Abstract
Seed weight, which is highly correlated to seed size, is a critical agronomic trait that determines the yield of Brassica napus. However, there have been limited researches on the genes involved in regulating seed size. In Arabidopsis thaliana, ENHANCER OF DA1 (EOD1), an E3 ubiquitin ligase gene, has been identified as a significant negative regulator in controlling organ size, but the function of its homologs in rapeseed remains unknown. Only two homologous of EOD1, BnaEOD1.A04 and BnaEOD1.C04, have been found in B. napus and were mutated using the CRISPR-Cas9 system. Three T-DNA-free lines, T2-157-1-C8, T2-390-2-B8, and T2-397-2-E2, were identified from the homozygous T2 mutant lines. The BnaEOD1.A04 showed a similar type of editing in these mutants, whereas the BnaEOD1.C04 in T2-397-2-E2 was only missing 26 amino acids, and the translation was not prematurely terminated, which was different from the other two mutants. In parallel, mutation of BnaEOD1s resulted in a noteworthy increase in both seed size and seed weight in the three editing lines. Additionally, there was a significant decline in the number of seeds per silique (SPS) and silique length (SL) in T2-157-1-C8 and T2-390-2-B8, but T2-397-2-E2 did not show any significant changes in the SPS and SL, possibly due to distinct types of editing in the three lines. The above results indicate the conserved function of EOD1 homologs and provides promising germplasm for breeding novel high-yield rapeseed varieties by improving seed size and thousand-seed weight.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-023-01430-z.
Keywords: Brassica napus, Seed weight, CRISPR/Cas9, Ubiquitin ligase, BnEOD1s
Introduction
Rapeseed is widely regarded as one of the most important oil crop worldwide and serves as a vital source of vegetable oil for the human consumption (Wang 2018). To meet the increasing world demand for rapeseed oil, enhancing production has always been a fundamental objective for rapeseed breeding. Seed size and seed weight are highly positively correlated, and augmenting yield per plant through large seeds is a crucial strategy to boost crop productivity (Li et al. 2015). The regulation of seed size has been extensively studied in model plants such as Arabidopsis and rice, in which the ubiquitin–protease pathway is thought to have a crucial role in regulating seed size (Li et al. 2019).
Ubiquitin is a highly conserved small globular protein consisting of 76 amino acids. Protein ubiquitination is an important post-translational modification that impacts protein stability, subcellular localization, molecular interaction, and activity (Li and Li 2014; Li et al. 2019). The ubiquitin-mediated signaling pathway performs a function in various aspects of plant life, particularly in the regulation of seed and organ size (Shu and Yang 2017). The ubiquitin receptor protein DA1 negatively regulates seed size by inhibiting seed epidermal cell division in Arabidopsis and may functionally conserved in B. napus (Li et al. 2008; Wang et al. 2017). Three genes—DA2, ENHANCER OF DA1 (EOD1, also known as BIG BROTHER), and Grain Width 2 (GW2)—all encode a RING type E3 ubiquitin ligase and interact with DA1 in Arabidopsis (Xia et al. 2013). The leaves of eod1 mutant plants exhibited greater area than the WT at early developmental stages, and the lifespan of the mutant leaves was prolonged at later developmental stages. Notably, the phenotypic differences in the da1-1_eod1-2 double mutants were even more striking, indicating that the EOD1 negatively regulates seed and organ size (Vanhaeren et al. 2017). The double mutant da1-1_da2-1 produces petals and seeds that are larger than those of the single mutants and WT, while the eod1_da2 double mutants showed an additive effect on organ size, indicating the independent functions of DA2 and EOD1 in the regulation of organ size (Li et al. 2008; Xia et al. 2013; Dong et al. 2017; Vanhaeren et al. 2017). EOD1 can ubiquitinated DA1 and its closely related family members DAR1 at multiple sites to activate DA1 and DAR1, which disassembles various growth regulators in the plant and regulates seed and organ size (Dong et al. 2017). Meanwhile, DA1 can also destabilize EOD1, indicating that these two genes may act as key nodes in the feedback regulation of the E3 ubiquitin ligase pathway in Arabidopsis (Li et al. 2019). The downregulation of BnDA1 has been shown to significantly increase seed weight and organ size in B. napus (Wang et al. 2017). Although the role of the ubiquitin pathway in the regulation of seed and organ size has been extensively studied in model plants, there are still many unknowns in B. napus.
