Abstract
Objective
The aim of the study is to describe the radiological spectrum of appearances of ovarian lymphoma (OL). The manuscript describes the radiological aspects of OL to assist the radiologist in achieving correct orientation of the diagnosis.
Methods
We conducted a retrospective evaluation of imaging studies of 98 cases of non-Hodgkin’s lymphoma, with extra-nodal localisation (ovaries) in three cases (1 primary, 2 secondary). A literature review was also performed.
Results
Of the three evaluated women, one had a primary ovarian involvement and two had a secondary ovarian involvement. The most common lesion characteristics were a well-defined, solid homogeneous and hypoechoic mass at US. CT depicts OL as a well-defined, non-infiltrating, homogeneous hypodense solid mass, with mild contrast enhancement. On T1-weight MRI, OL appears as a homogeneous mass of low signal intensity, which enhances avidly following intravenous gadolinium.
Conclusion
Clinical and serological presentation of OL can be similar to that of primary ovarian cancer. As imaging plays a central role in the diagnosis of OL, the radiologist should be familiar with US, CT and MRI appearances of this condition to correctly orient the diagnosis and so avoid unnecessary adnexectomy.
Keywords: Ovarian lymphoma, Ovarian neoplasms, Lymphoma, Lead vessel
Introduction
Ovarian involvement by malignant lymphoma occurs with a frequency of 7–26%, with up to 25% of women dying with lymphomas having ovarian infiltration at autopsy [1]. A normal ovary contains no lymphoid tissue, and it has been suggested that chronic inflammation (e.g. endometriosis, pelvic inflammatory disease) [8] can predispose women to the development of primary OL, which presumably originates from lymphocytes in supplying blood vessels, dispersed in ovarian stroma, within ovarian follicles and related to the corpus luteum. Involvement of the ovary in the lymphomatous process can occur in two ways, either primary or secondary. Frequently, the ovarian mass is a manifestation of a widely disseminated lymphomatous disease, whereas the initial clinical manifestation of an occult lymphoma as an ovarian mass is uncommon [2, 3]. Primary OL is rare, accounting for only 0.5% of all non-Hodgkin’s lymphomas (NHL), and 1.5% of all ovarian neoplasms [4]. Clinical presentation is non-specific (abdominal pain and distension), and physical examination may reveal a pelvic mass [3]. Imaging techniques such as transvaginal sonography (TVS), CT and MR are usually performed in the work-up of women with abdominal complaints [2]. A proper differential diagnosis between OL and primary ovarian cancer is essential for treatment, because urgent chemotherapy is the optimal treatment for the former, while surgical resection with debulking is the treatment of choice for the latter [3–7].The aim of this work is to describe clinical and ultrasound features of patients with OL. A review of the literature is also provided. We hope that this article will familiarise specialists with the US, CT and MRI appearances of OL, and assist them in correctly orienting the diagnosis.
Clinical features
Clinical presentation of OL can be similar to those of more usual ovarian cancers [3] or other ovarian metastasis [9]. OL may occur at any age, but mostly women in their 40 s are affected. The most common histological types in NHL are diffuse large B-cell lymphoma, follicular lymphoma and Burkitt lymphoma [9]. Symptoms such as abdominal pain and distension, a pelvic mass revealed by physical examination, and serology can be misleading because they are common but non-specific for ovarian epithelial tumours [5, 10]. Serum CA-125 levels are elevated in ovarian and uterine cancers, and in many benign diseases with peritoneal serosal involvement (endometriosis, pelvic inflammation, ovarian cysts, fibroids and pregnancy) [5, 11]. Similarly, this marker is elevated in lymphomas, with 43% of patients with NHL having high CA-125 levels [5, 12]. Some authors propose the dosage of CA-125 as a prognostic factor in NHL, with high levels correlating with advanced disease, poor response to treatment and poor survival rates [13, 23].
The dosage of serum LDH may be helpful in discriminating between diagnoses because elevated LDH levels are often found in lymphoma, but not in ovarian cancers [13].
Methods
Women who were referred over a period of about 2 years to the Department of Obstetrics and Gynecology at the University of Sant’Orsola Hospital in Bologna for the evaluation of a gynaecological mass were included in the study. The patients were evaluated and managed by the Department of Obstetrics and Gynecology, and were referred to our department for imaging studies (US, CT, MRI, PET-CT).
