Abstract
Purpose
We aimed to study the relationship between aging and increased parathyroid hormone (PTH) values.
Methods
We performed a retrospective cross-sectional study with data from patients who underwent outpatient PTH measurements performed by a second-generation electrochemiluminescence immunoassay. We included patients over 18 years of age with simultaneous PTH, calcium, and creatinine measurements and 25-OHD measured within 30 days. Patients with glomerular filtration rate < 60 mL/min/1.73 m2, altered calcemia, 25-OHD level < 20 ng/mL, PTH values > 100 pg/mL or using lithium, furosemide or antiresorptive therapy were excluded. Statistical analyses were performed using the RefineR method.
Results
Our sample comprised 263,242 patients for the group with 25-OHD ≥ 20 ng/mL, that included 160,660 with 25-OHD ≥ 30 ng/mL. The difference in PTH values among age groups divided by decades was statistically significant (p < 0.0001), regardless of 25-OHD values, ≥ 20 or ≥ 30 ng/mL. In the group with 25-OHD ≥ 20 ng/mL and more than 60 years, the PTH values were 22.1–84.0 pg/mL, a different upper reference limit from the reference value recommended by the kit manufacturer.
Conclusion
We observed a correlation between aging and PTH increase, when measured by a second-generation immunoassay, regardless of vitamin D levels, if greater than 20 ng/mL, in normocalcemic individuals without renal dysfunction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-023-02107-2.
Keywords: PTH value, Aging and PTH, Normal PTH, Parathyroid hormone
Introduction
Parathyroid hormone (PTH) is a polypeptide produced by the parathyroid gland that plays a crucial role in the maintenance of adequate levels of calcium and phosphorus required for regular neuromuscular activity, bone mineralization, and countless other cellular and metabolic processes. The interaction between plasma levels of calcium, PTH, 1,25-dihydroxyvitamin, and phosphorus keeps calcemia within very narrow limits, even with a wide variation in food intake.
Under normal physiological conditions, PTH secretion is regulated by a complex interplay among several factors, including plasma levels and renal excretion of calcium and phosphorus, 25-hydroxyvitamin D (25-OHD) and 1–25-OHD levels, glomerular filtration rate, thyroid function, and FGF-23 (Fibroblast Growth Factor-23) [1, 2]. In clinical practice, certain medications, such as diuretics and lithium, also influence PTH levels [3, 4].
Appropriate reference values (RVs) for each specific test model of PTH directly affect the interpretation of results and, consequently, the diagnostic accuracy [5]. The diagnosis of normocalcemic hyperparathyroidism or secondary hyperparathyroidism are clinical conditions that depend on accurate PTH reference values.
Current RV levels provided by manufacturers of commercial PTH assays were obtained from assessments with a limited number of patients, primarily young and with little-known vitamin D status [6–9]. One possible reason is that recruiting and selecting volunteers in specific population segments, such as the elderly, is quite laborious. Different groups have studied PTH values in recent years. Although several studies published in the literature demonstrated a positive correlation between aging and PTH increases [6–13], most were carried out with a limited number of participants. The Fourth International Workshop on Asymptomatic Primary Hyperparathyroidism [10] highlighted that current PTH reference values might not be adequate, and assessment of larger populations with vitamin D sufficiency and age stratification was necessary.
Data mining studies, also called indirect approaches, explore large amounts of data searching for consistent patterns and have shown usefulness in special populations, such as the elderly, children, and pregnant women, and are being increasingly used [14, 15]. Therefore, we performed a real-world big data study of PTH values behavior in a Brazilian population.
We studied the values obtained for this hormone relative to age among outpatient adult individuals presenting with vitamin D sufficiency using the database of a private clinical laboratory. In addition to evaluate the correlation between PTH levels and age, we calculated the percentage of patients classified as having elevated PTH by the current reference values but who had results classified as normal using our results.
Methods
Subjects
We carried out a cross-sectional retrospective study and data mining with data analysis of patients who underwent parathyroid hormone (PTH) measurement in the laboratories of the Fleury Group in Brazil from 01/01/2017 to 12/31/2020. Patients between 18 and 100 years old who underwent simultaneous PTH, calcium, and creatinine measurements were included. We also included data from 25-OHD tests performed within a month of PTH collection.
