Abstract
Purpose
Doses delivered to the urethra have been associated with an increased risk to develop long-term urinary toxicity in patients undergoing stereotactic body radiotherapy (SBRT) for prostate cancer (PCa). Aim of the present systematic review is to report on the role of urethra-sparing SBRT (US-SBRT) techniques for prostate cancer, with a focus on outcome and urinary toxicity.
Method
A systematic review of the literature was performed on the PubMed database on May 2023. Based on the urethra-sparing technique, 13 studies were selected for the analysis and classified in the two following categories: “urethra-steering” SBRT (restriction of hotspots to the urethra) and “urethra dose-reduction” SBRT (dose reduction to urethra below the prescribed dose).
Results
By limiting the urethra Dmax to 90GyEQD2 (α/β = 3 Gy) with urethra-steering SBRT techniques, late genitourinary (GU) grade 2 toxicity remains mild, ranging between 12.1% and 14%. With dose-reduction strategies decreasing the urethral dose below 70 GyEQD2, the risk of late GU toxicity was further reduced (< 8% at 5 years), while maintaining biochemical relapse-free survival rates up to 93% at 5 years.
Conclusion
US-SBRT techniques limiting maximum doses to urethra below a 90GyEQD2 (α/β = 3 Gy) threshold result in a low rate of acute and late grade ≥ 2 GU toxicity. A better understanding of clinical factors and anatomical substructures involved in the development of GU toxicity, as well as the development and use of adapted dose constraints, is expected to further reduce the long-term GU toxicity of prostate cancer patients treated with SBRT.
Keywords: Prostate cancer, SBRT, Radiotherapy, Hypofractionation, Urethra-sparing, Toxicity
Introduction:
Radiation therapy (RT) represents one of the mainstay treatments for men diagnosed with localized prostate cancer (PCa) [1, 2]. The technological improvements of the past decade, with both implementation of intensity-modulated radiation therapy (IMRT) or rotational techniques [3, 4] and image-guided radiation therapy (IGRT) [5], have enabled the mitigation of genitourinary (GU) and gastrointestinal (GI) toxicity.
With a better understanding of the radiobiology of PCa [6], hypofractionation has become a new standard for the treatment of localized disease, including extreme hypofractionated schedules in 5 or fewer fractions delivered with stereotactic body radiation therapy (SBRT) [7–11]. Although toxicity results seem acceptable, further efforts to minimize long-term toxicities of SBRT are constantly explored. While GU toxicity after prostate SBRT appears multifactorial and associated with age, baseline urinary function, and prostate size, emerging data emphasize the role of SBRT doses delivered to urinary substructures.
In the recent literature, the urethra has been identified as a new organ at risk potentially influencing the long-term toxicity of patients treated with definitive radiotherapy, in analogy with data reported in brachytherapy (BT) series [12–15]. As doses delivered to urethra have been associated with the development of GU toxicity in SBRT studies, radiotherapy techniques aiming to optimize and reduce dose delivered at this level have been developed and implemented in several trials [16–18]. Nevertheless, urethra-sparing radiotherapy techniques suffers from a great variability, both with respect to the anatomical definition of the urethra as organ at risk for treatment planning and use of dedicated dose constraints for treatment optimization.
In order to shed light on the available evidence on this emerging topic, in the present study, we aim to present a systematic review of the literature regarding urethra-sparing SBRT (US-SBRT) techniques for PCa, with a focus on outcomes and urinary toxicity of two different urethra optimization approaches, the “urethra-steering” and the “urethra dose-reduction.”
Material and methods
Eligibility criteria.
All trials reporting either toxicity or oncological outcomes after prostate SBRT were considered for inclusion. Studies were deemed eligible if they reported urethral dose-constraints either within their protocol or the manuscript. Studies were deemed to conduct urethra-sparing if they performed either “urethra-steering” (restriction of hotspots to the urethra) or “urethra dose-reduction” (maximal doses delivered to the urethra inferior to the dose of prescription to the prostate gland or dose-prescription on the urethra lower than dose of prescription to the target volume).
Information sources and search strategy.
A systematic search of the literature was performed in May 2023 on the PubMed database, using the MeSH term “urethra sparing.” Due to the scarcity of evidence on this topic, a broad search was voluntarily performed. There was no period restriction.
Selection process.
Two reviewers independently screened the articles, both at identification and screening process (J.L.G and T.Z.). Disagreements were discussed and resolved through consensus. For every study, the following data were retrieved: publication year, number of included patients, study design, radiation technique, dose delivered to the prostate gland, dose delivered to the urethra, median follow-up, toxicity outcomes (assessed by either RTOG or CTCAE grading scale), and oncological outcomes.
Synthesis method.
All studies meeting the inclusion criteria were selected for narrative synthesis. The results have been reported narratively, and summarized in tables when deemed appropriate. This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [19].
Results
Study selection
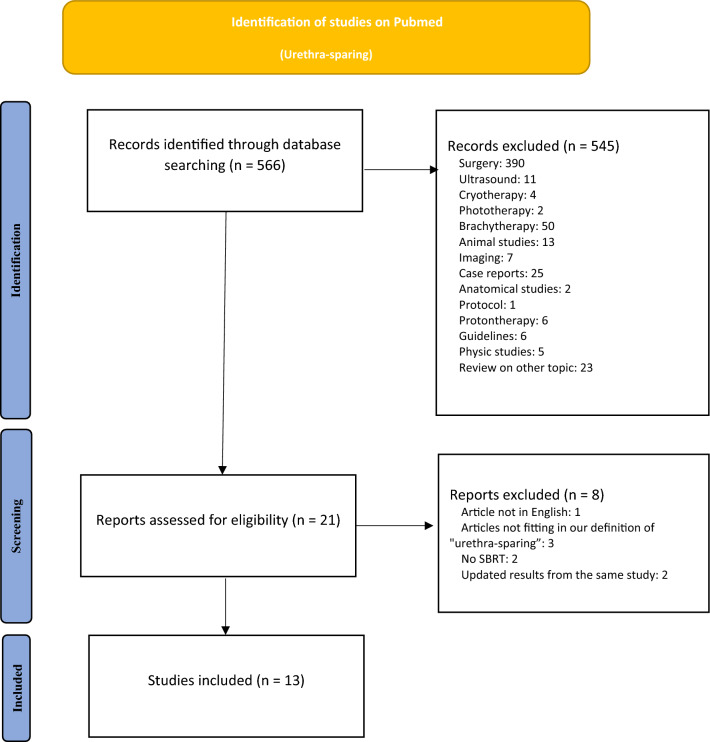
A flow chart of the literature screening is shown in Fig. 1. The search allowed to retrieve a total of 566 articles. After screening, 545 records that did not address the issue of urethra-sparing for PCa RT were excluded (surgical trials, brachytherapy trials, reviews…) leaving a total of 21 articles assessed for eligibility. After full-text reading, 8 additional reports were excluded (articles not in English, articles that did not fit our definition of urethra-sparing, treatment not performed using SBRT). A total of 13 articles were included in the present review.