Patients who agreed to participate to the study gave written informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients underwent biopsy, and histological diagnoses were obtained.
After consent and diagnoses were established, we retrospectively analysed all the 98 images (US, CT, MRI) and found 1 case of primary ovarian lymphoma and 2 cases of secondary ovarian involvement.
Results
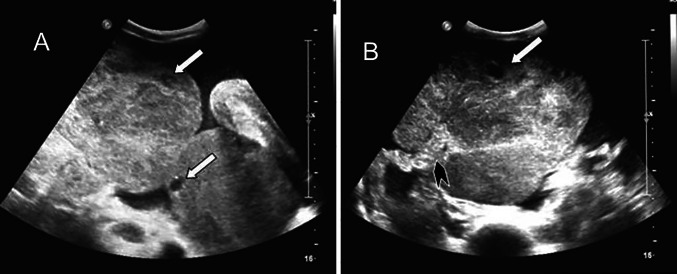
We documented a case of occult NHL (Burkitt type) in a 44-year-old patient presenting with abdominal pain, distension and bilateral solid ovarian masses. A Blumberg test was negative. Serological tests showed LDH (2590 U/L; n.v. 230-480 U/L) and CA 125 (442 U/mL; n.v. < 35). Sovrapubic US showed small anechoic areas of cystic appearance lining the periphery of the solid ovarian masses, indicative of preserved ovarian follicles. Colour Doppler TV US showed a left ovarian mass with the ‘lead vessel’ sign: a main vessel with many thinner branching vessels entering from the periphery to the core of the mass; two anechoic follicles were visible at the periphery of the mass (Figs. 1, 2, 3).
Fig. 1.
A 44 years old woman with occult NHL (Burkitt type) presenting with abdominal pain and distension, and bilateral solid ovarian masses (same patient as Fig. 1). Sovrapubic US shows small anechoic areas of cystic appearance lining at the periphery of the ovarian solid masses, indicative of preserved ovarian follicles (A, B). The vascular pedicle is clearly visible entering from the border to the core of the ovarian mass (B). (White arrow: ovarian follicle. Black arrowhead: vascular pedicle)
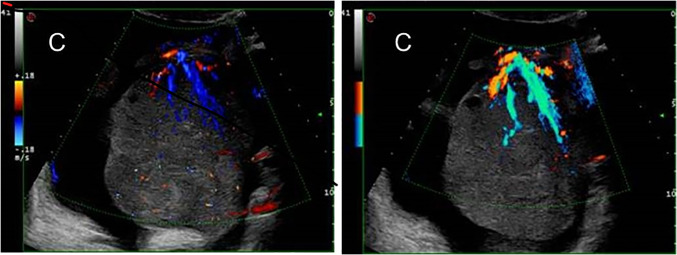
Fig. 2.
TV Colour and Power Doppler TVS clearly depict the main vessel (the “lead vessel”) entering from the periphery to the centre of the ovarian mass, with many branching vessels of thinner width. (White arrow: ovarian follicle)
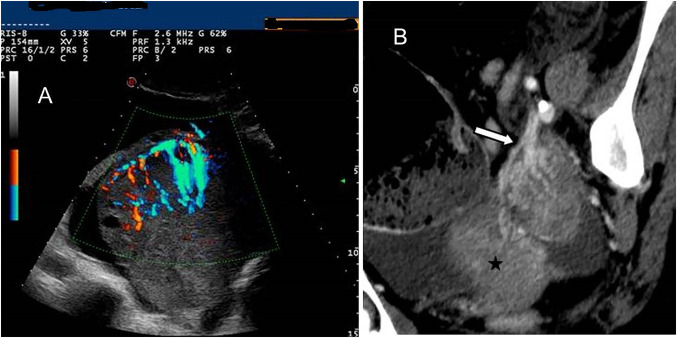
Fig. 3.