All tests were performed in outpatient facilities, excluding hospital units. In the private health system in Brazil, the measurement of calcium, PTH and 25OHD is common, even in the absence of signs or symptoms of disorders of mineral metabolism. Patients with a glomerular filtration rate < 60 mL/min/1.73 m2 calculated using the CKD-EPI equation were excluded, and all individuals were considered nonblack for this calculation. In Grupo Fleury’s laboratories, the patient is questioned about medication use, and the information is registered in the system. Patients with a history of using lithium, furosemide, denosumab, zoledronic acid or teriparatide were excluded from the analysis. Only the first result was used if the patient had more than one PTH analysis. The cutoff values for exclusion from the study, for the dosages of 25-OHD and PTH levels, were arbitrarily selected at > 60 ng/mL and > 100 pg/mL, respectively.
Finally, all patients with 25-OHD levels ≤ 20 ng/mL, total calcium > 10.3 mg/dL were excluded.
The initial database contained 356,297 records of outpatients who underwent PTH testing between 2017 and 2020. After applying all the predetermined filters, our population sample consisted of 263,242 patients. It is interesting to point out that 5813 (1.6%) individuals of the initial database were excluded because they had total calcium above 10.3 mg/dL. All 263,242 patients had 25-OHD values ≥ 20 ng/mL (50 nmol/L) and of these, 160,660 had 25-OHD values ≥ 30 ng/mL (75 nmol/L). Then, patients were stratified by age: 18–29, 30–39, 40–49, 50–59, + 60 years (Table 1).
Table 1.
Number of patients in each age group according to 25-OHD levels, ≥ 20 and ≥ 30 ng/mL
| Age | 25-OHD ≥ 20 | 25-OHD ≥ 30 |
|---|---|---|
| 18–29 | 22,169 | 12,448 |
| 30–39 | 58,846 | 33,460 |
| 40–49 | 72,783 | 42,357 |
| 50–59 | 53,239 | 34,457 |
| 60 + | 56,205 | 37,938 |
| Total | 263,242 | 160,660 |
The Fleury Ethics Committee approved this study, and there was no need to use a consent form, as the survey was retrospective and only used anonymized data.
Serum measurements
The measurement of PTH was performed by a second-generation electrochemiluminescence immunoassay (ECLIA) (Roche Elecsys, on the Cobas e602 device) on serum that uses a capture antibody directed against the C-terminus of PTH and a signal antibody directed against the N-terminus of PTH (Roche Diagnostics, Indianapolis, IN, USA). The reference values reported by the manufacturer are 15–65 pg/mL. The total coefficient of variation was 5%.
The measurement of 25-OHD was performed in serum by a competitive chemiluminescent assay (CLIA) using the DiaSorin LIAISON 25 OH Vitamin D Total assay (DiaSorin Inc., Stillwater, MN, USA) in the Liaison XL device. The total coefficient of variation was 8%.
Serum creatinine measurement was performed using a Roche CREJ2 assay (Roche Diagnostics, Indianapolis, IN, USA) on the Cobas c. The manufacturer reference values for women are from 0.50 to 0.90 mg/dL and for men are from 0.70 to 1.20 mg/dL.
Total serum calcium measurement was performed by colorimetric assay using the CA2 Calcium Gen 2 assay by Roche (Roche Diagnostics, Indianapolis, IN, USA) on the Cobas c. The manufacturer suggests as RV, for adults aged 18–60 years, 8.6–10.0 mg/dL and for adults aged 60–90 years, 8.8–10.2 mg/dL.
Statistical analysis
Statistical analyses were performed using the R program, version 4.1.1 [16], RefineR method. Wilcoxon's multiple tests, with Benjamini–Hochberg correction, were used to investigate the differences in the mean PTH values among age groups, the relationship between PTH and serum calcium values and between PTH and creatinine values. ANOVA with Cohen's D factorial test was used to verify whether the 25-OHD values varied with age. The PTH values found in this study was calculated by bootstrapping, and a confusion matrix was used to analyze the agreement between the usual values and the values found in this study.
