Summary
Background
The various subcategories under the overarching term of steatotic liver disease (SLD) have been recently proposed by the nomenclature consensus group and endorsed by international academic liver societies. Our aim was to investigate the association between each subtype of SLD and incident cardiovascular disease (CVD) in a nationwide Korean cohort.
Methods
From a nationwide health screening database from Korea, 351,068 individuals aged 47–86 years between January 1, 2009 and December 31, 2010 were included and followed until December 31, 2019 for a median of 9.0 years. Individuals were categorised into no SLD, metabolic dysfunction-associated steatotic liver disease (MASLD), MASLD with increased alcohol intake (MetALD), and alcohol-related liver disease (ALD). Hepatic steatosis was defined as fatty liver index ≥60. The primary outcome was a composite CVD, which includes non-fatal and fatal myocardial infarction and stroke. The subdistribution hazard ratio (SHR) was calculated using the Fine–Gray model with treating non-CVD-related death as a competing risk.
Findings
There were 199,817 male (56.9%) and 151,251 female (43.1%) with a median age of 55 years (interquartile range, 50–61). The prevalence of no SLD, MASLD, MetALD, and ALD was 44.3%, 47.2%, 6.4%, and 2.1%, respectively; and the incidence rate of CVD in each subcategory was 6.2, 8.5, 8.5, and 9.6 per 1000 person-years, respectively. MASLD (SHR, 1.19; 95% confidence interval [CI], 1.15–1.24), MetALD (SHR, 1.28; 95% CI, 1.20–1.36), and ALD (SHR, 1.29; 95% CI, 1.18–1.41) increased the risk of CVD compared to no SLD, which increment was in consecutive order (Ptrend < 0.001).
Interpretation
Individuals with MASLD, MetALD, or ALD are at an increased risk of developing incident CVD. Higher risk of CVD observed in MetALD compared to MASLD suggests the additive impact of alcohol consumption in conjunction with cardiometabolic risk factors on CVD development. These findings support and validate the utility of the new consensus criteria for SLD in predicting CVD.
Funding
The National Research Foundation of Korea and the Korea Centers for Disease Control and Prevention.
Keywords: Metabolic dysfunction-associated steatotic liver disease, Cardiovascular disease, Nonalcoholic fatty liver disease, Cardiovascular risk factors
Research in context.
Evidence before this study
Cardiovascular disease (CVD) is the main cause of death in individuals with nonalcoholic fatty liver disease (NAFLD) or metabolic dysfunction-associated fatty liver disease (MAFLD). Recently, a new nomenclature, metabolic dysfunction-associated steatotic liver disease (MASLD), was proposed as an alternative to NAFLD or MAFLD. Moreover, a new subcategory of metabolic dysfunction-associated steatotic liver disease with increased alcohol intake (MetALD) was also suggested along with MASLD. However, there is no data on the association of the newly suggested steatotic liver disease (SLD) subtypes with CVD risk.
Added value of this study
This nationwide cohort study demonstrated that individuals with SLD subtypes had a higher risk of developing CVD than did those with no SLD. MASLD and MetALD had a higher risk of CVD compared to no SLD with an increasing trend, suggesting that cardiometabolic risk factors and alcohol consumption may additively contribute to CVD risk enabling more granular risk stratification. Moreover, the increasing trend of CVD risk from no SLD to MASLD and further to MetALD was primarily attributed to a higher risk of stroke rather than coronary heart disease.
Implications of all the available evidence
This study supports and validates the utility of the new consensus criteria for SLD in predicting CVD. Therefore, we provide a basis for clinical utility and further research of the new consensus definition for SLD in predicting CVD.
Introduction
International experts have recently published a consensus statement on new fatty liver disease nomenclature, “steatotic liver disease” (SLD).1,2 The term, “nonalcoholic fatty liver disease” (NAFLD), which has been most widely used, is a negative diagnosis of excluding secondary causes of liver disease (e.g., alcohol and viral), while another recently proposed term “metabolic dysfunction-associated fatty liver disease” (MAFLD) comprised all SLD subcategories of different aetiologies without classifying each of them by cause.3 In this statement, SLD is classified into metabolic dysfunction-associated SLD (MASLD), MASLD with increased alcohol intake (MetALD), alcohol-related liver disease (ALD), SLD with specific aetiology, and cryptogenic SLD.1,2 MASLD is defined as the presence of hepatic steatosis along with at least one of five cardiometabolic risk factors that correspond to the components of metabolic syndrome.1,2
Previous studies have demonstrated the utility of both NAFLD and MAFLD in predicting liver-related and non-liver-related outcomes.4, 5, 6, 7, 8 Amongst the outcomes, cardiovascular disease (CVD) is the main cause of death in patients with SLD, and numerous studies have indicated that NAFLD or MAFLD is a significant predictor of CVD events.4,5,9, 10, 11, 12 The updated diagnostic criteria for SLD should be validated in terms of the prediction of clinical outcomes and the determination of long-term prognoses. In this nationwide cohort study, we compared the incidence rate and relative risk of CVD across the different subcategories of SLD.
Methods
Study participants
The study participants of this nationally representative cohort were derived from the Korea National Health Insurance Service (NHIS) Health Screening Cohort. The Korea NHIS provides mandatory health-care insurance service that covers medical health care for all Korean citizens.13 The NHIS collects individualised data regarding sociodemographic information, anthropometric measurements, health screening records, questionnaires on lifestyles, treatment records, hospital visits, and medication prescription records. A number of large epidemiological studies used the NHIS database, and the validity of this database is described in detail elsewhere.13, 14, 15
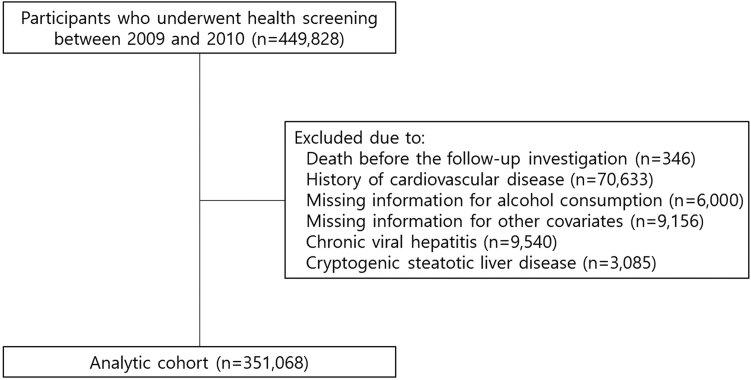
Among a total of 449,828 participants who underwent health screening examination between January 1, 2009 and December 31, 2010, those with death before follow-up investigation, a history of CVD, missing information for alcohol consumption and other covariates were excluded. To focus on the main aetiological factors of the new consensus criteria, namely cardiometabolic risk factors and alcohol consumption, we excluded individuals with chronic viral hepatitis or cryptogenic SLD who had the distinct etiological backgrounds. The amount of alcohol consumption was quantified based on the alcohol use disorder identification test questionnaire. Cryptogenic SLD was defined as SLD without cardiometabolic risk factors and significant alcohol consumption. The final remaining 351,068 participants were included in the analytic cohort (Fig. 1).
