Abstract
The determination of volatile compounds is essential for the chemical characterisation of honey's aroma and its correlation to its sensory profile and botanical origin. The present study describes the development, optimization and validation of a new, simple and reliable method for the determination of volatile compounds in honey using headspace solid-phase microextraction combined with gas chromatography/mass spectrometry (HS-SPME-GC-MS). The optimization of the SPME conditions showed that the ratio of honey: water (2:1) and the incubation temperature (60 °C) are the most critical parameters. Gas chromatography was performed with medium polar Varian CP-Select 624 column and the experimental Retention Index for a number of compounds was determined as an additional identification feature for suspect analysis. The simultaneous use of four internal standards chlorobenzene, benzophenone, 2-pentanol and 4-methyl-2-pentanone and matrix matched calibration enhanced method accuracy achieving recoveries 73–114 % and repeatability ranging between 3.9 and 19 % relative standard deviations. Furthermore, the superiority of the HS-SPME to static head space technique was verified exhibiting four-to nine-fold higher sensitivity. Target and suspect screening were applied to 30 Greek honey samples and 53 volatile compounds belonging to different chemical classes, such as alkanes, aldehydes, ketones, alcohols, and esters were identified with quantified concentrations ranging between 3.1 μg kg−1 (Limonene) up to 20 mg kg−1 (Benzeneacetaldehyde). Among the new findings is the detection of Myrtenol in Greek pine honey and 2,3-butanediol in Greek oak honey. The developed analytical protocol can be a valuable tool in order to chemically characterize honey based on the volatile content.
Keywords: Greek honey, Volatile compounds, Retention index, Target screening, Suspect screening, Multivariate experimental design
Graphical abstract
Highlights
-
•
Honey/water ratio and incubation temperature are critical HS-SPME parameters.
-
•
Determination of Retention Indexes of honey volatiles for medium-polar GC column.
-
•
Multiple internal standard normalization improves HS-SPME-GC-MS quantification.
-
•
Determination of 53 volatile compounds in Greek honey varieties.
1. Introduction
Honey is a natural sweetener produced by Apis Mellifera bees [1]. It mainly consists of sugars and water, along with other ingredients in minor quantities such as vitamins, minerals, amino acids, flavonoids, carotenoids, phenolic acids, and volatile compounds. The volatile profile of honey includes alcohols, aldehydes, ketones, esters, hydrocarbons, furans, pyrans, terpenes and benzene derivatives, and provides honey with a unique aroma [2]. Thus, the volatile composition substantially influences honey's quality and consumers' preferences in national and worldwide markets. In Greece, honey is among the most important foods and is considered a premium product. Its importance for the Greek economy is undeniable. Among the countries of the European Union (EU), Greece was in third place regarding the number of beehives per beekeeper in 2021, showing an increase of 33.8 % in comparison to the previous year, while its annual production reached 21000 tons, corresponding to a consumption rate of 1.7 kg per person [3].
Honey's nutritional and commercial value render the product susceptible to adulteration. Authenticity assessment can decrease such incidents by verifying its botanical and geographical origin to avoid mislabeling [4]. Consequently, reliable and validated analytical methodologies are required for honey characterization and authenticity verification. Several research studies have been conducted to establish honey's authenticity, and different analytical techniques have been applied to determine honey's constituents. Indicatively, honey's phenolic composition has been mainly studied using Liquid Chromatography coupled to Mass Spectrometry (LC-MS) [[5], [6], [7]], while Inductively Coupled Plasma Spectroscopy (ICP-MS) and Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) have been used for the determination of mineral content [8,9].
Gas chromatography coupled to Mass Spectrometry (GC-MS) has been worldwide used in a number of studies for the authentication of the botanical, geographical and entomological origin of honey, as well as for the authentication of honey production practices [10]. This analytical technique is characterized by high sensitivity and selectivity as well as by high degree of identification ability through the mass spectral information provided for each eluted chromatographic peak. GC-MS has been combined with both static headspace (SHS) and Headspace Solid Phase Microextraction (HS-SPME) for the determination of honey volatile and semi-volatile compounds. Static headspace was used in the comparative analysis of the volatile composition of honeys from Brazilian stingless bees [11], while HS-SPME, which is a powerful technique as it is a simple, sensitive, rapid, and low-cost, has been applied to a wider extent [[12], [13], [14], [15], [16], [17], [18]]. Although it is well-known that HS-SPME is more sensitive than SHS, comparison studies between these two techniques based on validation criteria are rare.