Results
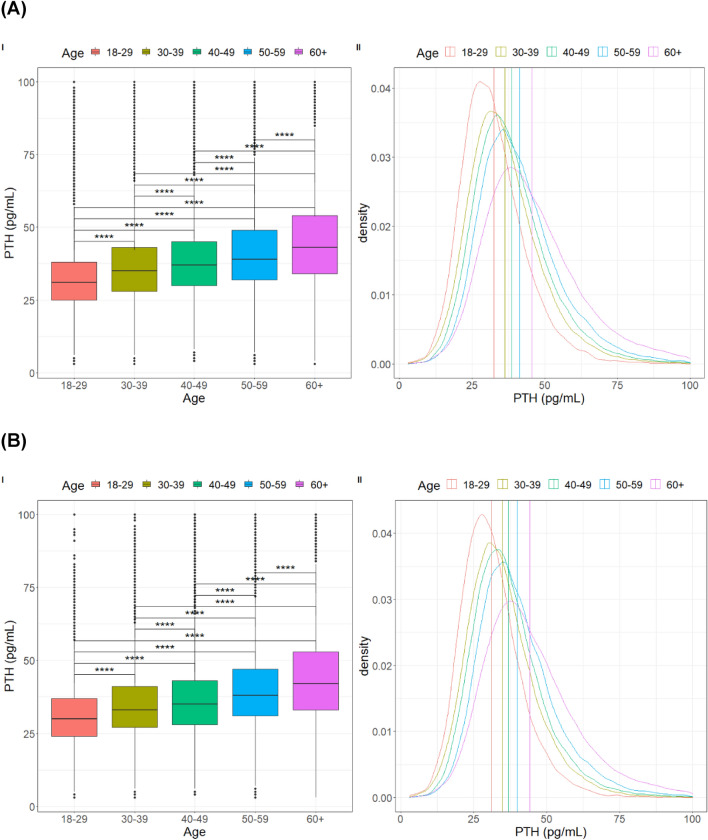
PTH distributions became less dense as age increased, presenting a greater range of values. The difference in PTH values among the age groups was statistically significant (p < 0.0001), regardless of whether 25-OHD values are greater than 20 or even 30 ng/mL (Figs. 1 and 2).
Fig. 1.
(I) Boxplot and (II) Density graph of PTH values (pg/mL) in different age groups in patients with 25-OHD above 20 ng/mL (A) and above 30 ng/mL (B). The vertical dashed lines represent the means of PTH in the different age groups. All comparisons between groups were statistically significant (p < 0.0001)
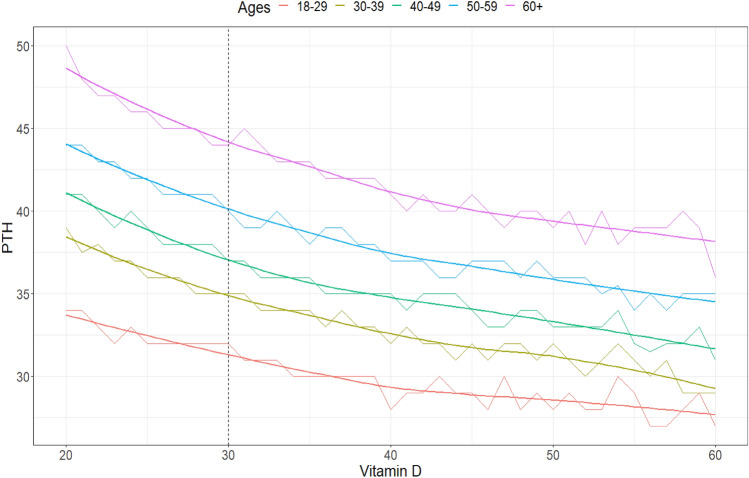
Fig. 2.
Line graph showing the relationship between vitamin D values (ng/mL) and the median PTH of individuals containing each possible analyte value for each age group. The fit line was created using the LOESS (LOcally Estimated Scatterplot Smoothing) technique. Dashed lines indicate the 25-OHD values of 20 and 30 ng/mL
In the sample studied, vitamin D levels varied slightly with age, being higher in individuals over 60 years of age, most likely due to the use of supplements with the hormone. To evaluate the importance of finding, Cohen's D test was used, and the results indicate the effects as small or insignificant.
The Mann–Whitney with Benjamini–Hochberg adjustment (alpha = 0.05) test was applied to investigate whether there was a gender difference in PTH levels. A small significant difference was observed between sexes in the younger groups, up to 39 years old, and in the group of individuals > 60 years, but Cohen’s D test pointed out that the magnitude of the effect is insignificant for all groups (Supplementary Results).