Fig. 1.
Flow diagram for inclusion of study participants. Study participants were derived from the National Health Insurance Service Health Screening Cohort after excluding participants with death, history of cardiovascular disease, missing information for alcohol consumption or other covariates, chronic viral hepatitis, and cryptogenic steatotic liver disease.
Ethics
This study was performed in accordance with the STROBE statement.16 The Institutional Review Board of the Seoul Metropolitan Government Boramae Medical Centre approved this study (07-2023-5). The requirement of the informed consents was waived because the NHIS database was strictly anonymized according to the Personal Data Protection Act guidelines.
Definitions of outcomes and follow-up investigation
The primary outcome was the composite CVD, including coronary heart disease (CHD) and stoke, with death without the onset of CVD as a competing risk. CVD was defined as the International Classification of Diseases 10th Revision (ICD-10) codes of I20–I25 and I60–I69 with at least 2 days of hospitalisation. CHD and stroke were defined as hospitalisation for at least 2 days using the ICD-10 codes of I20–I25 and I60–I69, respectively.9,14 All participants were followed from 1 January 2011 until the date of CVD, death, or 31 December 2019, whichever occurred first.
Definitions of SLD and its subtypes
SLD was defined as fatty liver index (FLI) ≥60, which is a widely accepted and validated non-invasive test for diagnosing hepatic steatosis with an area under the receiver operating characteristic curve of 0.844, positive predictive values of 83.2% and 84.8% and negative predictive values of 65.3% and 87.4% for Asian populations of males and females, respectively.17,18 The evaluation of the FLI is described in detail elsewhere.9,19
MASLD was defined as the presence of at least one of five cardiometabolic risk factors (i.e., the components of metabolic syndrome)1,2,20: (i) body mass index (BMI) ≥23 kg/m2 or waist circumference ≥90 cm for male and ≥85 cm for female21; (ii) fasting serum glucose ≥100 mg/dL or type 2 diabetes or treatment for type 2 diabetes; (iii) blood pressure ≥130/85 mmHg or antihypertensive drug treatment; (iv) triglycerides ≥150 mg/dL or lipid lowering treatment; and (v) high-density lipoprotein (HDL) cholesterol ≤40 mg/dL for male and ≤50 mg/dL for female or lipid lowering treatment, on the basis of the presence of hepatic steatosis. ALD was defined as significant alcohol consumption (>60 g/day for male and >50 g/day for female) on the basis of hepatic steatosis regardless of metabolic features. MetALD was defined as MASLD with concomitant moderate alcohol consumption (30–60 g/day for male and 20–50 g/day for female).
Statistical analysis
The continuous variables are presented as mean (standard deviation) if normally distributed or median (interquartile ranges) if not normally distributed. The differences between groups were evaluated by either independent t test or Mann–Whitney U test, respectively. Categorical data are expressed as the number (%), and the differences between groups were determined by the chi-squared test. For calculation of the prevalence of MASLD, MetALD, and ALD, biennial health screening records of 2010–2011, 2012–2013, 2014–2015, and 2016–2017 periods were used after excluding participants with missing information for the covariates that are involved in the evaluation of MASLD, MetALD, and ALD. Age-standardised prevalence was calculated according to the Korean Standard (https://jumin.mois.go.kr/ageStatMonth.do). The multivariable-adjusted Fine and Gray’s model was used to calculate the subdistribution hazard ratio (SHR) to estimate the marginal probability of CVD in the presence of death without the onset of CVD in the evaluation of the association of SLD subtypes with the risk of incident CVD after adjustments for age, sex, household income, BMI, hypertension, type 2 diabetes, dyslipidaemia, smoking, alcohol consumption, moderate-to-vigorous physical activity, and Charlson comorbidity index.22 We additionally used the Cox proportional hazards regression cause-specific model to evaluate the association of SLD subtypes with the risk of incident CVD as secondary analyses. The proportional hazards assumption was confirmed using the Kolmogorov-type supremum test. The incidence was calculated by events (n)/1000 person-years.
The first sensitivity analysis was performed by excluding participants with a history of prescription for steatogenic drugs, including combined antiretroviral therapy (e.g., didanosine, zidovudine, and stavudine) for human immunodeficiency virus infection, tamoxifen, tetracyclines, amiodarone, methotrexate, valproic acid, and amphetamines to preclude drug-induced SLD.23 The second sensitivity analysis was performed by excluding CVD cases that occurred within 1, 2, and 3 years from the first date of follow-up to preclude already incident cases before the first date of follow-up. Restricted cubic splines were generated with 4 knots to visualise the risk of CVD across continuous variables. Cumulative incidence function was generated using the R Project for Statistical Computing (version 4.3.0; https://www.r-project.org/). All data collection, mining, and statistical analyses were performed using SAS Enterprise Guide (version 7.3, SAS Institute, Cary, NC, USA).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Baseline characteristics of each subtype of SLD
A total of 351,068 participants were finally included in the analytic cohort with a median age of 55 years (interquartile range, 50–61) and a male proportion of 56.9% (n = 199,817; Supplementary Table S1). Detailed information on overall participants is presented (Supplementary Table S1). Table 1 provides baseline characteristics of 155,477 (44.3%), 165,654 (47.2%), 22,521 (6.4%), and 7416 (2.1%) participants with no SLD, MASLD, MetALD, and ALD, respectively. Participants with MetALD tended to be younger males with a higher proportion of current smokers and a lower proportion of having comorbidities. Liver injury markers, such as aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transpeptidase (GGT) levels, in addition to fasting glucose levels, were higher in participants with MASLD, MetALD, or ALD than in those with no SLD. The prevalence of hypertension and type 2 diabetes was also higher in participants with MASLD, MetALD, and ALD as compared to no SLD (Table 1).
Table 1.