Most of the HS-SPME methods reported in the literature have been optimized in order to determine the optimum extraction conditions for the volatiles [12,13]. However, it is noted that in the majority of these studies limited validation data are provided, rendering the quantification results of increased uncertainty, except for few studies such as the one which dealt with the establishment of authenticity and typicality of sugarcane honey, where a comprehensive validation of the developed methodology was performed [14]. Moreover, in the available HS-SPME-GC-MS methods for honey volatile determination, either a single internal standard (IS) is used for the normalization of the analytes, usually benzophenone and 2-pentanol [[15], [16], [17]] or even no internal standard may be reported [10]. This could lead to biased quantified results and/or of increased uncertainty since the volatile analytes belong to different chemical groups and the correction with the same IS is not always appropriate.
In order to perform a thorough characterization of honey and widen the chemical space of the applied methods, suspect screening is often performed since not all the eluted peaks can be usually identified with available analytical standards. Retention Index (RI) is an identification feature that increases reliability of the findings and has been widely used in GC analysis. RI values are correlated with system stationary phases. In the majority of studies on the volatile content of honey, non-polar analytical columns, such as HP-5 MS, Rtx-5MS, Rtx-1 or DB-5MS have been used [[17], [18], [19]] as well as polar columns, like DB-WAX and Rtx-WAX [20,21], whereas there is limited information on the application of alternative medium polar columns such as DB-624 for the determination of volatile content in honey [22].
The aim of this study was to address the identified gaps in the literature regarding the GC-MS determination of volatile and semi-volatile compounds in honey and propose a reliable, validated, low-cost analytical method that can contribute to the identification of compounds responsible for honey's aroma and serve as a useful tool for authentication studies. Therefore, a novel HS-SPME-GC-MS method was developed, after a stepwise optimization, that allows the determination of 53 volatiles in honey through target and suspect screening approaches. In addition, a static headspace SHS GC-MS method was developed and validated, and the figures of merit of both techniques, HS-SPME and SHS were compared. The developed HS-SPME-GC-MS method uses multiple internal standard normalization, in order to achieve more accurate quantification of all the volatiles. The applicability of the proposed methodology was assessed by analysing 30 honey samples belonging to 10 different botanical origins collected from various geographical regions of Greece.
2. Material and methods
2.1. Honey samples
A total of 30 Greek honey samples from 10 different botanical origins, namely pine (n = 3), fir (n = 4), oak (n = 2), thyme (n = 4), heather (n = 3), chestnut (n = 2), orange (n = 3), arbutus (n = 4), sage (n = 3), and carob (n = 2) were collected directly from beekeepers from all over Greece during the harvesting period of 2019. The botanical origin was firstly assessed by the producers and confirmed by physicochemical and melissopalynological analysis. All samples were stored in closed glass containers and maintained in a dark room at 4 °C until analysis in order to prevent unnecessary exposure to the sun and high temperatures, that may lead to honey degradation or affect the quality of the samples. Details about both the botanical and geographical origins of each sample are given in Table S1.
2.2. Chemicals and standard solutions
All reagents used in this study were of analytical grade. Specifically, LC-MS grade methanol (MeOH) was purchased from Merck (Darmstadt, Germany), ultra-pure water (18.2 MΩ cm−1) was provided by a Milli-Q water purification system (Direct-Q UV, Millipore, Bedford, MA, USA), while sodium chloride (NaCl) was supplied by Lach:ner (Neratovice, Central Bohemia, Czech Republic).
Standard solutions of a mix of carboxylic acids with purity >97.5 % (pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid, and tridecanoic acid), a mix of alkanes with purity >97.0 % (hexane, heptane, octane, nonane, decane, undecane, dodecane, tetradecane, pentadecane, hexadecane, heptadecane, and octadecane), isophorone, 3-pentanone, 1-hexanol, 1-octanol, 1-nonanol, ethyl octanoate, ethyl nonanoate, β-damascenone, and the IS chlorobenzene, benzophenone, 2-pentanol and 4-methyl-2-pentanone were purchased from CPAchem (Bogomilovo, Bulgaria). Standards of undecanol, ethyl hexanoate along with the aldehydes, butanal, pentanal, hexanal, heptanal, octanal, nonanal, decanal, and benzaldehyde were purchased from Sigma Aldrich (Oakville, ON, Canada). The purity of all the previously mentioned analytical standards was >96.0 %. A mixture of the four IS, benzophenone, chlorobenzene, 2-pentanol and 4-methyl-2-pentanone (purity >99.0 %) was prepared at a concentration of 100 μg mL−1 in MeOH by appropriate dilutions of the individual standards.
