Abstract
We report a case of an osteoid osteoma at the scapular glenoid that was treated with cryoablation. The patient presented with shoulder pain with subsequent CT and MRI imaging findings suspicious for an osteoid osteoma. The patient complained of persistent pain despite medical treatment and was referred to interventional radiology for consideration of ablation. CT-guided biopsy confirmed the diagnosis of osteoid osteoma and the patient underwent cryoablation of the lesion in the same session. The patient reported a substantial relief in her pain at a follow-up 4 weeks after the procedure. MRI performed 8 weeks after the procedure also confirmed radiographic remission of the lesion.
Keywords: Cryoablation, Glenoid, Interventional radiology, Musculoskeletal radiology, Osteoid osteoma
Case report
A 24-year-old woman was referred to the orthopedics clinic complaining of right shoulder pain for a year. The pain was worse at night and was not related to movement. It was initially managed with daily paracetamol and etoricoxib, but worsened in the past few months. She underwent physiotherapy and traditional Chinese medical treatment without improvement. The patient had good past health and unremarkable family history.
On physical examination, there was tenderness at the posterior shoulder at the area of the infraspinatus muscle. No tenderness was elicited over the supraspinatus, acromion or coracoid. There was no pain on passive movement of the shoulder. Rotator cuff muscle power was preserved. No myelopathic hand signs demonstrated.
