Abstract
Foot pain is one of the most common presenting complaints in orthopaedic clinical practice and can be attributed to a multitude of pathologies in the various osseous structures, ligaments, and tendons of the foot. The spring ligament complex (SLC) between the calcaneum and navicular supports the talus and plays a major role in the static stability of the medial longitudinal arch of the foot. Although calcific ligamentous enthesopathy around the ankle has been described in the literature, we report the first case of its kind affecting the SLC in a 51-year-old male with medial foot pain and no history of trauma. We highlight the role of radiological interventions in the diagnosis and effective management using ultrasound (US)-guided barbotage.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40477-023-00781-9.
Keywords: Ankle pain, Spring ligament, Calcification, Radiology, Ultrasound, Barbotage
Introduction
The spring ligament complex (SLC), also called the plantar calcaneonavicular ligament complex, is integral to the static stability of the medial longitudinal arch of the foot. It is made of three components: the superomedial calcaneonavicular ligament (SM-CNL), the medioplantar oblique calcaneonavicular ligament (MPO-CNL), and the inferoplantar calcaneonavicular ligament (IPL-CNL) [1]. These components are shown in Fig. 1.
Fig. 1.
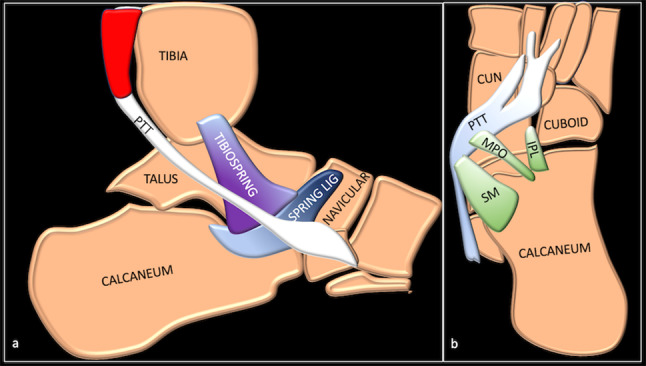
Sagittal (a) and axial (b) schematic showing the various components of SLC. PTT tibialis posterior tendon, CUN cuneiform, SPRING LIG spring ligament and its three components SM superomedial calcaneonavicular component, MPO medial plantar oblique calcaneonavicular component, IPL inferoplantar longitudinal calcaneonavicular component
A spectrum of injuries or pathologies can affect the SLC, resulting in pain, weakness, and foot deformity with the consequent collapse of the medial longitudinal arch that leads to pes planus [2].
Hydroxyapatite deposition disease (HADD) typically involves periarticular soft tissues, particularly tendons and ligaments. Calcific ligamentous involvement or tendinopathy is a condition of unknown aetiology caused by the pathological deposition of calcium hydroxyapatite (CHA) crystals in ligaments or tendons [3]. This condition can be asymptomatic. Symptomatic soft tissue calcification has been reported predominantly around the shoulder region and other unusual sites (wrist, elbow, hip, and knee) [4, 5]. Periarticular deposition of CHA crystals (believed to be due to diminished oxygen tension) within or around the tendons and ligaments induces a cell-mediated inflammatory response, resulting in a painful presentation for the patient [6].
Calcific ligamentous involvement or tendinopathy is uncommon around the foot and ankle, with few reports in the published literature [7, 8]. We present a case of CHA crystal deposition with isolated involvement of the SLC of the foot successfully treated with ultrasound (US)-guided barbotage. To our knowledge, this is the first case of its kind to be described in the literature.
Case description
A 51-year-old male presented with a 6-month history of gradual onset left midfoot pain. There was no history of antecedent trauma, but he was being treated with oral hypoglycaemic medication for his type II diabetes mellitus. The pain was localised to the medial aspect of the left foot. It worsened with walking and was present even when he was at rest, occasionally waking him up at night.
The clinical examination revealed no obvious deformity but a mild swelling of the midfoot distal to the tip of the medial malleolus at the talonavicular articulation. The skin surrounding the ankle and midfoot was of a normal colour. There was focal tenderness at this site, corresponding to the tuberosity of the navicular bone. The ankle demonstrated a full range of motion with no indications of midfoot instability. The medial longitudinal arch was preserved, and the patient was able to perform a single heel-rise test to evaluate the integrity of the tibialis posterior (TP) tendon. The dorsalis pedis and posterior tibial vessels were palpable, with intact sensation in the foot. Normal values were observed for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and serum uric acid (SUA).