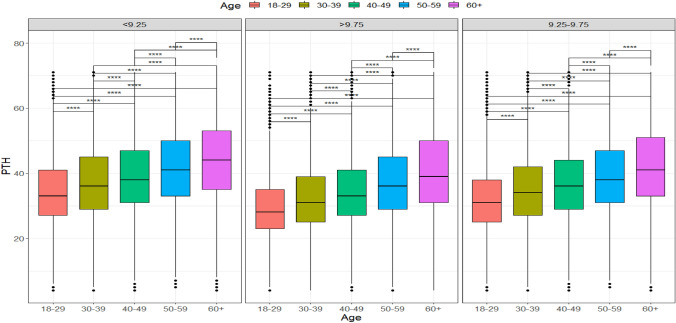
We also analyzed the relationship between PTH and serum calcium values using results within the laboratory's normal range of 8.6 and 10.3 mg/dL. Patients were segmented into three groups of different calcium values: less than 9.25 mg/dL, between 9.25 and 9.75 mg/dL, and above or equal to 9.76 mg/dL, and up to 10.3 mg/dL. These ranges were chosen arbitrarily to balance the number of patients in each group. We found a negative correlation (− 0.087) between calcium values and PTH. We observed that for the same range of calcium values, the PTH maintained the increase with age (Fig. 3).
Fig. 3.
Boxplots show PTH values (pg/mL) of the total sample of individuals with 25-OHD ≥ 20 ng/mL, in different groups of calcium values (mg/dL), separated by age groups (p < 0.0001)
We also analyzed the relationship between PTH and creatinine levels within the normal range, noting that all included patients had glomerular filtration rate > 60 mL/min/1.73 m2 calculated using the CKD-EPI formula. We selected patients whose creatinine values ranged from 0.7 to 1.3 mg/dL for men and from 0.6 to 1.10 mg/dL for women. Individuals were also segregated into arbitrarily chosen creatinine ranges: less than 0.75 mg/dL, between 0.75 and 0.9 mg/dL, and greater than or equal to 0.9 mg/dL. We did not observe a statistically significant difference between PTH and average creatinine values. Therefore, we concluded that PTH values are little influenced by creatinine when this parameter is within the reference range, even in elderly patients.
Then, due to the behavior of PTH according to age, we calculated reference values for PTH, obtained with the refineR algorithm, for different age groups and with specific cut-offs of vitamin D, ≥ 20 or ≥ 30 ng/mL (Table 2). In the group with 25-OHD ≥ 20 ng/mL and more than 60 years, the range was 22.1–84.0 pg/mL. We classified as false-positive results (FP) individuals with a PTH value above the upper limit of normality (ULN) by the standard RV and that were classified as normal when using the new range. A confusion matrix was performed to assess agreement between the two classifications (Table 3). When we considered patients over 60 years and with 25-OHD ≥ 20 ng/mL, the proportion of FP was 9.53%; even in individuals over 60 years and 25-OHD ≥ 30 ng/mL, we observed 7.84% of FP.
Table 2.
Reference values for PTH (pg/mL) obtained with the refineR algorithm
| Age | C.I. | PTH | |
|---|---|---|---|
| 25OHD > = 20 | 18–29 | 2.5 | 16.4 |
| 97.5 | 57.4 | ||
| 30–39 | 2.5 | 18.6 | |
| 97.5 | 63.7 | ||
| 40–49 | 2.5 | 20.1 | |
| 97.5 | 66.5 | ||
| 50–59 | 2.5 | 21.4 | |
| 97.5 | 71.4 | ||
| 60 + | 2.5 | 22.1 | |
| 97.5 | 84.0 | ||
| 25OHD > = 30 | 18–29 | 2.5 | 15.1 |
| 97.5 | 51.5 | ||
| 30–39 | 2.5 | 17.0 | |
| 97.5 | 57.5 | ||
| 40–49 | 2.5 | 18.5 | |
| 97.5 | 60.4 | ||
| 50–59 | 2.5 | 20.9 | |
| 97.5 | 68.5 | ||
| 60 + | 2.5 | 21.6 | |
| 97.5 | 81.8 |
Table 3.