2.3. GC-MS analysis
The chromatographic system was a SCION 456-EVOQ TQ from Bruker Daltonics. The injection was performed in splitless mode. A Varian CP-Select 624 column (30 m × 32 mm i.d. × 1.80 μm film thicknesses) was used to separate the analytes. The oven program was as follows: initially, the temperature was set at 35 °C for 2 min, then it was increased to 140 °C with a rate of 10 °C min−1 and held for 5 min, and finally it was increased to 240 °C and held for another 5 min. Overall, the total time of the chromatographic separation was 32.5 min. Helium was used as the carrier gas (purity 99.999 %) at a flow rate of 1.5 mL min−1. The mass spectra of the analytes were recorded in 35–500 m/z range. During the analysis, the injector was maintained at 240 °C, while the transfer line temperature was 250 °C. The EI ionization source was held at 240 °C with ionization energy 70 eV. Data processing was performed using Bruker Daltonics MS workstation.
2.4. Optimization of HS-SPME method
The optimization experiments were performed using one of the pine honey samples of the present study. The untreated honey sample and the same sample spiked with the target analytes, presented in Section 2.2, at a concentration of 100 μg kg−1 was analysed under different SPME conditions. The evaluation of the optimum method parameters was based on the peak areas and relative standard deviation (RSDs %) of specific target analytes and some suspect analytes that were detected in this pine sample through suspect screening.
In detail, the effect of the following parameters was evaluated: (i) honey:water ratio, (ii) addition of NaCl, (iii) incubation time, (iv) incubation temperature, and (v) sampling time. The fiber coating selected for the SPME was divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS). Its selection was based on previous studies according to which this mixed coating provided high extraction efficiencies regarding a great range of volatiles with different polarities and molecular weights, and exhibited better extraction properties in comparison to other types, such as polyacrylate (PA), polydimethylsiloxane (PDMS), divinylbenzene (DVB), and mixture of them (CAR/PDMS, PDMS/DVB) [2,14,23].
The method optimization started by testing of different honey:water ratios since sample dilution is a factor that should be investigated in order to obtain reproducible results and avoid problems related to sample viscosity [14]. Three different levels of honey:water ratios were tested in triplicate i) 1:2, ii) 1:1, and iii) 2:1 ratio. Higher levels of honey:water ratio were not investigated in order to avoid the possibility of saturation effects. Based on the results, the ratio 2:1 was adopted as the optimum, derived from a dilution of 4 g of honey with 2 mL of water.
As a second step, the addition of NaCl was investigated. In general, the equilibration between the liquid and gas phases is affected by the addition of inorganic salts. Their presence can change the nature of the molecular interactions and increase the ionic activity, leading to a higher concentration of volatile compounds in the gas phase. The presence of an inorganic salt, such as NaCl, may affect extraction efficiency in terms of repeatability and sensitivity [12]. During the optimization experiments, NaCl was added for this purpose in two different quantities: i) 0.25 g and ii) 0.50 g. The final selected value was 0.25 g.
Subsequently, multivariate experimental design for the optimization of incubation time, incubation temperature and sampling time was conducted in order to evaluate their potential interferences using the statistical software Develve. It is noted that several studies have indicated the importance of these parameters in determining the volatile content of honey and following either a univariate or a multivariate optimization procedure, different values of these factors have been proposed as optimum [2,13,23]. Therefore, the tested values for the incubation time were 15 min, 20 min, and 30 min, for the incubation temperature 45 °C and 60 °C and for the sampling time 10 min, 20 min and 30 min. The multivariate experimental design resulted in 18 experiments which are presented in Table S2. For every experiment, 4 g of honey and 2 mL of aqueous solution of NaCl water (6.25 % w/v) were added in 20-mL vials, the vials were vortexed for 1.5 min and the defined values of the three tested parameters were applied. Each experiment was performed in triplicate.
2.5. Final HS-SPME protocol
Four (4) g of honey, 2 mL of distilled water and 0.25 g NaCl were added into a 20-mL screw-cap vial. Next, 10 μL of IS mix was spiked and the vial was immediately sealed. The honey sample was vortexed for 1.5 min to be well-homogenized, and then it was incubated for 15 min at 60 °C under continuous stirring. After that, a preconditioned and thermally cleaned fiber of DVB/CAR/PDMS 50/30 μm thick (Supelco) was introduced into the vial and exposed for 10 min to absorb the volatile compounds. Finally, the fiber was transferred to the GC injector port and desorbed for 8 min.
