Abstract
Susac syndrome is a likely autoimmune microangiopathy affecting the brain, retina and inner ear. Due to the rarity of this condition, diagnosis and treatment can be challenging. Diagnosis is based on the presence of the clinical triad of central nervous system dysfunction, branch retinal artery occlusions and sensorineural hearing loss. Typical MRI findings of callosal and peri-callosal lesions may assist in diagnosis. Clinical course can be monophasic, polycyclic or chronic continuous. It is important to look out for red flags to attain an accurate diagnosis and follow a therapeutic algorithm based on severity of the disease and response to treatment. Patients are treated with steroids and immunosuppressive agents with a variable response. Early aggressive treatment especially in severe cases, may help in preventing relapses and morbidity/disability. This study highlights important diagnostic features and proposes a treatment algorithm based on clinical experience from management of 16 patients from 2 neuroscience centres in the UK since 2007, who were followed up over a long period of 3–15 years.
Keywords: Susac syndrome, Encephalopathy, Sensorineural hearing loss, Branch retinal artery occlusion, Visual loss
Introduction
Susac Syndrome is a likely autoimmune condition consisting of a triad of encephalopathy, branch retinal artery occlusions (BRAO) and sensorineural hearing loss, first described in 1979 by Susac et al. [1]. Since then, 450 cases have been reported (case reports and a few case series) up until 2021 [2]. It is due to a microangiopathy affecting the precapillary arterioles of the brain, retina, and inner ear (cochlea and semicircular canals). The various components of the triad may present sequentially and often incompletely, leading to a delay in the diagnosis. Current understanding of this condition has been enhanced over recent years with more cases appearing in the literature and attempts to rationalise treatment strategies. Here we present an update based on our experience of managing patients, with an aim to shed further insight into the differences, challenges and vigilance required in the diagnosis and management.
Methods
This study was a retrospective analysis of 16 patients diagnosed and managed across 2 tertiary level hospitals in the UK. All patients have been on regular follow-up since the time of diagnosis. Data was acquired from the institutional medical records.
The study was approved as a retrospective audit not requiring participant consent at the respective institutions (University Hospitals Birmingham Clinical Audit Registration and Management System, CARMS 13111, 08 November 2016 and CARMS 13667, 16 August 2017 and Nottingham University Hospitals, audit approval number 19-321Ca, completed on 9 December 2019).
Results
Demographics
Mean age of our patients was 35.6 years (SD = 10.1) ranging between 18 and 60 years. The female:male ratio was 3:1. Given the extreme rarity of diagnosis, we have consciously given the age at presentation in decades to avoid any inadvertent un-blinding of patients.
Presenting complaints and investigations
Clinical and laboratory features are summarised in Tables 1 and 2. 75% of patients presented to the hospital with a subacute onset of headache and focal neurological deficits. More than half of the patients had either hearing or visual symptoms at onset. The complete clinical triad of symptoms involving the brain, eye and inner ear was seen only in 4 patients at presentation. Six more patients were found to have auditory or visual involvement either over time or after specialist investigations. 50% (8/16) of our patients fulfilled the definite and 43.75% (7/16) the probable diagnostic criteria [3] of Susac syndrome. Time from the onset of clinical symptoms to diagnosis ranged from 2 weeks to 10 months. A history of travel prior to onset of symptoms was present in a quarter of patients and a background history of mental health problems was noted in less than a third of patients.
Table 1.
Clinical features, diagnostic tests, treatment and prognosis of sixteen patients with Susac syndrome
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Age (in decade) | 31–40 | 41–50 | 31–40 | 41–50 | 31–40 | 21–30 | 21–30 | 11–20 |
| Sex (M/F) | F | F | F | M | F | F | F | F |
| Presenting complaints | Three weeks intermittent headache with right hemi-paraesthesia preceded by scintillating spectra | Headache, persistent visual symptoms, memory problems | Six-week history of severe vertigo, headaches and vomiting | Vomiting, dizziness, confusion, headaches and ataxia following a viral prodromal illness | Five month history of episodic headaches, unsteadiness, episodic flashes, floaters and blurred vision, unilateral HL | Headache, facial numbness, confusion and diplopia | Intermittent episodes of unsteadiness, popping sensation in ears and blurred vision | Dizziness, forgetfulness |
| History of travel | Yes | Yes | Yes | Yes | No | No | No | No |
| Other associated medical conditions | Psoriasis, Chronic fatigue syndrome and anorexia | Chronic back pain, depression, epilepsy, diabetes and gastric banding | Depression and bipolar disorder | Rheumatoid Arthritis | IBS, cholelithiasis | Several infections in Dec 2016 | ||
| Diagnosis at presentation | ADEM | Migraine | Probable Susac | Viral/autoimmune encephalitis and Marchiafava Bignami | Susac | Probable Susac | Susac | Probable Susac |
| Triad at presentation | No | No | No | No | Yes | No | Yes | No |
| Triad at Diagnosis | No | No | No | No | Yes | Yes | Yes | Yes |
| Time to diagnosis | 8 months | 12 months | 1 month | 12 months | 5 months | 1.5 months | 3 months | 1 month |
| CNS symptoms | Headache, encephalopathy, ataxia, dystonia | Headache, encephalopathy, focal limb and cranial nerve weakness | Headache, encephalopathy, dysphasia, memory loss | Headache, encephalopathy, focal signs, memory loss | Headache | Headache, encephalopathy, focal limb and cranial nerve weakness | Encephalopathy | Headache, Encephalopathy |
| Visual symptoms | Scintillating spectra | Persistent visual symptoms, diplopia | Nil | Nil | Flashes and floaters | Double vision | Visual loss | Nil |