B. napus (AACC, 2n = 38) is a typical allotetraploid with a complex genome structure, for which only limited genes related to seed size regulation are currently available for use. Previous studies have demonstrated the significant role of EOD1/BB in the regulation of organ size in Arabidopsis, but its functionality in B. napus requires further investigation. In this study, we identified the homologous gene of EOD1 in B. napus using gene sequence BLAST and verified the functions of BnaEOD1s in Bnaeod1s mutant plants that generated using the CRISPR-Cas9 system. In addition, we screened for homozygous mutant lines that was stably inherited and without T-DNA insertion, which will provide promising germplasm for the studying the genetic basis of seed size variation in B. napus and the improvement of yield.
Materials and method
Materials
The spring B. napus Xiaoyun was used for genetic transformation in this study. B. napus plants were cultivated in the growth chamber at 22°C, light 22 h/dark 2 h, humidity 60–70%, the light source was LED (red, green; blue, 6:2:2), and the light intensity was maintained at approximately 500 μmol/m2/s.
Bioinformatics analyses of EOD1 homologous genes in the B. napus genome
Previously reported EOD1 gene sequences of Arabidopsis were used for BLAST in BnGDXY (http://localhost/BnGDXY/index.html). The expression profile of all copies of the BnaEOD1s can be analyzed in BnIR database (http://yanglab.hzau.edu.cn/BnTIR/). CDS and amino acid information were collected from BnGDXY. The molecular weight and theoretical pI were analyzed using ProParam (https://web.expasy.org/cgi-bin/protparam/protparam) (Wilkins et al. 1999). WOLF PSORT (http://www.genscript.com/wolf-psort.html) and ProtComp v. 9.0 (http://linux1.softberry.com/) were employed to predict the subcellular localization of EOD1 proteins in rapeseed (Horton et al. 2007; Tong et al. 2020).
Phylogenetic and sequence feature analysis of EOD1 homologous genes
The full length of EOD1 homologous proteins was collected from NCBI and BnGDXY. The phylogenetic tree of EOD1 homologous proteins was constructed using MEGA 11.0 software (https://www.megasoftware.net/) with the neighbor-joining (NJ) method, and the bootstrap test was performed with 1000 iterations (Li et al. 2021).
The exon–intron organizations of EOD1 homologous genes were presented by GSDS2.0 (http://gsds.cbi.pku.edu.cn/) based on the aligning results of the corresponding genomic DNA sequences. The MEME (http://meme-suite.org/tools/meme) was utilized for identifying the conserved motifs in EOD1 homologous protein. The motif distribution type was set as zero or one occurrence per sequence. The number of motifs was set as 20, and the motif width was between 6 and 50 amino acids (Li et al. 2021).
Vector construction, gene transformation, mutation identification, and phenotypic screening of transgenic plants
Firstly, two single guide RNA (sgRNA) that targeted the CDS sequence of all BnEOD1s was designed through CRISPR-P (http://crispr.hzau.edu.cn/CRISPR2/) (Table S1) (Liu et al. 2017). PCR was performed using primers XX-DT1-BsF, XX-DT1-F0, XX-DT2-R0, and XX-DT2-BsR (Table S1) to construct a DNA fragment containing two sgRNAs (Xing et al. 2014). Subsequently, the DNA fragment was connected to the pSKE401 skeleton vector using Golden Gate reactions to obtain the gene editing vector BnaEOD1-A4/C4-pKSE401 as previously described (Xing et al. 2014). The resultant gene editing vector was introduced into Xiaoyun via Agrobacterium-mediated genetic transformation as previously described by Dai et al. (2020).
To identify the mutant plants, genomic DNA was isolated from the leaves of both transgenic and non-transgenic B. napus plants using the CTAB protocol with minor modification (Doyle 1987). Subsequently, positive transgenic plants were screened using the positive detection primers 505F/505R and U626-IDF/U629-IDR. Only a plant with positive PCR results from both primer pairs was designated as a positive transgenic plant. The identification of gene editing for target gene was then conducted in positive transgenic plants through Sanger and Hi-TOM analyses using primers provided in Table S1 as described by Liu et al. (2019).
The homozygous T2 generation lines were planted in both a greenhouse and the field for phenotype investigation. In the greenhouse, three T2 generation edited lines were planted with 9 plants per line, and 6 WT plants were used as control. The field experiment was conducted at Huazhong Agricultural University, Wuhan, China, where five edited lines were planted in a three-row plot with 8–10 plants per row and 24 cm spacing between lines. Fifteen plants per line were used for the measurement of agronomic traits, including seedling growth status, organ size, plant architecture, SL, SPS, TSW, and seed germination. SL refers to the average length of 10 siliques on the main stem, which was measured and counted for each plant. SPS refers to the average seed number of 10 siliques on the main stem, which was also measured and counted for each plant. TSW was determined by collecting all seeds of each plant and removing impurities and then weighing the seeds on a SC-G seed detector (Hangzhou Wanshen detection technology Co., Ltd.). Curating the raw data, analyzing phenotypic data using a t-test, and creating charts were all performed using WPS Office.