2.6. IS selection
The suitability of (i) chlorobenzene, (ii) benzophenone, (iii) 2-pentanol, and (iv) 4-methyl-2-pentanone as IS was investigated for each target analyte. These compounds were selected because they do not naturally occur in honey, they belong to different chemical classes and they cover a wide range of retention times and molecular masses, enabling the normalization of different kinds of volatiles present in honey. The effect of each IS on method's performance characteristics, namely correlation coefficient of standard addition curve (R2), RSD %, Recovery %, limit of detection (LOD), and limit of quantification (LOQ) were examined separately for each compound in order to select the most appropriate one.
2.7. Method validation
Validating the optimized analytical methodοlogy was a crucial step in order to confirm that it is fit-for-purpose. The method validation focused on linearity, matrix effect, LODs, and LOQs calculation, as well as accuracy (trueness and precision) evaluation, according to the recommendations of the Association of Official Analytical Chemists.
Linearity was evaluated by preparing standard addition curves. More specifically, an untreated pine honey sample was spiked with each analyte and then calibration curves were created by plotting the ratio “analyte peak area/IS peak area” versus the corresponding concentration, and the least squares regression coefficients (R2) were calculated. For the analytes that were determined in the unspiked pine sample, their calibration curves were constructed by subtracting the peak area of the unspiked sample from the spiked ones. Matrix effects were calculated for each analyte according to Equation (1) where “Peak area mm” is the response in the matrix-matched standard, “Peak area unspiked sample” is the analyte's response in the untreated sample, and “Peak area standard” is the analyte's response in a standard solution prepared in solvent.
| (1) |
Precision was assessed through repeatability and intermediate precision experiments. The repeatability of the method was determined by analyzing six replicates of a spiked sample at 3 different concentration levels (10 μg kg−1, 50 μg kg−1 and 200 μg kg−1) under the same conditions, on the same day, while for intermediate precision, the same experiment was performed on two consecutive days. Precision was expressed in terms of RSD %.
Trueness was determined in terms of recovery, and was calculated as the ratio of the spiked sample concentration calculated from the calibration curves versus the nominal concentration [14,24]. The LODs and LOQs for each analyte were calculated as the concentration for which the signal-to-noise ratio was 3 and 10, respectively.
2.8. Static headspace method
In the framework of the present work a static headspace method was also developed and validated following the guidance of the AOAC, similarly to HS-SPME. The optimized parameters from HS-SPME method were kept the same. Thus, 4 g of honey, 2 mL of distilled water, and 0.25g of NaCl were introduced into a 20-mL screw-cap vial. A volume of 10 μL of IS mix was then spiked into the vial, and the sample was vortexed for 1.5 min to be well-homogenized. Next, the sample was incubated for 15 min at 60 °C under continuous stirring. Finally, 1000 μL of sample from the vial headspace obtained using the gas-tight syringe autosampler and were injected into the chromatographic system.
2.9. Target and suspect screening of honey samples
2.9.1. Target screening
A target compound database was created and used for the evaluation of the method's optimization, validation, and applicability in real honey samples. This database included 38 volatiles belonging to different chemical classes, and more specifically, 12 alkanes, 3 esters, 7 aldehydes, 4 ketones, 4 alcohols, and 8 organic acids (Table S3). For these analytes, analytical standards were available, as presented in Section 2.2. From each of these analytical standards, a working solution with a concentration of 1000 μg mL−1 was prepared and analysed to obtain individual chromatograms and full-scan mass spectra. Extracted Ion Chromatograms (EICs) were generated for each analyte using the molecular ions (M+), and retention time, along with the three most abundant ions, were recorded. The most abundant ion was used for the quantification of the volatiles in the samples (quantifier ion), while the other two were used for compound identification (qualifier ions).
Matrix-matched calibration curves prepared by adding known amounts of the target analytes and the mix of the internal standards in pine honey sample were used for the quantification of volatiles. The calibration curves plotted the ratio “analyte peak area/IS peak area” against the corresponding concentration. For the normalization of each volatile, the most suitable IS was used based on the derived results from the IS selection procedure (Section 3.2 and Table S8 in supplementary material). In terms of quality assurance one level recovery tests were performed in the rest of the examined honey varieties during the analysis of the samples. The recoveries obtained were in agreement with those obtained with the pine variety.