| VA | Decreased | Decreased in left eye | Decreased | Could not be done as patient encephalopathic | Decreased | Normal | Normal | Normal |
| Fundus/FFA | FFA- no occlusions | FFA not done as fundus examination was normal | Blurred disc margins, microaneurysms, BRAO | FFA not done as fundus examination was normal | BRAO and venous occlusions | Not cooperative for FFA, OCT done | BRAO | BRAO |
| Vestibulo-cochlear symptoms | Hearing loss | Hearing loss, tinnitus | Hearing loss R, vertigo | Nil | Hearing loss | Nil | Hearing loss | Hearing loss |
| Audiogram | SNHL | Low frequency SNHL | Normal | Not done | R. SNHL | Low frequency HL | Low frequency HL | Mid frequency HL |
| MRI brain changes | ||||||||
| Callosal | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pericallosal | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Infratentorial | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Enhancement | Yes | Yes | Yes | Yes | Yes | Yes | ||
| DWI restriction | Yes | Yes | Yes | |||||
|
CSF protein g/L (normal range 0.15–0.45 g/L) OCB |
1.35 Negative |
0.26 Negative |
1.02 Negative |
1.1 Negative |
No data |
1.47 Negative |
1.39 Negative |
0.92 Negative |
| Other investigations | ANA 1:25, brain biopsy | RF, dsDNA | Positive serum EBV PCR | ANA 1:50, Anti GAD, (post-mortem brain histology) | EBV and CMV IgG | |||
| Treatment | Steroids, IVIG, cochlear implant | Steroids, AZA, CYC | Steroids, CYC | Steroids, IVIG, PLEX, CYC | Steroids, IVIG, CYC, MMF | Steroids, IVIG, CYC, RTX | Steroids, IVIG, AZA, RTX, cochlear implant | Steroids, IVIG, MMF |
| Disease course | Relapsing—stable | Relapsing—stable | Monophasic | Relapsing—Death | Relapsing—stable | Monophasic | Relapsing—stable | Monophasic |
| Follow-up | 13 years | 3 years | 8 years | 1 year | 5 years | 5 years | 7 years | 4 years |
| Residual FNDs | Chronic Vertigo | Suicidal ideation, HL | Minimal cognitive deficits and occasional Vertigo | Full recovery | Walks with aid, low mood | Full recovery |
Good recovery Occasional tinnitus |
|
| Patient 9 | Patient 10 | Patient 11 | Patient 12 | Patient 13 | Patient 14 | Patient 15 | Patient 16 | |
|---|---|---|---|---|---|---|---|---|
| Age (in decades) | 31–40 | 31–40 | 31–40 | 31–40 | 31–40 | 31–40 | 51–60 | 41–50 |
| Sex (M/F) | M | F | M | F | F | F | M | F |
| Presenting complaints at first presentation | Left visual loss | Headaches, sensory symptoms and bladder dysfunction | Headaches, encephalopathy, bilateral vision loss | Headache, encephalopathy | Headache, monocular field defect, tinnitus, unilateral hearing deficits, vertigo | Tinnitus, vertigo, hearing deficit, headache | Headache, tinnitus, right sided hearing loss | Tinnitus, right sided hearing loss |
| History of travel | No | No | No | No | No | No | No | No |
| Other associated medical conditions | Type 2 DM | Depression | Depression | Depression | Hypertension, Hypercholesterolemia | |||
| Diagnosis at presentation | ON | MS | HSV | HSV | Susac | Susac | Probable Susac | Systemic vasculitis |
| Triad at presentation | No | No | No | No | Yes | Yes | No | No |
| Triad at final diagnosis | Yes | Yes | No | No | No | Yes | No | Yes |
| Time to diagnosis | 8 months | 4 months | 1 year | 7 months | ||||
| CNS symptoms | Headache | Headache, L paraesthesia | Headache, encephalopathy, visual hallucinations | Headache, encephalopathy, gait apraxia | Headache, | Headache | Seizures, personality changes, cognitive deficits | Headache, Encephalopathy |
| Visual symptoms | L Visual loss | Nil | R blurred vision | Nil | Field defect | Nil | Nil | Nil |
| VA | Decreased | Normal | Decreased | Normal | Normal | Normal | Decreased | Normal |
| Fundus/FFA | BRAO | AWH | BRAO | AWH | Cotton wool spots | BRAO | Not done | Cotton wool spots |
| Vestibulo-cochlear symptoms | BL Hearing loss, tinnitus, vertigo | Hearing loss, tinnitus, vertigo | Nil | Nil | Hearing loss, tinnitus | BL hearing loss | Hearing loss, tinnitus | Hearing loss, tinnitus |
| Audiogram | Bilateral SNHL | Bilateral SNHL | Normal | Normal | Normal (not done at onset) | SNHL | Severe SNHL | Moderate to severe SNHL |
| MRI brain changes | ||||||||
| Callosal | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pericallosal | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Infratentorial | Yes | Yes | Yes | |||||
| Enhancement | ||||||||
| DWI restriction | Yes | Yes | Yes | |||||
|
CSF protein g/L (normal range 0.15–0.45 g/L) OCB |
0.724 Negative |
0.436 Negative |
3.3 Negative |
0.8 Negative |
0.48 Negative |
Not performed Not done |
0.744 Negative |
5.0 Not done |
| Other investigations | Skin biopsy | |||||||
| Treatment | Steroids, MMF, aspirin, hearing aids | Steroids, AZA, aspirin | Steroids, aspirin | Steroids, aspirin | Steroids, aspirin | Steroids, aspirin | Clopidogrel | Steroids, CYC, aspirin, cyclosporin |
| Disease course | Monophasic (improved after higher dose of MMF) | Monophasic (improved after 1 year of AZA) | Monophasic | Monophasic | Monophasic | Monophasic | Monophasic (not offered steroids because of late presentation and patient choice) | Monophasic |
| Residual FNDs | Vision and hearing impairment | Depression, mild cognitive deficits, headaches, overactive bladder | Full recovery | Full recovery | Full recovery | Full recovery | Bilateral HL, mild cognitive deficit | Right sided HL |
Raised CSF protein was defined as > 0.45 g/L, based on the laboratory range
ADEM acute disseminated encephalomyelitis, ANA anti-nuclear antibody, AWH arteriolar wall hyperintensity, AZA azathioprine, BRAO branched retinal artery occlusion, CMV cytomegalovirus, CYC cyclophosphamide, DM diabetes mellitus, DWI diffusion weighted imaging, EBV Epstein–Barr Virus, FFA fundus fluorescein angiogram, FND focal neurological deficits, FU follow-up, GAD glutamic acid decarboxylase, HL hearing loss, HSV Herpes Simplex Virus, IBS Irritable Bowel Syndrome, IVIG intravenous immunoglobulin, MMF mycophenolate mofetil, MS multiple sclerosis, OCT optical coherence tomography, ON optic neuritis, RTX rituximab, SNHL sensorineural hearing loss, VA visual acuity
Table 2.