Descriptive characteristics of the participants in the National Health Insurance Service Health Screening Cohort across the subtypes of SLD.
| No SLD (n = 155,477) | MASLD (n = 165,654) | MetALD (n = 22,521) | ALD (n = 7416) | |
|---|---|---|---|---|
| Age, years | 56.9 (8.5) | 56.9 (8.0) | 54.6 (6.8) | 56.0 (7.9) |
| Sex, n (%) | ||||
| Male | 59,673 (38.4) | 111,498 (67.3) | 21,412 (95.1) | 7234 (97.5) |
| Female | 95,804 (61.6) | 54,156 (32.7) | 1109 (4.9) | 182 (2.5) |
| Household incomea, n (%) | ||||
| Upper half | 93,943 (60.4) | 109,637 (66.2) | 15,713 (69.8) | 4956 (66.8) |
| Lower half | 61,534 (39.6) | 56,017 (33.8) | 6808 (30.2) | 2460 (33.2) |
| Body mass index, kg/m2 | 22.2 (2.2) | 25.5 (2.5) | 25.0 (2.6) | 24.7 (2.8) |
| Waist circumference, cm | 76.2 (6.3) | 86.0 (6.6) | 86.4 (6.6) | 86.5 (7.1) |
| Systolic blood pressure, mmHg | 121.2 (14.7) | 127.4 (14.5) | 129.6 (14.4) | 130.4 (15.2) |
| Diastolic blood pressure, mmHg | 75.2 (9.6) | 79.3 (9.6) | 81.3 (9.7) | 81.4 (9.8) |
| Total cholesterol, mg/dL | 196.4 (34.8) | 205.7 (37.8) | 202.1 (36.9) | 199.5 (39.9) |
| HDL-cholesterol, mg/dL | 57.9 (23.9) | 52.0 (26.7) | 54.8 (23.2) | 57.2 (32.7) |
| Triglycerides, mg/dL | 99.7 (49.8) | 170.2 (100.2) | 190.4 (125.6) | 194.6 (133.8) |
| Fasting serum glucose, mg/dL | 95.3 (19.2) | 103.9 (27.1) | 107.5 (29.4) | 108.7 (30.6) |
| Alanine aminotransferase, IU/L | 18.9 (9.3) | 29.7 (21.4) | 31.1 (23.7) | 32.7 (25.2) |
| Aspartate aminotransferase, IU/L | 23.2 (8.0) | 28.0 (19.7) | 31.8 (25.4) | 35.4 (27.4) |
| γ-glutamyl transpeptidase, IU/L | 17.8 (7.7) | 47.5 (51.4) | 83.6 (92.4) | 110.3 (129.5) |
| Median (interquartile range) | 16.0 (13.0–21.0) | 34.0 (24.0–52.0) | 56.0 (37.0–93.0) | 70.0 (44.0–120.0) |
| Alcohol consumption, n (%) | ||||
| No | 110,769 (71.2) | 90,061 (54.4) | 0 (0) | 0 (0) |
| Yes | 44,708 (28.8) | 75,593 (45.6) | 22,521 (100) | 7416 (100) |
| Mildb | 38,519 (24.8) | 75,593 (45.6) | 0 (0) | 0 (0) |
| Moderatec | 5059 (3.3) | 0 (0) | 22,521 (100) | 0 (0) |
| Severed | 1130 (0.7) | 0 (0) | 0 (0) | 7416 (100) |
| Cigarette smoking, n (%) | ||||
| Never smoker | 117,991 (75.9) | 92,617 (55.9) | 5161 (22.9) | 1652 (22.3) |
| Past smoker | 19,035 (12.2) | 38,312 (23.1) | 7730 (34.3) | 2364 (31.9) |
| Current smoker | 18,451 (11.9) | 34,725 (21.0) | 9630 (42.8) | 3400 (45.8) |
| MVPA, n (%) | ||||
| No | 75,291 (48.4) | 73,376 (44.3) | 8623 (38.3) | 3451 (46.5) |
| 1–2 times/week | 25,137 (16.2) | 30,308 (18.3) | 4265 (18.9) | 1226 (16.5) |
| 3–4 times/week | 21,399 (13.8) | 25,147 (15.2) | 3849 (17.1) | 931 (12.6) |
| ≥5 times/week | 33,650 (21.6) | 36,823 (22.2) | 5784 (25.7) | 1808 (24.4) |
| Hypertension, n (%) | 37,323 (24.0) | 67,815 (40.9) | 9125 (40.5) | 2977 (40.1) |
| Type 2 diabetes, n (%) | 9439 (6.1) | 24,166 (14.6) | 3104 (13.8) | 1114 (15.0) |
| Dyslipidaemia, n (%) | 24,608 (15.8) | 41,332 (25.0) | 4435 (19.7) | 1281 (17.3) |
| Charlson comorbidity index, n (%) | ||||
| 0 | 34,505 (22.2) | 34,393 (20.8) | 5756 (25.6) | 1719 (23.2) |
| 1 | 48,161 (31.0) | 48,227 (29.1) | 7075 (31.4) | 2205 (29.7) |
| ≥2 | 72,811 (46.8) | 83,034 (50.1) | 9690 (43.0) | 3492 (47.1) |
| Statin use, n (%) | 27,782 (17.9) | 47,725 (28.8) | 5231 (23.2) | 1566 (21.1) |
| Aspirin use, n (%) | 20,523 (13.2) | 36,157 (21.8) | 4655 (20.7) | 1545 (20.8) |
| NSAIDs use, n (%) | 131,591 (84.6) | 137,203 (82.8) | 17,641 (78.3) | 5881 (79.3) |
Continuous data are presented as mean (standard deviation) (normally distributed) or medians (interquartile ranges) (not normally distributed). Categorical data are expressed as the number (%).
HDL, high-density lipoprotein; MVPA, moderate-to-vigorous physical activity; SLD, steatotic liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction-associated steatotic liver disease with increased alcohol intake; ALD, alcohol-related liver disease; NSAIDs, non-steroidal anti-inflammatory drugs.
Proxy for socioeconomic status based on the insurance premium of the National Health Insurance Service.
<30 g/day for male and <20 g/day for female.
30–60 g/day for male and 20–50 g/day for female.
>60 g/day for male and >50 g/day for female.
Temporal trend in the prevalence of SLD subtypes according to sex
The prevalence of SLD subtypes according to sex was investigated using a 5-year age stratification. We compared the prevalence of SLD subtypes across each 5-year age stratum in our sample group: 50–54, 55–59, 60–64, 65–69, 70–74, and 75–79 years. The prevalence of MASLD, MetALD, and ALD between 2010 and 2017 across sex and age strata is shown (Supplementary Fig. S1). The prevalence of MASLD was higher in the 50–54, 55–59, and 60–64 age groups for male, whereas it was higher in the 65–69 and 70–74 age groups for female. Age-standardised prevalence of MASLD increased from 51.7% in 2010–2011 to 54.3% in 2016–2017 for male (Supplementary Fig. S2).
Risk of incident CVD across different subtypes of SLD
The cumulative incidence rates for CVD, CHD, and stroke were significantly higher in MASLD, MetALD, and ALD than in no SLD (P < 0.001 by the Gray's test; Supplementary Fig. S3).