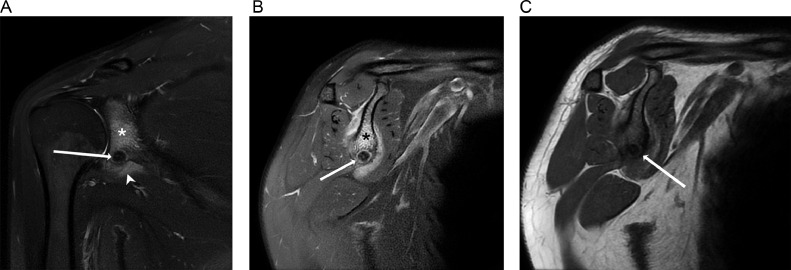
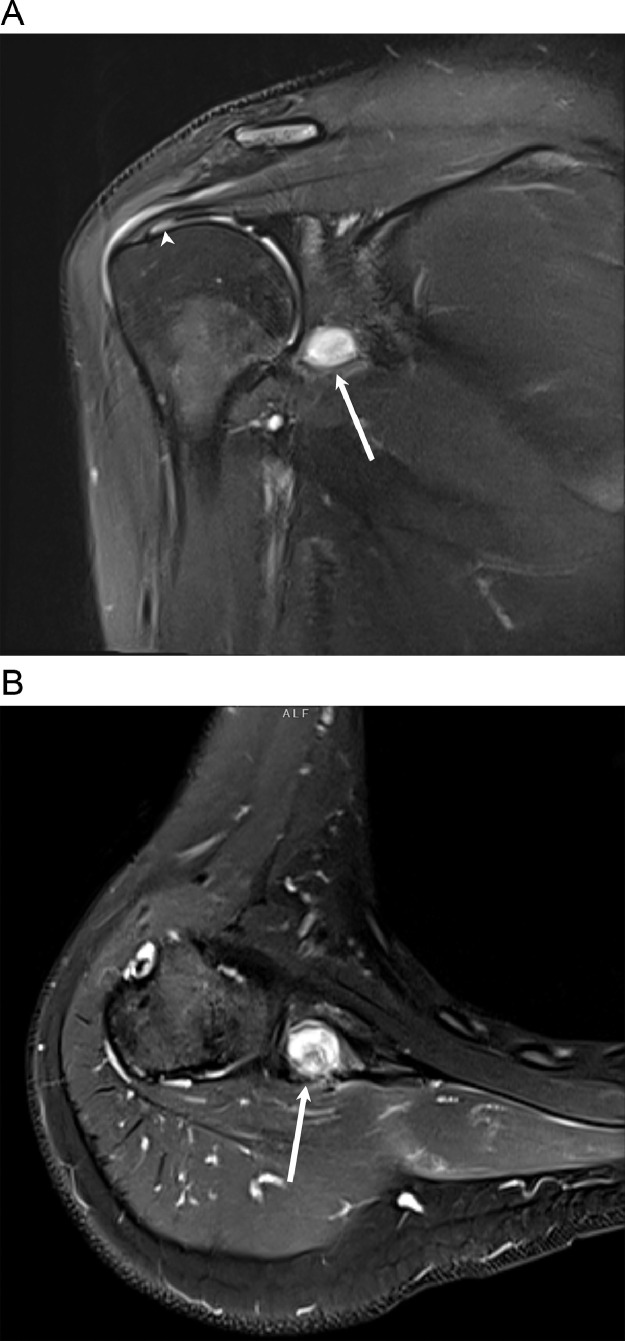
Magnetic resonance imaging (MRI) of the shoulder without contrast (Fig. 1) demonstrated a well-defined T2 hypointense focus with a thin rim of T2 hyperintense signal at the inferior glenoid. There were T2 hyperintense signals seen in the adjacent marrow and soft tissue. Thickening of the adjacent cortex was evident on T1 sequences. There was no associated extraosseous soft tissue mass or joint effusion demonstrated. The rotator cuff muscles and tendons were unremarkable.
Fig. 1.
Coronal (A) and sagittal (B) fat-suppressed T2-weighted MR image shows a well-defined hypointense focus with a thin rim of hyperintense signal at the inferior glenoid (arrow). Reactive edema is noted in the adjacent bone marrow (*) and soft tissue (arrowhead). (C) Sagittal T1-weighted MR images shows the low-signal-intensity nidus (arrow) with adjacent cortical thickening.
Given these findings, the possibility of osteoid osteoma was raised and further workup with plain radiography and computed tomography (CT) scan were performed.
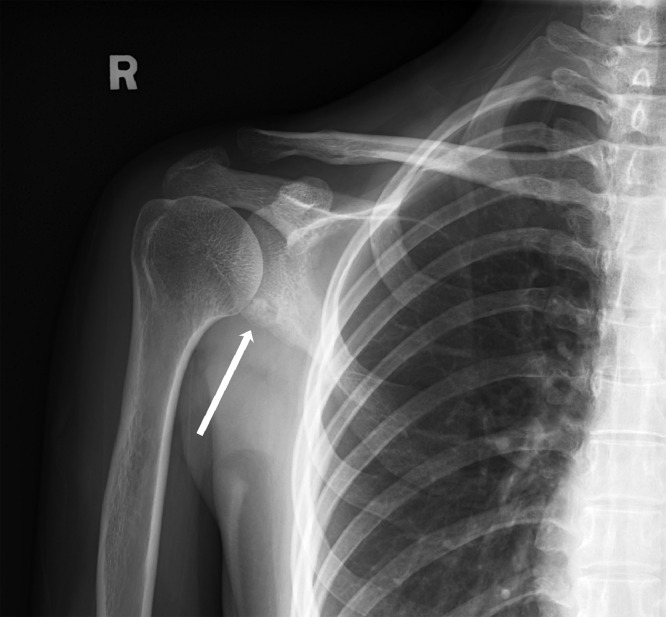
Plain radiograph of the right shoulder (Fig. 2) demonstrated a lucent lesion at the inferior glenoid with internal sclerotic focus. No definite periosteal reaction or soft tissue component to suggest aggressive lesion.
Fig. 2.
Plain radiograph of the right shoulder demonstrates a lucent lesion at the inferior glenoid with internal sclerotic focus. No definite periosteal reaction or soft tissue component to suggest aggressive lesion.