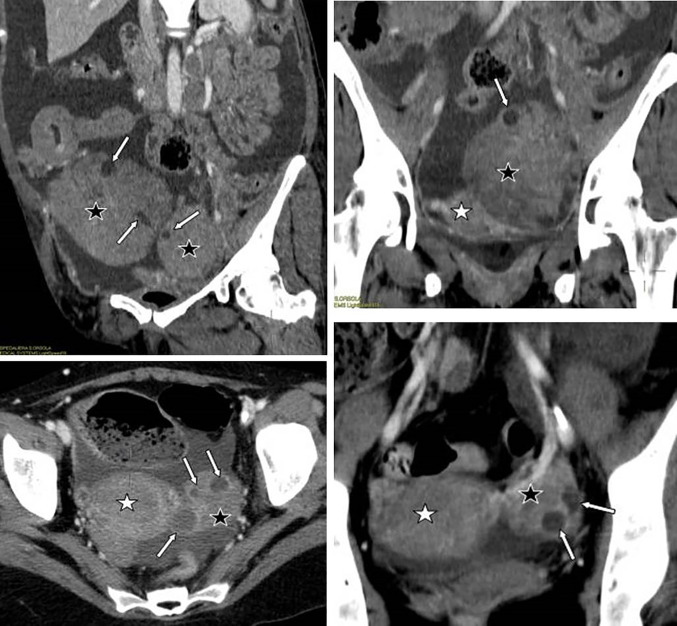
Colour Doppler TV US shows a left ovarian mass with the “lead vessel” sign: a main vessel with many thinner branching vessels entering from the periphery to the core of the mass; two an-hecoic follicles are visible at the periphery of the mass (A). Contrast enhanced oblique reformatted CT image (B) depicts the left ovarian mass and his vascular pedicle at the edge (white arrow): a tree-shaped main vessel with thinner branching vessels goes from the edge through the centre of the mass (the “lead vessel”). (Black star: uterus)
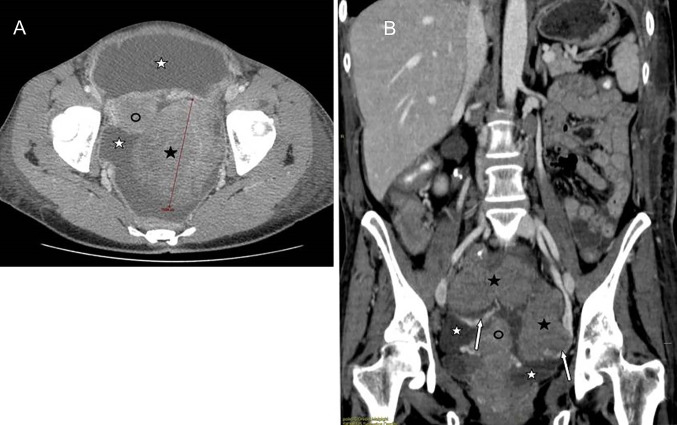
The second case showed a secondary ovarian involvement (primary gastric adenocarcinoma) in a 45-year-old woman with peritoneal and ovarian high-grade NHL presenting with ascites and bilateral ovarian masses. All the serological exams were normal (LDH 352 U/L, CA125 39U/L), with a moderate increase in ESR, leukopenia and thrombocytopenia. At contrast-enhanced CT, the ovarian masses appeared as well-defined, non-infiltrating lobulated hypodense solid lesions, with a homogeneous structure and with mild contrast enhancement (Figs. 4, 5).
Fig. 4.
A 45 years old woman with peritoneal and ovarian high grade NHL presenting with ascites and bilateral ovarian masses. Contrast enhanced CT, axial (A) and coronal (B) images: the ovarian masses (black star) appear as well-defined lobulated hypodense solid lesions, with a homogeneous structure, non-infiltrating, with mild contrast enhancement. Coronal reformatted image (B) depicts the vascular pedicles of both masses (white arrows). Ascites (white star): hypodense fluid circumscribing ovarian masses and uterus (black circle)
Fig. 5.