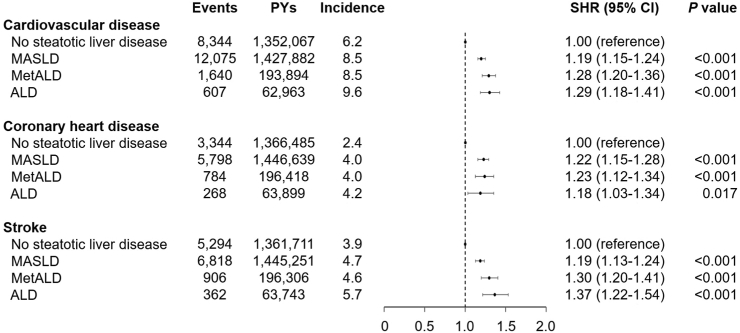
The cumulative incidence of CVD was 8.5, 8.5, and 9.6 per 1000 person-years for MASLD, MetALD, and ALD, respectively, which was higher than that for no SLD (6.2 per 1000 person-years; Fig. 2). MASLD (SHR, 1.37; 95% CI, 1.34–1.41; P < 0.001) and MetALD (SHR, 1.37; 95% CI, 1.30–1.45; P < 0.001) revealed a higher risk of incident CVD compared to no SLD in the crude model (Supplementary Table S2). After adjustments for age, sex, household income, BMI, hypertension, type 2 diabetes, dyslipidaemia, smoking, alcohol consumption, moderate-to-vigorous physical activity, and Charlson comorbidity index, MASLD (SHR, 1.19; 95% CI, 1.15–1.24; P < 0.001), MetALD (SHR, 1.28; 95% CI, 1.20–1.36; P < 0.001), and ALD (SHR, 1.29; 95% CI, 1.18–1.41; P < 0.001) had a higher risk of CVD compared to no SLD (P for trend < 0.001 for the risk of CVD, CHD, and stroke, respectively; Fig. 2). In addition, MetALD showed a higher risk of CVD than did MASLD (SHR, 1.07; 95% CI, 1.02–1.14; P = 0.011; Supplementary Table S3).
Fig. 2.
Association of steatotic liver disease subtypes with the risk of incident cardiovascular disease. Subdistribution hazard ratios (95% CIs) were calculated using the Fine and Gray’s model after adjustments for age, sex, body mass index, household income, hypertension, diabetes, dyslipidaemia, smoking, alcohol consumption, moderate-to-vigorous physical activity, Charlson comorbidity index, aspirin, and non-steroidal anti-inflammatory drugs. Acronyms: PY, person-year; SHR, subdistribution hazard ratio; CI, confidence interval; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction-associated steatotic liver disease with increased alcohol intake; ALD, alcohol-related liver disease.
The subgroup analyses showed that younger adults, males, and individuals without obesity, without hypertension, without type 2 diabetes, without dyslipidaemia, without comorbidities, and who did not take statin, aspirin, or non-steroidal anti-inflammatory drugs were apparently more affected by the SLD status in regard to a future risk of CVD (Table 2). Excluding participants with a prescription history of steatosis-inducing drugs showed similar results to those of primary analysis in terms of the SHRs of the subtypes of SLD (Supplementary Table S4). Furthermore, the sensitivity analyses considering the latent periods also revealed similar results to the primary results (Supplementary Table S5).
Table 2.
Subgroup analyses on the risk of incident cardiovascular disease across the subtypes of SLD.
| No SLD | MASLD | MetALD | ALD | Ptrend | Pinteraction | |
|---|---|---|---|---|---|---|
| Age, years | <0.001 | |||||
| Younger adults < 65 | 1.00 (reference) | 1.22 (1.16–1.27)c | 1.30 (1.21–1.39)c | 1.33 (1.20–1.48)c | <0.001 | |
| Older adults ≥ 65 | 1.00 (reference) | 1.14 (1.07–1.21)c | 1.22 (1.07–1.39)b | 1.17 (0.99–1.38) | <0.001 | |
| Sex | 0.012 | |||||
| Male | 1.00 (reference) | 1.26 (1.20–1.32)c | 1.35 (1.26–1.45)c | 1.38 (1.26–1.51)c | <0.001 | |
| Female | 1.00 (reference) | 1.10 (1.04–1.16)c | 1.16 (0.90–1.50) | 0.78 (0.38–1.57) | 0.003 | |
| Body mass index | 0.033 | |||||
| <25 kg/m2 | 1.00 (reference) | 1.20 (1.15–1.25)c | 1.29 (1.19–1.40)c | 1.25 (1.11–1.39)c | <0.001 | |
| ≥25 kg/m2 | 1.00 (reference) | 1.10 (1.02–1.19)a | 1.17 (1.05–1.31)b | 1.24 (1.07–1.44)b | 0.015 | |
| Hypertension | <0.001 | |||||
| No | 1.00 (reference) | 1.26 (1.20–1.32)c | 1.41 (1.30–1.54)c | 1.43 (1.27–1.61)c | <0.001 | |
| Yes | 1.00 (reference) | 1.08 (1.03–1.14)b | 1.10 (1.01–1.21)a | 1.10 (0.97–1.26) | 0.014 | |
| Type 2 diabetes | <0.001 | |||||
| No | 1.00 (reference) | 1.20 (1.15–1.25)c | 1.31 (1.23–1.41)c | 1.30 (1.17–1.43)c | <0.001 | |
| Yes | 1.00 (reference) | 1.09 (1.00–1.19) | 1.07 (0.92–1.24) | 1.18 (0.97–1.44) | 0.210 | |
| Dyslipidaemia | 0.005 | |||||
| No | 1.00 (reference) | 1.23 (1.18–1.28)c | 1.33 (1.24–1.42)c | 1.34 (1.22–1.48)c | <0.001 | |
| Yes | 1.00 (reference) | 1.06 (0.99–1.15) | 1.09 (0.95–1.24) | 1.06 (0.86–1.30) | 0.295 | |
| Smoking | <0.001 | |||||
| Never | 1.00 (reference) | 1.14 (1.09–1.19)c | 1.33 (1.19–1.49)c | 1.30 (1.09–1.54)b | <0.001 | |
| Past | 1.00 (reference) | 1.20 (1.11–1.31)c | 1.35 (1.20–1.53)c | 1.57 (1.33–1.85)c | <0.001 | |
