Abstract
Purpose
Chest x-ray (CXR) is the standard imaging used to evaluate children in acute respiratory distress and failure. Our objective was to compare the lung-imaging techniques of CXR and lung ultrasound (LUS) in the evaluation of children with acute respiratory failure (ARF) to quantify agreement and to determine which technique identified a higher frequency of pulmonary abnormalities.
Methods
This was a secondary analysis of a prospective observational study evaluating the sensitivity and specificity of LUS in children with ARF from 12/2018 to 02/2020 completed at the University of Wisconsin-Madison (USA). Children > 37.0 weeks corrected gestational age and ≤ 18 years of age admitted to the PICU with ARF were evaluated with LUS. We compared CXR and LUS completed within 6 h of each other. Kappa statistics (k) adjusted for maximum attainable agreement (k/kmax) were used to quantify agreement between imaging techniques and descriptive statistics were used to describe the frequency of abnormalities.
Results
Eighty-eight children had LUS completed, 32 with concomitant imaging completed within 6 h are included. There was fair agreement between LUS and CXR derived diagnoses with 58% agreement (k/kmax = 0.36). Evaluation of imaging patterns included: normal, 57% agreement (k = 0.032); interstitial pattern, 47% agreement (k = 0.003); and consolidation, 65% agreement (k = 0.29). CXR identified more imaging abnormalities than LUS.
Conclusions
There is fair agreement between CXR and LUS-derived diagnoses in children with ARF. Given this, clinicians should consider the benefits and limitations of specific imaging modalities when evaluating children with ARF. Additional studies are necessary to further define the role of LUS in pediatric ARF given the small sample size of our study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40477-023-00827-y.
Keywords: Pediatrics, Acute respiratory disease, Point-of-care ultrasound, Chest radiograph, Lung ultrasound
Introduction
Chest x-ray (CXR) is among the most commonly utilized tools in the assessment pediatric respiratory diseases and is a standard imaging technique to evaluate children in acute respiratory distress and failure [1]. Respiratory illness accounts for between 20 and 30% of pediatric intensive care unit (PICU) and ward admissions [2]. CXR is estimated to be obtained in 50% of PICU admissions [3]. Despite ubiquitous usage, CXRs accuracy and reliability has never been rigorously evaluated (i.e., compared to chest computerized tomography (CT)) [4]. While chest CT is considered the gold standard pulmonary imaging modality, CXR is the standard against which newer imaging techniques, such as lung ultrasound (LUS), are evaluated in pediatrics to limit exposure to the high ionizing radiation of CT [5–7].
Few studies have evaluated the diagnostic accuracy of CXR compared with CT and have demonstrated low to modest sensitivity (range 40–79%) and specificity (range 39–92%) in identifying pulmonary pathology [8–11]. Clinicians still highly value CXRs despite their low accuracy and continue to regularly utilize them in clinical practice [12]. Recently published guidelines support the use of LUS in pediatric respiratory diseases, yet cited studies include few children with acute respiratory failure (ARF), such as children requiring ancillary respiratory therapies, high supplemental oxygen support (hypoxemic respiratory failure), invasive or non-invasive mechanical support (hypercapnic respiratory failure), or an escalation from baseline respiratory support (acute on chronic respiratory failure) [13]. Many studies of specific disease processes have found LUS has high sensitivity (range 89–97%) and specificity (range 56–99%) identifying findings consistent with the underlying disease process; however, it has also been consistently described that children with a worse clinical course (defined by the requirement of more hospital resources) demonstrate more ultrasound abnormalities [14–17]. Therefore, our study objective was to compare lung imaging techniques (CXR and LUS) in the evaluation of children with undifferentiated ARF to quantify imaging agreement and determine which technique identifies a higher frequency of pulmonary abnormalities. We hypothesized that LUS would identify substantially more abnormalities than CXR in children with ARF. If borne out, clinicians may be able to reduce the financial cost and radiation exposure associated with CXR utilization while affording a better understanding of pulmonary pathophysiology and allowing for more specific treatments.
Methods
The Institutional Review Board at the University of Wisconsin-Madison (USA) approved this pre-designed secondary analysis of a prospective observational study evaluating the sensitivity and specificity of LUS in children admitted to the pediatric intensive care unit (PICU) with ARF (IRB 2018-071) [18]. ARF for the study was defined by the clinical needed for PICU admission and at least one of the following: (1) non-invasive (high flow nasal cannula (HFNC) ≥ 1 L/kg/min; continuous positive airway pressure; or bilevel positive airway pressure delivered by nasal cannula, nasal mask, or full face mask) or invasive respiratory support; (2) supplemental oxygen with FiO2 > 35% while on HFNC < 1 L/kg/min or nonrebreather/oxymask to maintain saturation ≥ 90%; (3) continuous nebulized therapy; or (4) chronic oxygen or ventilatory support and any increase in baseline support [18, 19]. Children > 37.0 weeks corrected gestational age and ≤ 18 years of age from whom signed, informed parent/guardian consent, and assent when appropriate, were enrolled from December 2018 to February 2020.