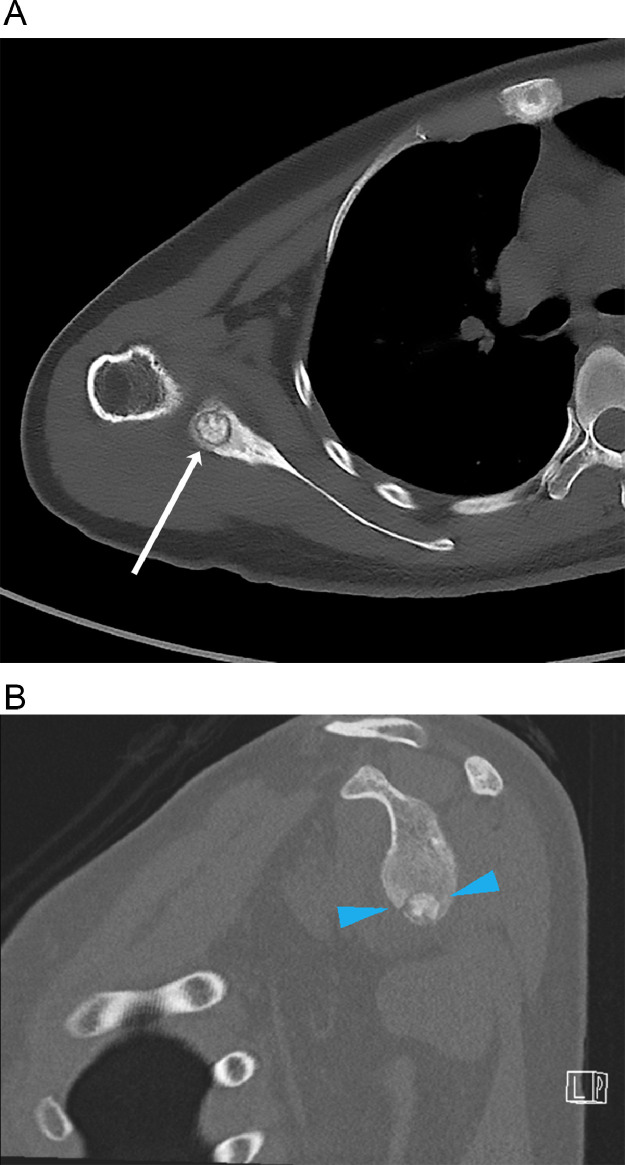
Nonenhanced computed tomography (CT) scan of the right shoulder (Fig. 3) revealed a well-defined lytic lesion with a central sclerotic focus, suggestive of a nidus with central mineralization. Adjacent sclerosis and cortical thickening were seen.
Fig. 3.
Axial (A) and sagittal (B) unenhanced CT image shows the nidus (arrow, blue arrowheads) with reactive sclerosis in the adjacent marrow.
The CT and MRI images were discussed in a multidisciplinary tumor board including orthopedic surgeons and musculoskeletal radiologists. Given the CT and MRI findings, in combination with the patient's symptoms, a diagnosis of osteoid osteoma was made. Since the patient's pain was not controlled with oral analgesics, the option of management by percutaneous CT-guided cryoablation was offered by the radiology team. Decision was made to perform the procedure under local anesthesia combined with conscious sedation so that patient's neurologic function could be assessed in real time.
The procedure was performed on a 64 detector-row dual-energy CT scanner (Revolution HD, General Electric Medical Systems, Waukesha, WI). Before the procedure, the patient was given 50 µg IV fentanyl for analgesia. The patient was positioned prone with her right arm to her side. A posterior approach was chosen with care to avoid the suprascapular and axillary nerves. 14 ml 1% lignocaine was injected into the surrounding soft tissue and periosteum as local anesthesia.