Results
Molecular characterization of EOD1 homologous genes in B. napus
A BLASTN searching was conducted to identify homologous genes of EOD1 in B. napus by using the DNA sequence of AtEOD1 (AT3G63530). It reveals that the rapeseed genome harbors two homologous genes for EOD1: BnaEOD1.A04 (BnaA04G0000200XY) and BnaEOD1.C04 (BnaC04G0257100XY). The CDS sequence for BnaEOD1.A04 and BnaEOD1.C04 is 729bp and 723bp, respectively, and both copies comprise seven exons and six introns, consistent with the AtEOD1 (Table 1; Fig. 1A). The predicted encoded products of BnaEOD1.A04 and BnaEOD1.C04 comprise 242 and 240 amino acids, respectively, and both possess a ZNF domain (Table 1; Fig. 1B–D). Upon comparing the amino acid sequences, it was found that AtEOD1 shared a similarity of 85.9% with BnaEOD1.A04 and 83.7% with BnaEOD1.C04. Additionally, there was a high similarity of 95.1% between BnaEOD1.A04 and BnaEOD1.C04 (Fig. 1B and C).
Table 1.
Physicochemical properties of the EOD1 homologous in the B. napus
| Gene name | Gene ID | CDS (bp) | Amino acids | MW (Da) | PI | Subcellular localization |
|---|---|---|---|---|---|---|
| BnEOD1.A04 | BnaA04G0000200XY | 729 | 242 | 27354.21 | 4.79 | Nucl |
| BnEOD1.C04 | BnaC04G0257100XY | 723 | 240 | 27215.99 | 4.8 | Nucl |
| AtEOD1 | AT3G63530 | 747 | 248 | 27982.6 | 4.47 | Nucl |
Fig. 1.
Gene structures and conserved domain analysis of EOD1s. A Exon–intron structures of AtEOD1 and BnEOD1s. B Amino acid sequence alignment and conserved domain analysis. C Motif locations. D Amino acid sequences of the motif conserved across rapeseed EOD1 proteins. The bigger the font size, the more likely the amino acid is at that location across all EOD1 proteins
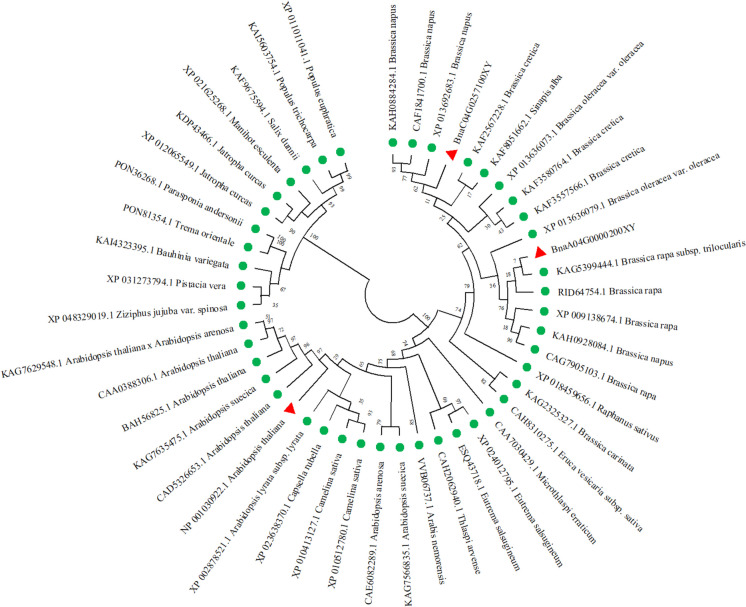
To comprehend the phylogenetic connections among EOD1 proteins in rapeseed and other species, we constructed an unrooted phylogenetic tree comprising members of EOD1 family. As shown in Fig. 2, two copies of BnaEOD1s are found to be closely related with EOD 1 in B. rapa and B. oleracea, respectively.
Fig. 2.
Phylogenetic connections of EOD1s. A total of 47 protein sequences was subjected to multiple sequence alignment in ClustalW prior to constructing a phylogenetic tree using the neighbor-joining method in MEGA 11. The aligned EOD1 proteins originating from A. thaliana and B. napus are marked by red triangles
Implement genetic editing for two BnaEOD1s using CRISPR-Cas9
The expression profile analysis of BnaEOD1s revealed that the expression levels of the two copies of BnaEOD1s were relatively high in the main stem, flower, and the early stages of silique and seed development (Figure S1). This suggests that BnaEOD1s may primarily regulate the developmental processes of seed in B. napus and there is evident functional redundancy between the two copies of BnaEOD1s (Figure S1). In order to validate the role of BnaEOD1s in the regulating seed size in B. napus, we constructed CRISPR-Cas9 target editing for these genes with dual targets (Fig. 3A and B), and then the gene editing vector was introduced into Xiaoyun to generate mutant plants.