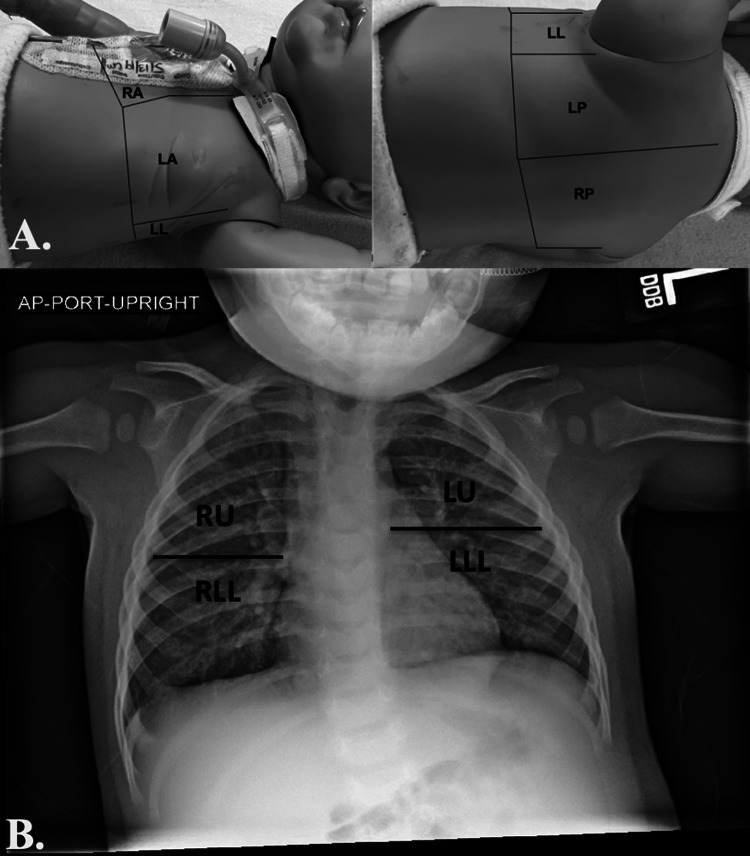
A 3-region per hemithorax LUS was completed using a modified BLUE (Bedside Lung Ultrasound in Emergency) protocol within 14 h of PICU admission by one of three pediatric intensivists with point-of-care ultrasound training and clinical LUS experience [20, 21]. The three lung regions were defined by: (1) the anterior chest wall between the anterior axillary line and sternum; (2) the lateral chest wall between the anterior and posterior axillary lines; and (3) the posterolateral chest wall between the posterior axillary line and the spine (Fig. 1A). For each region, the most abnormal ultrasound pattern was identified and a representative 4-s video recorded and uploaded to Q-path imaging system (Telexy Healthcare; British Columbia, Canada). In Q-path, an assessment of ultrasound artifacts was made for each individual region and an overall diagnosis determined (e-Table 1A). For comparison with CXR, A lines were considered a normal ultrasound pattern; multiple B lines and confluent B lines were considered an interstitial disease pattern; and sub-pleural consolidation and lobar consolidation were considered consolidation patterns [21, 22]. All patients/images were examined by a senior intensivist with extensive experience in LUS acquisition and interpretation and blinded to patient historical and clinical information. A second physician (ultrasound fellowship-trained emergency medicine physician) independently reviewed all images to determine an ultrasound diagnosis, but did not document individual ultrasound artifact findings due to loss of documented lung region location during a Q-path system upgrade. Differences in diagnoses between the two were resolved by consensus.
Fig. 1.
Lung ultrasound (A) and chest radiograph (B) regions. LA left anterior, LL left lateral, LLL left lower lobe, LP left posterior, LU left upper lobe, RA right anterior, RLL right lower (right middle lobe and right lower lobe), RP right posterior, RU right upper lobe
Clinical CXRs completed within 6 h of LUS were evaluated concurrently by two pediatric radiologists who were blinded to patient historical, clinical, and LUS information. CXRs were evaluated for the presence of interstitial lung disease, atelectasis, consolidations, pleural effusions, and pneumothoraces across four lung regions and a radiographic diagnosis was determined [22]. Differences in CXR interpretation were resolved in real-time by consensus (Fig. 1B; e-Table 1B). The six LUS regions were combined into four to correspond with the four CXR regions, approximating the five lung lobes (e-Table 2) [22]. A LUS sub-pleural consolidation (SPC) artifact was considered a consolidation for the primary study analysis [22]. A separate analysis was conducted that did not include SPC as a consolidation, as small consolidations may not be identified on CXR. A CXR finding of atelectasis was considered a consolidation for comparisons. Given the relatively long duration between imaging studies, sub-analyses of studies completed within 4 h and within 2 h of each other were additionally performed.
All LUS exams were performed using a Philips Sparq ultrasound machine (Koninklijke Philips N.V; Amsterdam, Netherlands) with a L12-4 linear probe (frequency 12–4 MHz) or S4-2 phased array probe (frequency 4–2 MHz) with children in a recumbent or semi-recumbent position and (when clinically feasible) rolled onto their side or placed into a sitting position to optimize posterior lung field scanning according to the previously published protocol [18]. In the PICU, a portable single view anterior–posterior (AP) CXR was obtained using a Fujifilm FDR Go Mobile radiograph machine (Fujifilm Medical Systems; Connecticut, USA). In the Emergency Department either a portable single view AP CXR was obtained using a Philips MobileDiagnost wDR 2.2 (Philips Healthcare; Massachusetts, USA) or a two-view AP and lateral CXR was obtained using Samsung GC85 radiograph machine (Samsung/Neurologica Corporation; Massachusetts, USA). All CXR imaging was obtained according to standard hospital thoracic imaging protocols, as requested for clinical care by the clinicians caring for the patient. The reference standard for the final patient diagnosis was an independent, standardized review of the medical record following hospital discharge by a physician not involved in the care of the patient (final diagnosis) and incorporated the history, clinical findings, laboratory tests, radiographic (including CXR) data, patient management, and course to determine the etiology of the ARF [18].