Summary of clinical features of sixteen patients with Susac syndrome
| History of travel prior to onset of symptoms | 4/16 | 25% |
| Presenting complaints | ||
| Headache | 12/16 | 75% |
| Eye symptoms | 8/16 | 50% |
| Ear symptoms | 9/16 | 56.25% |
| Confusion/forgetfulness | 6/16 | 37.5% |
| Encephalopathy (at any stage) | 11/16 | 68.75% |
| Psychiatric comorbidities | 5/16 | 31.25% |
| CSF—raised protein | 12/14 | 85.7%% |
| MRI | ||
| Callosal lesions | 16/16 | 100.0% |
| Pericallosal lesions | 14/16 | 87.5% |
| Infratentorial lesions | 9/16 | 56.25% |
| Enhancement | 6/16 | 37.5% |
| DWI | 6/16 | 37.5% |
| Hearing loss (at any stage) | 12/16 | 75% |
| Audiometry abnormalities (at any stage) | 11/14 | 78.6% |
| Objective evidence of retinal ischaemia, arterial occlusions on FFA/Fundus (at any stage) | 11/16 | 68.75% |
| Monophasic course | 11/16 | 68.75% |
Encephalopathy was the most common central nervous system (CNS) manifestation after headache, seen at some point during the illness, although only 6/16 (37.5%) patients had it at onset. The ophthalmologic involvement was clinically silent and subtle on dilated retinal examination in some patients, in whom fundus fluorescein angiography (FFA) and optical coherence tomography (OCT) aided the diagnosis.
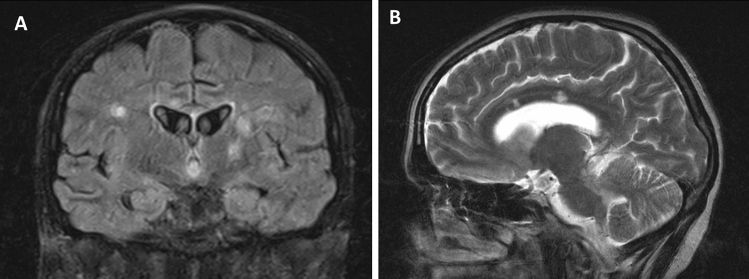
Callosal and/or peri-callosal lesions on brain MRI were seen in all patients (Fig. 1). Only 1/14 patient had CSF leukocytosis (26 lymphocytes) and 12/14 patients had raised CSF protein. Oligo-clonal bands were absent in all 13 patients who were tested for it.
Fig. 1.
MRI changes in Susac syndrome MRI sequences of showing callosal (arrows), periventricular and thalamic lesions in coronal FLAIR (A) and sagittal T2W (B) images
Histological findings
One of our patients (patient 1) underwent stereotactic brain biopsy whilst another patient (patient 4) had an autopsy, both of which showed multiple microinfarcts involving grey/white matter, deep grey nuclei, brain stem and corpus callosum with endothelial cell necrosis and perivascular lymphocytic infiltration (Fig. 2A, B). C4d immunostaining showed complement deposition in the capillaries and venules in 30% of the vessels, suggesting humoral mediated microangiopathy. Patient 4 presented with encephalopathy and cognitive impairment with a compatible MRI but had no accompanying retinal or vestibulo-cochlear symptoms. Histology showed evidence of micro-infarcts and histopathological evidence of microangiopathy in the brain on postmortem study.
Fig. 2.
Pathological changes in Susac syndrome—brain histology showing multiple microinfarcts (arrows) in cerebral cortex (A, H&E × 40) with higher magnification (H&E, × 100) showing micro angiopathy with thickening of wall of arteriolar wall and sparse perivascular lymphocytic infiltrate (arrow) (B). H&E haematoxylin and eosin staining
Treatment
The various treatments used for our patients are demonstrated in Table 1. Of the 15/16 patients who were treated with steroids, 10 received Intravenous Methylprednisolone (IVMP) at presentation, and 3 others at some point during the course of illness. 2/16 patients received oral steroids at presentation. Intravenous immunoglobulin (IVIg) was more frequently used in relapsing disease course.
Not all patients were given long-term immunosuppression. All except 2 patients who had encephalopathy had a protracted course despite early treatment with IVMP. The relapses stopped after regular immunosuppression either with regular IVIG as in patient 1, or prolonged course of steroid-sparing agents (Patients 2, 3, 5, 6). Patients had fewer relapses on Cyclophosphamide and Mycophenolate mofetil as compared to Azathioprine. Interestingly, all patients who had Aspirin or Clopidogrel had a monophasic course (Table 1). Our experience allowed serial monitoring of some patients for over a decade (for up to 15 years) and despite best immunotherapy, several (> 50%) patients developed brain atrophy and cognitive impairment, very similar to what is often seen in other autoimmune central nervous system diseases like multiple sclerosis (MS).
Discussion and neurological update
Epidemiology
Susac syndrome has a female preponderance with a female-to-male ratio of 2.2:1 and a mean age of onset of 30.5 years (± 9.6 years) [3] very similar to our observations. In a review by Dorr et al., mean age of presentation was 31.6 years with 81% falling between the ages of 16 and 40 years [4]. First presentation between ages 9 and 72 years was reported [5].
5% of the women had symptoms during or in the post-partum period [4]. Patient 12 in our series was 30 weeks pregnant at presentation. Cases have been reported from all continents. However, in a review of all reported cases, only 25% reported ethnicity, and 81% of these were white Caucasians [4].
Pathophysiology
Autoimmune endotheliopathy seems to be the most likely pathologic process. One patient in our series had a probable clinical diagnosis of Susac, but had histopathological findings compatible with the diagnosis. Brain biopsies have shown micro-infarcts with microglial activation suggesting a T cellcell-mediated process [6]. CD8 T cell adherence to microvasculature (causing endothelial damage, vessel narrowing and occlusion) leading to microinfarcts was seen in transgenic mouse models and patients with Susac syndrome [7]. A recent study suggested similar pathological mechanisms between Susac, Rasmussens encephalitis and Narcolepsy type 1 [8]. Earlier reports supported the similarity to the pathogenesis of juvenile dermatomyositis where a different group of tissues were involved [9].
Neuropathologic studies are limited: findings reported so far include peri-arteriolar mononuclear infiltrate and collagen deposition, thickened arteriolar wall and basement membrane and microvascular fibrosis [10] [11], similar to what we have observed (Fig. 2). Petty et al. also found similar findings in the muscle of patients with Susac Syndrome [12].
Attempts at elucidating the pathophysiology of the condition were first made with brain biopsy in one of Dr Susac’s original patients, which demonstrated scarring representing a healed angiitis [1]. Other biopsies have found similar findings [13].
Preceding history of travel, insect bite, infection and serum Epstein–Barr virus (EBV)/cytomegalovirus (CMV) positivity in a few of our cases are pointers towards possible infectious triggers. Although not proven, the possibility of an infectious trigger has been proposed [14]. Petty et al., reported that 50% of patients in their study were CMV positive [12]. Four patients had low serum folate levels—the significance of this finding remains unestablished.
Clinical features and diagnosis
All features may not be present at the initial presentation making the diagnosis challenging [3, 7, 9]. Clinical triad was fulfilled only in 13% patients at initial presentation and 80% of patients eventually, as reported in a review [4]. Although we had a higher percentage fulfilling the diagnostic criteria at onset, a lesser number of patients completed the triad on follow-up (Tables 1 and 2). Patients may present to ENT specialists and ophthalmologists at onset [15], who may not always be familiar with Susac Syndrome. The literature reports frequent misdiagnoses, often related to a lack of knowledge of this condition, both by physicians and radiologists, attributing MRI findings to multiple sclerosis or ADEM [16, 17].