Fig. 3.
Schematic diagram of CRISPR-Cas9 vector structure and target site sequence and location. A Structure of pKSE401 vector. B sgRNA sequence and the target site in BnaEOD1s. S1 and S2 are 2 sgRNA sequences, with arrows indicating the direction and the red base for the PAM sequence
A total of 118 T0 positive plants was obtained, generating 59 editing plants with various types of editing at the targeted site of BnaEOD1.A04 and BnaEOD1.C04. Of these, 58 plants underwent simultaneous editing at two copies, mainly involving three editing types: single-base insertion, single-base deletion, and multiple-base deletion (Table S2). T0-149 and T0-293 had the longest base deletions, with both having a 77bp base deletion in the copy on chromosome C4, and their DNA fragments between sgRNA1 and sgRNA2 were all deleted (Table S2). Editing events mostly observed 3–4 bases upstream of the PAM (Fig. 4). Hi-TOM sequencing analysis of target sequences from the T0 edited plants of BnaEOD1s revealed up to six different editing types in the T0-226 plant, which had simultaneous editing events with single-base insertion, single-base deletion, two-base deletion, three-base deletion, four-base deletion, and five-base deletion at the sgRNA2 position of BnaEOD1.A04 (Table S2).
Fig. 4.
Target editing and amino acid sequence analysis of homozygous editing plants for BnaEOD1s. A Analysis of target editing of T1 edited plants of BnaEOD1s. B Amino acid sequence analysis of T2 edited plants. S1 and S2 are sgRNA1 and sgRNA2, respectively; WT is wild-type; a means BnaEOD1.A04 on A4 chromosome; c means BnaEOD1.C04 on C4 chromosome. C Identification of T-DNA free lines. Y127 and H2O, negative control for identification; +, positive control for identification
Subsequently, we conducted in-depth analysis on the editing situation of 36 randomly selected editing lines (Table 2); the results revealed that the probabilities of editing by the two sgRNAs were 75.00% and 97.22% for BnaEOD1.A04. Additionally, the probability of base insertion and base deletion was 72.22% and 11.11% for sgRNA1 and 38.89% and 63.89% for sgRNA2. Similarly, we observed that the probability of editing by the two sgRNAs was 83.33% and 100.00% for BnaEOD1.C04. Furthermore, the probability of base insertion and base deletion was 66.67% and 16.67% for sgRNA1 and 47.22% and 80.56% for sgRNA2.
Table 2.
Statistics of editing events of BnaEOD1s in T0 edited plants
| Gene | sgRNA | GC (%) | NP | Base insertion | Percentage | Base deletion | Percentage | WT | Editing rate |
|---|---|---|---|---|---|---|---|---|---|
| BnaEOD1.A04 | S1 | 52.60% | 36 | 26 | 72.22% | 4 | 11.11% | 9 | 75.00% |
| S2 | 47.40% | 36 | 14 | 38.89% | 23 | 63.89% | 1 | 97.22% | |
| BnaEOD1.C04 | S1 | 52.60% | 36 | 24 | 66.67% | 6 | 16.67% | 6 | 83.33% |
| S2 | 47.40% | 36 | 17 | 47.22% | 29 | 80.56% | 0 | 100.00% |
The results demonstrate that base editing in the T0 editing plants could be efficiently inherited to the next generation, and homozygous mutants with different types of editing were isolated (Fig. 4). The T1 offspring of T0-157, T0-315, T0-390, and T0-397 lines were used for selecting homozygous edited lines. The descendants of T0-157 presented two types of single-base insertion at the sgRNA1 position of BnaEOD1.C04, one with the insertion of adenine (A) and the other with thymine (T). Similarly, the offspring of T0-315 had two types of single-base insertion at the sgRNA1 position of BnaEOD1.C04, with insertion of cytosine (C) and thymine (T), respectively. They also presented two types of multi-base deletion at the sgRNA2 target site, with a deletion of 2 bases and 12 bases, respectively. The descendants of T0-390 presented two types of multi-base deletion at the sgRNA2 target site of BnaEOD1.C04, with deletion of 2 bases and 15 bases, respectively. The offspring of T0-397 isolated two types of single-base insertion with one guanine (G) and one adenine (A) at the sgRNA1 position of BnaEOD1.A04, while a new type of editing was isolated in the offspring of T0-397, with a 78-base deletion between sgRNA1 and sgRNA2, possibly due to the CRISPR-Cas9 tool still works in the edited plants (Fig. 4A).