Descriptive statistics were used to define the frequency of imaging abnormalities. Kappa statistics (k) adjusted for maximum attainable agreement (kmax) were used to quantify agreement between techniques [23–25]. The 95% confidence intervals (95% CI) for k and kmax were computed by bootstrap replications (B = 5000) [26]. The k/kmax was used when the sparsity of diagnoses made the default maximum of one unattainable, as the upper and lower limits of k are a function of the frequency [24]. Kappa statistical agreement (for both k and k/kmax) was considered: k ≤ 0.00, less than chance agreement; k > 0.00–0.20, slight agreement; k > 0.20–0.40, fair agreement; k > 0.40–0.60, moderate agreement; k > 0.60–0.80, substantial agreement; and k ≥ 0.80, near perfect agreement [25]. Statistical analysis was performed using R version 3.6.3 (R Project for Statistical Computing; Vienna, Austria). The study was registered with ClinicalTrials.gov and data are reported using Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Results
Eighty-eight children had LUS completed for the primary study [18]; 32 of these 88 (36%) had LUS and CXR completed within 6 h of each other and are included in this study (e-Fig. 1). The study cohort includes 2 (6%) patients with status asthmaticus, 17 (53%) with bronchiolitis/viral pneumonitis, 12 (37%) with pneumonia, and 1 (3%) patient with multiple concurrent final diagnoses. CXR was completed a median of 1.25 h (IQR − 2.0 to 5.81) following PICU admission while LUS was completed a median of 4.67 h (IQR 2.70–7.63) following PICU admission and a median of 3.64 h (IQR 1.85–5.88) from CXR. Demographics of the study cohort are presented in Table 1.
Table 1.
Demographics of study population, n = 32
| Patient characteristics | Values |
|---|---|
| Age (median, IQR) | 1.59 years (0.55–3.62) |
| < 2 years (n, %) | 19 (59%) |
| ≥ 2 years (n, %) | 13 (41%) |
| Gender (n, %) | |
| Male | 20 (62%) |
| Female | 12 (38%) |
| Medical history (n, %)1 | |
| None | 20 (62%) |
| Asthma | 8 (25%) |
| Bronchopulmonary dysplasia2 | 3 (9%) |
| Chronic aspiration | 2 (6%) |
| Baseline pulmonary medications | 13 (41%) |
| Home respiratory support device | 5 (16%) |
| *Respiratory support (n, %) | |
| HFNC | 14 (44%) |
| CPAP | 3 (9%) |
| BiPAP | 8 (25%) |
| Mechanical ventilation | 5 (16%) |
| Continuous nebulized therapy alone | 2 (6%) |
| *Respiratory Therapies (n, %)1 | |
| B-agonist | 21 (66%) |
| Corticosteroid | 8 (25%) |
| Hypertonic saline | 4 (12%) |
| Ipratropium | 8 (25%) |
| Magnesium | 2 (6%) |
| Racemic epinephrine | 2 (6%) |
| Antibiotics | 21 (66%) |
| *Respiratory Viral Panel (n, %) | |
| Positive3 | 20 (87%) |
| Negative3 | 3 (13%) |
| Not obtained4 | 9 (28%) |
| Length of stay (median, IQR) | |
| PICU (days) | 2.2 days (1.3–4.6) |
| Hospital (days) | 4.6 days (2.7–7.9) |
BiPAP bilevel positive airway pressure, CPAP continuous positive airway pressure, HFNC high flow nasal cannula, IQR interquartile range, m months, PICU pediatric intensive care unit, y years
*Patient receiving at the time POC-LUS performed
1Does not sum to 100% given patients with multiple underlying medical conditions
2Bronchopulmonary dysplasia, chronic lung disease, and/or chronic respiratory failure diagnosis
3Percentage of obtained tests
4Percentage of total patients
There was fair agreement between LUS and CXR derived diagnoses with 58% agreement (k/kmax = 0.36, 95% CI − 0.01 to 0.80) (Table 2A). CXR identified more abnormalities (104 abnormal lung regions across 123 examined regions) compared with LUS (71 abnormal regions) (Table 3A; e-Table 3). Evaluation of specific imaging patterns included: normal, 57% agreement (k = 0.032, 95% CI − 0.11 to 0.17); interstitial pattern, 47% agreement (k = 0.003, 95% CI − 0.20 to 0.20); consolidation, 65% agreement (k = 0.294, 95% CI 0.13–0.46); pleural effusion, 94% agreement (k = NA; one imaging modality did not find an effusion); and pneumothorax, 99% agreement (k = NA; one imaging modality did not find a pneumothorax) (Table 3B).
Table 2.