Lymphoma involves ovaries but preserves their normal structure (images from different patients having ovarian lymphomatous involvement from NHL): contrast enhanced CT shows monolateral or bilateral solid omogeneous ovarian masses (black star) with some centimetric cystic areas in a linear arrangement in the periphery, corresponding to anechogenic cysts at US, and referred to preserved ovarian follicles in the cortex. (White star: uterus)
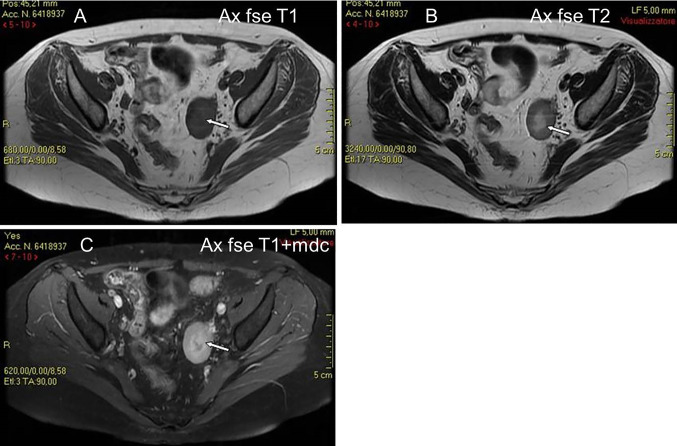
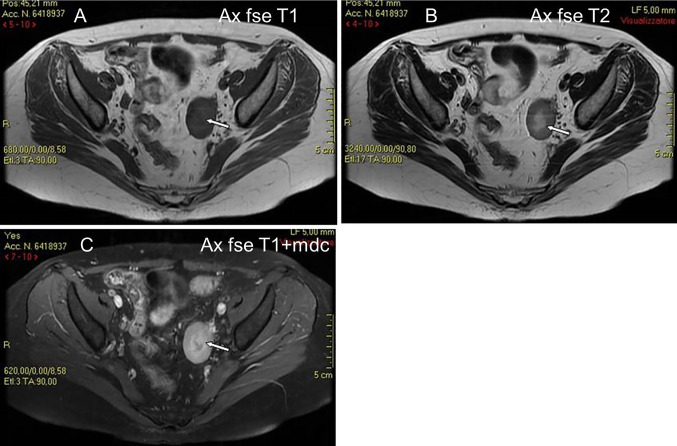
The last case was one of secondary ovarian involvement in a 32-year-old woman with NHL (DLBC type) that relapsed to the left ovary (ovarian, pancreatic and renal involvement of a disseminated lymphoma in progression). Serological exams showed leukocytosis (23,000/mm/c), neutrophilia (neutrophils 96%), amylase (724 U/L), lipase (510 U/L), PCR (20,30) and LDH (1323U/L). TVS showed a well-defined, solid hypoechoic mass with a central area of higher echogenicity. Contrast-enhanced axial and coronal reformatted CT images showed a well-defined left ovarian mass with smooth borders, mild enhancement and a central area of hypodensity, but with no contrast enhancement, indicative of necrosis (Fig. 6).
Fig. 6.
A 32 years old woman with NHL (DLBC type) relapsed to the left ovary (same patient as Fig. 6). MR shows a solid ovarian mass of low signal intensity on T1W images (A), mildly low signal intensity on T2W images (B), and with mildly high signal intensity on gadolinium enhanced T1W images (C). The mass has a central area of low signal intensity on T1W images (A) and mildly high signal intensity on T2W images (B), and without enhancement on gadolinium T1W images (C) (white arrows), suggestive of necrotic tissue
Discussion
Non-Hodgkin’s lymphoma in the female genital tract is unusual. The ovary is the most common site for NHL in the gynaecological tract, either as a primary neoplasm, or as a secondary involvement in a systemic NHL [26].
Our main results show that whenever an ovarian mass is discovered, it is important to take into account the differential diagnosis between OL and another primary ovarian neoplasm, to avoid unnecessary surgery.
We reported one case of occult NHL (Burkitt type) where colour Doppler TV US showed a left ovarian mass with the ‘lead vessel’ sign, a case of secondary ovarian involvement (primary gastric adenocarcinoma) in which ovarian masses appeared as non-infiltrating, well-defined lobulated hypodense solid lesions with mild contrast enhancement at contrast-enhanced CT, and one case of secondary ovarian involvement NHL (DLBC type) that relapsed to the left ovary. CT images showed a well-defined left ovarian mass with smooth borders, mild enhancement and a central area of hypodensity, but with no contrast enhancement, indicative of necrosis.
Sovrapubic and transvaginal US represent the first diagnostic step in the work-up for pelvic and abdominal complaints (Table 1). Ovarian masses identified by US are further characterised by CT and/or MR. 18F-FDG PET/CT is routinely performed for staging and assessment of therapeutic response in systemic lymphoma, and it seems to be useful in the management of OL as well [14], but literature is still scarce [25].