Fig. 1.
PRISMA flow chart
Urethra-steering SBRT
Most of trials performing urethra-steering implemented a whole gland prostate irradiation schedule with a simultaneous integrated boost (SIB) on the dominant intraprostatic lesion (DIL) (Table 1). McDonald et al. recruited 26 patients within a prospective pilot study to receive 36.25 Gy on the prostate gland with dose escalation up to 40 Gy to the DIL [20]. Within a 3-month follow-up, acute grade 2 toxicity occurred in 52% of patients, mostly consisting in dysuria and frequency. Two patients required catheterization for acute retention. Within the Hypo-FLAME trial, 35 Gy in 5 fractions was prescribed on the whole prostate gland, with dose escalation up to 50 Gy on the DIL [21]. While dose constraints on the urethra were limited to a Dmax of 42 Gy as per protocol (equivalent to 96.6 Gy in standard fractionation using a α/β of 3 Gy for late toxicity – EQD2), the median delivered Dmax reported in the study was 85.4 Gy EQD2. After a median follow-up of 18 months, acute and late grade 2 GU toxicity were reported in 34% and 14% of the patients, respectively. No patient experienced grade 3 toxicity. While patients received RT treatment once-weekly within the HYPO-FLAME trial, the same team assessed the safety of a reduction in overall treatment time from 29 to 15 days within the HYPOFLAME 2.0 trial [22]. Patients treated within the once-weekly arm experienced significantly less acute grade 2 GU toxicity than patients treated within the semi-weekly schedule (34% vs 47.5%, p = 0.01). No significant difference was observed with regard to acute grade 2 GI toxicity. Cloitre et al. led a phase I-II dose escalation trial with CyberKnife®, prescribing doses of 36.25 Gy in 5 fractions to the whole prostate, while simultaneously escalating doses to the DIL up to 50 Gy [23, 24]. Acute grade 2 GU toxicity was reported in 15% of men. Despite a toxicity flare observed 1 month after SBRT as assessed by the EORTC quality of life (QoL) PR-25 questionnaire and IPSS score, a return to the baseline status was observed at month 3. Late grade 2 GU toxicity was observed in 12.1% of the patients, consisting mostly in urinary frequency and urgency. No grade 3 GU toxicity occurred over the complete course of follow-up. These results were consistent with a median urethra delivered dose to 0.1 cc and 1 cc (D0.1 cc and D1cc) of 83.5 Gy EQD2 and 77.7 Gy EQD2, respectively, lower than the maximal doses accepted as per protocol (91.8 Gy EQD2 and 84.2 Gy EQD2, respectively). Within a median follow-up of 61 months, 5-year biochemical relapse-free survival (bRFS) was 70%, including a 30% of intraprostatic (30%) relapse. Omission of androgen deprivation therapy (ADT) in the majority of intermediate- and high-risk patients may explain these results.
Table 1.
Toxicity and oncological outcomes after urethra-steering protocols (limitation of maximal doses, with maximal doses delivered to the urethra lower than maximal doses delivered within the target volume)
| Author | Patients | Study design | RT technique | RT dose | Urethral maximum dose (EQD2, α/β = 3 Gy) |
ADT | Median follow-up | Toxicity scale | Acute GU toxicity | Acute GI toxicity | Late GU toxicity | Late GI toxicity | Oncological outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Herrera et al., 2019 [24] Cloitre et al., 2023 [23] |
33 pts IR = 42% HR = 55% |
Phase I/II prospective trial |
CyberKnife (82%) Tomotherapy |
36.25 Gy / 5 fx Dose escalation up to 50 Gy / 5 fx to the DIL |
D1cc < 39 Gy (84.2 Gy EQD2) D0.1 cc < 41 Gy (91.8 Gy EQD2) |
3% (IR) |
61 months | CTCAE | G2: 15% | G2: 6.1% | G2: 12.1% | G2: 3% | bRFS: 70% |
| Draulans et al., 2020 [21] [22] |
100 pts IR = 25% HR = 75% |
Phase II prospective trial (HYPO-FLAME) |
VMAT |
35 Gy / 5fx Dose escalation to 50 Gy / 5 fx to the DIL |
Dmax < 42 Gy (96.6 Gy EQD2) Dmax delivered (85.4 Gy EQD2) |
31% short-term 31% long-term |
18 months | CTCAE |
Cumulative at 3 months: G2: 34% G ≥ 3: 0% |
Cumulative at 3 months: G2: 5% G ≥ 3: 0% |
Prevalence at 6 months G2: 14% G3: 0% |
Prevalence at 6 months G2: 4% G3: 0% |
NR |
|
Fuller et al., 2018 [28] Fuller et al., 2022 [29] |
259 pts LR = 53% IR = 57% |
Phase II prospective trial | CyberKnife | 38 Gy / 4fx |
Dmax < 45.6 Gy (131.3 Gy EQD2) D10% < 41.8 Gy (112.4 Gy EQD2) D50% < 39.9 Gy (103.6 Gy EQD2) |
No | 5.5 years | CTCAE |
G2: 35.1% G3: 1.1% |
G2: 6.9% G3: 0% |
Cumulative incidence G ≥ 2: 19.2% |
Cumulative incidence G2: 4.1% |
10-year bRFS: LR: 100% IR-favorable: 84.3% IR-unfavorable: 68.4% |
| McDonald et al., 2018 [20] |
26 pts LR = 33% IR = 77% |
Prospective pilot trial | VMAT |
36.25 Gy /5fx Dose escalation to 40 Gy /5fx to the DIL |
Dmax ≤ 38.78 Gy (83.5 Gy EQD2) | 30% | At least 3 months | NR |
G2: 7.7% G3: 0% |
G2: 7.7% G3: 0% |
NR | NR | NR |
|
Brand et al., 2019 [25] Tree et al., 2022 [7] |
874 pts (414 pts in the SBRT arm) |
Phase III prospective randomized trial (PACE-B) |
VMAT IMRT CyberKnife |
36.25 Gy and 40 Gy/5fx |
V42Gy (95.7 Gy EQD2) < 50% V44Gy (103.8 Gy EQD2) < 20% |
No | 24 months | CTCAE |
Worst acute toxicity: G ≥ 2: 30.9% |
Worst acute toxicity: G ≥ 2: 15.6% | Cumulative incidence G ≥ 2: 32.3% | Cumulative incidence G ≥ 2: 12.5% | NR |
| Pryor et al., 2019 [27] |
135 pts IR = 76% HR = 26% |
Phase II prospective trial (PROMETHEUS) |
VMAT / IMRT |
Prostate: 46 Gy /23fx Followed by a boost: 19-20 Gy /2fx |
D0.1 cc < 20.9 Gy (total of 102 Gy EQD2) – 22 Gy (total of 107.6 Gy EQD2) |
36% short-term 18% long-term |
24 months | CTCAE |
G2: 26.6% G3: 0% |
G2: 4.4% G3: 0% |
Cumulative incidence G ≥ 2: 24.9% G ≥ 3: 2.2% |
Cumulative incidence G ≥ 2: 4.5% G ≥ 3: 2% |
NR |
| Kishan et al., 2023 [36] |
156 pts IR = 61% HR = 23% vHR = 9% N + = 7% |
Prospective randomized phase III trial (MIRAGE) | MR-guided vs CT-guided SBRT | 40 Gy /5fx | Dmax < 42 Gy (95.7 Gy EQD2) |
CT-arm: 74% MRgRT: 62% |
At least 3 months | CTCAE |
MRgRT: G ≥ 2: 24.4% CT-guided: G ≥ 2: 43.4% |
MRgRT: G ≥ 2: 0% CT-guided: G ≥ 2: 10.5% |
NR | NR | NR |