Mean time to diagnosis was 3 months in our series, whereas it was 3 weeks in a case series of 7 patients [14] and up to 3 months in another study [15]. Diagnostic delay depends on the organs involved. A recent study reported a mean time to diagnosis of 3 months when the diagnostic criteria were met, 10 months with brain and retina involvement, 3 months with brain and inner ear involvement and 2.5 months with brain involvement alone [15].
Clinical features seen outside of the diagnostic clinical triad include skin manifestations like livedo reticularis and confusion [18], emotional disturbances, personality changes and psychiatric symptoms [19]. Studies are needed to evaluate the relevance of history of travel prior to onset of symptoms (Table 2) and a background history of mental health problems as seen 6/16 patients in our series (Table 1).
Brain symptoms
Encephalopathy and migraine-like headaches are the most common brain manifestations of Susac syndrome. However, encephalopathy may not always be present especially at disease onset [4, 7]. Presence of encephalopathy may mask the other symptoms involving the eye and ear (as seen in patient 7) and therefore a targeted search for their involvement is warranted in patients presenting with subacute onset of migraine-like headache and encephalopathy, especially in the presence of corpus callosal lesions.
CNS symptoms were the most common first clinical manifestation followed by an equal proportion of eye or ear symptoms. Migraine-like headaches may be present at onset in up to 80% of patients [4], quite similar to our cohort. Headaches have been associated with the imaging finding of leptomeningeal enhancement, suggesting meningeal inflammation as one of the possible causes for headache [20].
Neuropsychiatric presentations have also been observed, including behavioural changes and dementia [20] [21]. A background of mental health problems may therefore make diagnosis challenging especially in patients with a predominant encephalopathic onset.
Ear symptoms
Hearing loss may be the only presenting feature of Susac syndrome and it may be sudden or insidious in onset [5]. We found 9/16 of our patients who had hearing loss at onset. Sensorineural hearing loss, vertigo and tinnitus is most likely related to vestibulo-cochlear damage, and reports have mainly found audiometry deficits in the low to medium frequency ranges, averaging ~ 40 dB [22], as also seen in our series. Hearing loss tends to be progressive and bilateral, but asymmetric and higher frequency involvement may be due to involvement of larger aspects of the cochlea, suggesting more aggressive disease [23].
Visual symptoms
Retinal involvement may manifest clinically (visual loss, change in visual fields, scintillating scotoma, etc.) or be subacute and asymptomatic [23, 24]. The diagnoses in patients 5 and 6 were confirmed with the aid of specialist ophthalmology input. Only half of our cohort reported visual symptoms. A possible explanation could be the fact that retinal involvement is often peripheral and patients presenting with encephalopathy may not be able to accurately report visual symptoms. We would therefore recommend all patients with suspected Susac Syndrome to be screened by a senior ophthalmologist. The hallmark of the ophthalmological findings in Susac are FFA findings of BRAO as well as vessel wall hyper-fluorescence [4]. Endothelial damage may lead to extravasation of lipids and blood leading to formation of atheromatous plaques or Gass plaques. These, unlike true emboli (Hollenhorst plaques) which typically centre on the arterial bifurcation, are usually in the mid-arteriole [25]. The Gass Plaques are refractile and are best seen on clinical examination or fundus photography [23].
Two of our patients had arteriolar wall hyper-fluorescence as the only finding which would be clinically undetectable without FFA. This arteriolar wall hyper-fluorescence can be seen at the site of infarction but also at the site of vessel wall damage where infarction has not yet occurred [26]. Therefore, in those with encephalopathy and auditory findings suggestive of Susac Syndrome, without obvious retinal findings, FFA is essential to detect the early disease. This, however, might be difficult in an uncooperative encephalopathic patient.
FFA, like most other procedures is not without clinical risk [27]. However, the benefit of FFA is the early detection of retinal changes which will aid accurate and early clinical management, preventing visual loss, long-term disability and possibly long-term relapse.
OCT in acute stages may cause thickening of the retinal nerve fibre layer reflecting oedema from BRAOs. This may later resolve leading to focal thinning of the retinal nerve fibre and abnormal foveal contour. The findings differ from MS where a more diffuse involvement is seen (affecting the temporal nerve fibre layer and with normal foveal contour) [7].
Advanced ocular imaging modalities such as wide-field imaging, which as the name suggests, captures a wider retinal field, are useful for long-term retinal monitoring [28]. FFA may need to be repeated if no retinal abnormalities are detected because of the temporal disparity of the clinical symptoms and signs seen with the Susac triad and also because BRAO may recur over the course of illness [5]. OCT angiography for the screening of Susac Syndrome remains a research tool, due to the limited field of view [29].
Retinal micro-aneurysm is a new ocular finding in Susac syndrome which suggests ischaemic damage to the retina [30]. Two of our patients had cotton wool spots on fundus examination, which may also suggest the same pathology [3].
Radiology
Rennebohm et al. have suggested that the microinfarcts or “snowball” (larger lesions) appearance of the mid-corpus callosum and/or the “string of pearls” (micro-infarcts of the internal capsule) is perhaps sufficient to diagnose Susac Syndrome without evidence of hearing impairment or BRAOs [9, 31]. Although callosal lesions are included in the diagnostic criteria [3], they are not always present [32]; they are typically seen in the central fibres and splenium without involving the undersurface unlike MS [33]. The callosal roof is often involved giving an ‘icicle’ look to the lesions [7]. Callosal lesions are usually small and as a consequence of occlusion of the small (less than 100 µm) precapillary arterioles [33]. The periventricular white matter as well as the deep grey matter is also affected [7]; the deep grey matter can be involved in up to 70% of cases [5].
Leptomeningeal involvement can be seen in up to 33% [5] (Fig. 1). The correlation to headache however could not be established in our series. A plausible explanation is the variable course of the condition. Moreover, the lack of sensitivity of post-contrast T1-weighted images as compared to post-contrast fluid-attenuated inversion recovery (FLAIR) sequences may provide a reasonable explanation [34]. Spinal involvement has rarely been reported in Susac Syndrome [35, 36]. Small vessel or perivascular enhancement on high-resolution intracranial vessel wall MRI has been reported in Susac syndrome [37]. Degradation of fibres in the genu of corpus callosum, using Diffusion Tensor Imaging (DTI), is thought to be specific for Susac syndrome [38] and can potentially be a future diagnostic tool.