Table 1.
Clinical and ultrasound features of cases of lymphoma involvement of the ovary for the literature review
| References | N° | Age | Subtype | Homogeneous | Lead vessels | LDH | CA 125 |
|---|---|---|---|---|---|---|---|
| A. Crasta et al. | 44 | NHL(Stage IV) DLCL | Yes | – | – | – | – |
| J Crawshaw et al. | 1 | 28 | NHL Burkitt Type | Yes | – | 905U/L | 1111U/ml |
| A.C. Testa, Mancari et al. | 31 | Median 56 | – | Yes | Yes | – | – |
| A.C. Testa, Ferrandina et al. | 67(3 lymphoma) | – | – | Yes | – | – | – |
| F. Ferrozzi et al. | 8 | 13 to 70 | 6 diffuse lymphomas, 2 large cell follicular lymphomas | Yes | Yes | – | – |
US performed with transabdominal and transvaginal probes has demonstrated an accuracy of up to 90% in the evaluation of ovarian masses [16]. The appearance of OL at TVS is that of a well-defined, solid, homogeneous and hypoechoic mass, with through-transmission of sound [5, 17, 18]. The prevalence of solid morphology has been reported to be higher in lymphoma and in metastatic versus primary ovarian carcinomas [5, 17, 18]. Some authors have described the presence of small anechoic areas of cystic appearance lined at periphery of ovarian solid masses [5, 19]. Mitsumori et al. [19] proved that the structure of the cortex was well preserved in the resected specimen of the ovary, and that the small cysts in a linear arrangement at the periphery of the ovarian mass were ovarian follicles in the cortex. In fact, in the process of growth, lymphoma involves the ovary but preserves its normal structure. At colour Doppler, US masses are highly vascularised, with a Doppler score of 4 [10, 17]. Sovrapubic US may detect a pedicle entering from the periphery to the centre of the ovarian mass. Colour Doppler TVS better depicts this pedicle as a main vessel with many branching vessels of thinner width (Fig. 1, 2). This tree-shaped vessel has been described by Testa et al. [20] as the ‘lead vessel’. It has a prevalence of 35.4% in solid metastatic ovarian tumours and of 100% in lymphomatous ovarian metastases, while it has been identified in only 0.01% of primary ovarian tumours. It has been proposed that this vessel can be the pedicle of the ovary (hardly visible in a normal ovary) or a result of a neoangiogenetic process of the tumour, appearing more commonly in solid ovarian metastasis (from stomach cancer, breast, lymphoma and uterine cancer) [20]. In some cases, we have seen the ‘lead vessel’ in OL by means of contrast-enhanced CT with multiplanar reformatted imaging (Fig. 3).
At CT scan, with mild contrast enhancement, ovarian lymphomas appear as well-defined, non- infiltrating, homogeneous and hypodense solid masses. Some centimetric cystic areas at the periphery of the tumours have been described, and referred to as ovarian follicles [10] (Figs. 4, 5).
At MRI evaluation of T1-weighted images, OL appears as a homogeneous mass of low signal intensity, greatly enhanced following intravenous gadolinium [5, 10, 19, 21]. On T2-weighted MR imaging, OL appears as a solid mass with intermediate signal intensity and hyperintense septa, showing moderate to marked enhancement on gadolinium-enhanced T1-weighted images [5, 10, 19].
In our study, TVS showed a well-defined, solid hypoechoic mass with a central area of higher echogenicity, which appeared hypodense and without contrast enhancement on CT. On MR imaging, the mass showed a central zone of low signal intensity on T1-weighted images and mildly high signal intensity on T2-weighted images, without enhancement on gadolinium T1-weighted images (Fig. 6) [22]. We referred this finding to a central zone of necrosis, which is a possible but unusual feature of lymphoma. Several necrotic centres on US and MR imaging were described by Weingertner et al. [6] in a case of primary OL. MR is better able to visualise the ovarian follicles at the periphery of the tumour on T2-weighted MR images than US and CT imaging [5, 19]. In addition, multiplanar CT and MR imaging shows that lesions have a cleavage fat plane preserved from surrounding structures.