Comparison between ultrasound and chest x-ray derived diagnoses (A) and between the final diagnosis, lung ultrasound, and chest x-ray (B)* Bolded numbers represent agreement between the two imaging modalities
| A. Agreement between lung ultrasound and chest x-ray derived diagnoses | |||
|---|---|---|---|
| Ultrasound diagnosis | Chest x-ray diagnosis | ||
| Status asthmaticus | Bronchiolitis1 | Pneumonia2 | |
| Asthma | 0 | 4 | 0 |
| Bronchiolitis1 | 0 | 12 | 3 |
| Pneumonia2 | 2 | 4 | 6 |
| B. Agreement between LUS and CXR derived diagnoses compared with the final diagnosis | ||||||
|---|---|---|---|---|---|---|
| Final diagnosis | Ultrasound diagnosis | Chest x-ray diagnosis | ||||
| SA3 | Bronchiolitis1 | Pneumonia2 | SA3 | Bronchiolitis1 | Pneumonia2 | |
|
Asthma n = 2 |
1 | 1 | 0 | 0 | 1 | 1 |
|
Bronchiolitis n = 17 |
2 | 11 | 4 | 2 | 12 | 6 |
|
Pneumonia n = 12 |
1 | 3 | 8 | 0 | 4 | 5 |
Table 2A. Fair agreement between the ultrasound diagnosis and the chest x-ray diagnosis with 58% absolute agreement (18/31; 95% CI 41–74%) k = 0.26 (95% CI − 0.01 to 0.53), kmax = 0.72, k/kmax = 0.36 (95% CI − 0.01 to 0.80)
Table 2B. Moderate agreement between the ultrasound diagnosis and final diagnosis with 65% absolute agreement (20/31; 95% CI 0.47–0.79), k = 0.38 (95% CI 0.09–0.66), kmax = 0.89, k/kmax = 0.43 (95% CI 0.12–0.84). Slight agreement between the chest x-ray diagnosis and final diagnosis with 55% absolute agreement (17/31; 95% CI 0.38–0.71), k = 0.15 (95% CI − 0.15 to 0.44), kmax = 0.82, k/kmax = 0.18 (95% CI − 0.21 to 0.65)
1Bronchiolitis or viral pneumonitis
2Unilateral or bilateral bacterial pneumonia
3Status asthmaticus
*Table compares 31 of 32 study patients to maintain consistency with the parent study (reference 18) in which one patient with multiple final diagnoses was removed from final analysis
Table 3.
Comparison of the number and frequency of lung imaging findings (A) and agreement between imaging modalities (B)
| A. Comparison of the number and frequency of lung imaging findings across all lung regions1 | |||
|---|---|---|---|
| Ultrasound finding | Lung ultrasound | Chest X-Ray | p value |
| Normal imaging | 52 (42%) | 19 (15%) | 0.003 |
| Abnormal imaging | |||
| Interstitial pattern | 30 (24%) | 69 (56%) | 0.009 |
| Consolidation | 58 (47%) | 49 (40%) | 0.235 |
| Pleural effusion | 0 (0%) | 8 (7%) | 0.765 |
| Pneumothorax | 1 (1%) | 0 (0%) | 0.778 |
| B. Comparison of agreement between lung ultrasound and chest x-ray findings | ||||
|---|---|---|---|---|
| Ultrasound finding | Agreement2 | 95% CI | Kappa Agreement | 95% CI |
| Normal imaging | 0.57 (70/123) | 0.46–0.68 | 0.032 | − 0.11 to 0.17 |
| Abnormal imaging | ||||
| Interstitial pattern | 0.47 (58/123) | 0.34–0.60 | 0.003 | − 0.20 to 0.20 |
| Consolidation | 0.65 (80/123) | 0.56–0.74 | 0.294 | 0.13 to 0.46 |
| Pleural effusion | 0.94 (115/123) | 0.88–0.99 | NA3 | NA3 |
| Pneumothorax | 0.99 (122/123) | 0.98–1.00 | NA3 | NA3 |
CI confidence interval
1One hundred twenty-three observations among 32 patients
2Number of observations in agreement divided by total number of observations
3One imaging modality did not identify abnormality
When comparing LUS and CXR with the final patient diagnosis, LUS demonstrated moderate agreement (k/kmax = 0.43, 95% CI 0.12–0.84) while CXR demonstrated slight agreement (k/kmax = 0.18, 95% CI − 0.21 to 0.65) (Table 2B). Additional analyses evaluating agreement between LUS and CXR when SPCs were not considered LUS determined consolidations and when comparing imaging obtained within 4 h and 2 h of each other, did not significantly increase agreement (Table 4).
Table 4.
Comparison of agreement between lung ultrasound and chest x-ray findings when sub-pleural consolidations are not considered a lung ultrasound consolidation, n = 32 (A); between lung ultrasound and chest x-ray findings when images obtained within 4 h of each other, n = 16 (B) and 2 h of each other, n = 9 (C)
| Imaging finding | Agreement1 | 95% CI | Kappa agreement | 95% CI |
|---|---|---|---|---|
| A. Agreement when sub-pleural consolidation not considered a LUS consolidation, n = 32 | ||||
| Normal imaging | 0.58 (71/123) | 0.47–0.68 | 0.054 | − 0.08 to 0.20 |
| Abnormal imaging | ||||
| Interstitial pattern | 0.48 (59/123) | 0.35–0.61 | 0.012 | − 0.19 to 0.21 |
| Consolidation | 0.68 (84/123) | 0.58–0.78 | 0.240 | 0.08 to 0.40 |
| Pleural effusion | 0.94 (115/123) | 0.88–0.99 | NA2 | NA2 |
| Pneumothorax | 0.99 (122/123) | 0.98–1.00 | NA2 | NA2 |
| B. Agreement when images obtained within 4 h of each other, n = 16 | ||||
| Normal imaging | 0.56 (35/62) | 0.41–0.72 | 0.036 | − 0.18 to 0.25 |
| Abnormal imaging | ||||
| Interstitial pattern | 0.37 (23/62) | 0.18–0.56 | − 0.120 | − 0.44 to 0.20 |
| Consolidation | 0.68 (42/62) | 0.56–0.80 | 0.355 | 0.12 to 0.59 |
| Pleural effusion | 0.92 (57/62) | 0.83–1.00 | NA2 | NA2 |
| Pneumothorax | 1.00 (62/62) | 0.81–1.00 | NA2 | NA2 |
| C. Agreement when images obtained within 2 h of each other, n = 9 | ||||
| Normal imaging | 0.49 (17/35) | 0.28–0.69 | − 0.179 | − 0.39 to 0.03 |
| Abnormal imaging | ||||
| Interstitial pattern | 0.26 (9/35) | 0.02–0.50 | − 0.321 | − 0.80 to 0.16 |
| Consolidation | 0.63 (22/35) | 0.45–0.81 | 0.270 | − 0.08 to 0.62 |
| Pleural effusion | 0.91 (32/35) | 0.79–1.00 | NA2 | NA2 |
| Pneumothorax | 1.00 (35/35) | 0.81–1.00 | NA2 | NA2 |
CI confidence interval
1Number of observations in agreement divided by total number of observations
2One imaging modality did not identify abnormality
There was substantial agreement between the intensivist and emergency medicine physician-derived ultrasound diagnoses with 67% observed agreement (k/kmax = 0.61, 95% CI 0.49–0.78) [27]. Agreement in CXR interpretation between radiologists was not determined as interpretations were performed concurrently with differences resolved in real-time.