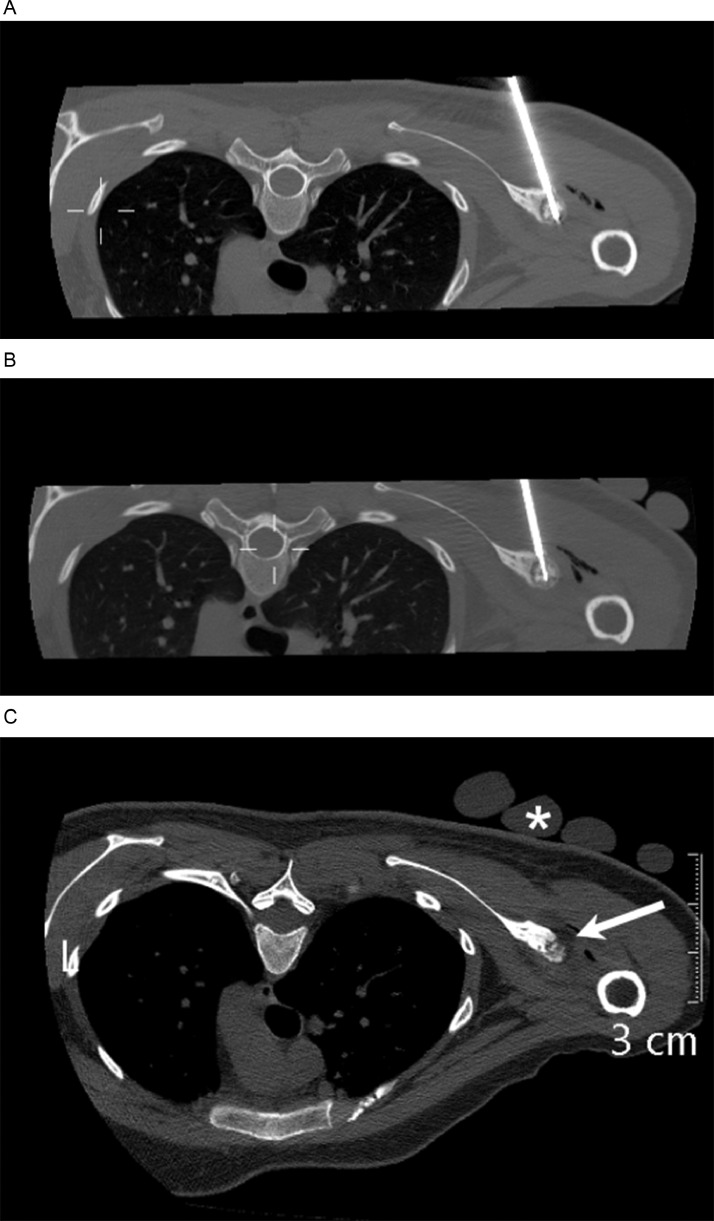
A 11-gauge Westbrook Bone Biopsy access needle (Merit Medical, South Jordan, UT) was inserted into the center of the lesion (Fig. 4A) under CT guidance. The trocar stylet was removed and the trephine biopsy needle was inserted coaxially to perform a core biopsy of the nidus. The biopsy specimen was placed in formalin and sent for histopathology, which was interpreted after the cryoablation.
Fig. 4.
(A) Axial unenhanced CT image obtained intraprocedurally shows the 11-gauge Westbrook Bone Biopsy access needle and trephine biopsy needle within the lesion. (B) Axial unenhanced CT image obtained intraprocedurally shows the cryoprobe placed within the nidus. (C) Axial unenhanced CT image obtained intraprocedurally shows iceball extension to the pericortical soft tissue (arrows). The sterile gloves filled with warm water can be visualised over the skin (*).
The 11-gauge access needle was left in place and subsequently, a 17-gauge IceSphere 1.5 CX cryoablation needle (Boston Scientific, Natick) was inserted into the lesion coaxially into the needle, with tip positioned at opposite cortex of the glenoid (Fig. 4B). Sterile gloves filled with warm water were placed over the patient's skin at the puncture site to avoid skin injury. The patient was examined regularly during the procedure to ensure she did not experience any neurologic symptoms.
Cryoablation was then initiated with two cycles of a 10-minute freeze at 70% power followed by 5 minutes thaw. During the ablation process, intermittent CT scanning was able to visualize the ice ball in the pericortical soft tissue (Fig. 4C), representing the ablation zone.
During the procedure, the patient complained of increasing pain during the thaw cycles. The patient described the pain as similar to her usual pain from the lesion. Intraprocedural CT did not demonstrate any obvious complication such as hematoma. A further 50 µg of intravenous fentanyl and 1 mg of intravenous midazolam was therefore administered. However, the patient only reported mild pain relief, but she was able to tolerate the pain until procedure completion.