The radiological aspects that are indicative of lymphomatous ovarian involvement and have clinical implications in our study are the presence of solid homogeneous ovarian masses, which have well-defined profiles and are cleaved from surrounding structures; mild-to-high vascularisation with arborised vessels on colour Doppler US (‘lead vessel sign’) and contrast-enhanced CT with multiplanar imaging; mild-to-high enhancement after administration of contrast material on CT and MR; and a pattern of growth that involves the ovary but preserves its normal structure, with evidence of well-preserved follicles at the periphery of the lesion.
In conclusion, the radiologist should be familiar with these features to avoid surgical errors, especially in young women.
Usefulness of 18 F-FDG PET
Several reports proved the usefulness of 18-FDG-PET in the management of systemic lymphoma. Komoto et al. [14, 15, 25] proved the utility of 18F-FDG PET in the staging and assessment of therapeutic response in a case of primary NHL of the ovary. They described a strong uptake by the abdominal masses (SUV max = 12.5), which were reduced significantly after the first day of chemotherapy. Similarly, in our experience, 18F-FDG PET revealed a strong uptake in lymphomatous ovarian masses, which were reduced after chemotherapy (Fig. 7).
Fig. 7.
A 33 years old woman with high grade NHL relapse presenting with abdominal pain. 18-FDG-PET CT shows high uptake (SUV: 26) within the right ovarian mass referred to lymphomatous involvement (white arrows) (A). After chemotherapy, both degree and extension of right ovarian mass FDG uptake decrease to standard values (B)
Differential diagnosis
The differential diagnosis for OL should include solid ovarian tumours such as epithelial ovarian neoplasms, which are rarely homogenously solid, and usually have a complex structure with cystic or necrotic areas and solid components that enhance after contrast administration [5, 10]. Predominantly solid ovarian tumours are fibromas, thecomas, Brenner cell tumours and granulosa cell tumours, but they rarely show homogeneous content at TVS. Moreover, fibromas and fibrotecomas do not grow as rapidly as lymphomas. Rapid growth is another feature more common in lymphoma than in other ovarian neoplasms, except germ cell tumours [5]. Most of the published literature has adopted the diagnostic criteria for primary ovarian non-Hodgkin’s lymphoma proposed by Fox et al. in 1976:
At the time of diagnosis, the lymphoma is clinically confined to the ovary and full investigation fails to reveal evidence of lymphoma elsewhere. A lymphoma can still, however, be considered as primary if spread to the immediately adjacent lymph nodes has occurred or if there has been direct spread to infiltrate immediately adjacent structures.
The peripheral blood and bone marrow should not contain any abnormal cells.
If further lymphomatous lesions occur at sites remote from the ovary, then at least several months should have elapsed between the appearance of ovarian and extraovarian lesions [24].
Imaging can reveal the presence of ascites or peritoneal implants, which are common in epithelial cancers [5, 16], or widespread lymphoadenomegalies and splenomegaly, which are suggestive for systemic lymphoma [5, 9].
Metastatic disease should also be included in differential diagnosis. Ovarian metastases from breast, stomach, and uterine cancer appear as solid homogeneous masses and may be highly vascularised at colour Doppler US, but those features are more frequently associated to OL [17]. Metastases derived from the colon, rectum, or biliary tract manifest more heterogeneous morphological patterns; most are multicystic, with irregular borders [17, 18].
Conclusions
Clinical and serological presentation of OL may be similar to that of primary ovarian cancers. Imaging plays a central role because some radiological aspects are indicative of lymphomatous ovarian involvement. These include: (1) the presence of solid homogeneous ovarian masses with well-defined profiles that are cleaved from surrounding structures; (2) mild-to-high vascularisation with arborised vessels on colour Doppler US (‘lead vessel sign’) and contrast-enhanced CT with multiplanar imaging; (3) mild-to-high enhancement after administration of contrast material on CT and MR; and (4) a pattern of growth that involves the ovary but preserves its normal structure, with evidence of well-preserved follicles at the periphery of the lesion. To assess the diagnosis, we suggest the use of US /MRI as first step, followed by CT scan to evaluate the extent of the disease or presence of metastases.