Discussion
Acute respiratory distress and failure are among the most common indications for pediatric hospitalization [2]. As such, there is great interest in improving diagnostic accuracy and treatment specificity given the high disease burden and associated costs [2]. Given frequent diagnostic uncertainty, many children presenting with respiratory concerns, especially when on the severe end of the disease spectrum, are initially treated with a multi-therapy approach until the underlying etiology becomes better defined, after which therapeutic de-escalation may occur [28–30]. LUS has proven useful in adult medicine where it demonstrates improved sensitivity and specificity in determining the etiology of ARF when compared with standard clinical evaluation (history, physical, and CXR imaging) [21, 22, 31, 32]. The few studies evaluating the ability of LUS to determine the underlying etiology in undifferentiated pediatric respiratory distress and failure have been less convincing [16, 18, 20, 33, 34]; though when evaluating a single suspected disease entity, LUS appears to have favorable operating characteristics [5, 6, 14, 17, 35]. Most pediatric studies use CXR as the standard against which LUS is compared [5–7] and most use children with a known or suspected disease process (and thus high pre-test probability). In a blinded assessment of temporally related imaging studies, we found only fair agreement between imaging derived diagnoses and fair to slight agreement in identifying specific imaging abnormality patterns. CXR detected more interstitial disease and pleural effusions while LUS detected more consolidations and pneumothoraces, though neither of these were statistically more than CXR.
Chest CT is considered the preeminent pulmonary imaging modality but in contrast to adult medicine, in which CT is considered very early in the disease course, it is hardly ever used for diagnosis of acute pediatric respiratory diseases because of the high ionizing radiation, difficulty in patient cooperation, and high cost [5, 6]. Instead, clinical examination and CXR remain standard of care despite relatively low sensitivity and specificity [5, 8, 9, 36]. In a study of 56 children being evaluated for complicated pneumonia that were unresponsive to therapy, CXR missed 110 CT findings, including parenchymal, pleural, and consolidative findings [36]. In another study of 949 children with suspected pneumonia who underwent CT, CXR demonstrated only 79% sensitivity and 60% specificity [8] and in a study of 36 children with suspected pneumonia who underwent CT, CXR demonstrated 62% sensitivity (specificity not reported) [9]. These two studies additionally evaluated LUS with CT and found that LUS demonstrated slightly improved sensitivity and similar or worse specificity when compared with CXR [8, 9]. Still, meta-analyses comparing LUS to CXR demonstrate pooled estimates of the area under the receiver operating curve of 98% [5, 6]. However, the utility of evaluating LUS compared with an imaging modality with known modest operating characteristics is unclear. A large (413 patients with 1002 CXR/LUS comparisons) recently published study similarly comparing imaging patterns in children with concomitantly obtained CXR and LUS found k agreement ranging from 0.51 (moderate agreement) in the detection of left sided pleural effusion to 0.98 (near perfect agreement) in the detection of right sided pneumothorax [37]; though agreement did differ significantly based on imaged region/location and only 19% of patients had underlying respiratory pathology. Another study of 81 children comparing LUS with CXR found k agreement of 0.64 (substantial agreement) for the diagnosis of pneumonia [38], though agreement for other disease processes are not specifically provided. Newer LUS studies are evaluating temporal trends and changes over time including using the lung ultrasound score to evaluate children with bronchiolitis [14, 39, 40] and pediatric acute respiratory distress syndrome [41], and to evaluate changes in lung aeration over time [42].
Pediatric researchers are also beginning to utilize alternative ultrasound modes (i.e., non-B mode) to more thoroughly define LUS specific patterns for individual disease processes [43]. M-mode has classically been utilized to detect pleural motion (or lack thereof) over time; now doppler ultrasound is being used to describe consolidation characteristics [43]. Color and pulsed wave doppler have been used to identify the presence and type of vessels within consolidations [44], helping to differentiate fluid or mucus filled bronchograms, lung necrosis, and abscesses [43]. Incorporation of lesion characteristics including size, number, and distribution of consolidations, position and motion of bronchograms, as well as pleural effusion characteristics and the distribution, movement, and characteristics of vertical artifacts can all significantly complement LUS evaluation [45, 46]. Contrast-enhanced ultrasound and microvascular flow imaging are additional techniques being studied which may provide benefit in LUS [47, 48]. Inclusion of real-time ultrasound during therapeutic trials and inclusion of clinical information may further inform LUS examination [33, 49].