| Current | 1.00 (reference) | 1.34 (1.24–1.45)c | 1.32 (1.20–1.48)c | 1.27 (1.10–1.46)c | <0.001 | |
| MVPA | 0.058 | |||||
| No | 1.00 (reference) | 1.18 (1.12–1.24)c | 1.29 (1.17–1.41)c | 1.14 (1.01–1.30)a | <0.001 | |
| 1–4 times/week | 1.00 (reference) | 1.19 (1.12–1.28)c | 1.25 (1.12–1.40)c | 1.49 (1.26–1.76)c | <0.001 | |
| ≥5 times/week | 1.00 (reference) | 1.23 (1.14–1.33)c | 1.31 (1.15–1.48)c | 1.39 (1.16–1.66)c | <0.001 | |
| CCI | 0.003 | |||||
| 0 | 1.00 (reference) | 1.27 (1.15–1.40)c | 1.41 (1.22–1.63)c | 1.41 (1.13–1.75)b | <0.001 | |
| 1 | 1.00 (reference) | 1.21 (1.12–1.30)c | 1.31 (1.17–1.48)c | 1.38 (1.17–1.64)c | <0.001 | |
| ≥2 | 1.00 (reference) | 1.16 (1.11–1.22)c | 1.22 (1.12–1.32)c | 1.21 (1.08–1.36)b | <0.001 | |
| Statin use | 0.003 | |||||
| No | 1.00 (reference) | 1.23 (1.18–1.28)c | 1.34 (1.25–1.44)c | 1.33 (1.20–1.47)c | <0.001 | |
| Yes | 1.00 (reference) | 1.08 (1.01–1.16)a | 1.09 (0.97–1.23) | 1.12 (0.93–1.34) | 0.094 | |
| Aspirin use | <0.001 | |||||
| No | 1.00 (reference) | 1.23 (1.18–1.28)c | 1.31 (1.22–1.41)c | 1.30 (1.17–1.44)c | <0.001 | |
| Yes | 1.00 (reference) | 1.07 (1.00–1.14)a | 1.18 (1.05–1.33)b | 1.22 (1.04–1.45)a | 0.022 | |
| NSAIDs use | 0.044 | |||||
| No | 1.00 (reference) | 1.30 (1.18–1.44)c | 1.47 (1.26–1.71)c | 1.28 (1.02–1.60)a | <0.001 | |
| Yes | 1.00 (reference) | 1.18 (1.13–1.22)c | 1.25 (1.17–1.34)c | 1.30 (1.18–1.43)c | <0.001 |
Subdistribution hazard ratios (95% CI) were calculated using the Fine and Gray’s model after adjustments for age, sex, body mass index, household income, hypertension, diabetes, dyslipidaemia, smoking, alcohol consumption, moderate-to-vigorous physical activity, and Charlson comorbidity index.
P for trend was calculated across the no SLD, MASLD, and MetALD groups.
SLD, steatotic liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction-associated steatotic liver disease with increased alcohol intake; ALD, alcohol-related liver disease; MVPA, moderate-to-vigorous physical activity; CCI, Charlson comorbidity index; NSAIDs, non-steroidal anti-inflammatory drugs.
P < 0.05.
P < 0.01.
P < 0.001.
Crude cause-specific HRs for incident CVD across SLD subtypes are shown (Supplementary Table S6), which revealed similar results to the Fine and Gray’s model, and the adjusted HRs (aHRs) are presented (Supplementary Table S7). Compared with MASLD, MetALD had a higher risk of CVD (aHR, 1.08; 95% CI, 1.02–1.14; P = 0.006) and no SLD had a lower risk of CVD (aHR, 0.84; 95% CI, 0.81–0.87; P < 0.001). Given that BMI and triglycerides are incorporated into the FLI, sensitivity analyses without adjustment for BMI and dyslipidaemia showed similar results to those derived from the primary fully adjusted model (Supplementary Table S8).
Among five cardiometabolic risk factors which were associated with a higher risk of CVD, abnormal blood pressure was associated with the highest risk of CVD (Supplementary Fig. S4). In addition, the number of cardiometabolic risk factors was positively correlated with a gradually increasing risk of CVD.
Cubic splines to model relationships between alcohol intake or cardiometabolic burdens and the risk of incident CVD among individuals with either MASLD or MetALD.
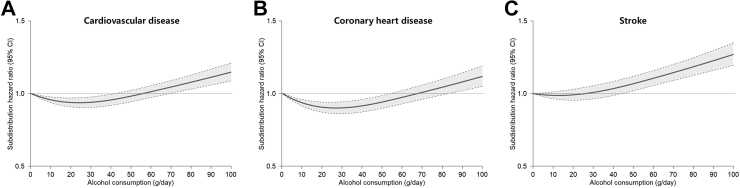
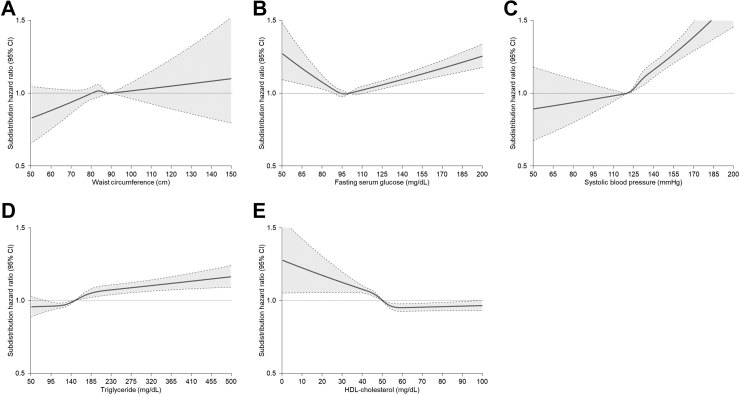
Restricted cubic splines for the association of alcohol consumption with CVD including CHD and stoke among participants with either MASLD or MetALD showed a J-shape curve for CVD (Fig. 3A) and CHD (Fig. 3B) but not for stroke (Fig. 3C). Restricted cubic splines for the association of cardiometabolic risk factors with CVD among participants with either MASLD or MetALD showed a right-upward curve for waist circumference (Fig. 4A) and a V-shape curve for fasting serum glucose (Fig. 4B). The risk of CVD gradually increased with blood pressure above 120 mmHg (Fig. 4C) and triglycerides above 150 mg/dL (Fig. 4D), which were thresholds for systolic blood pressure and serum triglycerides levels, respectively. In addition, the risk of CVD accordingly increased as HDL-cholesterol level decreased below the threshold of 60 mg/dL (Fig. 4E). Restricted cubic splines for the association of cardiometabolic risk factors with the risk of CHD and stroke are presented (Supplementary Figs. S5 and S6).
Fig. 3.