2.9.2. Suspect screening
A suspect list of 31 volatile compounds was created based on a literature review on the volatile compounds identified in Greek and international honey samples (Table S4) [3,7,15]. Using the program NIST MS SEARCH, the spectra of these selected compounds were exported to a created file, which was imported into the final method by using the Bruker MS Workstation and Method Editor programs. Thus, the identification of these volatiles was achieved by matching their mass spectra, derived from the analysed honey samples, with those of the National Institute of Standards and Technology library (NIST search 2.3). For the identification, a similarity threshold higher than 80 % was necessary [14,19].
Furthermore, the experimental Retention Indexes (RIs) of the detected volatiles were calculated using the mixture of alkanes (C4–C20) according to equation (2) [25]. Measurement of RIs of chemical compounds and comparison with available retention-data collections represents the usual approach in compound identification. These calculated RIs were compared with corresponding values obtained from literature, using a column with a similar polarity. Regarding RI's it has to be noted that, in the vast majority of studies presenting the determination of honey volatiles, non-polar columns, such as HP-5 MS, Rtx-5MS or DB-5MS, have been used [13,17,19]. Moreover, the use of polar columns like DB-WAX and Rtx-WAX, has also been reported [20,21,26]. None of these columns is equivalent to CP-Select 624 which was used in the present study. The published references on the determination of volatiles in honey with this type of column is limited to only one [22]. For this reason, we extended our search in other food matrices [27,28]. However, there were still no available data for some compounds.
| (2) |
where,
tx: Retention time of suspect analyte
tn: Retention time of n-alkanes eluting immediately before the compound
t(n+1): Retention time of n-alkanes eluting immediately after the compound.
Semi-quantification was achieved based on the ratio of peak areas of the detected volatiles to those of the selected IS multiplied by the final concentration of the IS, considering a response factor (RF) equal to 1 for all the compounds.
3. Results and discussion
3.1. Method optimization
The results of the honey:water ratio optimization revealed that the 2:1 ratio was more effective than 1:1 and 1:2 achieving higher sensitivity in terms of peak area and better precision in terms of RSD% as summarized in Table S5 for nine target analytes. This is also illustrated in Fig. S1 where the peak areas of benzeneacetaldehyde, isophorone and nonane are compared for the different ratios honey: water. In more detail it is shown that:
-
(a)
Benzeneacetaldehyde was not detected at all when the honey:water ratio was 1:2, with its peak area being higher (1.4 × 106) when the ratio was 2:1 than the corresponding one under the 1:1 phase ratio (7.3 × 105). RSDs % were similar under 1:1 (4.5 %) and 2:1 (4.4 %) ratios.
-
(b)
The mean peak area of isophorone was 5.5 × 105 (RSD 7.8 %) under a honey:water ratio of 1:2. When the ratio was increased to 1:1, the peak area was almost doubled (1.2 × 106 with RSD 6.9 %), and a further increase to 2:1 led to ever better sensitivity and precision (1.5 × 106, RSD 6.0 %).
-
(c)
Nonane detection was significantly increased under a honey:water ratio of 2:1 (3.4 × 106 and 3.5 %) in comparison with both 1:1 (2.4 × 106 and 7.5 %), and 1:2 (2.2 × 106 and 9.9 %).
Regarding the effect of NaCl content on analytes' responses, no statistically significant difference was observed between the peak areas obtained with the two tested values 0.25 g and 0.50 g of NaCl (Fig. S2 and Table S6). Based on these results, the addition of 0.25 g of NaCl was selected. These results are in agreement with those of other studies, which demonstrated that adding extra salt did not significantly affect the extraction of volatile compounds [11].
The results of the multivariate experimental design for the incubation time, incubation temperature and sampling time are summarized in Table 1. Incubation temperature was coded as A, incubation time as B and sampling time as C. For the evaluation of results, the under-optimization analytes were divided into six groups based on their chemical class. Evaluating the derived p-values, incubation temperature is the most important factor of the optimization procedure (p « 0.05). More specifically, an incubation temperature of 60 °C improved the response of all analytes. This observation is in accordance with previous scientific research, in which the leading role of the incubation temperature was highlighted [2]. On the other hand, incubation time was not statistically important since the p-values for all the tested chemical classes were greater than 0.05. Another factor that was statistically important for some compounds (p < 0.05) was sampling time, as it had a positive effect on the extraction of ketones, aldehydes, alcohols and especially organic acids. However, the less volatile alkanes seemed to be unaffected by sampling time, while the response of esters expressed as peak areas were reduced when the sampling time was higher.