LUS also has proposed benefits outside of its diagnostic abilities, including its ubiquity in patient care units, it does not rely on another provider for performance (it is interpreted by a provider with clinical knowledge of the patient), it promotes provider time spent at the bedside, it is readily repeatable, that allows immediate integration into treatment plans, and that it has a rapid time to acquisition (typically < 10–15 min but shorter with a directed clinical question) [13, 18, 33, 50]; though in certain instances or circumstances these may not be considered beneficial. LUS has additionally been used to guide therapeutic intervention and patient reassessment including in guiding fluid administration/diuresis, lung recruitment, and chest tube insertion [13, 51]; in emergent or time-sensitive situations such as determination of the presence of a pneumothorax [13, 51, 52]; it has been combined with point-of-care cardiac exams to evaluate for reversible causes of cardiopulmonary arrest [51]; and it may be helpful in rapid assessment of reflux/aspiration risk [53]. Our data and similar recently published studies [37–39, 41, 42] suggest that it is likely that LUS plays a complimentary role to CXR in children with respiratory distress and ARF given differences in strengths and weaknesses [54], though our study was not specifically designed to test this. CXR provides an overall picture of the patient at a moment in time, is well established in clinical care, and is completed by another provider allowing for quick examination of multiple patients, while LUS provides more regionalized information, is performed by providers caring for the patient, and can readily be repeated following changes in therapeutic intervention or patient pathophysiology.
A major limitation in the use of LUS is in its inability to detect peri-hilar lesions which are especially common in pediatrics, as it requires findings which extend to the pleural surface [49, 50]. Additional limitations specific to our study include: failure to evaluate LUS and CXR operating characteristics with CT (the gold standard pulmonary imaging modality); evaluation of only the most abnormal LUS pattern in each lung region which may have decreased agreement; evaluation of only 3 LUS regions per hemithorax and inclusion of single view AP CXR which likely decreased the number of identified pathology and thus imaging sensitivity, though chosen for the study design given its clinically pragmatic approach; utilization of approximate lung anatomy (lobes) and corresponding imaging location which may not have correlated with actual anatomy; imaging interpretation being blinded to the patient’s history (and interpretation by different providers) which likewise may have decreased agreement (and is different from clinical practice, though chosen for our study design to reduce confirmation bias and maximize reliability); inability to use a LUS expert consensus of ultrasound artifact findings (due to loss of documented lung region location during a Q-path system upgrade) which may have increased or decreased lesion and location specific agreement; failure to compare agreement in lesion size which may influence a patient’s clinical presentation and course; failure to include patients with pulmonary edema, pneumothorax, or pleural effusion which may have increased agreement; utilization of standard clinical imaging (CXR) in the final diagnosis; and the 6-h duration between imaging which may have been sufficient for changes in pulmonary pathophysiology (either due to progression or improvement with treatment) so as to change imaging patterns (though sub-analyses completed at shorter intervals did not significantly increase agreement). Though chest CT is considered the “gold standard” lung imaging modality, given its limitations, it is not the “standard of [clinical] care” used in pediatrics; future studies should consider evaluating imaging modalities in the context of clinical care (rather than using a blinded interpretation as in this study), as well as evaluating how image acquisition and interpretation influences clinical management.
Conclusion
There is fair agreement between CXR and LUS-derived imaging diagnoses in children with ARF when imaging is completed within 6 h of each other. Given this, clinicians should consider the benefits and limitations of specific imaging modalities when evaluating children with ARF. Additional studies are necessary to further define the role of LUS in children with ARF given the small sample size of our study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Drs. Hagen and Allen for their guidance in the production of this project, the nurses for their enthusiasm and support of this research, and the families and patients at the University of Wisconsin-Madison for their willingness to participate.
Prior presentation
Study data were presented at the Society of Critical Care Medicine (SCCM) Annual Congress (2022 April 18; virtual conference).
Abbreviations
- AP
Anterior–posterior
- ARF
Acute respiratory failure
- BLUE
Bedside lung ultrasound in emergency protocol
- CT
Computerized tomography
- CXR
Chest x-ray
- HFNC
High flow nasal cannula
- LUS
Lung ultrasound
- PICU
Pediatric intensive care unit
- SPC
Subpleural consolidation
Author contributions
Ryan DeSanti, Awni Al-Subu, and Pierre Kory contributed to the conception of the study, data acquisition, analysis and interpretation, work drafting and gave final approval of the version to be published. Kara Gill; Jonathan Swanson, Jessica Schmidt, and Eileen Cowan contributed to data acquisition, work drafting and gave final approval of the version to be published. Michael Lasarev contributed to data analysis and interpretation, work drafting and gave final approval of the version to be published. Ryan DeSanti is the guarantor of the paper, had full access to all study data, and takes responsibility for the integrity of the data and accuracy of analysis, from inception to published article.
Funding
This project was supported by the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS), Grant UL1TR002373. The content of the work and manuscript are solely the responsibility of the authors and do not represent the views of the NIH. The funding source had no involvement in the study design; collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the article for publication. The authors have declared no other sources of funding related to this work.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Dr. Al-Subu has a consulting agreement with Edwards Lifesciences LLC. The remaining authors have declared no conflicts of interest related to this work.
Study location, ethical approval, and consent to participate
This study was completed at The American Family Children’s Hospital, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA. The Institutional Review Board at the University of Wisconsin-Madison approved this study (IRB 2018-071). Signed, informed parent/guardian consent, and assent when appropriate was obtained for participants. The study was registered with ClinicalTrials.gov (NCT03744169).