Restricted cubic splines for the association of alcohol consumption with the risk of incident cardiovascular disease among individuals with metabolic dysfunction-associated steatotic liver disease. Subdistribution hazard ratios (95% CIs) were calculated using the Fine and Gray’s model after adjustments for age, sex, body mass index, household income, hypertension, diabetes, dyslipidaemia, smoking, alcohol consumption, moderate-to-vigorous physical activity, Charlson comorbidity index, aspirin, and non-steroidal anti-inflammatory drugs. (A) Association of alcohol consumption with cardiovascular disease, (B) Association of alcohol consumption with coronary heart disease, and (C) Association of alcohol consumption with stroke.
Fig. 4.
Restricted cubic splines for the association of cardiometabolic risk factors with the risk of incident cardiovascular disease among individuals with metabolic dysfunction-associated steatotic liver disease. Subdistribution hazard ratios (95% CIs) were calculated using the Fine and Gray’s model after adjustments for age, sex, body mass index, household income, hypertension, diabetes, dyslipidaemia, smoking, alcohol consumption, moderate-to-vigorous physical activity, Charlson comorbidity index, aspirin, and non-steroidal anti-inflammatory drugs. (A) Association of waist circumference with cardiovascular disease, (B) Association of fasting serum glucose with cardiovascular disease, (C) Association of systolic blood pressure with cardiovascular disease, (D) Association of triglycerides with cardiovascular disease, and (E) Association of HDL-cholesterol with cardiovascular disease.
Discussion
In this nationwide cohort study including 351,068 participants, we demonstrated that individuals with SLD had a higher risk of incident CVD, and its risk was found to gradually increase from no SLD to MASLD and further to MetALD. This supports the significance of not only SLD itself, but also the presence of metabolic dysfunction in terms of cardiovascular risk.9 The amount of alcohol consumption showed a J-shaped association with CHD and a linear association with stroke, which was consistent with previous findings.24 Indeed, the amount of alcohol consumption served as an additional risk for CVD in SLD with metabolic dysfunction. In subgroup analyses, individuals without metabolic dysfunction (e.g., obesity, hypertension, type 2 diabetes, and dyslipidaemia) and who did not take statin were appeared to be more affected by the SLD status regarding their future CVD risk. This indicates that the new definition of SLD could be more useful for predicting CVD in metabolically healthy adults compared to those with metabolic dysfunction. Overall, each subgroup of SLD (i.e., MASLD, MetALD, and ALD) demonstrated its predictive utility of CVD compared to no SLD.
The new proposed criteria for metabolic SLD require at least one cardiometabolic risk factor to be considered to have metabolic dysfunction. In this study, ∼92.7% of individuals had at least one cardiometabolic risk factor, leading to fewer individuals with cryptogenic SLD or other specific aetiology SLD including ALD. Thus, the possible negative impact of the new criteria is that it may overlook the importance of the amount of alcohol consumption on SLD development and its complications. These criteria are less stringent than those used to define metabolic perturbations in MAFLD which require at least two cardiometabolic risk factors (∼73.7% had ≥2 metabolic dysfunctions in this study). Considering the less stringent criteria for MASLD that require fewer cardiometabolic risk factors, it is crucial to thoroughly assess whether this definition may result in a great number of individuals diagnosed with MASLD, without being able to accurately identify individuals at a higher risk of advanced liver disease or other complications including CVD. Previous studies have demonstrated that each component of metabolic dysfunction (e.g., obesity, higher fasting glucose or triglycerides, and lower HDL-cholesterol) is associated with future development of SLD,25, 26, 27 but the degree of metabolic dysfunction and the number of cardiometabolic risk factors required to induce hepatic steatosis have not yet been determined, and merit further investigation in a prospective setting. In contrast to MAFLD definition, insulin resistance and high-sensitivity C-reactive protein that may reflect the underlying pathophysiology of SLD were omitted from cardiometabolic risk factor for diagnosing MASLD.28
The association between SLD and incident CVD was more pronounced in male than in female as shown in the current study. Previous studies have shown that conventional metabolic risk factors, such as type 2 diabetes, hypertension, and dyslipidaemia, have a stronger effect on the risk of CVD in female compared with male.29, 30, 31 Male tend to engage in more unhealthy behaviours, including smoking, alcohol consumption, and higher red meat consumption, which are known to contribute to increased CVD morbidity and mortality, and these factors may obscure the association between conventional metabolic risk factors and incident CVD in male.30,32 Therefore, the more apparent association between SLD and CVD in male observed in this study warrants further investigation to determine whether sex differences have a direct causal effect on the contribution of SLD to the development of CVD. Otherwise, this discrepancy might be attributed to a higher statistical power in male, which could stem from a higher prevalence of SLD (70.1% in male vs. 36.7% in female) and CVD (7.0% in male vs. 5.7% in female) in male than in female. Irrespective of the variations in odds by sex, individuals with SLD should prioritise diligent management of their classical CVD risk factors.
SLD and chronic metabolic diseases, such as type 2 diabetes and hypertension, share common pathophysiological mechanisms, including insulin resistance and chronic inflammation, which ultimately contribute to the development of atherosclerosis and CVD.25 It is noteworthy that SLD accompanied by cardiometabolic risk factors (i.e., MASLD) remained significantly associated with the future risk of CVD even after adjusting for chronic metabolic diseases. However, the observed decreasing trend in the risk of CVD after adjusting for other metabolic diseases suggests a complex and intertwined relationship among these factors in association with SLD and the development of CVD.33 Additional research is necessary to determine the relative contribution of SLD compared with other metabolic factors to the development of CVD.
In the current study, individuals with MetALD carried a higher risk of CVD than did those with MASLD, suggesting that higher alcohol consumption may further elevate the risk of CVD in individuals with existing cardiometabolic risk factor(s). The contribution of metabolic dysfunction and alcohol intake to the development and progression of SLD is complex and influenced by various factors. Especially, the minimal threshold of alcohol consumption that could result in liver damage within the context of MASLD remains uncertain. While some studies have suggested potential protective effects associated with mild alcohol consumption,34,35 others have shown that there is no safe level of alcohol intake.36,37 Mechanistically, metabolic dysfunction and alcohol consumption exhibit distinct yet interconnected processes, synergistically driving the progression of SLD. Metabolic dysfunction, often accompanied by insulin resistance and increased adipose tissue lipolysis, results in excessive fat accumulation within the liver. Excessive fat accumulation in hepatocytes, in turn, leads to steatohepatitis and fibrosis.38 Alcohol increases blood endotoxin level, inducing oxidative stress and endoplasmic reticulum stress responses, which exacerbates both hepatic steatosis and fibrosis.39 Indeed, beyond contributing to advanced liver disease, metabolic dysfunction and alcohol consumption can directly foster endothelial dysfunction, contributing to the increased risk of CVD. One representative mechanism involved in cardiometabolic comorbidities, such as hypertension, dyslipidaemia, diabetes, and obesity, is endothelial dysfunction due to reduced nitric oxide synthesis.40,41 Alcohol consumption itself further promotes endothelial dysfunction through increased inflammation and oxidative stress.42, 43, 44 A comprehensive understanding of how these two factors shape the development of vascular complications in SLD would provide valuable insights into the precise diagnoses and prognoses prediction of each SLD subtype.