RT radiation therapy, GU genitourinary, GI gastrointestinal, VMAT volumetric arc therapy, pts patients, LR low-risk, IR intermediate-risk, HR high-risk, NR non reported, DIL dominant intraprostatic lesion, IMRT intensity-modulated radiation therapy, G grade, bRFSbiochemical relapse-free survival
Other trials implemented a urethra-steering technique for a whole prostate gland SBRT irradiation. Tree et al. recently reported the 2-year toxicity results of patients randomized in the PACE-B phase III clinical trial comparing a 5-fraction SBRT schedule versus moderate or standard fractionation [7]. For the 5-fraction schedule, the constraints to the urethra were optional, with a V44Gy < 20% amended successively in favor of a V42Gy < 50%. The 2-year cumulative incidence of grade ≥ 2 GU toxicity was acceptable, raising to 32.3%. The most frequent grade ≥ 2 GU toxicity was an increased urinary frequency, peaking at 15 months and observed in 10% of patients. Worst acute grade ≥ 2 GU toxicity was reported earlier and raised up to 30.9% of men with a peak 2 weeks after the start of SBRT [25]. Kishan et al. recently reported the early toxicity results of the randomized phase III MIRAGE trial, comparing a magnetic resonance (MR)-guided with a computed tomography (CT)-guided prostate SBRT [26]. The clinical target volume was expanded by 4 mm in case of CT-guided SBRT and 2 mm for MR-SBRT and the delivered dose was 40 Gy in 5 fractions, including an additional boost on the DIL or pelvic lymph node radiotherapy. The maximal dose delivered to the urethra was limited to 42 Gy (95.7 Gy EQD2). MR-based SBRT with reduced margins enabled a significant reduction of both acute grade ≥ 2 GU and GI toxicities compared to a CT-based SBRT (24.4% vs 43.4%, p = 0.01 and 0% vs 10.5%, p = 0.03). Of note, the maximal dose delivered to the urethra was similar between the two arms (41.6 Gy and 41.7 Gy for CT-guidance and MRI-guidance, respectively), while MR-based SBRT was associated with a significant reduction in the volume of bladder receiving 40 Gy (0.3 cc vs 0.7 cc, p = 0.001) and 39 Gy (1.9 cc vs 3.7 cc, p < 0.001). Pryor et al. reported the results of the PROMETHEUS trial, evaluating the use of a high-dose ultra-hypofractionated SBRT boost as dose-escalation strategy for men with either intermediate-risk or high-risk PCa [27]. Prostate SBRT boost consisted of either 19 Gy or 20 Gy in two fractions, followed by a prostate radiotherapy at a dose of 46 Gy in 23 fractions. The D0.1 cc delivered to the urethra was limited to < 110% of the total SBRT dose. Acute and cumulative rates of grade ≥ 2 GU toxicities were reported in 26.6% and 27.1%, respectively, with a peak observed at 18 months. Fuller et al. reported the outcomes of 259 low- and intermediate-risk PCa patients treated within a phase II trial of “high-dose rate (HDR)-like” SBRT [28, 29]. The prescribed dose was 38 Gy in 4 fractions, with planning target volume receiving at least 150% of the prescription dose. The maximal dose constraint imposed on the urethra (Dmax < 131.3 GyEQD2) was significantly higher than what was previously reported [7, 21, 23]. A deterioration of QoL scores was noted at one month, with the appearance of obstructive complaints and weak stream in 15% and 8% of patients, respectively, returning to baseline by 6 months. A 36.2% and 19.2% rate both acute of late grade ≥ 2 GU toxicity were reported, including up to 10% of the patients reporting the use of incontinence pads after 5 years of follow-up. Of note, one patient required a total cysto-prostatectomy for a grade 4 cysto-urethritis. While this study recruited a 4% rate of patients with prior transurethral resection of the prostate (TURP), authors suggest a cautious patient selection when high biologically effective dose are delivered to the whole prostate gland. Excellent biochemical control was demonstrated in men with low-risk PCa, with a 10-year biochemical relapse free-survival (bRFS) of 100%. However, the 10-year bRFS reached only 68.4% for unfavorable intermediate-risk PCa patients, probably attributable to the lack of ADT prescription.
Urethra dose-reduction SBRT
A urethra dose-reduction strategy was tested in two prospective phase II SBRT trials [30, 31] (Table 2). While a total dose of 36.25 Gy in 5 fractions was prescribed to the whole prostate gland, a dose reduction to 32.5 Gy was delivered to a 2-mm planning organ-at-risk volume (PRV) generated around the urethra. This dose reduction was adopted as best dose compromise in an attempt to minimize GU toxicity while maintaining an acceptable tumor control to the possible microscopic periurethral disease (74 Gy EQD2, α/β = 1.5 Gy). In a phase II multicenter randomized clinical trial, Zilli et al. tested this optimization strategy using two different schedules, delivered every-other-day (EOD) or once-a-week (QW) [31, 32]. Among the 165 patients treated, mostly diagnosed with either low- or intermediate-risk PCa, acute toxicity was mild or absent, with no differences between arms. With a follow-up of more than 70 months, the incidence of CTCAE grade 2 GU toxicity was below 10% for both arms, respectively, corresponding to a 5-year grade 2 or greater GU toxicity-free survival of 75.9% and 76.1% for patients treated EOD versus QW, respectively (P = 0.945). Together with a minimal impact on QoL, oncological outcomes were encouraging, with a 5-year bRFS exceeding 90% for both fractionations. Of note, the trial reported dosimetry protocol deviations in 31% of the cases, consisting mainly of underdosing of urethral PRV (12% of the patients with D98% < 30.2 Gy), particularly when using an IMRT technique [33]. Using the same fractionation schedule delivered with adaptive Magnetic Resonance-guided Radiotherapy (MRgRT), Bruynzeel et al. reported a 19.8% of grade 2 CTCAE GU toxicity at the end of SBRT, decreasing to 7.9% at 6-weeks and remaining between 3.1% and 5.1% thereafter [30, 34]. This trial recruited a majority of patients with high-risk PCa. To date, no-long-term oncological results are available.