After completion of the ablation, the introducer and cryoablation needles were removed. A final CT scan performed did not demonstrate any obvious complications. Dressing was applied to the needle wound. The patient was able to tolerate the postprocedure pain and was discharged with oral pain killers the same day.
The histopathology report showed trabeculae of woven bone partly surrounded by plump osteoblasts and occasional osteoclasts. No atypia detected. This was confirmed to be compatible with an osteoid osteoma in the subsequent clinicopathologic meeting.
At a follow-up 1 month after the procedure, the patient reported complete resolution of pain a few days after the procedure and a return to normal activity.
A follow-up MRI performed around 8 weeks after the procedure (Fig. 5) demonstrated a T1 hypointense T2 hyperintense focus at the ablation site, compatible with post ablation changes. The previously identified edema in the adjacent bone marrow of the glenoid and soft tissue had resolved. However, there was a new T2 hyperintense signal seen over the articular side of the supraspinatus tendon, suspicious for a partial thickness tear, which was assumed to be incidental.
Fig. 5.
Coronal (A) and axial (B) fat-suppressed T2-weighted MR image shows a well-defined hyperintense focus at the previous site of the osteoid osteoma, compatible with post-ablation changes. Previously seen reactive edema in the adjacent bone marrow and soft tissue is no longer seen. Hyperintense fluid filled defect at the articular side of the distal supraspinatus tendon is suspicious for a partial thickness tear.
Discussion
Osteoid osteomas are relatively common bone lesions that represent around 12% of all benign bone lesions [1]. They most commonly present in the cortices of the shafts of long bones such as the femurs and tibia, but can present in any bone and in an intramedullary location [1]. Osteoid osteomas are rarely seen in the scapula [2], with only a few case reports of osteoid osteomas presenting in the scapula glenoid [3].
They most commonly present with pain, typically worse at night and relieved with nonsteroidal anti-inflammatory drugs (NSAIDs). High levels of prostaglandin production in the nidus have been suggested as the pathophysiology behind the pain, and therefore the good response to NSAIDs.
Classical imaging findings of osteoid osteomas are of a nidus with cortical thickening, reactive sclerosis, and bone marrow edema [2]. Central mineralization may be present.
This case presents atypically given the location in the scapular glenoid, as well as its intramedullary location, but the patient's symptoms are typical for an osteoid osteoma, and the CT and MRI findings are also suggestive of the diagnosis of osteoid osteoma.
Conservative treatment with nonsteroid anti-inflammatory drugs is the first line of treatment. Traditionally, those who fail conservative treatment undergo surgical resection. However, this has been largely replaced by image-guided procedures, most commonly percutaneous radiofrequency ablation [4].
There have been increasing reports in literature which demonstrate successful treatment of osteoid osteomas with cryoablation [[5], [6], [7], [8]–9], with a clinical success rate of up to 96%-100% [5,6]. Cryoablation has several advantages over radiofrequency ablation in treating osteoid osteomas. Most importantly, the ice ball can be visualized intraprocedurally to accurately assess the ablation zone [5]. This allows for certainty in complete ablation of the lesion, as well as making sure critical nearby structures are preserved. Secondly, ice ball formation can be conducted across sclerotic bone [5], which is especially helpful when treating bone-forming tumors such as osteoid osteoma. This also means that the cryoablation probe can be placed at an extraosseous location and still ablate the tumor. Finally, the pain from cryoablation is better tolerated by patients and can therefore be performed under conscious sedation rather than general anesthesia [5,10], and patients report less postprocedural pain [10].
However, our patient developed pain during the thaw cycles as well as postoperatively, despite the use of intravenous fentanyl. We postulate that this was due to a combination of the loss of analgesic effect from the ice ball, as well as the sudden release of large amounts of prostaglandins from the nidus after its destruction. In a review of the literature regarding ablation of osteoid osteomas, it was noted that one author directly administered 10 mL of bupivacaine, a long acting local anesthetic, into the nidus [11]. Another author administered high dose ketoprofen intravenously before the procedure for a complete prostaglandin blockade [6] to avoid postprocedural pain. This may suggest that these specific measures can be helpful in the management of periprocedural pain during ablation of osteoid osteoma, where they may not be necessary in the ablation of other musculoskeletal tumors.