Knock-out of BnaEOD1 by CRISPR-Cas9 significantly enhanced the seed size and TSW
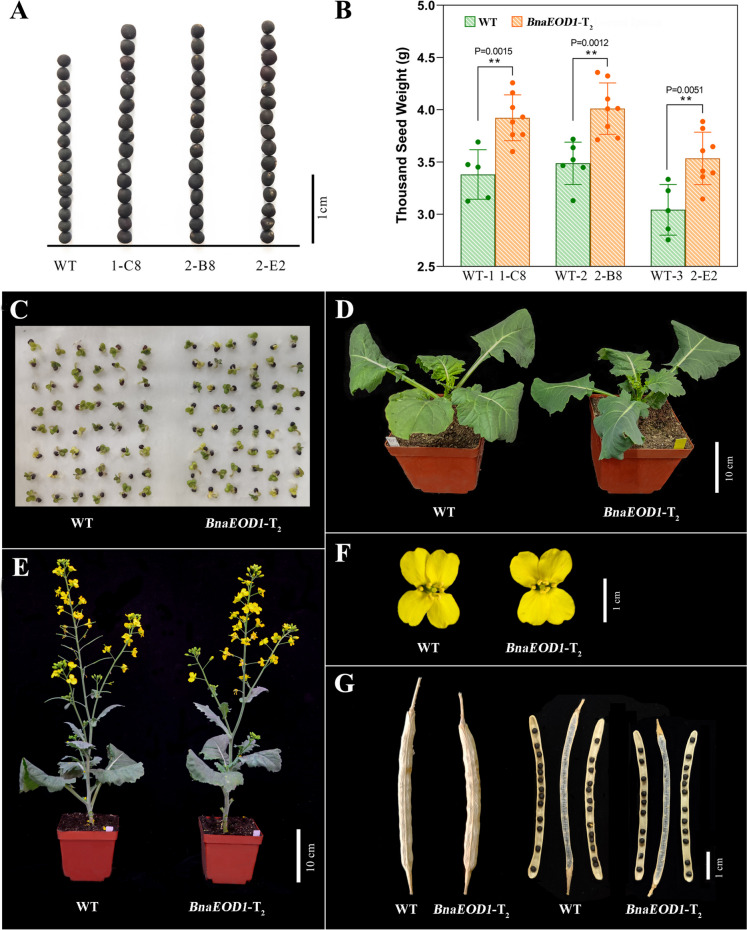
To obtain stable inheritable mutant lines, homozygous mutant lines without T-DNA insertion were screened from 27 T2 edited plants. Three T-DNA-free plants (T2-157-1-C8, T2-390-2-B8, and T2-397-2-E2) with significant mutations in the BnaEOD1.A04 and BnaEOD1.C04 were successfully obtained from these homozygous T2 mutants (Fig. 4B and C). Subsequently, the three homozygous edited lines were further examined for their phenotype. Notably, all three homozygous edited lines exhibited significantly larger seeds and an increased TSW compared with WT (Fig. 5A and B). Specifically, the TSW of three edited lines was approximately 15.98% (P = 0.0015), 14.90% (P = 0.0012), and 16.12% (P = 0.0051) higher than WT, respectively (Table 3; Fig. 5B).
Fig. 5.
Phenotypic analysis of BnEOD1 edited lines. A Seed size; B TSW; C–G represent results for germination of seeds, plant architectural at seedling, and the flowering stage, flowers, and silique. Bar represents 10 cm in D and E. Bar represent 1 cm in A, F, and G. 1-C8, 2-B8, and 2-E2 represents T2-157-1-C8 and T2-390-2-B8 and T2-397-2-E2, respectively. WT-1, WT-2, and WT-3 represent the wild-type plants that were cultivated under the same environment as the mutant lines they compared, but these WT plants were planted in different pots
Table 3.