Confusion matrix for patients > 60 years accounting for the agreement of the classification of patients if their PTH values are below, above, or within normal values according to the previously established ranges (10–65 pg/mL) and according to the ranges defined in Table 2
| (I) | New classification | |||
|---|---|---|---|---|
| Under | Normal | Above | Total | |
| Old classification | ||||
| Under | 56 (0.1%) | 0 (0%) | 0 (0%) | 56 (0.1%) |
| Normal | 2203 (3.92%) | 46,931 (83.5%) | 0 (0%) | 49,134 (87.42%) |
| Above | 0 (0%) | 5357 (9.53%) | 1658 (2.95%) | 7015 (12.48%) |
| Total | 2259 (4.02%) | 52,288 (93.03%) | 1658 (2.95%) | 56,205 (100%) |
| (II) | New classification | |||
|---|---|---|---|---|
| Under | Normal | Above | Total | |
| Old classification | ||||
| Under | 45 (0.12%) | 0 (0%) | 0 (0%) | 45 (0.12%) |
| Normal | 1317 (3.47%) | 32,513 (85.7%) | (0%) | 33,830 (89.17%) |
| Above | 0 (0%) | 2976 (7.84%) | 1087 (2.87%) | 4063 (10.71%) |
| Total | 1362 (3.59%) | 35,489 (93.54%) | 1087 (2.87%) | 37,938 (100%) |
(I) Patients over 60 years and 25OHD > 20 ng/mL (II) Patients over 60 years and 25OHD > 30 ng/mL
Discussion
Our study on PTH values is probably the largest in terms of the number of participants, 263,242 Brazilian adults, with an analysis controlled by serum calcium, vitamin D values and renal function.
Although RV reported by manufacturers of commercial kits for PTH assays are established based on results obtained from apparently healthy volunteers, there is no correction for other parameters that are known to interfere with the metabolism of the analyte in question, and the population sample recruited is generally younger than 60 years. Even fundamental parameters such as vitamin D status and renal function are usually absent from the analysis.
We demonstrated a correlation between aging and an increase in PTH values in a population with vitamin D sufficiency, normal serum calcium, and without renal dysfunction. Furthermore, this correlation did not differ between genders, with a slight and negligible higher value of PTH in younger women and those over 60 years of age, compared to men of the same age group. As we did not evaluate the weight of patients, perhaps this variable may influence the slight difference in PTH levels seen between men and women in certain age groups.
Previous research, using different PTH assays, observed higher upper limit values of PTH in the elderly, corroborating our findings [9, 17]. Data mining was also used by Farrel et al. [11], demonstrating that older age was associated with higher PTH concentrations among patients with optimal 25-OHD status. Age-related reference intervals showed a 63% increase in the upper and lower reference limits between the youngest (18–29 years of age) and the oldest (80 years of age or older) individuals. Delgado et al. [17] also observed that the reference intervals for PTH were age-dependent.
Touvier et al. [18] obtained PTH RV for middle-aged adults between 35 and 65 years, which were lower than those currently used. Nonetheless, we observed a gradual and significant increase in PTH values with age. Our study has some differences in terms of the evaluated subjects, PTH measurement methodology and study design. The Touvier study did not include individuals over 65 years old, while our study had a significant percentage of patients (27.16%) aged ≥ 60 years, a point that deserves to be highlighted. We did not consider the patient's weight variation or calcium intake. Another difference is methodological; we used serum instead of plasma to measure PTH, and variations in preanalytical procedures may cause variability in the results [19, 20].
Our study excluded patients with a glomerular filtration rate (GFR) < 60 mL/min/1.73 m2, calculated by the CDK-EPI formula. The evaluated samples come from a population in the Brazilian southeast region, highly mixed (European, black, indigenous, and Asian ancestry), with an insignificant percentage of blacks defined as children from a black father and mother. In September 2021, the National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Diseases released a report that outlines a new race-free approach to diagnose kidney disease. In the report, the NKF-ASN Task Force recommends the adoption of the new eGFR 2021 CKD EPI creatinine equation that estimates kidney function without a race variable [21–24].
According to our results, the RVs presented by the PTH assay manufacturer (in this case Roche) are only suitable for adults up to 49 years of age. This finding is relevant, as some patients can be classified as having elevated PTH and be misdiagnosed as normocalcemic or secondary hyperparathyroidism. In our study, in the population over 60 years of age with 25-OHD ≥ 20 mg/mL, 9.53% of patients had high PTH levels using the usual RVs but had normal PTH results using the values we found. This percentage of false positives has a great impact on the clinical evaluation of the elderly population, which can lead to misdiagnosis and even to poorly indicated surgeries for normocalcemic hyperparathyroidism.