Whenever an ovarian mass with features resembling those of OL is found, a systemic lymphoma should be ruled out before surgical adnexectomy is performed [1, 6]. The presence of splenomegaly or disseminated lymphadenomegalies and a history of previous lymphoma may orient the diagnosis. Hystocitopathological evidence remains mandatory for the definitive diagnosis of OL [6]. OL must be considered a localised manifestation of a systemic disease, and treatment and prognosis are the same as that of other nodal lymphomas [7, 16]. A conservative treatment based on appropriate chemotherapy is the management of choice for OL, and a correct diagnosis is essential to avoid radical surgery in women of young age desiring pregnancy [3, 5–7, 27].
Funding
The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors have not disclosed any competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/3/2023
A Correction to this paper has been published: 10.1007/s40477-023-00799-z
References
- 1.Ferry JA, Young RH. Malignant lymphoma, pseudolymphoma and hemopoeitic disorders of the female genital tract. Pathol Annu. 1991;26:227–263. [PubMed] [Google Scholar]
- 2.Crasta JA, Vallikad E. Ovarian lymphoma. Indian J Med Pediatric Oncol. 2009;30:28–30. doi: 10.4103/0971-5851.56333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun J, Kim SJ, Won JH, Choi CW, Eom HS, Kim JS, Kim MK, Kwak J, Suh WSK. Clinical features and prognostic relevance of ovarian involvement in non-Hodgkin's lymphoma: a consortium for improving survival of lymphoma (CISL) report. Leuk Res. 2010;34:1175–1179. doi: 10.1016/j.leukres.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos MA, Daliani D, Pugh W, Gershenson D, Cabanillas F, Sarris AH. Primary ovarian non-Hodgkin’s lymphoma: outcome after treatment with combination chemotherapy. Gynecol Oncol. 1997;64:446–450. doi: 10.1006/gyno.1996.4583. [DOI] [PubMed] [Google Scholar]
- 5.Crawshaw J, Sohaib SA, Wotherspoon A, Shepherd J. A primary non-Hodgkin's lymphoma of the ovaries: imaging findings. Br J Radiol. 2007;80:155–158. doi: 10.1259/bjr/35049074. [DOI] [PubMed] [Google Scholar]
- 6.Weingertner AS, Roedlich MN, Hamid D, Baldauf JJ. Non-Hodgkin malignant lymphoma revealed by an ovarian tumor: a case report and review of the literature. Gynecol Oncol. 2004;95:750–754. doi: 10.1016/j.ygyno.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Signorelli M, Maneo A, Cammarota S, et al. Conservative management in primary genital lymphomas: the role of chemiotherapy. Gynecol Oncol. 2007;104:416–421. doi: 10.1016/j.ygyno.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Skodras G, Fields V, Kragel PJ. Ovarian lymphoma and serous carcinoma of low malignant potential arising in the same ovary: a case report with review of literature of 14 primary ovarian lymphomas. Arch Pathol Lab Med. 1994;118:647–650. [PubMed] [Google Scholar]
- 9.Koyama T, Mikami Y, Saga T, Tamai K, Togashi K. Secondary ovarian tumors: spectrum of CT and MR features with pathologic correlation. Abdom Imagingb. 2007;32:784–795. doi: 10.1007/s00261-007-9186-4. [DOI] [PubMed] [Google Scholar]
- 10.Ferrozzi F, Catanese C, Uccelli M, Bassi P. Ovarian Lymphoma findings with ultrasonography, computerized tomography and magnetic resonance. Radiol Med. 1998;95:493–497. [PubMed] [Google Scholar]
- 11.De Gregorio N, Schmitt W, Kreienberg R, Wulff C. Ovarian metastasis of a lymphoma presenting as primary ovarian cancer. Oncologie. 2009;32:752–753. doi: 10.1159/000252968. [DOI] [PubMed] [Google Scholar]
- 12.Zidan J, Hussein O, Basher W, Zohar S. Serum CA125: a tumor marker for monitoring response to treatment and follow-up in patients with non-Hodgkin’s lymphoma. Oncologist. 2004;9:417–421. doi: 10.1634/theoncologist.9-4-417. [DOI] [PubMed] [Google Scholar]