Phenotype data of T2 edited plants for BnaEOD1s in greenhouse
| Lines | NP | TSW (g) | SPS | SL (mm) |
|---|---|---|---|---|
| WT-1 | 5 | 3.38 ± 0.21 | 25.90 ± 3.22 | 59.30 ± 4.43 |
| T2-157-1-C8 | 8 | 3.92 ± 0.21** | 18.78 ± 2.75** | 50.77 ± 2.24*** |
| WT-2 | 6 | 3.49 ± 0.19 | 28.10 ± 1.32 | 65.52 ± 1.48 |
| T2-390-2-B8 | 8 | 4.01 ± 0.23** | 16.73 ± 2.34**** | 54.53 ± 3.71*** |
| WT-3 | 5 | 3.04 ± 0.22 | 22.27 ± 5.39 | 56.63 ± 3.97 |
| T2-397-2-E2 | 8 | 3.53 ± 0.23** | 22.52 ± 2.01ns | 61.13 ± 2.50* |
The statistical value is the mean ± t-test standard deviation; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.WT-1, WT-2, and WT-3 represent the wild-type plants that were grown under the same environment as the mutant lines they compared, but these WT plants were cultivated in different pot
Alongside the significant alterations in seed size and TSW, other agronomic traits closely related to B. napus development, including seed germination, cotyledons, true leaves, flowers, and plant architectural, were non-significant differences with WT (Fig. 5C–F). However, two edited lines (T2-157-1-C8 and T2-390-2-B8) exhibited notably shorter and wider silique, along with considerably lower SPS compared with WT (Table 3; Fig. 5G). Specifically, SL was reduced by 14.38% and 16.77%, and SPS was reduced 27.39% and 40.46%, respectively. In contrast, the edited line T2-397-2-E2 did not show any significant change in SPS (Table 3). This disparity in outcomes may have resulted from distinct types of editing in the three lines, whereby the encoded product of BnaEOD1.C04 in T2-397-2-E2 missing only 26 amino acids and the translation was not prematurely terminated, possibly retaining partial gene function (Fig. 4B).
We also conducted a phenotype comparison of five homozygous T3 editing lines and WT under field conditions. Consistent with previous results in greenhouse, all five edited lines displayed larger seeds and a noticeable increase in TSW while also showing shorter silique and fewer SPS (Table 4). In comparison to WT, the TSW of these edited lines experienced a significant uplift ranging from 0.72 g to 1.16 g (Table 4).
Table 4.
Phenotypic data of T3 homozygous edited lines for BnaEOD1s under field
| Lines | NP | TSW (g) | SPS | SL (mm) |
|---|---|---|---|---|
| WT | 15 | 3.84±0.26 | 19.35±1.45 | 58.51±1.38 |
| Bnaeod1-1 | 15 | 4.71±0.43**** | 16.58±2.71** | 53.67±3.55**** |
| Bnaeod1-2 | 15 | 5.00±0.48**** | 16.34±2.96** | 55.36±2.99** |
| Bnaeod1-3 | 15 | 4.56±0.63*** | 14.16±3.21**** | 53.69±3.24**** |
| Bnaeod1-4 | 15 | 4.60±0.65*** | 14.86±2.57**** | 54.13±1.99**** |
| Bnaeod1-5 | 15 | 4.71±0.71*** | 13.09±2.28**** | 52.42±2.17**** |
The statistical value is the mean ± t-test standard deviation; ns, no significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Bnaeod1-1, Bnaeod1-2, and Bnaeod1-3 are the offsprings of T2-157-1-C8; Bnaeod1-4 and Bnaeod1-5 are the offsprings of T2-390-2-B8
Discussion
Seed size shows a strong correlation with the TSW, which is a major determinant of crop yield. Extensive research has been conducted on the regulatory mechanisms of seed size in model plants like rice and Arabidopsis. EOD1 is recognized as a key negative regulator in controlling organ size in Arabidopsis thaliana, while investigations into the function and regulatory mechanisms of this gene in rapeseed are lacking (Dong et al. 2017; Vanhaeren et al. 2017). In this study, we identified two EOD1 homologous genes in B. napus through sequence homology BLAST, namely, BnaEOD1.A04 (BnaA04G0000200XY) and BnaEOD1.C04 (BnaC04G0257100XY). Evaluation of the gene and protein sequences proved that these homologous copies shared the same gene structure and exhibited significant sequence similarity with AtEOD1 (Fig. 1). Moreover, the proteins encoded by these copies also displayed very similar physicochemical properties and subcellular localization (Table 1). Additionally, transcriptome data indicated highly similar expression profiles of the two homologous copies (Figure S1, Liu et al. 2021, http://yanglab.hzau.edu.cn/). These findings suggest that the function of BnEOD1s may be conserved with AtEOD1, without any evident sub-functionalization between the two copies.
The CRISPR-Cas9 system has demonstrated high efficacy as a gene editing tool in B. napus, leading to the creation of numerous new rapeseed accessions (Zhai et al. 2020; Zhang et al. 2019; Wu et al. 2020; Khan et al. 2021). In order to investigate the function of BnaEOD1s in B. napus, we developed a double sgRNA editing vector using the pSKE401 vector to simultaneously edit BnaEOD1.A04 and BnaEOD1.C04. Assays of the editing events demonstrated that the vector successfully achieved simultaneous and efficient editing of two copies in B. napus, with single-copy editing being detected in only one T0 generation line (T0-110) (Table S2). Furthermore, the editing efficiency of sgRNA2 was found to be higher than that of sgRNA1 in all T0 generation editing plants. The editing rates of sgRNA2 are against two homologous copies being 97% and 100%, while sgRNA1 targeted two homologous copies at 75% and 83%, respectively. Additionally, the types of editing occurring at the two sgRNA target sites were significantly different. Base insertions were more frequent at the sgRNA1 target site, while base deletions are more frequent at the sgRNA2 target site. These results indicate that sgRNA1 and sgRNA2 exhibits distinct differences in editing efficiency and preference for editing types. However, it is still unclear whether these differences are related to the vector or the position of the sgRNA in the vector.