Schini et al., in an interesting study on normocalcemic hyperparathyroidism (NPHPT), have observed greater individual variability of calcemia in patients with elevated PTH compared to control subjects. A percentage of patients with elevated PTH, according to the manufacturer’s RV, was considered normal after performing a bivariate statistical approach considering the variability of calcemia. Besides that, the authors noticed that PTH increases with age in normal control subjects, as seen in our study. This data stress the importance of discussing PTH values as a function of several variables, particularly in the diagnostic definition of NPHPT [25].
The serum PTH increase with age in both men and women has generally been attributed to an age-related decline in renal function. However, the most recent data indicate that this is not the only explanation. It has already been established that impaired intestinal calcium absorption occurs with age, partly by a decrease in the number of intestinal vitamin D receptors, leading to decreased responsiveness to 1–25-OHD [26, 27]. Additionally, PTH can be oxidized on methionine residues located at positions 8 and 18, rendering it less biologically active but still measurable by second-generation assays [28]. The presence of increased levels of reactive oxygen species (ROS) increases with age, and oxidizing PTH can also interfere with PTH receptor signaling and trafficking [29]. These two effects can contribute to the finding of higher PTH levels with increasing age.
It is not yet clear whether the increase in PTH with age is just an adaptative phenomenon with no harmful consequences or is a detrimental event. Several studies have suggested a correlation between PTH levels and mortality rates in the elderly. Domiciano et al. [30] observed that PTH levels and low bone mineral density, are independent risk factors for mortality in older adults. This may result from the noncalcemic effects of PTH, such as atherogenesis by vascular calcification, left ventricular hypertrophy, and direct effects on vascular smooth muscle cells. Suriyaarachchia et al. [31] demonstrated that older persons with high levels of serum PTH have a higher prevalence of falls and fractures, in addition to a strong association with osteosarcopenia, even in the absence of vitamin D deficiency or impaired renal function.
The large population sample, 263,242 Brazilian adults, and the adjustment for related parameters are the greatest strengths of our study, but there are also some limitations. First, the lack of medical history of the participants was minimized by the exclusion of patients who reported using medication known to influence PTH values, such as loop diuretics, lithium, denosumab, zoledronic acid and teriparatide, and the use of data from outpatient collections only. In our country, in medical private practice, the criteria for requesting exams are flexible and patients with no history of metabolic bone diseases routinely perform PTH measurement.
Another limitation could be the measurement of 25-OHD levels within a month of PTH collection. However, we believe that there is little impact because of the total of 263,242 individuals, just 20,112 reported using cholecalciferol continuously, which represents only 7.64% of the sample.
We also do not have information on patients’ weight, body mass index, dietary calcium intake or urinary calcium excretion, which are which are variables affecting hormonal outcomes [18]. As hypercalciuria and intestinal calcium malabsorption were not evaluated in our study, and we included patients with PTH values up to 100 pg/mL, a certain number of cases of Secondary Hyperparathyroidism, due to these causes, were probably included in our sample.
Finally, as our population consisted of approximately 263,000 people with outpatient follow-up and in a private laboratory, some patients with disorders of mineral metabolism would have little impact on the overall analysis.
Another issue to be addressed when discussing PTH value is the variability between different assays. Souberbille et al. [20] compared the PTH results obtained through 15 different assays from the same serum sample and showed discrepant values. Furthermore, other studies have shown variation in PTH results measured by the same assay when serum and plasma samples are used [32]. Additionally, significant differences exist in values obtained with assays provided by different manufacturers [33]. This aspect can be even more important when 3rd generation assays are used, since they report lower values due to their specificity, limited to the bioactive PTH form [34]. Thus, we must emphasize that we cannot generalize our data to all assays, and it only applies to the 2nd Generation Roche assay, measured on a Roche Cobas e602 platform, using serum samples.
In conclusion, we showed that age influences PTH values and that clinical laboratories should establish specific RVs for elderly individuals according to the PTH assays used. This effort will help in understanding the role of PTH in aging and in obtaining an accurate diagnosis of metabolic bone diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Fleury Group for the support.
Author contributions
All authors contributed to the study conception and design. LBCPC wrote the original article. LBCPC, CMAB, JGHV were the main responsible for drafting this paper and had fundamental participation in the conception and design of this work. JGHV also coordinated all steps. PSTR and JPMM were the responsible for the statistical analysis. CMAFF: acquisition of data for the work. MIC, RPMB and JVLJ: reviewing, and editing, acquisition of financial support. All authors read and approved the final manuscript.