In the current study, the increasing trend of CVD risk from no SLD to MASLD and further to MetALD was primarily attributed to a higher risk of stroke rather than CHD. This can be partly explained by the interaction between the amount of alcohol consumption and the risk of individual CVD. Indeed, the association between alcohol consumption and CHD exhibited a J-shaped curve, with moderate alcohol consumption (>30 g/day, the lower cutoff for moderate consumption) associated with a lower risk of CHD. In contrast, the association between alcohol consumption and stroke showed a more linear trend, suggesting that even smaller amounts of alcohol consumption might contribute to an increased risk of stroke. This differential interaction aligns with previous reports analysing data from the Emerging Risk Factors Collaboration, EPIC-CVD, and the UK Biobank,24 accounting for the more pronounced increase in the risk of stroke in individuals with MetALD compared with those with MASLD. On the contrary, the risk of CHD did not show such a significant difference between MASLD and MetALD (Fig. 2). Further investigation is warranted to explore the potential underlying mechanisms by which alcohol contributes to a higher risk of CVD in individuals with cardiometabolic risk factors.
The main strength of this study is the utilisation of representative nationwide claims data, as approximately 97% of Koreans are enrolled in the NHIS and most receive medical services at least once a year. This large sample size allowed us to construct a prospective cohort and demonstrate the role of metabolic SLD in developing CVD.
There are several limitations to consider in this study. First, hepatic steatosis was defined using the FLI which is an indirect measurement unlike histological or radiological methods. Extensive epidemiological data on the diagnostic and prognostic performance of the FLI supports its utility as an acceptable surrogate marker for hepatic steatosis.3,17,19,45, 46, 47, 48, 49 However, since the FLI was validated in Asian populations with alcohol consumption of less than 60 g/day17 and heavy alcohol use can raise GGT levels used to calculate the FLI, the FLI might not be an appropriate surrogate marker for ALD. Second, we set competing risk analysis as a main analytic model. The risk of CVD in a setting of non-CVD-related mortality as a competing risk may differ from the risk of CVD estimated through conventional Cox proportional hazards regression that treats death as a censoring event. Third, the NHIS database has the advantage of representing the population compared to other big databases, but variables on health behaviours are limited since the data were obtained from self-reported questionnaires which can be subject to recall bias. Forth, we excluded individuals with missing values without imputation, which may have potentially introduced selection bias. Finally, due to the observational nature of the current study, we could not confirm the causal relationship or exclude residual confounding effects.
In conclusion, the new consensus criteria for SLD emphasise that individuals with MASLD and MetALD are at an increased risk of developing incident CVD. In particular, MetALD confers a higher risk of CVD than does MASLD, indicating the pathogenic role of alcohol consumption in the development of CVD when combined with cardiometabolic risk factors. These findings provide a basis for future investigations into the prognostic value of the new consensus criteria for MASLD, encompassing not only CVD, but also other clinical outcomes, such as liver-related outcomes, extrahepatic cancer, and all-cause mortality.
Contributors
The authors contributed to: Conception or design: JHM, SJ, and WK; Acquisition, analysis, or interpretation of data: JHM, SJ, HJ, BKK, and WK; Drafting the work or revising: JHM, SJ, HJ, BKK, and WK. SJ had full access to all the data and all authors verified the underlying data presented and accept responsibility to submit for publication. All authors read and approved the final version of the manuscript.
Data sharing statement
Data are available from the National Health Insurance Service (NHIS), which owns the data. Requests for data can be sent to the data owners, NHIS (http://www.nhiss.nhis.or.kr/).
Declaration of interests
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (2021R1C1C1009875 and RS-2023-00222910 to Joon Ho Moon, RS-2023-00237783 to Seogsong Jeong, and 2021R1A2C2005820, 2021M3A9E4021818 and RS-2023-00223831 to Won Kim) and the Research Program funded by the Korea Centers for Disease Control and Prevention (2022ER090200 to Won Kim).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102292.
Appendix ASupplementary data
References
- 1.Rinella M.E., Lazarus J.V., Ratziu V., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023 doi: 10.1016/j.jhep.2023.06.003. S0168-8278(23)00418-X (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Rinella M.E., Lazarus J.V., Ratziu V., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023 doi: 10.1097/HEP.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 3.Eslam M., Newsome P.N., Sarin S.K., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A., Csermely A., Petracca G., et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–913. doi: 10.1016/S2468-1253(21)00308-3. [DOI] [PubMed] [Google Scholar]
- 6.Kim D., Konyn P., Sandhu K.K., Dennis B.B., Cheung A.C., Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75(6):1284–1291. doi: 10.1016/j.jhep.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Jeong S., Oh Y.H., Choi S., et al. Association of non-alcoholic fatty liver disease with incident dementia later in life among elder adults. Clin Mol Hepatol. 2022;28(3):510–521. doi: 10.3350/cmh.2021.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon J.H., Kim W., Koo B.K., Cho N.H. Innovative target exploration of Nc. Metabolic dysfunction-associated fatty liver disease predicts long-term mortality and cardiovascular disease. Gut Liver. 2022;16(3):433–442. doi: 10.5009/gnl210167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong S., Oh Y.H., Choi S., et al. Metabolic dysfunction-associated fatty liver disease better predicts incident cardiovascular disease. Gut Liver. 2022;16(4):589–598. doi: 10.5009/gnl210256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H.L., Koo B.K., Joo S.K., Kim W. Association of arterial stiffness with the histological severity of nonalcoholic fatty liver disease. Hepatol Int. 2020;14(6):1048–1056. doi: 10.1007/s12072-020-10108-z. [DOI] [PubMed] [Google Scholar]
- 11.Park J.H., Koo B.K., Kim W., Kim W.H. Innovative Target Exploration of NC. Histological severity of nonalcoholic fatty liver disease is associated with 10-year risk for atherosclerotic cardiovascular disease. Hepatol Int. 2021;15(5):1148–1159. doi: 10.1007/s12072-021-10209-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen B., Tang W.H.W., Rodriguez M., et al. NAFLD in cardiovascular diseases: a contributor or comorbidity? Semin Liver Dis. 2022;42(4):465–474. doi: 10.1055/s-0042-1757712. [DOI] [PubMed] [Google Scholar]