Consent to publish
All study authors have reviewed the final manuscript and gave approval for the version to be published.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Price MB, Grant MJ, Welkie K. Financial impact of elimination of routine chest radiographs in a pediatric intensive care unit. Crit Care Med. 1999;27:1588–1593. doi: 10.1097/00003246-199908000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Leyenaar JK, Ralston SL, Shieh M-S, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11:743–749. doi: 10.1002/jhm.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta R, Nallasamy K, Williams V, et al. Prescription practice and clinical utility of chest radiographs in a pediatric intensive care unit: a prospective observational study. BMC Med Imaging. 2021;21:44. doi: 10.1186/s12880-021-00576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurney JW. Why chest radiography became routine. Radiology. 1995;195:245–246. doi: 10.1148/radiology.195.1.7892479. [DOI] [PubMed] [Google Scholar]
- 5.Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714–722. doi: 10.1542/peds.2014-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21:183–195. doi: 10.1007/s40477-018-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin H, Li J, Hu H-Y. Is lung ultrasound useful for diagnosing pneumonia in children?: A meta-analysis and systematic review. Ultrasound Q. 2018;34:3–10. doi: 10.1097/RUQ.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 8.Yan C, Hui R, Lijuan Z, Zhou Y. Lung ultrasound vs. chest X-ray in children with suspected pneumonia confirmed by chest computed tomography: a retrospective cohort study. Exp Ther Med. 2020;19:1363–1369. doi: 10.3892/etm.2019.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambroggio L, Sucharew H, Rattan MS, et al. Lung ultrasonography: a viable alternative to chest radiography in children with suspected pneumonia? J Pediatr. 2016;176:93–98. doi: 10.1016/j.jpeds.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Maughan BC, Asselin N, Carey JL, et al. False-negative chest radiographs in emergency department diagnosis of pneumonia. R I Med J (2013) 2014;97:20–23. [PubMed] [Google Scholar]
- 11.Winkler MH, Touw HR, van de Ven PM, et al. Diagnostic accuracy of chest radiograph, and when concomitantly studied lung ultrasound, in critically Ill patients with respiratory symptoms: a systematic review and meta-analysis. Crit Care Med. 2018;46:e707–e714. doi: 10.1097/CCM.0000000000003129. [DOI] [PubMed] [Google Scholar]
- 12.Tolsma M, van der Voort PHJ, van der Meer NJM. Why intensivists want chest radiographs. Crit care. 2015;19:100. doi: 10.1186/s13054-015-0816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) Crit Care. 2020;24:65. doi: 10.1186/s13054-020-2787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basile V, Di Mauro A, Scalini E, et al. Lung ultrasound: a useful tool in diagnosis and management of bronchiolitis. BMC Pediatr. 2015;15:63. doi: 10.1186/s12887-015-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueno-Campaña M, Sainz T, Alba M, et al. Lung ultrasound for prediction of respiratory support in infants with acute bronchiolitis: a cohort study. Pediatr Pulmonol. 2019;54:873–880. doi: 10.1002/ppul.24287. [DOI] [PubMed] [Google Scholar]
- 16.Varshney T, Mok E, Shapiro AJ, et al. Point-of-care lung ultrasound in young children with respiratory tract infections and wheeze. Emerg Med J. 2016;33:603–610. doi: 10.1136/emermed-2015-205302. [DOI] [PubMed] [Google Scholar]
- 17.Dankoff S, Li P, Shapiro AJ, et al. Point of care lung ultrasound of children with acute asthma exacerbations in the pediatric ED. Am J Emerg Med. 2017;35:615–622. doi: 10.1016/j.ajem.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 18.DeSanti RL, Al-Subu AM, Cowan EA, et al. Point-of-care lung ultrasound to diagnose the etiology of acute respiratory failure at admission to the PICU. Pediatr Crit Care Med. 2021;22:722–732. doi: 10.1097/PCC.0000000000002716. [DOI] [PubMed] [Google Scholar]
- 19.Tynan M (2015) Pediatric respiratory failure: the need for specific definitions. Assoc Clin Doc Improv Spec 1–9. https://acdis.org/
- 20.Tripathi S, Ganatra H, Martinez E, et al. Accuracy and reliability of bedside thoracic ultrasound in detecting pulmonary pathology in a heterogeneous pediatric intensive care unit population. J Clin Ultrasound. 2019;47:63–70. doi: 10.1002/jcu.22657. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tierney DM, Huelster JS, Overgaard JD, et al. Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med. 2020;48:151–157. doi: 10.1097/CCM.0000000000004124. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 24.Cook RJ (2005) Kappa. In: Armitage P, Colton T (eds) Encyclopedia of Biostatistics, 2nd edn. John Wiley & Sons, Hoboken
- 25.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 26.Banjanovic ES, Osborne JW. Confidence intervals for effect sizes: applying bootstrap resampling. Pract Assess Res Eval. 2016 doi: 10.7275/dz3r-8n08. [DOI] [Google Scholar]
- 27.DeSanti RL, Cowan EA, Kory PD, et al. The inter-rater reliability of pediatric point-of-care lung ultrasound interpretation in children with acute respiratory failure. J Ultrasound Med. 2022;41:1159–1167. doi: 10.1002/jum.15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florin TA, Byczkowski T, Ruddy RM, et al. Variation in the management of infants hospitalized for bronchiolitis persists after the 2006 American Academy of Pediatrics bronchiolitis guidelines. J Pediatr. 2014;165:786–792. doi: 10.1016/j.jpeds.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss AK, Hall M, Lee GE, et al. Adjunct corticosteroids in children hospitalized with community-acquired pneumonia. Pediatrics. 2011;127:e255–e263. doi: 10.1542/peds.2010-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baan EJ, Janssens HM, Kerckaert T, et al. Antibiotic use in children with asthma: cohort study in UK and Dutch primary care databases. BMJ Open. 2018;8:e022979. doi: 10.1136/bmjopen-2018-022979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xirouchaki N, Magkanas E, Vaporidi K, et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med. 2011;37:1488–1493. doi: 10.1007/s00134-011-2317-y. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Özkaya AK, Başkan Vuralkan F, Ardıç Ş. Point-of-care lung ultrasound in children with non-cardiac respiratory distress or tachypnea. Am J Emerg Med. 2019;37:2102–2106. doi: 10.1016/j.ajem.2019.05.063. [DOI] [PubMed] [Google Scholar]
