMed] [Google Scholar]
- 45.Muir CA, Munsif A, Blaker K, Feng Y, Tewari MDS. Antenatal thyroid function does not increase risk of gestational diabetes mellitus in a multi-ethnic pregnancy cohort. Korean Thyroid Assoc. 2020;13(1):13–18. [Google Scholar]
- 46.Daly B, Toulis KA, Thomas N, Gokhale K, Martin J, Webber J, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. PLoS Med. 2018;15(1):e1002488. doi: 10.1371/journal.pmed.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumfeld Y, Novack L, Wiznitzer A, Sheiner E, Henkin Y, Sherf M, et al. Pre-Conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS ONE. 2015;10(10):e0139164. doi: 10.1371/journal.pone.0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Malley EG, Reynolds CME, Killalea A, O’Kelly R, Sheehan SR, Turner MJ. Maternal obesity and dyslipidemia associated with gestational diabetes mellitus (GDM) Eur J Obstet Gynecol Reprod Biol. 2020;246:67–71. doi: 10.1016/j.ejogrb.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Rahnemaei FA, Pakzad R, Amirian A, Pakzad I, Abdi F. Effect of gestational diabetes mellitus on lipid profile: a systematic review and meta-analysis. Open Med (Wars) 2022;17(1):70–86. doi: 10.1515/med-2021-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diboun I, Ramanjaneya M, Majeed Y, Ahmed L, Bashir M, Butler AE, et al. Metabolic profiling of pre-gestational and gestational diabetes mellitus identifies novel predictors of pre-term delivery. J Transl Med. 2020;18(1):366. doi: 10.1186/s12967-020-02531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross GP, Falhammar H, Chen R, Barraclough H, Kleivenes O, Gallen I. Relationship between depression and diabetes in pregnancy: a systematic review. World J Diabetes. 2016;7(19):554–571. doi: 10.4239/wjd.v7.i19.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee KW, Ching SM, Devaraj NK, Chong SC, Lim SY, Loh HC, et al. Diabetes in pregnancy and risk of antepartum depression: a systematic review and meta-analysis of cohort studies. Int J Environ Res Public Health. 2020;17(11):E3767. doi: 10.3390/ijerph17113767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(Suppl 3):S173–211. doi: 10.1016/S0020-7292(15)30007-2. [DOI] [PubMed] [Google Scholar]
- 54.Strandberg RB, Iversen MM, Jenum AK, Sørbye LM, Vik ES, Schytt E, et al. Gestational diabetes mellitus by maternal country of birth and length of residence in immigrant women in Norway. Diabetes Med. 2021;38(6):e14493. doi: 10.1111/dme.14493. [DOI] [PubMed] [Google Scholar]
- 55.Kragelund Nielsen K, Andersen GS, Damm P, Andersen A-MN. Gestational diabetes risk in migrants. A nationwide, register-based study of all births in Denmark 2004 to 2015. J Clin Endocrinol Metab. 2020;105(3):024. doi: 10.1210/clinem/dgaa024. [DOI] [PubMed] [Google Scholar]
- 56.Shah NS, Wang MC, Freaney PM, Perak AM, Carnethon MR, Kandula NR, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011–2019. JAMA. 2021;326(7):660–669. doi: 10.1001/jama.2021.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strandberg RB, Iversen MM, Jenum AK, Sørbye LM, Vik ES, Schytt E, Aasheim V, Nilsen RM. Gestational diabetes mellitus by maternal country of birth and length of residence in immigrant women in Norway. Diabet Med. 2021;38(6):4493. doi: 10.1111/dme.14493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Unidentified survey data will be provided by sending an email request to miriam_oros@hotmail.com.