- 13.Gui W, Wang T, Wang J, Wang L, He J, Yang B, Zhao Z, Zhang H, Zhang Q. An improved prognostic parameter for non-Hodgkin's lymphoma based on the combination of three serum tumor markers. Int J Biol Markers. 2008;23:207–213. doi: 10.1177/172460080802300402. [DOI] [PubMed] [Google Scholar]
- 14.Komoto D, Nischiyama Y, Monden T, Yamamoto Y, Sasakawa Y, Toyama Y, Satho K, Ohono M, Kanenischi K, Ohkawa M. A case of Non-Hodgkin's lymphoma of the ovary: usefulness of 18F-FDG PET for staging and assessment of therapeutic response. Ann Nucl Med. 2006;20:157–160. doi: 10.1007/BF02985629. [DOI] [PubMed] [Google Scholar]
- 15.Gu J, Chan T, Zhang J, Leung AYH, Kwong Y, Khong P. Whole-body diffusion-weighted imaging: the added value to whole-body MRI at initial diagnosis of lymphoma. AJR Am J Roentgenol. 2010;197:384–391. doi: 10.2214/AJR.10.5692. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Xu Y, Wang J. Ultrasonography, computer tomography and magnetic resonance imaging for diagnosis of ovarian carcinoma. Eur J Radiol. 2007;62:328–334. doi: 10.1016/j.ejrad.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Testa AC, Ferrandina G, Timmerman D, Savelli L, Ludovisi M, Holsbeke CV, Malaggese M, Scambia G, Valentin L. Imaging in gynecological disease: ultrasound features of metastases in the ovaries differ depending on the origin of primary tumor. Ultrasound Obstet Gynecol. 2007;29:505–511. doi: 10.1002/uog.4020. [DOI] [PubMed] [Google Scholar]
- 18.Guerriero S, Alcazar JL, Pascual MA, Ajossa S, Olartecoechea B, Hereter L. The pre-operative diagnosis of metastatic ovarian tumors is related to the origin of primary tumor. Ultrasound Obstet Gynecol. 2012;39:581–586. doi: 10.1002/uog.10120. [DOI] [PubMed] [Google Scholar]
- 19.Mitsumori A. MR appearance of non-Hodgkin's Lymphoma of the Ovary. AJR Am J Roentgenol. 1999;173:245. doi: 10.2214/ajr.173.1.10397144. [DOI] [PubMed] [Google Scholar]
- 20.Testa AC, Mancari R, Di Legge A, Mascilini F, Salutari V, Scambia G, Ferrandina G. The "lead vessel": a vascular ultrasound features of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218–221. doi: 10.1002/uog.5251. [DOI] [PubMed] [Google Scholar]
- 21.Slonimsky E, Korach J, Perri T, Davidson T, Apter S, Inbar Y. Gynecological lymphoma: a case series and review of the literature. J Comput Assist Tomogr. 2018;42(3):435–440. doi: 10.1097/RCT.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 22.Ferrozzi F, Tognini G, Bova D, Zuccoli G. Non-Hodgkiqn lymphomas of the ovaries: MR findings. J Comput Assist Tomogr. 2000;24(3):416–420. doi: 10.1097/00004728-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Hou Z, Huang J, Xu W, Wang C, Ma X, Lu N, Liu J, Mao Y, Qian Y. Successful pregnancy via in vitro fertilization in a primary infertile woman with primary lymphoma of the fallopian tube after surgery: a case report and literature review. Medicine (Baltimore) 2022;101(30):e29353. doi: 10.1097/MD.0000000000029353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung YW, Lin YS, Chen YT, Yeh LS. Non-Hodgkin's B-cell lymphoma of the ovary: a case report and review of the literature. Taiwan J Obstet Gynecol. 2022;61(3):539–543. doi: 10.1016/j.tjog.2022.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Saini VK, Mammoottil AE, Nazar AH, Pavecha P, Ora M, Gambhir S. Utero-ovarian involvement in non-Hodgkin's lymphoma on 18F-fluorodeoxyglucose positron emission tomography/computed tomography: a case series and literature review. Indian J Nucl Med. 2022;37(1):64–67. doi: 10.4103/ijnm.ijnm_88_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IslimyeTaskın M, Gokgozoglu L, Kandemır B. Primary ovarian large B-cell lymphoma. Case Rep Obstet Gynecol. 2013;2013:493836. doi: 10.1155/2013/493836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trenhaile TR, Killackey MA. Primary pelvic non-Hodgkin's lymphoma. Obstet Gynecol. 2001;97(5 Pt 1):717–720. doi: 10.1016/s0029-7844(00)01225-4. [DOI] [PubMed] [Google Scholar]