Table 2.
Toxicity and oncological outcomes after urethra dose-reduction protocols (prescription of a lower dose on the whole urethra, or with maximal doses delivered to the urethra lower than the dose of prescription to the prostate gland)
| Author | Patients | Study design | RT technique | RT dose | Urethral dose prescription | Dose constraints (EQD2, α/β = 3 Gy) | ADT | Median follow-up | Toxicity scale | Acute GU toxicity | Acute GI toxicity | Late GU toxicity | Late GI toxicity | Oncological outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Bruynzeel et al., 2020 [30] Tetar et al., 2021 [34] |
101 pts LR = 4% IR = 37% HR = 59% |
Phase II prospective trial | MRgRT | 36.25 Gy / 5 fx | 32.5 Gy /5fx | D2% < 34.8 Gy (69.2 Gy EQD2) |
41% short-term 41% long-term |
At least 3 months | CTCAE | G ≥ 2: 19.8% | G ≥ 2: 3% | G2: 3.1–5.1% | 0% | 2-year bRFS: 96.7% |
| Greco et al., 2022 [37] |
444 pts LR = 4.1% IR = 84% HR = 11.9% |
Phase II prospective trial | VMAT | 45 Gy /5fx | None | D1cc < 36 Gy (73.4 Gy EQD2) | 36% | 58 months | RTOG |
G2: 6.8% G3: 0% |
G2: 0.5% G3: 0% |
Cumulative incidence: G ≥ 2: 5.3% |
Cumulative: G ≥ 2: 1.1% |
7-year bRFS: 86.2% |
| Greco et al., 2021 [38] |
30 pts IR = 100% |
Phase II prospective randomized trial (PROSINT) |
VMAT | 45 Gy /5fx | None |
Dmax < 42.75 Gy (98.7 Gy EQD2) D1cc < 36 Gy (73.4 Gy EQD2) |
No | 48 months | RTOG | G ≥ 2: 0% | G ≥ 2: 0% |
Cumulative incidence: G2: 17% |
G ≥ 2: 0% | 4-year bRFS: 85.7% |
| 24 Gy /1fx |
Dmax < 22.8 Gy (117.6 Gy EQD2) D1cc < 19.2 Gy (85.2 Gy EQD2) |
G ≥ 2: 0% | G ≥ 2: 0% |
Cumulative incidence G2: 11.4% Urethral strictures: 6.6% |
G ≥ 2: 0% | 4-year bRFS: 77.1% | ||||||||
| Magli et al., 2021 [36] | 59 pts | Phase II prospective trial | IMRT | 40 Gy / 3 fx | 33 Gy /3fx | D0.1 cc < 33 Gy (92.4 Gy EQD2) | 3 months if prostate size > 80cm3 | At least 12 months | CTCAE | G ≥ 2: 13.8% | G2: 8.5% |
Prevalence at 12 months: G2: 0% |
G2: 0% | NR |
| Parsai et al., 2020 [35] |
35 pts LR = 9% IR = 40% HR = 51% |
Prospective pilot study | VMAT |
50 Gy /5fx 36.25 Gy /5fx to HDAZ |
36.25 Gy /5fx |
Dmax < 50 Gy (130 Gy EQD2) D1cc < 45 Gy (108 Gy EQD2) |
No: 45% Short-term: 50% Long-term: 5% |
46 months | CTCAE |
G2: 19.4% G4: 2.9% |
G2: 0% G4: 2.9% |
12-months incidence: G2: 25% G4: 2.9% |
12-months incidence: G2: 5.6% G4: 2.9% |
3-year bRFS: 88% LR: 100% IR: 89.5%-100% HR: 82.3% |
| Zilli et al., 2019 [39] | 6 pts |
Phase I prospective trial (ONE SHOT) |
VMAT | 19 Gy /1fx | 17 Gy /1fx | D2% < 18.2 Gy (77.1 Gy EQD2) | No | At least 3 months | CTCAE |
G2: 33% G ≥ 3: 0% |
G2: 0% | NR | NR | NR |
|
Zilli et al., 2020 [32] Zilli et al., 2023 [31] |
170 pts LR = 22% IR = 64% HR = 14% |
Phase II randomized trial (once a week vs every other day) |
IMRT / VMAT | 36.25 Gy /5fx | 32.5 Gy /5fx | D2% < 34.8 Gy (69.2 Gy EQD2) | 45% | 77/78 months | CTCAE | Worst G2: 17/19% | Worst G2: 2/0% |
Cumulative: G2: 21.6% G3: 0.6% Incidence: G2 8.3% and 7.3% (5-yr) |
Cumulative: G2: 9.3% G3: 0% Incidence: G2 < 2% (5- yr) |
5-year bRFS: 92.2% (every other day) – 93% (once a week) |
RT radiation therapy, GU genitourinary, GI gastrointestinal, MRgRT magnetic resonance guided radiation therapy, NR non reported, bRFS biochemical relapse-free survival, pts patients, LR low-risk, IR intermediate-risk, HR high-risk, IMRT intensity modulated radiation therapy, HDAZ high dose avoidance zone, NS non specified, G grade, bRFS biochemical relapse-free survival
Parsai et al. further implemented urethra dose-reduction within a “high-dose avoidance zones” (HDAZ) protocol, defined as a 3-mm expansion around rectum, urethra, and bladder [35]. A dose of 50 Gy in 5 fractions was prescribed to the target volume, with a dose reduction as low as 36.25 Gy on the prostate gland in close proximity with organs at risk. Urethral Dmax and D1cc were deemed to be less than 130 GyEQD2 and 108GyEQD2, respectively. At a median follow-up of 46 months, a 19.4% and 25% rate of acute and late grade 2 GU toxicity were observed, respectively, consisting mostly of urinary irritation and obstructive symptoms. One patient developed Fournier gangrene after implantation of radiofrequency transponders, requiring multiple surgeries for debridement. For the whole cohort, the 3-year bRFS was 88%, while the same rate decreased to 82.3% in patients with high-risk disease. In a dose escalated phase II trial, Magli et al. tested a three fractions SBRT schedule up to 40 Gy, with a dose reduction to 33 Gy to the urethral PRV [36]. Acute grade ≥ 2 toxicity was reported in 13.8% of the patients, consisting mostly in irritative symptoms rapidly improving 1 month after treatment end. At 1 year, no patient experienced persistent grade 2 GU toxicity. Greco et al. recently reported the outcomes of 444 men treated within a phase II trial at a dose of 45 Gy in 5 fractions on the prostate gland [37]. Most patients were diagnosed with intermediate-risk PCa (84%), with only a small proportion of men presenting with high-risk PCa (11.9%). The maximal dose delivered to the urethra was limited to 36 Gy (73.4 Gy EQD2). A Foley catheter loaded with 3 electromagnetic transponders was used for both urethra visualization and tracking. Only 6.8% of the patients experienced grade 2 toxicity, with 4 cases of acute retention requiring catheterization. Excellent oncological outcomes were demonstrated in the whole population with a 7-year bRFS of 86.2%, yet reaching only 73.5% in the high-risk population. Of the 34 patients with positive positron emission tomography/computed tomography (PET/CT) findings at relapse, 73.5% showed evidence of intraprostatic relapse at the site of the pre-treatment DIL.