Conclusion
Osteoid osteoma of the scapular glenoid is a rare cause for shoulder pain, but imaging findings in combination with a patient's clinical symptoms can help clinch the diagnosis. The successful use of cryoablation in our case suggests that cryoablation is a safe and effective method for the percutaneous treatment of osteoid osteomas. However, periprocedural pain management may need to be tailored specifically to osteoid osteomas when compared with other musculoskeletal tumor ablation.
Patient consent
An informed consent for the case report titled “A rare cause of shoulder pain: Osteoid osteoma of the scapular glenoid treated with cryoablation” was taken on July 6, 2023 with the patient herself. A signed informed consent form has been obtained and retained by the first author.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Greenspan A. Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Skelet Radiol. 1993;22:485–500. doi: 10.1007/BF00209095. [DOI] [PubMed] [Google Scholar]
- 2.Chai JW, Hong SH, Choi J-Y, Koh YH, Lee JW, Choi J-A, et al. Radiologic diagnosis of osteoid osteoma: from simple to challenging findings. Radiographics. 2010;30(3):737–749. doi: 10.1148/rg.303095120. [DOI] [PubMed] [Google Scholar]
- 3.Amin ZHF, Al-Rasheedi RS, Sairafi MH, Alrashidi Y. Osteoid osteoma of scapular glenoid: a case report. Int J Surg Case Rep. 2020;77:143–146. doi: 10.1016/j.ijscr.2020.10.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815–821. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Santiago E, Pauly V, Brun G, Guenoun D, Champsaur P, Le Corroller T. Percutaneous cryoablation for the treatment of osteoid osteoma in the adult population. Eur Radiol. 2018;28(6):2336–2344. doi: 10.1007/s00330-017-5164-6. [DOI] [PubMed] [Google Scholar]
- 6.Le Corroller T, Vives T, Mattei JC, Pauly V, Guenoun D, Rochwerger A, et al. Osteoid osteoma: percutaneous CT-guided cryoablation is a safe, effective, and durable treatment option in adults. Radiology. 2022;302(2):392–399. doi: 10.1148/radiol.2021211100. [DOI] [PubMed] [Google Scholar]
- 7.Wu B, Xiao YY, Zhang X, Zhao L, Carrino JA. CT-guided percutaneous cryoablation of osteoid osteoma in children: an initial study. Skelet Radiol. 2011;40(10):1303–1310. doi: 10.1007/s00256-011-1119-1. [DOI] [PubMed] [Google Scholar]
- 8.Coupal TM, Mallinson PI, Munk PL, Liu D, Clarkson P, Ouellette H. CT-guided percutaneous cryoablation for osteoid osteoma: initial experience in adults. AJR Am J Roentgenol. 2014;202(5):1136–1139. doi: 10.2214/AJR.13.11336. [DOI] [PubMed] [Google Scholar]
- 9.Santiago E, Pauly V, Brun G, Guenoun D, Champsaur P, Le Corroller T. Percutaneous cryoablation for the treatment of osteoid osteoma in the adult population. Eur Radiol. 2018;28(6):2336–2344. doi: 10.1007/s00330-017-5164-6. [DOI] [PubMed] [Google Scholar]
- 10.Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases: why cryoablation? Skelet Radiol. 2009;38(9):835–839. doi: 10.1007/s00256-009-0736-4. [DOI] [PubMed] [Google Scholar]
- 11.Marquardt B, Gebert C, Gosheger G, Steinbeck J, Lindner NJ. Percutaneous radiofrequency ablation of an osteoid osteoma of the scapula: a case report. J Shoulder Elbow Surg. 2005;14(4):447–449. doi: 10.1016/j.jse.2004.07.005. [DOI] [PubMed] [Google Scholar]