Differential diagnosis
The prodrome of migraines, subacute onset and characteristic MRI lesions in the brain help differentiate Susac syndrome from remitting relapsing MS. The absence of oligo-clonal bands may be a useful marker to differentiate it from MS [4]. A common differential diagnosis at presentation is acute demyelinating encephalomyelitis (ADEM) and the presence of micro-infarcts (restriction on diffusion weighted imaging) may be beneficial in pointing towards a diagnosis of Susac syndrome in patients who do not meet the full diagnostic criteria. As Susac syndrome is a vasculopathy with clinical features similar to primary CNS vasculitis, it can often be misdiagnosed as the latter. MRI/CT angiogram and formal cerebral angiography can be beneficial in such patients.
Clinical course
Clinical course was described as monophasic (fluctuating disease lasting less than 2 years), polyphasic or chronic continuous by Renneboum et al. [5], and in the 114 patients stratified in this manner 54% had monophasic, 42% had polyphasic and 4% had chronic continuous course [4].
A previous study suggested that clinical course may be self-limiting in those who develop encephalopathy within the first two years. MRI and FFA findings do not seem to be helpful in predicting the course of the illness [5]. There was no correlation between encephalopathy at onset and monophasic course in our series. In our experience, in terms of prognosis, those who had asymptomatic retinal findings of either arteriolar wall hyper-fluorescence or branch artery occlusions, tended to have a monophasic illness, which may indicate that early treatment has the potential to change the course of the disease by inducing remission. Conversely, those who reported photopsia symptoms, had a relapsing illness, which may indicate that these symptoms confer a worse prognosis. From our experience, in terms of prognosis, those who had asymptomatic retinal findings of either arteriolar wall hyper-fluorescence or branch artery occlusions and received appropriate immuno-suppression tended to have a monophasic illness.
Remissions have been noted spontaneously, [16] although patients can have a variable course and sometimes recurrence may occur after prolonged stability for several years [39].
A previous study found that most patients had a good recovery and brain atrophy seen on MRI may not always correlate with cognitive decline [40].
Treatment
Randomised trials and prospective studies are lacking. Hence, all recommendations are from case series and expert opinions. Variable response to immunosuppressive treatment has been previously reported [5].
Most patients will require immunomodulation either in the form of steroids, IVIg ± steroid-sparing drugs. Only one patient from our cohort did not receive immunomodulation.
Moderate to severe encephalopathic patients may need more aggressive treatment (RTX or CYC) in comparison to those with only ear and/or eye involvement (MMF with or without MTX for 2 years after the relapse resolved). [9] Relapses have been reported during steroid taper and seem to improve with escalating the steroid dose [5].
A variety of treatment approaches have been tried to our patients (Table 1 and 2). The difference in therapeutic strategies reflects the variable response seen in this patient group and the variation to the severity of the disease. Other patients in the literature were treated in a similar fashion [3, 41]. Frequent Intravenous immunoglobulin (IVIg) may be of benefit in relapsing disease course.
We recommend early and aggressive therapy to prevent relapses because of the unpredictable course of the disease, and the potentially devastating neurological sequelae. There is limited experience of monoclonal antibody therapies for Susac, but some patients demonstrated remarkable improvement following their use [42]. The use of Rituximab in our series (patients 6, 7) was associated with a relatively good clinical response. There was complete return of visual acuity following treatment and patients remained relapse-free for a period of 4 years and 15 months, respectively. More studies on anti-endothelial cell antibodies may help justify treatment with B cell-targeted therapies [43]. Natalizumab in one report led to worsening symptoms [44], whereas off-label use in another study on 4 patients proved promising; however, the disease relapsed in 2/4 patients in the study when it was discontinued [45]. Infliximab may potentially be useful in refractory cases of Susac Syndrome [6].
We have changed our practice to include Aspirin in all new diagnoses over the last few years. Although anti-platelets were not officially included in the proposed treatment guidelines [9], they are commonly used in clinic practice [3, 46]. Their anti-inflammatory and antiplatelet properties may have a role to play. Longer-term prospective studies are needed to confirm or refute this observation.
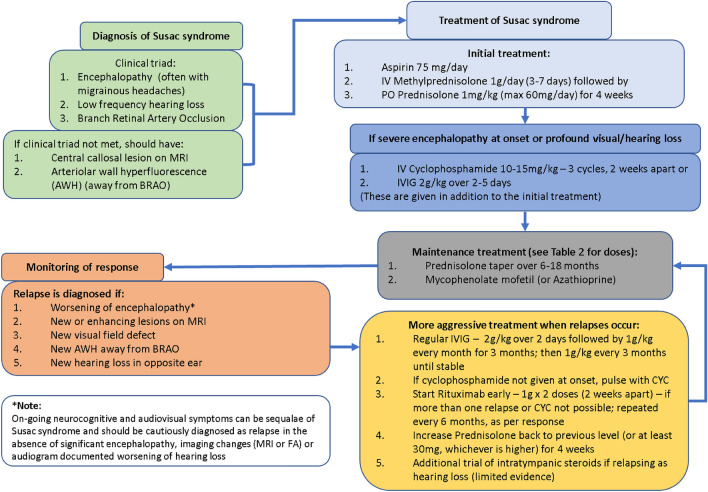
Based on our experience, we have summarised the medical management of Susac Syndrome in Fig. 3 with the details of medications in Table 3. A consensus guideline for the management of Susac Syndrome has been recently published, which is not too dissimilar to our own recommendations [9]. Given the relapse potential of Susac Syndrome, strict follow-up is paramount [47].
Fig. 3.
Proposed algorithm for managing Susac syndrome algorithm for diagnosis, treatment and monitoring of Susac Syndrome
Table 3.