Through our comprehensive breeding platform, we have successfully obtained T2 and T3 generation lines with homozygous mutations in BnaEOD1.A04 and BnaEOD1.C04. Phenotype analysis has demonstrated that the loss of function in BnaEOD1s leads to seed enlargement, indicating that BnaEOD1s play a negative role in regulating seed size in B. napus. Additionally, we have discovered that mutations in BnaEOD1s also have a significant impact on various important agronomic traits, including shorter silique and lower SPS. The phenotype of silique in the mutants varies depending on the types of editing, with lines edited to have premature termination of amino acid translation showing more dramatic phenotypic changes than lines edited for amino acid deletion (Fig. 4, Table 3). Previous research in Arabidopsis has shown that loss of function mutations in EOD1 result in smaller organs like flowers and leaves (Li et al. 2008). However, our study in B. napus did not find any significant difference in the size of other organs. These results imply that BnaEOD1s might have distinct functional differentiation from AtEOD1, even though they still maintain their conserved function in regulating seed and silique size. Moreover, the function of BnaEOD1s seems to be dependent on the sequences beyond exon 5, and different types of mutations in this region distinctly affect the function of BnaEOD1s. Furthermore, we have managed to acquire T2 and T3 generation mutants with a diverse array of homozygous mutations in BnaEOD1.A04 and BnaEOD1.C04 within a mere year. This remarkable achievement was accomplished through the efficient utilization of both gene editing and comprehensive breeding platform. Ultimately, our success serves as a testament to the immense potential of combining these innovative techniques and their ability to generate new germplasm for future breeding endeavors.
In conclusion, our study has demonstrated that BnaEOD1s play a vital role in regulating the growth and development of seed and silique. However, further investigation is required to elucidate the precise regulatory mechanisms of these genes in B. napus. Furthermore, we have successfully identified T-DNA-free homozygous edited mutants from the T2 generation lines, which will serve as excellent material for future research on the genetic basis influencing seed size variation and enhancing yield in rapeseed.
Supplementary information
(DOCX 29 kb)
Abbreviations
- B. napus
Brassica napus
- WT
wild-type
- TSW
thousand-seed weight
- T-DNA
transfer DNA
- SL
silique length
- SPS
number of seeds per silique
- ZNF
RING type zinc finger
- CDS
coding sequence
- MW
molecular weight
- PI
isoelectric point
- Nucl
nuclear
- NP
number of plants used for phenotype analysis
- NS
no significant difference
Author contribution
Material preparation, data collection, and analysis were performed by J.C. The first draft of the manuscript was written by J.G., and all authors commented on previous versions of the manuscript. D.H. participated in research design and manuscript proof. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD1200400) and the National Natural Science Foundation of China (32072099, 31971977)
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Dai C, Li Y, Li L, Du Z, Lin S, Tian X, Li S, Yang B, Yao W, Wang J, Guo L, Lu S (2020) An efficient agrobacterium-mediated transformation method using hypocotyl as explants for Brassica napus. Mol Breeding 40:96. 10.1007/s11032-020-01174-0
- Dong H, Dumenil J, Lu FH, Na L, Vanhaeren H, Naumann C, Klecker M, Prior R, Smith C, McKenzie N, Saalbach G, Chen L, Xia T, Gonzalez N, Seguela M, Inze D, Dissmeyer N, Li Y, Bevan MW. Ubiquitylation activates a peptidase that promotes cleavage and destabilization of its activating E3 ligases and diverse growth regulatory proteins to limit cell proliferation in Arabidopsis. Genes Dev. 2017;31(2):197–208. doi: 10.1101/gad.292235.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15. 10.2307/4119796
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:585–587. doi: 10.1385/1-59259-584-7:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MHU, Hu LM, Zhu MS, Zhai YG, Khan SU, Ahmar S, Amoo O, Zhang KP, Fan CC, Zhou YM. Targeted mutagenesis of EOD3 gene in Brassica napus L. regulates seed production. J Cell Physiol. 2021;236(3):1996–2007. doi: 10.1002/jcp.29986. [DOI] [PubMed] [Google Scholar]