Two studies assessed the safety and efficacy of a single-dose SBRT for men with localized PCa. Using the same tracking approach, Greco et al. randomized in the PROSINT trial 30 men to receive either 45 Gy in 5 fractions or 24 Gy in one fraction [38]. In the single-dose arm, urethral Dmax and D1cc were respectively constraints to 22.8 Gy (117.6 GyEQD2) and 19.2 Gy (85.2 GyEQD2). While no patient experienced grade 2 GU toxicity in the acute setting, one patient out of fifteen experienced urethral stricture at 30 months of follow-up. The 4-year bRFS reached only 75% and 64% for men with unfavorable intermediate-risk disease in the 5 fraction and single-fraction arm, respectively. Zilli et al. also explored in a single-arm multicenter phase I/II trial the safety and efficacy of a single-fraction SBRT for men presenting with low- or intermediate-risk PCa [39]. In the “ONE SHOT” trial, the prostate gland was planned to receive a dose of 19 Gy, while a dose-reduction at 17 Gy was performed to the urethral PRV. In the phase I, fifty percent of men reported grade 2 toxicity at 1 week after treatment, returning to baseline at 12 weeks. No grade 3 toxicity was reported with a minimal dosimetric impact of intrafraction prostate motion by using real-time electromagnetic tracking combined with beam gating [40].
Discussion
The urethra can be considered as a tubular “serial” structure, and most US-SBRT trials investigated the association between the maximum doses to the urethra and the onset of late GU toxicity. The moderate hypofractionation FLAME trial (77 Gy in 35 fractions with or without a 95 Gy dose escalation on the DIL) reported an exponential dose-toxicity relationship, with a strong correlation with urethra D0.1 cc metrics [41]. The authors proposed to implement a dose constraint of 80 Gy when SIB optimization is performed (D0.1 cc ≤ 91.2GyEQD2). These constraints are broadly similar to those published by Zhang et al. for a 38 Gy/4 fractions HDR-like SBRT schedule, advising in favour of the implementation of a urethral maximal dose constraint of 38/42 Gy (Dmax < 80.6 – 95.8 GyEQD2) [42]. More recently, the meta-analysis of 23 SBRT prospective trials led by Leeman et al. demonstrated a significant association between urethral doses and onset of late GU toxicity, with each increase in 1 Gy in maximal urethral doses corresponding to a 0.8% and 1% increase in acute and late grade ≥ 2 GU toxicity [16]. According to this model, a maximal urethral dose of 100 GyEQD2 would result in a 10% probability to experience late grade ≥ 2 GU toxicity. The vast majority of the urethra-sparing trials have imposed urethral Dmax below this threshold within their protocol [20, 21, 24, 30, 31, 36, 36, 37, 43] and demonstrated the efficiency of this approach in reducing high-grade late toxicities. In trials restricting urethra Dmax to 90 GyEQD2, the rates of late grade 2 toxicity ranged from 12.1% [20] to 14% [21], with no report of urethral stenosis. On the other hand, trials imposing less stringent urethral constraints (Dmax > 100GyEQD2) [7, 25, 27–29, 35, 38] attested significantly higher rates of late grade 2 GU toxicity, with cumulative incidences ranging from 17 to 32%, together with the onset of severe toxicity consisting either in urethral strictures [38] or urethritis requiring cysto-prostatectomy [28]. Although both US-SBRT techniques have been associated with promising mitigation of GU toxicity, a strict comparison between the two optimization strategies remains difficult. While dose reduction strategies with strict urethral dose constraints may represent a valid option when the prostate is treated with a homogeneous dose and the dominant tumor is not located closely to the transition zone, urethra-steering may be the technique of choice when dose-escalation on the DIL is performed.
While most SBRT studies assessed the impact of dose-volume parameters delivered to the intraprostatic urethra, the dose delivered to other urethral segments has also been suggested to be associated with the late onset of GU toxicity. Several retrospective series with HDR BT suggested the bulbo-membranous urethra to be the most radiosensitive segment, after reporting this portion as the one most frequently affected by stenosis [14, 44]. Additionally, Mohammed et al. showed a significant association between the risk to develop a urethral stricture and the maximal doses delivered to the bulbo-membranous urethra [45]. Low-to-intermediate radiotherapy doses delivered to the bulbo-membranous urethra were also associated with the occurrence of late onset dysuria in a voxel-based analysis performed in patients treated within the RADAR and CHHiP trials [46]. The development of predictive pixel and voxel approaches also led to the development of the hypothesis of a heterogeneous intra-organ radiosensitivity. Mylona et al. recently identified the volume of bladder trigone receiving > 72 Gy as a predictor of acute urinary retention [47]. Also using a voxel-based analysis, Improta et al. found an association between the dose delivered to the bladder trigone and the risk of acute GU toxicity [48]. Last but not least, Ghadjar et al. demonstrated an association between various trigone dose-parameters and the occurrence of overall grade ≥ 2 GU toxicity and late obstructive voiding symptoms [49]. Future trials delineating the different urinary sub-structures separately and investigating dose–volume relationships in these regions are awaited to further characterize their dose sensitivity.
Beyond the dose parameters delivered to urinary structures, the occurrence of late GU toxicity is known to be multifactorial. While transurethral resection of the prostate (TURP) has long been associated with the onset of urethral strictures after BT [14], the impact of surgical treatments of benign prostatic hyperplasia has been poorly studied in SBRT trials. In a retrospective study including 47 patients treated with SBRT, Pepin et al. reported late grade 2 and 3 GU toxicity raising up to 48.9% and 6.4% of the patients, respectively, consisting mostly in haematuria in relation to necrosis occurring in the bladder neck or TURP defect [50]. Huck et al. also reported late grade 2 and 3 GU toxicity in 33% and 17% of patients with a previous history of surgical treatment for benign prostatic hyperplasia, occurring more frequently in patients with prior adenomectomy, multiple TURP and/or large volumes of the intraprostatic resection cavity [51]. Up to 42% of the patients experienced at least one episode of hematuria. Prostate volume (> 50–60 cc) has also been suggested to be a predictor of both acute and late GU toxicity, without any threshold being formally identified [52, 53]. In men presenting with large prostate (> 50 cc), a late urinary flare consisting mostly in dysuria and retention has been observed up to two years after prostate SBRT, yet with no impact on quality of life [54]. In a population of patient with a prostate size > 100 cc, Haas et al. also demonstrated a transient decline in EPIC scores at 1 and 3 months after SBRT, resolving by 1 year after treatment completion [55]. Although discrepancies still exist between studies, prostate size may not be one of the strongest determinants of urinary toxicity after SBRT.