Medications used in management of Susac syndrome
| Medication | Dosage | Summary of usage |
|---|---|---|
| IV methylprednisolone (IVMP) | 1000 mg/day 3–7 days | All patients should have pulsed IVMP at onset for at least 3 days, with longer course for patients with more severe encephalopathic onset |
| Oral prednisolone | 1 mg/kg/day (max 60 mg)—see titration suggestions | All patients should start oral Prednisolone at onset after the initial dose of intravenous steroids and maintained for at least 4 weeks, following which this can be reduced by 5 mg every 2 weeks until on 30 mg OD, then reduce by 5 mg every 4 weeks until on 15 mg OD, provided there are no relapses on dose reduction. Further tapering by 2 mg every month until on 9 mg OD and then by 1 mg every month and withdraw, especially if maintained on steroid-sparing drugs. In monophasic illness the dose can be tapered and stopped between 12 and 18 months |
| IV immunoglobulin (IVIg) | 2 g/kg/day × 5 days | Our practice is to use IVIG in very severe encephalopathic onset or after first relapse. Many authors consider IVIG should be used as first line therapy along with corticosteroids |
| IV Cyclophosphamide (CYC) | 10–15 mg/kg × three doses (2 weeks apart) | Unless major contraindications (eg: renal or concerns regarding ovarian failure), CYC can be used in refractory cases c. i. We generally prefer to maintain patients on other immunosuppressants (AZA/MMF etc.) in the longer term than oral cyclophosphamide maintenance to minimise side effects |
| Oral mycophenolate mofetil (MMF) | Start at 500 mg BD and increase to 1000 mg BD after 2–4 weeks | We find that MMF is better than other steroid-sparing immunosuppressants because of the faster onset of action needed to minimise longer term side effects. However, given the potential caution during pregnancy, this is probably avoided in young women when azathioprine or rituximab should be tried (If giving CYC, the lower dose can be started at onset with the dose increased to the maintenance level 2 weeks after the third cycle of CYC) |
| Oral azathioprine (AZA) | 2.5 mg/kg/day | This was traditionally the first steroid-sparing drug used, although its slower onset of action and increasing availability of Rituximab has made this a less popular drug recently. However, this is still the drug of choice in young women because of the safety data in pregnancy |
| IV rituximab (RTX) | 1 g/day × two doses (2 weeks apart) | Rituximab is now becoming an increasingly common drug especially if there is a relapse on any of the above treatments. CD19 levels can be monitored, and further doses given, if required |
| Oral aspirin (ASA) | 75 mg/day | Although less commonly used, our experience has revealed that this is a safe drug |
Organ-specific treatments (inner ear and retina)
Generally hearing impairment has been permanent with mild improvement at follow-up despite aggressive immunotherapy [48]. The higher susceptibility of the inner ear for irreversible damage due to ischaemia may account for lack of improvement.in hearing loss with treatment [7]. There are also previous reports of some improvement in hearing with intra-tympanic steroids [49]. Cochlear implantation in patients with hearing loss has been beneficial [50] as in 2 of our patients (patient 1 and 7). Retinal neovascularization may occur as a result of recurrent ischaemia [51] and photocoagulation, vascular endothelial growth factor inhibitor alone or together may be treatment options to prevent further complications [7].
Conclusion
Susac Syndrome is a rare, under-recognised condition. Diagnosis is challenging as the complete clinical triad is not often seen at presentation, leading to misdiagnosis [3, 7]. Although the diagnostic criteria insist on the presence of symptoms suggesting vestibulo-cochlear impairment, patients presenting with encephalopathy may not be able to give a history suggestive of the same. Therefore, a detailed auditory and/or visual examination (including FFA and OCT) in suspected patients (e.g. migraine-like headaches, or/and encephalopathy with corpus callosum MRI lesions) is strongly recommended [3, 7] in order to assist in diagnosis. In our experience, FFA remains superior in terms of arterial wall hyper-fluorescence and evidence of fluorescence leakage distal to the wall hyper-fluorescence (Fig. 4A–D). In those with CNS symptoms and auditory compromise, a dilated ophthalmic examination, supported by wide-field retinal photography and OCT imaging should be performed [52]. Where there are CNS symptoms, no auditory compromise and where no retinal clinical signs are detected, the risk and the benefit of FFA should be discussed.
Fig. 4.
Fundal pictures in Susac syndrome optical coherence tomography (OCT) infrared image of the right eye (A) showing inferior retinal branch involvement (black arrows); the black arrows on the right of the image show occlusion, whereas on the left there is incomplete occlusion. Fundus fluorescein angiography (FFA), late phase of the left eye (B) in the same patient seen in A. There is arteriolar wall hyper-fluorescence seen along with frank leakage. FFA of the left eye (late phase, C) shows the requirement for peripheral views to gauge the extent of the retinal vascular involvement. FFA of the right eye (D, late phase) shows superior arterial tree involvement in the same patient
Early diagnosis and prompt treatment of patients with Susac Syndrome can result in resolution of symptoms, potentially curbing disease progression [3, 7]. In our experience, the disease severity, response to treatment and long-term prognosis are variable amongst patients, as previously described [3, 7, 9]. The mainstay of treatment is early prompt immunosuppression, as inadequate treatment might lead to irreversible damage [9]. On the other hand, there are cases (like our patients 11–14) that had a monophasic course with full recovery with steroids only. An individualised treatment approach based on severity at presentation [9] and response to first line immunosuppressive treatment is recommended based on our observations. Moreover, serial brain MRI imaging, OCT/FFA and audiometry for comparison is crucial in decision-making about acute and long-term immunosuppression.
Even though treatment is fundamentally by immunosuppression, optimal protocols and duration have not been clearly elucidated.
The role of RTX (or other similar B cell-targeted therapies) as a promising treatment in refractory patients hints towards the need for more studies evaluating its possible role in preventing relapses and progressive cerebral atrophy which may influence cognition in the long term.
The importance of starting Aspirin early-on to prevent micro-infarcts is also worth considering. Given the rarity of the disease, it is not surprising that randomised controlled trial evidence is lacking. The approach of utilising experience of treatment from other similar conditions is fundamental to current practice and allows anecdotal evidence and expert opinion to guide management. Even though the retrospective nature of the study is a limitation, the highlights of our study are the large cohort of patients with long-term follow-up. This patient group was diverse; the youngest patient was in the late teens and the oldest was in their 6th decade at presentation. Two patients had successful pregnancies post diagnosis. One patient had an aggressive disease leading to death, whereas 6 patients had a monophasic course not requiring long-term immunosuppression.
Acknowledgements
The authors would like to acknowledge all patients who allowed us to access the clinical data; Dr Santhosh Nagaraju, Consultant Neuropathologist for help with the histology; Prof Vijay Sawlani, Consultant Neuroradiologist for the neuroimages and all clinicians who referred the patients.
Abbreviations
- ADEM
Acute disseminated encephalomyelitis
- AWH
Arteriolar wall hyperfluorescence
- AZA
Azathioprine
- BRAO
Branch retinal artery occlusions
- CMV
Cytomegalovirus
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CYC
Cyclophosphamide
- FFA
Fundus fluorescein angiography
- H&E
Haematoxylin and eosin
- HSV
Herpes simplex virus
- IVIg
Intravenous immunoglobulin
- IVMP
Intravenous methylprednisolone
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- OCT
Optical coherence tomography
- PCR
Polymerase chain reaction
- PLEX
Plasma exchange
- RTX
Rituximab
Funding
None relevant to this study.