Data availability
Data are available in: https://grupofleury.sharepoint.com/:f:/s/ProjetosdeDados/EoU888nXfUpFnFvRd6rWbFwBadsaWnfMk1rKJXtV1F26fg. This link will be available for a limited period of time, only for peer review and referee analysis. This dataset will not be used in any other paper or study.
Declarations
Conflict of interest
All authors are consultants for Fleury Group. Jose Gilberto Henriques Vieira owns stocks of Fleury Group.
Ethical approval
The Fleury Ethics Committee approved this study.
Informed consent
There was no need to use a consent form, as the survey was retrospective and only used anonymized data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gardella TJ, Nissenson RA, Jüppner H, et al. Parathyroid hormone. In: Bilezikian JP, et al., editors. Primer on the metabolic bone diseases and disorders of mineral metabolism. 9. Wiley; 2018. pp. 205–211. [Google Scholar]
- 2.Andrukhova O, Streicher C, Zeitz U, Erben RG. FGF-23 and parathyroid hormone signaling interact in kidney and bone. Mol Cell Endocrinol. 2016;436:224–239. doi: 10.1016/j.mce.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;2:16033. doi: 10.1038/nrdp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souberbielle JC, Friedlander G, Cormier C. Practical considerations in PTH testing. Clin Chim Acta. 2006;366:81–89. doi: 10.1016/j.cca.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem Med (Zagreb) 2016;26:5–16. doi: 10.11613/BM.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souberbielle JC, Cormier C, Kindermans C, Gao P, Cantor T, Baulieu EE, et al. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J Clin Endocrinol Metab. 2001;86:3086–3090. doi: 10.1210/jcem.86.7.7689. [DOI] [PubMed] [Google Scholar]
- 7.Aloia JF, Feuerman M, Yeh JK. Reference range for serum parathyroid hormone. Endocr Pract. 2006;12:137–144. doi: 10.4158/EP.12.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souberbielle JC, Massart C, Brailly-Tabard S, Cormier C, Cavalier E, Delanaye P, Chanson P. Serum PTH reference values established by an automated third-generation assay in vitamin D-replete subjects with normal renal function: consequences of diagnosing primary hyperparathyroidism and the classification of dialysis patients. Eur J Endocrinol. 2016;174:315–323. doi: 10.1530/EJE-15-0595. [DOI] [PubMed] [Google Scholar]
- 9.Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L. Determinants of plasma PTH and their implication for defining a reference interval. Clin Endocrinol (Oxf) 2011;74:37–43. doi: 10.1111/j.1365-2265.2010.03894.x. [DOI] [PubMed] [Google Scholar]
- 10.Eastell R, Brandi ML, Costa AG, D'Amour P, Shoback DM, Thakker RV. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2015;99(10):3570–3579. doi: 10.1210/jc.2014-1414. [DOI] [PubMed] [Google Scholar]
- 11.Farrell CL, Nguyen L, Carter AC. Parathyroid hormone: data mining for age-related reference intervals in adults. Clin Endocrinol (Oxf) 2018;88(2):311–317. doi: 10.1111/cen.13486. [DOI] [PubMed] [Google Scholar]
- 12.Arabi A, Baddoura R, El-Rassi R, El-Hajj Fuleihan G. Age but not gender modulates the relationship between PTH and vitamin D. Bone. 2010;47:408–412. doi: 10.1016/j.bone.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Carrivick SJ, Walsh JP, Brown SJ, Wardrop R, Hadlow NC. Brief report: does PTH increase with age, independent of 25-hydroxyvitamin D, phosphate, renal function, and ionized calcium? J Clin Endocrinol Metab. 2015;100:2131–2134. doi: 10.1210/jc.2014-4370. [DOI] [PubMed] [Google Scholar]
- 14.CLSI (2012) Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline – Third Edition. CLSI document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Browning T. Vitamin D. http://www.traineecouncil.org/pearl (Clinical Chemistry Trainee Council, Pearls of Laboratory Medicine)
- 15.Ma C, Wang X, Wu J, Cheng X, Xia L, Xue F, Qiu L. Real-world big-data studies in laboratory medicine: current status, application, and future considerations. Clin Biochem. 2020;84:21–30. doi: 10.1016/j.clinbiochem.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 16.R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