The development of urethra-sparing techniques for PCa has initially been discouraged due to the report of unusually high rates of biochemical failure within the first phase II trial led by Vainshtein et al. [56]. More recent urethra-dose reduction trials reported encouraging results in terms of biochemical control, despite restrictions due to a short follow-up. Zilli et al. recently reported a 5-year bRFS of 92.2% with 36.25 Gy in 5 fractions schedule performed EOD, in a population of patients mostly represented by low-risk or intermediate-risk PCa [31]. Greco et al. demonstrated a 7-year bRFS of 86.2% with dose-escalation up to 45 Gy, with a cumulative incidence rate of PSA failure of 2%, 16.6%, and 27.2% in the low- and favorable intermediate-, unfavorable intermediate-risk, and high-risk groups, respectively [37]. Excellent oncological results were also demonstrated by Fuller et al., with a 10-year bRFS reaching 100% and 84.3% in the low-risk and the favorable intermediate-risk cohort, respectively, with only 3 reports of biopsy-proven local recurrence [28]. While data support the safety of implementation of US-SBRT for low- to intermediate-risk PCa, data remain scarce with regard to high-risk PCa. Parsai et al. reported a 3-year bRFS of 82.3% in men presenting with high-risk disease, which compares favorably with a pooled meta-analysis published by King et al. showing a 5-year bRFS of 81% in this population of patients [57]. None of these studies required a minimum distance between the urethra and the intra-prostatic tumor, and to date only Cloitre et al. deemed a 3-mm minimal distance between the tumor and the urethra to safely adopt urethra-sparing techniques. [23].
Several ongoing SBRT trials are implementing urethra-sparing techniques to mitigate long-term GU toxicity (Table 3). Precise definition of the urethra represents one the major limitations to the implementation of this technique in clinical practice. Although use of a Foley catheter is the standard technique used to define the urethra [58], the invasive nature of this technique and the risk of plan uncertainties due urethral displacements [59] limit its widespread application in clinical practice. The use of MRI with dedicated sequences and automatic segmentation based on artificial intelligence (AI) are promising tools increasingly used to improve the accuracy in the definition of the urethra. Integration of these technologies into modern MRI-linacs, makes MR-guided SBRT an appealing treatment option to treat PCa patients. The definition of the urethra on dedicated MRI sequences, the use of adaptive treatment delivery with reduced PTV margins [26], and the possibility of optimization on other structures involved in GU toxicity (trigone, bladder neck, bulbous and membranous portions of the urethra) constitute the main advantage of this technology compared to standard CT-guided SBRT techniques.
Table 3.
Ongoing prostate SBRT trials implementing urethra-sparing techniques
| Trial | Design | Technique | Dose delivered to the target volume | Dose delivered to the urethra | Primary outcome |
|---|---|---|---|---|---|
|
(Proseven) |
Single arm prospective trial | MRgRT |
PTV: 36 Gy/5fx (90% isodose line) Prostate gland: 40 Gy /5fx DIL: 42 Gy/5fx |
V40Gy: < 1 cc | Acute toxicity (CTCAE and RTOG) |
|
(PRO-FAST) |
Single arm prospective trial | NR | PTV: 24 Gy /1fx | NR | Acute toxicity (CTCAE) |
|
(SAFO) |
Single arm prospective trial | NR |
PTV: 36.25 Gy /5fx DIL: 50 Gy /5fx |
NR |
bRFS Local PFS |
|
(SUPR-SABR) |
Single arm prospective phase II trial | NR | PTV: 40 Gy /5fx | Dmax: 36.25 Gy | Toxicity (EPIC score) |
|
(ARTIA- prostate) |
Single arm prospective trial | Adaptive RT | PTV: 40 Gy /5fx | Prescription dose: 35-36 Gy/5fx | Acute toxicity (EPIC) |
| NCT02470897 | Randomized prospective trial | IMRT | PTV: 37.5 Gy or 40 Gy /5fx | NR |
Acute and late toxicity bRFS |
PTV planning target volume, DIL dominant intraprostatic lesion, MRgRT magnetic resonance-guided radiotherapy, fx fractions, bRFS biochemical relapse-free survival, PFS progression-free survival, RT radiotherapy
This systematic review has several limitations. First, a comprehensive overview of studies performing urethra-sparing radiotherapy remains difficult to be conducted, due to the lack in some cases of information on urethra-sparing procedures. Toxicity evaluation was also heterogeneous among studies, including use of different grading scales (either RTOG and/or CTCAE). Also, protocol violations or “real-life” doses delivered to the urethra were not reported in most trials, which represents a limitation in the interpretation of toxicity outcomes. Moreover, delineation of urethra has been performed using either a Foley catheter [31, 35, 36, 38, 39, 43] or a co-registration with the diagnostic MRI [23, 30]. Last but not least, some studies implemented a 2–3-mm PRV margin around the urethra [21, 30, 31, 35, 36, 39], while other did not [23, 29, 38], leading to a large variation in the sparing and treatment optimization of this structure.
Conclusions
In patients with localized prostate cancer, US-SBRT techniques limiting maximum doses to urethra below a 90-GyEQD2 (α/β = 3 Gy) threshold represent a promising strategy to mitigate acute and long-term grade ≥ 2 GU toxicity, while maintaining at the same time acceptable rates of local disease control. Dose-reduction to urethra below 70 GyEQD2 (α/β = 3 Gy) may enable a further reduction in long-term GU toxicity in selected patients with no tumour in the transition zone. A better understanding of the clinical factors and anatomical substructures involved in the development of urinary toxicity, as well as the development and use of adapted dose constraints, will help to further reduce the long-term GU toxicity of patients undergoing SBRT for prostate cancer.
Funding
Open access funding provided by Università della Svizzera italiana.
Declarations
Conflict of interest:
No competing interests concerning the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References:
- 1.Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology : Prostate Cancer. V 3.2022.