Declarations
Conflicts of interest
Prof Jacob has served as an international advisory board member for Alexion, Alnylam, ArgenX, Immunovant, Janssen, Regeneron and UCB pharmaceuticals, is currently an expert panel member of Myasthenia Gravis consortium for Argenx pharmaceuticals, and has received speaker fees from Terumo BCT and Eisai pharmaceuticals. None of these are relevant to the submitted manuscript. Prof. Mollan reports advisory board membership for Invex Therapeutics and Janssen. She is currently a consultant for Neurodiem and has received speaker fees from Heidelberg Engineering, and Chugai-Roche Ltd. All outside the submitted work. Dr Appleton is supported in part by a National Institute of Health Research (NIHR) Health and Care Research Scholarship.
Footnotes
Smriti Bose, Athanasios Papathanasiou and Sameep Karkhanis have contributed equally to the work.
References
- 1.Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29:313–316. doi: 10.1212/wnl.29.3.313. [DOI] [PubMed] [Google Scholar]
- 2.David C, Sacré K, Henri-Feugeas M-C, et al. Susac syndrome: a scoping review. Autoimmun Rev. 2022;21:103097. doi: 10.1016/j.autrev.2022.103097. [DOI] [PubMed] [Google Scholar]
- 3.Kleffner I, Dörr J, Ringelstein M, et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry. 2016;87:1287–1295. doi: 10.1136/jnnp-2016-314295. [DOI] [PubMed] [Google Scholar]
- 4.Dörr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013;9:307–316. doi: 10.1038/nrneurol.2013.82. [DOI] [PubMed] [Google Scholar]
- 5.Rennebohm RM, Egan RA, Susac JO. Treatment of Susac’s syndrome. Curr Treat Options Neurol. 2008;10:67–74. doi: 10.1007/s11940-008-0008-y. [DOI] [PubMed] [Google Scholar]
- 6.Hardy TA, O’Brien B, Gerbis N, et al. Brain histopathology in three cases of Susac’s syndrome: implications for lesion pathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2015;86:582–584. doi: 10.1136/jnnp-2014-308240. [DOI] [PubMed] [Google Scholar]
- 7.Marrodan M, Fiol MP, Correale J. Susac syndrome: challenges in the diagnosis and treatment. Brain. 2022;145:858–871. doi: 10.1093/brain/awab476. [DOI] [PubMed] [Google Scholar]
- 8.Wiendl H, Gross CC, Bauer J, et al. Fundamental mechanistic insights from rare but paradigmatic neuroimmunological diseases. Nat Rev Neurol. 2021 doi: 10.1038/s41582-021-00496-7. [DOI] [PubMed] [Google Scholar]
- 9.Rennebohm RM, Asdaghi N, Srivastava S, Gertner E. Guidelines for treatment of Susac syndrome—an update. Int J Stroke. 2020;15:484–494. doi: 10.1177/1747493017751737. [DOI] [PubMed] [Google Scholar]
- 10.Agamanolis DP, Prayson RA, Asdaghi N, et al. Brain microvascular pathology in Susac syndrome: an electron microscopic study of five cases. Ultrastruct Pathol. 2019;43:229–236. doi: 10.1080/01913123.2019.1692117. [DOI] [PubMed] [Google Scholar]
- 11.Agamanolis DP, Klonk C, Bigley K, Rennebohm RM. neuropathological findings in Susac syndrome: an autopsy report. J Neuropathol Exp Neurol. 2019;78:515–519. doi: 10.1093/jnen/nlz031. [DOI] [PubMed] [Google Scholar]
- 12.Petty GW, Engel AG, Younge BR, et al. Retinocochleocerebral vasculopathy. Medicine (Baltimore) 1998;77:12–40. doi: 10.1097/00005792-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro ML, Swanson RA, Coppeto JR, et al. A microangiopathic syndrome of encephalopathy, hearing loss, and retinal arteriolar occlusions. Neurology. 1985;35:1113–1121. doi: 10.1212/wnl.35.8.1113. [DOI] [PubMed] [Google Scholar]
- 14.Wilf-Yarkoni A, Elkayam O, Aizenstein O, et al. Increased incidence of Susac syndrome: a case series study. BMC Neurol. 2020;20:332. doi: 10.1186/s12883-020-01892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triplett JD, Qiu J, O’Brien B, et al. Diagnosis, differential diagnosis and misdiagnosis of Susac syndrome. Eur J Neurol. 2022;29:1771–1781. doi: 10.1111/ene.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Susac JO. Susac’s syndrome. Am J Neuroradiol. 2004;25:351–352. [PMC free article] [PubMed] [Google Scholar]
- 17.Susac JO, Egan RA, Rennebohm RM, Lubow M (2007) Susac’s syndrome: 1975–2005 microangiopathy/autoimmune endotheliopathy. J Neurol Sci 257(1–2):270-272. 10.1016/j.jns.2007.01.036 [DOI] [PubMed]
- 18.Gertner E, Rosenbloom MH. Susac syndrome with prominent dermatological findings and a prompt response to intravenous immunoglobulin, steroids, and rituximab: a case report. J Med Case Rep. 2016;10:137. doi: 10.1186/s13256-016-0917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alshaqi O, Moodie T, Alchaki A. Involuntary crying episodes with Susac’s syndrome—a rare presentation of a rare disease: a case report. BMC Neurol. 2022;22:155. doi: 10.1186/s12883-022-02639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleffner I, Duning T, Lohmann H, et al. A brief review of Susac syndrome. J Neurol Sci. 2012;322:35–40. doi: 10.1016/j.jns.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Saux A, Niango G, Charif M, et al. Susac’s syndrome, a rare, potentially severe or lethal neurological disease. J Neurol Sci. 2010;297:71–73. doi: 10.1016/j.jns.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Roeser MM, Driscoll CLW, Shallop JK, et al. Susac syndrome–a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009;30:34–40. doi: 10.1097/mao.0b013e31818b6ac2. [DOI] [PubMed] [Google Scholar]
- 23.Ohalloran HS, Pearson PA, Lee WB, Susac JO, Berger JR. Microangiopathy of the brain, retina, and cochlea (Susac syndrome). A report of five cases and a review of the literature. Ophthalmology. 1998;105(6):1038–1044. doi: 10.1016/S0161-6420(98)96005-5. [DOI] [PubMed] [Google Scholar]
- 24.Murata Y, Inada K, Negi A. Susac syndrome. Am J Ophthalmol. 2000;129:682–684. doi: 10.1016/s0002-9394(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 25.Egan RA, Hills WL, Susac JO. Gass plaques and fluorescein leakage in Susac Syndrome. J Neurol Sci. 2010;299:97–100. doi: 10.1016/j.jns.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 26.Martinet N, Fardeau C, Adam R, et al. Fluorescein and indocyanine green angiographies in Susac syndrome. Retina Phila Pa. 2007;27:1238–1242. doi: 10.1097/IAE.0b013e31809ff824. [DOI] [PubMed] [Google Scholar]