- 34.Hegazy LM, Rezk AR, Sakr HM, Ahmed AS. Comparison of Efficacy of LUS and CXR in the Diagnosis of Children Presenting with Respiratory Distress to Emergency Department. Indian J Crit care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2020;24:459–464. doi: 10.5005/jp-journals-10071-23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amatya Y, Russell FM, Rijal S, et al. Bedside lung ultrasound for the diagnosis of pneumonia in children presenting to an emergency department in a resource-limited setting. Int J Emerg Med. 2023;16:2. doi: 10.1186/s12245-022-00474-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donnelly LF, Klosterman LA. The yield of CT of children who have complicated pneumonia and noncontributory chest radiography. Am J Roentgenol. 1998;170:1627–1631. doi: 10.2214/ajr.170.6.9609186. [DOI] [PubMed] [Google Scholar]
- 37.Sachdev A, Khatri A, Saxena KK, et al. Chest sonography versus chest radiograph in children admitted to paediatric intensive care—a prospective study. Trop Doct. 2021;51:296–301. doi: 10.1177/00494755211016650. [DOI] [PubMed] [Google Scholar]
- 38.Phung NTN, Vo TTT, Hon KLE. The role of lung ultrasonography in etiologic diagnosis of acute dyspnea in a resource limited setting. Bull Emerg Trauma. 2020;8:121–124. doi: 10.30476/BEAT.2020.46453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobillo-Perez S, Sorribes C, Gebellí P, et al. Lung ultrasound to predict pediatric intensive care admission in infants with bronchiolitis (LUSBRO study) Eur J Pediatr. 2021;180:2065–2072. doi: 10.1007/s00431-021-03978-4. [DOI] [PubMed] [Google Scholar]
- 40.Gori L, Amendolea A, Buonsenso D, et al. Prognostic role of lung ultrasound in children with bronchiolitis: multicentric prospective study. J Clin Med. 2022 doi: 10.3390/jcm11144233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Wang C, Wang F, et al. Lung ultrasound in pediatric acute respiratory distress syndrome received extracorporeal membrane oxygenation: a prospective cohort study. Front Pediatr. 2022;10:798855. doi: 10.3389/fped.2022.798855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fochi O, Bronco A, Nacoti M, et al. Modified pediatric lung ultrasound score compared with computed tomography for assessment of lung aeration in children. Minerva Anestesiol. 2021;87:675–683. doi: 10.23736/S0375-9393.21.15155-7. [DOI] [PubMed] [Google Scholar]
- 43.Musolino AM, Tomà P, De Rose C, et al. Ten years of pediatric lung ultrasound: a narrative review. Front Physiol. 2021;12:721951. doi: 10.3389/fphys.2021.721951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acosta CM, Tusman G, Costantini M, et al. Doppler images of intra-pulmonary shunt within atelectasis in anesthetized children. Crit Ultrasound J. 2016;8:19. doi: 10.1186/s13089-016-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buonsenso D, Musolino A, Ferro V, et al. Role of lung ultrasound for the etiological diagnosis of acute lower respiratory tract infection (ALRTI) in children: a prospective study. J Ultrasound. 2021 doi: 10.1007/s40477-021-00600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buonsenso D, De Rose C, Ferro V, et al. Lung ultrasound to detect cardiopulmonary interactions in acutely ill children. Pediatr Pulmonol. 2022;57:483–497. doi: 10.1002/ppul.25755. [DOI] [PubMed] [Google Scholar]
- 47.Rumolo M, Santarsiere M, Menna BF, et al. Color doppler and microvascular flow imaging to evaluate the degree of inflammation in a case of hidradenitis suppurativa. J Vasc Ultrasound. 2022;46:67–70. doi: 10.1177/15443167211066491. [DOI] [Google Scholar]
- 48.Tufano A, Flammia RS, Antonelli L, et al. The value of contrast-enhanced ultrasound (CEUS) in differentiating testicular masses: a systematic review and meta-analysis. Appl Sci. 2021;11:8990. doi: 10.3390/app11198990. [DOI] [Google Scholar]
- 49.Tusman G, Acosta CM, Nicola M, et al. Real-time images of tidal recruitment using lung ultrasound. Crit Ultrasound J. 2015;7:19. doi: 10.1186/s13089-015-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conlon TW, Nishisaki A, Singh Y, et al. Moving beyond the stethoscope: diagnostic point-of-care ultrasound in pediatric practice. Pediatrics. 2019 doi: 10.1542/peds.2019-1402. [DOI] [PubMed] [Google Scholar]
- 51.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 52.Scialanga B, Buonsenso D, Scateni S, et al. Lung ultrasound to detect pneumothorax in children evaluated for acute chest pain in the emergency department: an observational pilot study. Front Pediatr. 2022;10:812246. doi: 10.3389/fped.2022.812246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minella R, Minelli R, Rossi E, et al. Gastroesophageal and gastric ultrasound in children: the state of the art. J Ultrasound. 2021;24:11–14. doi: 10.1007/s40477-020-00471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conlon T, Keim G. Pathophysiology versus etiology using lung ultrasound: clinical correlation required. Pediatr Crit Care Med. 2021;22:761–763. doi: 10.1097/PCC.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iuri D, De Candia A, Bazzocchi M. Evaluation of the lung in children with suspected pneumonia: usefulness of ultrasonography. Radiol Med. 2009;114:321–330. doi: 10.1007/s11547-008-0336-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.