- 3.Hatano K, Tohyama N, Kodama T, et al. Current status of intensity-modulated radiation therapy for prostate cancer: History, clinical results and future directions. Int J Urol Off J Jpn Urol Assoc. 2019;26:775–784. doi: 10.1111/iju.14011. [DOI] [PubMed] [Google Scholar]
- 4.Bauman G, Rumble RB, Chen J, et al. Intensity-modulated radiotherapy in the treatment of prostate cancer. Clin Oncol R Coll Radiol G B. 2012;24:461–473. doi: 10.1016/j.clon.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Latorzeff I, Mazurier J, Boutry C, et al. Benefit of intensity modulated and image-guided radiotherapy in prostate cancer. Cancer Radiother J Soc Francaise Radiother Oncol. 2010;14:479–487. doi: 10.1016/j.canrad.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-Fractionation Sensitivity of Prostate Cancer Deduced From Radiotherapy Outcomes of 5,969 Patients in Seven International Institutional Datasets: α/β = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol. 2012;82:e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 7.Tree AC, Ostler P, van der Voet H, et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2022;23:1308–1320. doi: 10.1016/S1470-2045(22)00517-4. [DOI] [PubMed] [Google Scholar]
- 8.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. The Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 9.Jackson WC, Silva J, Hartman HE, et al. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int J Radiat Oncol. 2019;104:778–789. doi: 10.1016/j.ijrobp.2019.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukka HR, Deshmukh S, Bruner DW, et al. Five-Year Patient-Reported Outcomes in NRG Oncology RTOG 0938, Evaluating 2 Ultrahypofractionated Regimens for Prostate Cancer. Int J Radiat Oncol Biol Phys. 2022;S0360–3016(22):03671–3679. doi: 10.1016/j.ijrobp.2022.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corkum MT, Achard V, Morton G, Zilli T. Ultrahypofractionated Radiotherapy for Localised Prostate Cancer: How Far Can We Go? Clin Oncol R Coll Radiol G B. 2022;34:340–349. doi: 10.1016/j.clon.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Merrick GS, Butler WM, Wallner KE, et al (2006) Risk factors for the development of prostate brachytherapy related urethral strictures. J Urol 175:1376–1380; discussion 1381. 10.1016/S0022-5347(05)00681-6 [DOI] [PubMed]
- 13.Merrick GS, Butler WM, Tollenaar BG, et al. The dosimetry of prostate brachytherapy-induced urethral strictures. Int J Radiat Oncol Biol Phys. 2002;52:461–468. doi: 10.1016/s0360-3016(01)01811-9. [DOI] [PubMed] [Google Scholar]
- 14.Hindson BR, Millar JL, Matheson B. Urethral strictures following high-dose-rate brachytherapy for prostate cancer: Analysis of risk factors. Brachytherapy. 2013;12:50–55. doi: 10.1016/j.brachy.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Panettieri V, Rancati T, Onjukka E, et al. External Validation of a Predictive Model of Urethral Strictures for Prostate Patients Treated With HDR Brachytherapy Boost. Front Oncol. 2020;10:910. doi: 10.3389/fonc.2020.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leeman JE, Chen Y-H, Catalano P, et al. Radiation Dose to the Intraprostatic Urethra Correlates Strongly With Urinary Toxicity After Prostate Stereotactic Body Radiation Therapy: A Combined Analysis of 23 Prospective Clinical Trials. Int J Radiat Oncol Biol Phys. 2022;112:75–82. doi: 10.1016/j.ijrobp.2021.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Zilli T, Achard V, Guevelou JL. Intraprostatic Urethra: The New Kid on the Block for Prostate Cancer Radiation Therapy? Int J Radiat Oncol. 2022;113:92–95. doi: 10.1016/j.ijrobp.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Groen VH, van Schie M, Zuithoff NPA, et al. Urethral and bladder dose-effect relations for late genitourinary toxicity following external beam radiotherapy for prostate cancer in the FLAME trial. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2022;167:127–132. doi: 10.1016/j.radonc.2021.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman Dg (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009 Jul 2167e1000097 [PMC free article] [PubMed]
- 20.McDonald AM, Dobelbower MC, Yang ES, et al. Prostate Stereotactic Body Radiation Therapy With a Focal Simultaneous Integrated Boost: Acute Toxicity and Dosimetry Results From a Prospective Trial. Adv Radiat Oncol. 2019;4:90–95. doi: 10.1016/j.adro.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draulans C, van der Heide UA, Haustermans K, et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother Oncol. 2020;147:92–98. doi: 10.1016/j.radonc.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 22.De Cock L, Draulans C, Pos FJ, et al (2023) From once-weekly to semi-weekly whole prostate gland stereotactic radiotherapy with focal boosting: primary endpoint analysis of the multicenter phase II hypo-FLAME 2.0 trial. Radiother Oncol J Eur Soc Ther Radiol Oncol 109713. 10.1016/j.radonc.2023.109713 [DOI] [PubMed]
- 23.Cloitre M, Valerio M, Mampuya A, et al. Toxicity, quality of life, and PSA control after 50 Gy stereotactic body radiation therapy to the dominant intraprostatic nodule with the use of a rectal spacer: results of a phase I/II study. Br J Radiol. 2023;96:20220803. doi: 10.1259/bjr.20220803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera FG, Valerio M, Berthold D, et al. 50-Gy Stereotactic Body Radiation Therapy to the Dominant Intraprostatic Nodule: Results From a Phase 1a/b Trial. Int J Radiat Oncol. 2019;103:320–334. doi: 10.1016/j.ijrobp.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20:1531–1543. doi: 10.1016/S1470-2045(19)30569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishan AU, Ma TM, Lamb JM, et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023;9:365–373. doi: 10.1001/jamaoncol.2022.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pryor D, Sidhom M, Arumugam S, et al. Phase 2 Multicenter Study of Gantry-Based Stereotactic Radiotherapy Boost for Intermediate and High Risk Prostate Cancer (PROMETHEUS) Front Oncol. 2019;9:217. doi: 10.3389/fonc.2019.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuller DB, Falchook AD, Crabtree T, et al. Phase 2 Multicenter Trial of Heterogeneous-dosing Stereotactic Body Radiotherapy for Low- and Intermediate-risk Prostate Cancer: 5-year Outcomes. Eur Urol Oncol. 2018;1:540–547. doi: 10.1016/j.euo.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Fuller DB, Crabtree T, Kane BL, et al (2022) High Dose “HDR-Like” Prostate SBRT: PSA 10-Year Results From a Mature, Multi-Institutional Clinical Trial. Front Oncol 12:935310. 10.3389/fonc.2022.935310 [DOI] [PMC free article] [PubMed]
- 30.Bruynzeel AME, Tetar SU, Oei SS, et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int J Radiat Oncol. 2019;105:1086–1094. doi: 10.1016/j.ijrobp.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Zilli T, Jorcano S, Bral S, et al. Every-Other-Day Versus Once-a-Week Urethra-Sparing Prostate Stereotactic Body Radiation Therapy: 5-Year Results of a Randomized Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2023;S0360–3016(23):00301–302. doi: 10.1016/j.ijrobp.2023.03.057. [DOI] [PubMed] [Google Scholar]