A standing radiograph revealed a type III accessory navicular with well-preserved joint articulations, and no discernible calcification was noted (Fig. 2). Initial treatment with non-steroidal anti-inflammatory medication and physiotherapy did not result in a significant improvement.
Fig. 2.

Dorsoplantar (a) and oblique (b) radiographs of the hindfoot does not show any calcification
The patient underwent magnetic resonance imaging (MRI) to evaluate the cause of medial foot pain. This demonstrated mild insertional tendinopathy of the TP with a small amount of fluid in the tendon sheath (Fig. 3). A low signal was observed in the spring ligament with no significant surrounding oedema. No osseous oedema of the navicular or talus was noted. The talonavicular joint and other joints were unremarkable. The flexor digitorum longus, flexor hallucis longus, peroneals, and anterior tendon complexes were intact. The medial and lateral ligament complexes, plantar fascia, Achilles tendon, and sinus tarsi were normal.
Fig. 3.
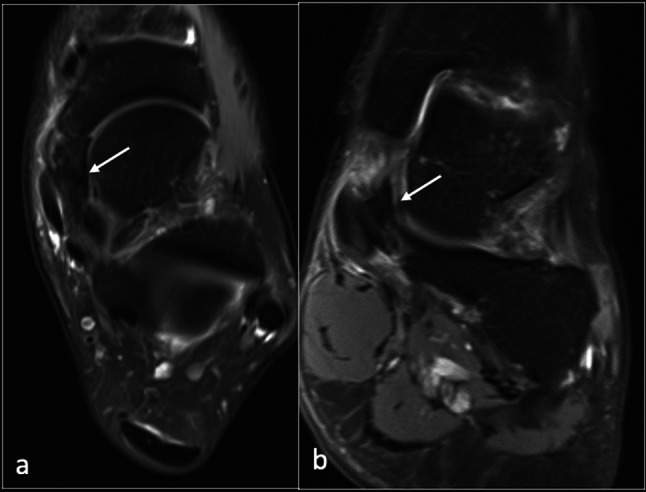
Axial (a) and coronal (b) PDFS (proton density fat suppressed) images showing low signal (arrow) within the spring ligament
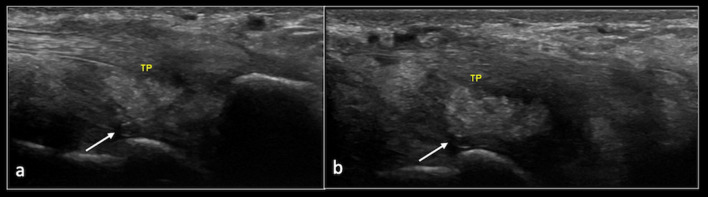
Furthermore, US was performed to assess the TP in detail. This revealed a 2 cm × 2 cm × 1 cm focus of type II and type III calcification within the SLC. The TP tendon demonstrated mild tendinopathy, but there was no evidence of a tear or insufficiency (Fig. 4).
Fig. 4.
Longitudinal ultrasound images showing calcification within the spring ligament (arrow). TP (Tibialis posterior tendon)
A dual-energy computed tomography (DECT) scan was suggested to determine the type of crystal deposition. This confirmed isolated CHA crystal deposition involving the SLC (Fig. 5).
Fig. 5.
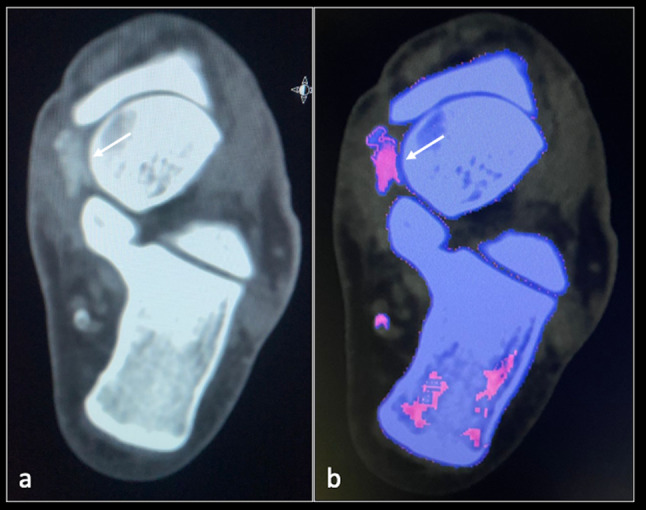
DECT axial (a, b) images showing calcification (arrow) within the spring ligament
A diagnosis of symptomatic CHA crystal deposition with isolated involvement of the SLC of the foot was made. Since oral analgesics were ineffective, the patient was successfully treated with US-guided barbotage using a 16G white needle after obtaining informed consent (Fig. 6; Video 1).