- 17.Delgado JA, Bauça JM, Pastor MI, Barceló A. Use of data mining in the establishment of age-adjusted reference intervals for parathyroid hormone. Clin Chim Acta. 2020;508:217–220. doi: 10.1016/j.cca.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Touvier M, Deschasaux M, Montourcy M, Sutton A, Charnaux N, Kesse-Guyot E, Fezeu LK, et al. Interpretation of plasma PTH concentrations according to 25OHD status, gender, age, weight status, and calcium intake: importance of the reference values. J Clin Endocrinol Metab. 2014;99:1196–1203. doi: 10.1210/jc.2013-3349. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Aa J, Wang G, Yan B, Zhang Y, Wang X, Zhao C, et al. Differences in metabolite profile between blood plasma and serum. Anal Biochem. 2010;406:105–112. doi: 10.1016/j.ab.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson-Body E, Massart C, et al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int. 2006;70:345–350. doi: 10.1038/sj.ki.5001606. [DOI] [PubMed] [Google Scholar]
- 21.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322:113–114. doi: 10.1001/jama.2019.5774. [DOI] [PubMed] [Google Scholar]
- 22.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 23.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–288.e1. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Schini M, Jacques RM, Oakes E, Peel NFA, Walsh JS, Eastell R. Normocalcemic hyperparathyroidism: study of its prevalence and natural history. J Clin Endocrinol Metab. 2020;105(4):e1171–e1186. doi: 10.1210/clinem/dgaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez Pardo V, Russo de Boland A. Age-related changes in the response of intestinal cells to 1α,25(OH)2-vitamin D3. Ageing Res Rev. 2013;12:76–89. doi: 10.1016/j.arr.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Nordin BE, Need AG, Morris HA, O'Loughlin PD, Horowitz M. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998–1002. doi: 10.1093/ajcn/80.4.998. [DOI] [PubMed] [Google Scholar]
- 28.Ursem SR, Vervloet MG, de Jongh RT, Heijboer AC. Oxidation of parathyroid hormone. Clin Chim Acta. 2020;506:84–91. doi: 10.1016/j.cca.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Ardura JA, Alonso V, Esbrit P, Friedman PA. Oxidation inhibits PTH receptor signaling and trafficking. Biochem Biophys Res Commun. 2017;482:1019–1024. doi: 10.1016/j.bbrc.2016.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domiciano DS, Machado LG, Lopes JB, Figueiredo CP, Caparbo VF, Oliveira RM, Scazufca M, et al. Bone mineral density and parathyroid hormone as independent risk factors for mortality in community-dwelling older adults: a population-based prospective cohort study in Brazil. The São Paulo Ageing & Health (SPAH) study. J Bone Miner Res. 2016;31:1146–1157. doi: 10.1002/jbmr.2795. [DOI] [PubMed] [Google Scholar]
- 31.Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Murthy L, Grill V, Duque G. High parathyroid hormone levels are associated with osteosarcopenia in older individuals with a history of falling. Maturitas. 2018;113:21–25. doi: 10.1016/j.maturitas.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Joly D, Drueke TB, Alberti C, Houillier P, Lawson-Body E, Martin KJ, Massart C, et al. Variation in serum and plasma PTH levels in second-generation assays in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2008;51(6):987–995. doi: 10.1053/j.ajkd.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 33.La`ulu SL, Roberts WL. Performance characteristics of six intact parathyroid hormone assays. Am J Clin Pathol. 2010;134:930–938. doi: 10.1309/AJCPLGCZR7IPVHA7. [DOI] [PubMed] [Google Scholar]
- 34.Bonancea TC, Ohe MN, Brandão CM, Ferrer CF, Santos LM, Lazaretti-Castro M, Vieira JGH. Experience with the third-generation parathyroid hormone assay (BIO-PTH) in the diagnosis of primary hyperparathyroidism in a Brazilian population. Arch Endocrinol Metab. 2016;60:420–425. doi: 10.1590/2359-3997000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in: https://grupofleury.sharepoint.com/:f:/s/ProjetosdeDados/EoU888nXfUpFnFvRd6rWbFwBadsaWnfMk1rKJXtV1F26fg. This link will be available for a limited period of time, only for peer review and referee analysis. This dataset will not be used in any other paper or study.