- Li J, Lin KY, Zhang S, Wu J, Fang YJ, Wang YP. Genome-wide analysis of myeloblastosis-related genes in Brassica napus L. and positive modulation of osmotic tolerance by BnMRD107. Front Plant Sci. 2021;12:678202. doi: 10.3389/fpls.2021.678202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Li YH. Ubiquitin-mediated control of seed size in plants. Front Plant Sci. 2014;5:332. doi: 10.3389/fpls.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Peng W, Shi JQ, Wang XF, Liu GH, Wang HZ. The natural variation of seed weight is mainly controlled by maternal genotype in rapeseed (Brassica napus L.) PLoS One. 2015;10:e0125360. doi: 10.1371/journal.pone.0125360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Xu R, Li YH. Molecular networks of seed size control in plants. Annu Rev Plant Biol. 2019;70:435–463. doi: 10.1146/annurev-arplant-050718-095851. [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng L, Corke F, Smith C, Bevan MW. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev. 2008;22(10):1331–1336. doi: 10.1101/gad.463608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Yu L, Wei L, Yu P, Wang J, Zhao H, Zhang Y, Zhang S, Yang Z, Chen G, Yao X. BnTIR: an online transcriptome platform for exploring RNA-seq libraries for oil crop Brassica napus. Plant Biotechnol J. 2021;19:1895–1897. doi: 10.1111/pbi.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ding YD, Zhou YQ, Jin WQ, Xie KB, Chen LL. CRISPR-P 2.0: an improved CRISPR/Cas9 tool for genome editing in plants. Mol Plant. 2017;10(3):530–532. doi: 10.1016/j.molp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang C, Jiao XZ, Zhang HW, Song LL, Li YX, Gao CX, Wang KJ. Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci China Life Sci. 2019;62(1):1–7. doi: 10.1007/s11427-018-9402-9. [DOI] [PubMed] [Google Scholar]
- Shu K, Yang W. E3 ubiquitin ligases: ubiquitous actors in plant development and abiotic stress responses. Plant Cell Physiol. 2017;58(9):1461–1476. doi: 10.1093/pcp/pcx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Walk TC, Han P, Chen L, Shen X, Li Y, Gu C, Xie L, Hu X, Liao X, Qin L. Genome-wide identification and analysis of high-affinity nitrate transporter 2 (NRT2) family genes in rapeseed (Brassica napus L.) and their responses to various stresses. BMC Plant Biol. 2020;20(1):464. doi: 10.1186/s12870-020-02648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaeren H, Nam YJ, De Milde L, Chae E, Storme V, Weigel D, Gonzalez N, Inzé D. Forever young: the role of ubiquitin receptor DA1 and E3 ligase BIG BROTHER in controlling leaf growth and development. Plant Physiol. 2017;173(2):1269–1282. doi: 10.1104/pp.16.01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HZ. New-demand oriented oilseed rape industry developing strategy. Chin J Oil Crop Sci. 2018;40(5):613–617. [Google Scholar]
- Wang JL, Tang MQ, Chen S, Zheng XF, Mo HX, Li SJ, Wang Z, Zhu KM, Ding LN, Liu SY, Li YH, Tan XL. Down-regulation of BnDA1, whose gene locus is associated with the seeds weight, improves the seeds weight and organ size in Brassica napus. Plant Biotechnol J. 2017;15(8):1024–1033. doi: 10.1111/pbi.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Yan GB, Duan ZQ, Wang ZJ, Kang CY, Guo L, Liu KD, Tu JX, Shen JX, Yi B, Fu TD, Li X, Ma CZ, Dai C. Roles of the Brassica napus DELLA protein BnaA6.RGA, in modulating drought tolerance by interacting with the ABA signaling component BnaA10.ABF2. Front Plant Sci. 2020;11:577. doi: 10.3389/fpls.2020.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Li N, Dumenil J, Li J, Kamenski A, Bevan MW, Gao F, Li YH. The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis. Plant Cell. 2013;25(9):3347–3359. doi: 10.1105/tpc.113.115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014;14:327. doi: 10.1186/s12870-014-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai YG, Yu KD, Cai SL, Hu LM, Amoo O, Xu L, Yang Y, Ma BY, Jiao YM, Zhang CF, Khan MHU, Khan SU, Fan CC, Zhou YM. Targeted mutagenesis of BnTT8 homologs controls yellow seed coat development for effective oil production in Brassica napus L. Plant Biotechnol J. 2020;18(5):1153–1168. doi: 10.1111/pbi.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Nie LL, Cheng QQ, Yin YT, Chen K, Qi FY, Zou DS, Liu HH, Zhao WG, Wang BS, Li MT. Effective editing for lysophosphatidic acid acyltransferase 2/5 in allotetraploid rapeseed (Brassica napus L.) using CRISPR/Cas9 system. Biotechnol Biofuels. 2019;12:225. doi: 10.1186/s13068-019-1567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 29 kb)
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.