Fig. 6.

Ultrasound image showing needle (arrow) barbotage of the HADD (arrow head) of spring ligament
Discussion
The SLC and its three component ligaments connect the calcaneum and navicular bones while supporting the talus in the form of a sling [9]. The SLC is the strongest and most significant medial longitudinal arch stabiliser of the foot [10]. Consequently, injury, failure, or pathological conditions affecting the SLC can result in midfoot pain and, over time, instability and pes planus deformity [2, 10, 11].
Although there have been reports of calcific ligamentous involvement or tendinopathy around the ankle and foot, to the best of our knowledge, isolated HADD of the SLC has not been previously documented. Identifying symptomatic CHA crystal deposition within soft tissues around the foot, such as the SLC, is essential for patient management.
The pathogenesis of CHA crystal deposition, which is part of the spectrum of HADD, is not well understood. It is believed that repetitive microtrauma, local ischaemia, or reduced oxygen tension may contribute to CHA deposition [6]. In addition, patients with associated co-morbidities such as end-stage renal disease, hyperparathyroidism, and metabolic disorders such as diabetes mellitus appear to have a higher predilection, possibly due to a derangement of the serum calcium profile. Women are more frequently affected than men (2:1), with the average age of onset being approximately 45 years [12].
HADD may manifest symptoms depending on the location and severity of calcium deposition [13]. Symptoms include pain, swelling, stiffness, and decreased range of joint movement [4, 5, 7]. When sufficiently large, it can cause symptoms of soft tissue impingement, particularly in the rotator cuff tendons. However, if these calcium crystals can spill into the adjacent bursa or joint, or if the calcific deposit erodes into the adjacent bone, it can mimic septic arthritis [14].
The main differential diagnosis of HADD includes other crystal deposition diseases such as gout or calcium pyrophosphate deposition disease (CPPD) [13, 14].
The following clinical characteristics distinguish HADD from CPPD arthropathy:
CHA typically involves tendons, bursae, and periarticular tissue, whereas CPPD typically deposits in hyaline and fibrocartilage.
The deposition of CHA may mimic acute inflammation.
CHA depositions are often monoarticular and more commonly found in the shoulders.
They usually present acutely and are tender to palpation.
Joints with fibrocartilage and hyaline cartilage, such as the knees and wrists, are more susceptible to CPPD arthropathy. On a hand radiograph, CPPD arthropathy is distinguished by the loss of joint space in the 2nd and 3rd metacarpophalangeal (MCP) joints and the presence of hook osteophytes [15]. Although CPPD is the most prevalent cause of radiographic manifestations in chondrocalcinosis, CHA crystals can also be present (Table 1).
Table 1.
Table showing the differentiating features between gout, CPPD and HADD
| Gender predilection and Age of onset | Commonest joint involved | Structures involved | Crystal type | Crystal characteristics | Salient Imaging features | |
|---|---|---|---|---|---|---|
| Gout |
Middle aged (> 40) > Men (20:1), |
1st MTP joint | Tendons, bursae, and periarticular tissue | Mono sodium urate (MSU) crystals, |
Large, needle shape, Seen on light microscopy Strongly negative birefringence No special stains needed |
Punched out juxta articular erosions with overhanging margins Dense soft tissue swelling Double contour sign on US |
| CPPD |
Elderly (> 60) > Women |
Knee joint | Hyaline and fibrocartilage | Calcium Pyrophosphate Dihydrate crystals, |
Large, Rhomboid shape Seen on light microscopy Weakly positive birefringence No special stains needed |
Background Osteoarthritis, Chondrocalcinosis (TFCC wrist, Menisci of knee, Labrum in shoulder) 2nd 3rd MCP joints involvement Hook osteophytes Large subchondral cysts |
| HADD |
Middle aged (> 45) > Women(2:1) |
Shoulder joint | Tendons, bursae, and periarticular tissue | Calcium Hydroxyapatite crystals, |
Small, not seen on light microscopy Seen on electron microscopy, Non-birefringent Needs Alizarin red staining |
Calcific deposits of varying sizes in tendons and periarticular soft tissues Can spill into bursae or joints or erode the adjacent bone causing significant inflammation/oedema |
On radiographs, HADD appears as a homogeneous, round-to-ovoid calcification in the soft tissue with well-defined or ill-defined margins. However, in our case, the calcific deposit was not visible on the plain film. Several classifications based on size, shape, morphology, and density have been proposed, but none of them is sufficiently reproducible [16].