- 27.Littlewood R, Mollan SP, Pepper IM, Hickman SJ. The utility of fundus fluorescein angiography in neuro-ophthalmology. Neuro-Ophthalmol Aeolus Press. 2019;43:217–234. doi: 10.1080/01658107.2019.1604764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giuffrè C, Miserocchi E, Marchese A, et al. Widefield OCT angiography and ultra-widefield multimodal imaging of Susac syndrome. Eur J Ophthalmol. 2020;30:NP41–NP45. doi: 10.1177/1120672119843281. [DOI] [PubMed] [Google Scholar]
- 29.Azevedo AGB, Lima LH, Müller L, et al. Anatomical and functional correlation in Susac syndrome: multimodal imaging assessment. Int J Retina Vitr. 2017;3:39. doi: 10.1186/s40942-017-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinah Z, Michaella G, Barequet D, et al. Susac’s syndrome—a new ocular finding and disease outcome. Eye. 2021 doi: 10.1038/s41433-021-01464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennebohm R, Susac JO, Egan RA, Daroff RB. Susac’s Syndrome—update. J Neurol Sci. 2010;299:86–91. doi: 10.1016/j.jns.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Susac JO, Murtagh FR, Egan RA, et al (2003) MRI findings in Susac’s syndrome. Neurology 61(12):1783–1787. 10.1212/01.WNL.0000103880.29693.48 [DOI] [PubMed]
- 33.Garg N, Reddel SW, Miller DH, et al. The corpus callosum in the diagnosis of multiple sclerosis and other CNS demyelinating and inflammatory diseases. J Neurol Neurosurg Psychiatry. 2015;86:1374–1382. doi: 10.1136/jnnp-2014-309649. [DOI] [PubMed] [Google Scholar]
- 34.Bellanger G, Biotti D, Adam G, et al. Leptomeningeal enhancement on post-contrast FLAIR images for early diagnosis of Susac syndrome. Mult Scler J. 2021 doi: 10.1177/13524585211012349. [DOI] [PubMed] [Google Scholar]
- 35.Allmendinger AM, Viswanadhan N, Klufas RA, Hsu L. Diffuse cauda equina enhancement in a middle aged male with Susac syndrome and symptomatic cauda equina syndrome. J Neurol Sci. 2013;333(1–2):25–28. doi: 10.1016/j.jns.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Hua LH, Donlon SL, Okuda DT (2014) A case of Susac syndrome with cervical spinal cord involvement on MRI. J Neurol Sci 337(1–2):228–231. 10.1016/j.jns.2013.11.040 [DOI] [PubMed]
- 37.Yahyavi-Firouz-Abadi N, Kiczek M, Zeiler SR, Wasserman BA. Imaging features of Susac syndrome on high-resolution intracranial vessel wall MRI. Neurol Neuroimmunol Neuroinflamm. 2021 doi: 10.1212/NXI.0000000000000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleffner I, Deppe M, Mohammadi S, et al. Neuroimaging in Susac’s syndrome: focus on DTI. J Neurol Sci. 2010;299:92–96. doi: 10.1016/j.jns.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Feresiadou A, Eriksson U, Larsen H-C, et al. Recurrence of Susac syndrome following 23 years of remission. Case Rep Neurol. 2014;6:171–175. doi: 10.1159/000362868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machado S, Jouvent E, Klein I, et al. Cognitive dysfunction and brain atrophy in Susac syndrome. J Neurol. 2020;267:994–1003. doi: 10.1007/s00415-019-09664-8. [DOI] [PubMed] [Google Scholar]
- 41.Do TH, Fisch C, Evoy F. Susac syndrome: report of four cases and review of the literature. AJNR Am J Neuroradiol. 2004;25:382–388. [PMC free article] [PubMed] [Google Scholar]
- 42.Hardy TA, Garsia RJ, Halmagyi GM, et al. Tumour necrosis factor (TNF) inhibitor therapy in Susac’s syndrome. J Neurol Sci. 2011;302:126–128. doi: 10.1016/j.jns.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Jarius S, Neumayer B, Wandinger KP, et al. Anti-endothelial serum antibodies in a patient with Susac’s syndrome. J Neurol Sci. 2009;285:259–261. doi: 10.1016/j.jns.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Zhovtis Ryerson L, Kister I, Snuderl M, et al. Incomplete Susac syndrome exacerbated after natalizumab. Neurol Neuroimmunol Neuroinflamm. 2015;2:e151. doi: 10.1212/NXI.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross CC, Meyer C, Bhatia U, et al. CD8 + T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat Commun. 2019;10(1):5779. doi: 10.1038/s41467-019-13593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mateen FJ, Zubkov AY, Muralidharan R, Fugate JE, Rodriguez FJ, Winters JL, Petty GW (2012) Susac syndrome: clinical characteristics and treatment in 29 new cases. Eur J Neurol 19(6):800–811. 10.1111/j.1468-1331.2011.03627.x [DOI] [PubMed]
- 47.Marie I, Guegan-Massardier E, Levesque H, et al. Susac’s syndrome or retinocochleocerebral vasculopathy: a misdiagnosed and overlooked disorder. Eur J Intern Med. 2000;11:108–111. doi: 10.1016/s0953-6205(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 48.Allmendinger AM, Spektor V, Destian S. CT and MR imaging of Susac syndrome in a young male presenting with acute disorientation. Clin Imaging. 2010;34:138–142. doi: 10.1016/j.clinimag.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Hardy TA, Taylor RL, Qiu J, et al. The neuro-otology of Susac syndrome. J Neurol. 2020;267:3711–3722. doi: 10.1007/s00415-020-10086-0. [DOI] [PubMed] [Google Scholar]
- 50.Pérez PL, McCall AA, Hirsch BE. Scoping review of cochlear implantation in Susac’s syndrome. World J Otorhinolaryngol Head Neck Surg. 2021;7:126–132. doi: 10.1016/j.wjorl.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar K, Jariwala B, Raj P, Agarwal A. Retinal neovascularisation in Susac syndrome: a rare complication. Semin Ophthalmol. 2017;32:492–495. doi: 10.3109/08820538.2015.1122068. [DOI] [PubMed] [Google Scholar]
- 52.Alba-Linero C, Liscombe-Sepúlveda JP, Llorenç V, et al. Use of ultra-wide field retinal imaging and optical coherence tomography angiography in the diagnosis of incomplete Susac syndrome. Eur J Ophthalmol. 2021;31:3238–3247. doi: 10.1177/1120672120965482. [DOI] [PubMed] [Google Scholar]