- 32.Zilli T, Jorcano S, Bral S, et al. Once-a-week or every-other-day urethra-sparing prostate cancer stereotactic body radiotherapy, a randomized phase II trial: 18 months follow-up results. Cancer Med. 2020;9:3097–3106. doi: 10.1002/cam4.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaccard M, Zilli T, Dubouloz A, et al. Urethra-Sparing Stereotactic Body Radiation Therapy for Prostate Cancer: Quality Assurance of a Randomized Phase 2 Trial. Int J Radiat Oncol. 2020;108:1047–1054. doi: 10.1016/j.ijrobp.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Tetar SU, Bruynzeel AME, Oei SS, et al. Magnetic Resonance-guided Stereotactic Radiotherapy for Localized Prostate Cancer: Final Results on Patient-reported Outcomes of a Prospective Phase 2 Study. Eur Urol Oncol. 2021;4:628–634. doi: 10.1016/j.euo.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Parsai S, Juloori A, Sedor G, et al. Heterogenous Dose-escalated Prostate Stereotactic Body Radiation Therapy for All Risk Prostate Cancer: Quality of Life and Clinical Outcomes of an Institutional Pilot Study. Am J Clin Oncol. 2020;43:469–476. doi: 10.1097/COC.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 36.Magli A, Farneti A, Faiella A, et al. Toxicity at 1 Year After Stereotactic Body Radiation Therapy in 3 Fractions for Localized Prostate Cancer. Int J Radiat Oncol. 2021;111:93–100. doi: 10.1016/j.ijrobp.2021.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Greco C, Pares O, Pimentel N, et al (2022) Urethra Sparing With Target Motion Mitigation in Dose-Escalated Extreme Hypofractionated Prostate Cancer Radiotherapy: 7-Year Results From a Phase II Study. Front Oncol 12:863655. 10.3389/fonc.2022.863655 [DOI] [PMC free article] [PubMed]
- 38.Greco C, Pares O, Pimentel N, et al. Safety and Efficacy of Virtual Prostatectomy With Single-Dose Radiotherapy in Patients With Intermediate-Risk Prostate Cancer: Results From the PROSINT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021;7:700–708. doi: 10.1001/jamaoncol.2021.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zilli T, Franzese C, Bottero M, et al. Single fraction urethra-sparing prostate cancer SBRT: Phase I results of the ONE SHOT trial. Radiother Oncol. 2019;139:83–86. doi: 10.1016/j.radonc.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Jaccard M, Ehrbar S, Miralbell R, et al. Single-fraction prostate stereotactic body radiotherapy: Dose reconstruction with electromagnetic intrafraction motion tracking. Radiother Oncol. 2021;156:145–152. doi: 10.1016/j.radonc.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Groen V Urethra and bladder dose-effect relations for genitourinary toxicity after EBRT for prostate cancer. ESTRO 2021 Abstr. Form
- 42.Zhang L, Johnson J, Gottschalk AR, et al. Receiver operating curves and dose-volume analysis of late toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol. 2017;7:e109–e116. doi: 10.1016/j.prro.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Zilli T, Scorsetti M, Zwahlen D, et al. ONE SHOT - single shot radiotherapy for localized prostate cancer: study protocol of a single arm, multicenter phase I/II trial. Radiat Oncol. 2018;13:166. doi: 10.1186/s13014-018-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan L, Williams SG, Tai KH, et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol. 2009;91:232–236. doi: 10.1016/j.radonc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Mohammed N, Kestin L, Ghilezan M, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:204–212. doi: 10.1016/j.ijrobp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Marcello M, Denham JW, Kennedy A, et al. Increased Dose to Organs in Urinary Tract Associates With Measures of Genitourinary Toxicity in Pooled Voxel-Based Analysis of 3 Randomized Phase III Trials. Front Oncol. 2020;10:1174. doi: 10.3389/fonc.2020.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mylona E, Ebert M, Kennedy A, et al. Rectal and Urethro-Vesical Subregions for Toxicity Prediction After Prostate Cancer Radiation Therapy: Validation of Voxel-Based Models in an Independent Population. Int J Radiat Oncol. 2020;108:1189–1195. doi: 10.1016/j.ijrobp.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Improta I, Palorini F, Cozzarini C, et al. Bladder spatial-dose descriptors correlate with acute urinary toxicity after radiation therapy for prostate cancer. Phys Med. 2016;32:1681–1689. doi: 10.1016/j.ejmp.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Ghadjar P, Zelefsky MJ, Spratt DE, et al. Impact of Dose to the Bladder Trigone on Long-Term Urinary Function After High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer. Int J Radiat Oncol. 2014;88:339–344. doi: 10.1016/j.ijrobp.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pepin A, Aghdam N, Shah S, et al. Urinary Morbidity in Men Treated With Stereotactic Body Radiation Therapy (SBRT) for Localized Prostate Cancer Following Transurethral Resection of the Prostate (TURP) Front Oncol. 2020;10:555. doi: 10.3389/fonc.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huck C, Achard V, Zilli T. Surgical Treatments of Benign Prostatic Hyperplasia and Prostate Cancer Stereotactic Radiotherapy: Impact on Long-Term Genitourinary Toxicity. Clin Oncol R Coll Radiol G B. 2022;34:e392–e399. doi: 10.1016/j.clon.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Seymour ZA, Chang AJ, Zhang L, et al. Dose-volume analysis and the temporal nature of toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol. 2015;5:e465–e472. doi: 10.1016/j.prro.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Katz AJ, Kang J (2014) Quality of Life and Toxicity after SBRT for Organ-Confined Prostate Cancer, a 7-Year Study. Front Oncol 4:. 10.3389/fonc.2014.00301 [DOI] [PMC free article] [PubMed]
- 54.Janowski E, Chen LN, Kim JS, et al. Stereotactic body radiation therapy (SBRT) for prostate cancer in men with large prostates (≥50 cm3) Radiat Oncol. 2014;9:241. doi: 10.1186/s13014-014-0241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haas JA, Mendez C, Witten MR, et al (2021) Stereotactic Body Radiation Therapy for Ultra-Large (> 100 cc) Prostate Glands: Oncologic, Toxicity and Patient-Reported Outcomes. Int J Radiat Oncol 111:e276. 10.1016/j.ijrobp.2021.07.892
- 56.Vainshtein J, Abu-Isa E, Olson KB, et al. Randomized phase II trial of urethral sparing intensity modulated radiation therapy in low-risk prostate cancer: implications for focal therapy. Radiat Oncol. 2012;7:82. doi: 10.1186/1748-717X-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217–221. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Kovács G, Pötter R, Loch T, et al. GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localised prostate cancer. Radiother Oncol. 2005;74:137–148. doi: 10.1016/j.radonc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Dekura Y, Nishioka K, Hashimoto T, et al. The urethral position may shift due to urethral catheter placement in the treatment planning for prostate radiation therapy. Radiat Oncol. 2019;14:226. doi: 10.1186/s13014-019-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]