On MRI, CHA deposits appear as a low signal on all sequences, making it difficult to distinguish the calcification from the intrinsic low signal in tendons or ligaments. Gradient echo sequences (GRE) or susceptibility-weighted imaging (SWI) can better identify calcific foci, with a sensitivity and specificity of 98% and 96%, respectively. MRI is useful for evaluating surrounding oedema and detecting other associated pathologies, such as tendon tears, which are proportional to the patient’s symptoms [16].
US is highly sensitive and considered the gold standard for identifying calcific deposits within tendons and ligaments. Depending on the calcium concentration, they appear as focal echogenic deposits of varying sizes with or without posterior acoustic shadowing. On US, four different calcification patterns can be identified [16]:
Type I: hyperechoic calcification with well-defined posterior acoustic shadowing due to the consistent amount of calcium.
Type II: hyperechoic calcification with mild posterior acoustic shadowing due to the reduced amount of calcium as a result of hydrolysis of the calcium deposits.
Type III: isoechoic calcification without posterior acoustic shadowing due to the softening of the deposit.
Type IV: fluid hypo/anechoic calcifications without posterior acoustic shadowing, which can lead to the formation of a pseudo-abscess. In our patient, type II and type III deposits were identified within the spring ligament.
Until recently, aspiration of the joint fluid or calcific deposit followed by microscopic analysis (polarised microscopy) has been the standard practice for identifying the type of crystal deposition disease (Table 1). However, it is not always possible to aspirate the joint in daily clinical practice due to various factors such as a lack of expertise, a lack of joint fluid, and patient compliance. Imaging techniques such as DECT have gained an increasingly important role in differentiating gout from other crystal arthropathies non-invasively. DECT has diagnostic potential for gout, as it can automatically colour-code monosodium urate (MSU) depositions based on pre-defined software settings. The technique is based on the simultaneous acquisition of images at two different levels of x-ray photon energies (high- and low-kV series). In addition, DECT is not only a non‐invasive method for the visualisation and characterisation of MSU crystals, but it also assists in the quantification, treatment, and monitoring of this condition. Dual-source or single-source CT scanners can perform DECT scans [17]. In our institute, a single-source CT scanner was used with a fast kV switching technique employing dynamic switching of the tube voltage between 140 and 80 kV at rapid intervals of < 0.5 ms in a single projection.
Depending on the severity of the condition, treatment for HADD may involve a combination of medications, physical therapy, image-guided barbotage, and surgery.
Medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids may be used to reduce inflammation and pain. Physical therapy may be beneficial for maintaining mobility and strength in the affected joint.
US-guided barbotage (breaking calcifications using a needle so that they can be absorbed by the body) is the primary method of pain relief in the treatment of HADD and is considered to be safe and effective. The two-needle technique involves a needling and lavage procedure. The needling procedure is performed to break down the hydroxyapatite crystals, while the lavage procedure is performed to remove the resultant fragmented crystals. For additional pain relief, the procedure is followed by local anaesthetic infiltration and corticosteroid injection [18].
As a last resort, surgery may be required to remove calcium deposits or repair damaged tissues.
Conclusion
Previously unreported isolated involvement of the SLC of the foot with CHA crystal deposition as a spectrum of HADD should be considered in the differential diagnosis of patients presenting with medial foot pain. The use of US is essential for accurate diagnosis and effective management with US-guided barbotage.
Learning points and take-home messages
HADD can be a very painful condition, and clinicians should be aware of the role of different imaging modalities in facilitating prompt diagnosis.
Clinicians should be aware of the differentiating features of various crystal arthropathies, such as CPPD arthropathy, gout, and HADD.
Isolated HADD of the SLC is a rare cause of medial foot pain.
US plays an important role in the diagnosis and management of HADD.
DECT is very useful for distinguishing gout from calcium deposition arthropathies (CPPD and HADD).
Supplementary Information
Below is the link to the electronic supplementary material.
Video showing barbotage of the HADD of spring ligament
Funding
No funding.
Data availability
The data that support the findings of this study are available from the corresponding author [RB], upon reasonable request.
Declarations
Conflict of interest
No conflict of interest to declare.
Ethical approval
Ethical committee approval not required.