- 13.Cheol Seong S., Kim Y.Y., Khang Y.H., et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46(3):799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son J.S., Choi S., Kim K., et al. Association of blood pressure classification in Korean young adults according to the 2017 American college of cardiology/American heart association guidelines with subsequent cardiovascular disease events. JAMA. 2018;320(17):1783–1792. doi: 10.1001/jama.2018.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seong S.C., Kim Y.Y., Park S.K., et al. Cohort profile: the national health insurance service-National health screening cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 17.Nomura T., Ono M., Kobayashi K., et al. Validation of fatty liver index as a predictor of hepatic steatosis in Asian populations: impact of alcohol consumption and sex. Hepatol Res. 2023;53(10):968–977. doi: 10.1111/hepr.13935. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S., Tanaka M., Higashiura Y., et al. Prediction and validation of nonalcoholic fatty liver disease by fatty liver index in a Japanese population. Endocr J. 2022;69(4):463–471. doi: 10.1507/endocrj.EJ21-0563. [DOI] [PubMed] [Google Scholar]
- 19.Bedogni G., Bellentani S., Miglioli L., et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti K.G., Eckel R.H., Grundy S.M., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y.S., Han B.D., Han K., Jung J.H., Son J.W. Taskforce team of the obesity fact sheet of the Korean society for the study of O. Obesity fact sheet in Korea, 2021: trends in obesity prevalence and obesity-related comorbidity incidence stratified by age from 2009 to 2019. J Obes Metab Syndr. 2022;31(2):169–177. doi: 10.7570/jomes22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 23.Liebe R., Esposito I., Bock H.H., et al. Diagnosis and management of secondary causes of steatohepatitis. J Hepatol. 2021;74(6):1455–1471. doi: 10.1016/j.jhep.2021.01.045. [DOI] [PubMed] [Google Scholar]
- 24.Wood A.M., Kaptoge S., Butterworth A.S., et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391(10129):1513–1523. doi: 10.1016/S0140-6736(18)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Targher G., Corey K.E., Byrne C.D., Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 26.Zheng R., Du Z., Wang M., Mao Y., Mao W. A longitudinal epidemiological study on the triglyceride and glucose index and the incident nonalcoholic fatty liver disease. Lipids Health Dis. 2018;17(1):262. doi: 10.1186/s12944-018-0913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou Y., Zhong L., Hu C., Zhong M., Peng N., Sheng G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: a 5-year longitudinal cohort study. Lipids Health Dis. 2021;20(1):28. doi: 10.1186/s12944-021-01457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim G.A., Moon J.H., Kim W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: implication of Janus-faced modernity. Clin Mol Hepatol. 2023;29(4):831–843. doi: 10.3350/cmh.2023.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juutilainen A., Kortelainen S., Lehto S., Ronnemaa T., Pyorala K., Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27(12):2898–2904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 30.Garcia M., Mulvagh S.L., Merz C.N., Buring J.E., Manson J.E. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin M.A., Hokanson J.E., Edwards K.L. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 32.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95(1):252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed H.S., Wang N., Carr J.J., et al. The association between hepatic steatosis and incident cardiovascular disease, cancer, and all-cause mortality in a US multicohort study. Hepatology. 2023;77(6):2063–2072. doi: 10.1097/HEP.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn W., Sanyal A.J., Brunt E.M., et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2012;57(2):384–391. doi: 10.1016/j.jhep.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon H.K., Greenson J.K., Conjeevaram H.S. Effect of lifetime alcohol consumption on the histological severity of non-alcoholic fatty liver disease. Liver Int. 2014;34(1):129–135. doi: 10.1111/liv.12230. [DOI] [PubMed] [Google Scholar]
- 36.Aberg F., Helenius-Hietala J., Puukka P., Farkkila M., Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67(6):2141–2149. doi: 10.1002/hep.29631. [DOI] [PubMed] [Google Scholar]
- 37.Chang Y., Cho Y.K., Kim Y., et al. Nonheavy drinking and worsening of Noninvasive fibrosis markers in nonalcoholic fatty liver disease: a cohort study. Hepatology. 2019;69(1):64–75. doi: 10.1002/hep.30170. [DOI] [PubMed] [Google Scholar]
- 38.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You M., Fischer M., Deeg M.A., Crabb D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277(32):29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 40.Fornoni A., Raij L. Metabolic syndrome and endothelial dysfunction. Curr Hypertens Rep. 2005;7(2):88–95. doi: 10.1007/s11906-005-0080-6. [DOI] [PubMed] [Google Scholar]
- 41.Petrie J.R., Ueda S., Webb D.J., Elliott H.L., Connell J.M. Endothelial nitric oxide production and insulin sensitivity. A physiological link with implications for pathogenesis of cardiovascular disease. Circulation. 1996;93(7):1331–1333. doi: 10.1161/01.cir.93.7.1331. [DOI] [PubMed] [Google Scholar]
- 42.Puddey I.B., Zilkens R.R., Croft K.D., Beilin L.J. Alcohol and endothelial function: a brief review. Clin Exp Pharmacol Physiol. 2001;28(12):1020–1024. doi: 10.1046/j.1440-1681.2001.03572.x. [DOI] [PubMed] [Google Scholar]
- 43.Hwang C.L., Muchira J., Hibner B.A., Phillips S.A., Piano M.R. Alcohol consumption: a new risk factor for arterial stiffness? Cardiovasc Toxicol. 2022;22(3):236–245. doi: 10.1007/s12012-022-09728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka A., Cui R., Kitamura A., et al. Heavy alcohol consumption is associated with impaired endothelial function. J Atheroscler Thromb. 2016;23(9):1047–1054. doi: 10.5551/jat.31641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong V.W., Chan W.K., Chitturi S., et al. Asia-pacific working party on non-alcoholic fatty liver disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33(1):70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 46.Khang A.R., Lee H.W., Yi D., Kang Y.H., Son S.M. The fatty liver index, a simple and useful predictor of metabolic syndrome: analysis of the Korea National Health and Nutrition Examination Survey 2010-2011. Diabetes Metab Syndr Obes. 2019;12:181–190. doi: 10.2147/DMSO.S189544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang X., Xu M., Chen Y., et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle-aged and elderly Chinese. Medicine (Baltimore) 2015;94(40) doi: 10.1097/MD.0000000000001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z., Li H., Tian S., et al. Blood biomarkers for the diagnosis of hepatic steatosis in metabolic dysfunction-associated fatty liver disease. J Hepatol. 2020;73(5):P1264–P1265. doi: 10.1016/j.jhep.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Chung G.E., Jeong S.M., Cho E.J., et al. Association of fatty liver index with all-cause and disease-specific mortality: a nationwide cohort study. Metabolism. 2022;133 doi: 10.1016/j.metabol.2022.155222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.