Informed consent
Informed patient consent was obtained from the patient for the Figures and the Video.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamada T, Matsubara H, Ohno N, Hikichi T, Shimokawa K, Miyati T, Ozaki N, Tsuchiya H. Comparison of each bundle of the spring ligament complex between the standing and supine positions: a multiposture magnetic resonance imaging study. Foot Ankle Surg. 2022;28(5):616–621. doi: 10.1016/j.fas.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Bastias GF, Dalmau-Pastor M, Astudillo C, Pellegrini MJ. Spring ligament instability. Foot Ankle Clin. 2018;23(4):659–678. doi: 10.1016/j.fcl.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Siegal DS, Wu JS, Newman JS, Del Cura JL, Hochman MG. Calcific tendinitis: a pictorial review. Can Assoc Radiol J. 2009;60(5):263–272. doi: 10.1016/j.carj.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Chianca V, Albano D, Messina C, Midiri F, Mauri G, Aliprandi A, et al. Rotator cuff calcific tendinopathy: from diagnosis to treatment. Acta Biomed. 2018;89(1-S):186–196. doi: 10.23750/abm.v89i1-S.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyengar KP, Yusta-Zato JARB. Calcific tendinopathy of the pronator quadratus muscle: A rare site and cause of ulnar sided wrist pain. J Clin Orthop Trauma. 2022;32:101968. doi: 10.1016/j.jcot.2022.101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman J, Le Goff B, De Lima J, Brion R, Chevalier C, Blanchard F, et al. Pro-inflammatory effects of human apatite crystals extracted from patients suffering from calcific tendinopathy. Arthritis Res Ther. 2021;23(1):131. doi: 10.1186/s13075-021-02516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah A, Iyengar KP, Hegde G, Ramos J, Botchu R. Calcific enthesopathy of the superior extensor retinaculum—an unusual cause of medial ankle pain. J Ultrason. 2022;22(91):e236–e239. doi: 10.15557/jou.2022.0038.PMID:36483788;PMCID:PMC9714275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harries L, Kempson S, Watura R. Calcific tendonitis of the tibialis posterior tendon at the navicular attachment. J Radiol Case Rep. 2011;5(6):25–30. doi: 10.3941/jrcr.v5i6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil V, Ebraheim NA, Frogameni A, Liu J. Morphometric dimensions of the calcaneonavicular (spring) ligament. Foot Ankle Int. 2007;28(8):927–932. doi: 10.3113/FAI.2007.0927. [DOI] [PubMed] [Google Scholar]
- 10.Orr JD, Nunley JA., 2nd Isolated spring ligament failure as a cause of adult-acquired flatfoot deformity. Foot Ankle Int. 2013;34(6):818–823. doi: 10.1177/1071100713483099. [DOI] [PubMed] [Google Scholar]
- 11.Mengiardi B, Pinto C, Zanetti M. Spring ligament complex and posterior tibial tendon: MR anatomy and findings in acquired adult flatfoot deformity. Semin Musculoskelet Radiol. 2016;20(1):104–115. doi: 10.1055/s-0036-1580616. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt LH, Ferrance RJ. The musculoskeletal effects of diabetes mellitus. J Can Chiropr Assoc. 2006;50(1):43–50. [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia GM, McCord GC, Kumar R. Hydroxyapatite crystal deposition disease. Semin Musculoskelet Radiol. 2003;7(3):187–193. doi: 10.1055/s-2003-43229. [DOI] [PubMed] [Google Scholar]
- 14.Bernier D, Marteau E, Roulet S, Antar H, Triki A, Laulan J, Bacle G. Hydroxyapatite deposits of the hand and wrist: a diagnosis not to be ignored. Pan Afr Med J. 2021;29(38):408. doi: 10.11604/pamj.2021.38.408.29253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamson TC, 3rd, Resnik CS, Guerra J, Jr, Vint VC, Weisman MH, Resnick D. Hand and wrist arthropathies of hemochromatosis and calcium pyrophosphate deposition disease: distinct radiographic features. Radiology. 1983;147(2):377–381. doi: 10.1148/radiology.147.2.6300958. [DOI] [PubMed] [Google Scholar]
- 16.Chianca V, Pietto FD, Albano D, Corvino A, Del Grande F. Ultrasound-guided percutaneous irrigation of rotator cuff calcific tendinosis. What radiologist should know. Pol J Radiol. 2022;87:e87–e92. doi: 10.5114/pjr.2022.113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou H, Chin TY, Peh WC. Dual-energy CT in gout—a review of current concepts and applications. J Med Radiat Sci. 2017;64(1):41–51. doi: 10.1002/jmrs.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tafti D, Byerly DW (2022) Ultrasound Guided Barbotage. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL). PMID: 34283462 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video showing barbotage of the HADD of spring ligament
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [RB], upon reasonable request.