Table 1.
Statistical analysis of the multivariate experimental design data of HS-SPME. (A: Incubation temperature, B: incubation time and C: sampling time).
| Esters | Alkanes | Aldehydes | Ketones | Acids | Alcohols | |
|---|---|---|---|---|---|---|
| R2 | 0.946 | 0.912 | 0.974 | 0.962 | 0.856 | 0.947 |
| Total SS | 5.4 × 108 | 1.4 × 1015 | 4.5 × 1013 | 1.9 × 1013 | 1.6 × 1013 | 2.5 × 1013 |
| Total df | 17 | 17 | 17 | 17 | 17 | 17 |
| p-value A | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| p-value B | 0.14 | 0.85 | 0.68 | 0.46 | 0.11 | 0.17 |
| p-value C | 0.05 | 0.08 | 0.01 | 0.02 | 0.01 | 0.02 |
| p-value A × B | 0.02 | 0.26 | 0.11 | 0.10 | 0.04 | 0.01 |
| p-value A × C | 0.66 | 0.02 | 0.29 | 0.02 | 0.45 | 0.63 |
| p-value B × C | 0.31 | 0.57 | 0.35 | 0.83 | 0.19 | 0.25 |
| Coefficient A | 5.4 × 108 | 3.7 × 108 | 1.6 × 107 | 9.8 × 106 | 2.6 × 106 | 1.2 × 107 |
| Coefficient B | −6.2 × 107 | 6.9 × 106 | 3.2 × 105 | −4.6 × 105 | 6.1 × 105 | 8.9 × 105 |
| Coefficient C | −8.8 × 107 | −7.4 × 107 | 2.7 × 106 | 1.7 × 106 | 1.4 × 106 | 1.7 × 106 |
| Coefficient A × B | 1.1 × 108 | 4.2 × 107 | 1.3 × 106 | −1.0 × 105 | 7.8 × 105 | 2.6 × 106 |
| Coefficient A × C | −1.7 × 107 | −1.0 × 108 | 8.5 × 105 | −1.8 × 106 | −2.7 × 105 | 3.0 × 105 |
| Coefficient B × C | 4.8 × 107 | 2.6 × 107 | 8.8 × 105 | −1.6 × 105 | −9.8 × 105 | 8.5 × 105 |
| Effect A | 17 | 12 | 25 | 19 | 9.1 | 24 |
| Effect B | −1.6 | 0.19 | 0.4 | −0.78 | 1.8 | 1.5 |
| Effect C | −2.3 | −2.0 | 3.5 | 2.8 | 4.1 | 2.7 |
| Effect A × B | 3.0 | 1.2 | 1.8 | −1.8 | 2.4 | 4.6 |
| Effect A × C | −0.5 | −2.8 | 1.1 | −3.0 | −0.79 | −0.51 |
| Effect B × C | 1.1 | 0.60 | 0.90 | −0.22 | −1.4 | −1.2 |
| Min Area | 1.3 × 107 | 1.2 × 109 | 3.1 × 107 | 2.0 × 107 | 1.7 × 106 | 3.2 × 107 |
| Max Area | 1.6 × 108 | 1.8 × 109 | 4.1 × 107 | 4.8 × 107 | 9.3 × 106 | 6.6 × 107 |
| Mean Area | 8.5 × 107 | 1.5 × 109 | 3.5 × 107 | 3.4 × 107 | 6.4 × 106 | 4.5 × 107 |
| Std Dev | 5.7 × 107 | 2.1 × 108 | 3.6 × 106 | 2.1 × 106 | 2.8 × 106 | 1.6 × 106 |
Regarding the interaction between the investigated factors, the p-value of incubation time × sampling time was >0.05 in all cases. A simultaneous increase of both incubation temperature and time provided an increase in the response of esters, alcohols, and organic acids, while the simultaneous increase of incubation temperature and extraction time had a negative effect on alkanes and ketones. R2 indicates how efficiently the data is fitting the regression model. The lower value was that of organic acids (0.856), while aldehydes had the highest R2 (0.974). The majority of chemical classes had a satisfactory R2 > 0.90.
Summarizing the results, the highest temperature (60 °C) was selected as the optimum one for the final protocol. The shortest incubation time (15 min) was selected since this parameter proved to be in all cases statistically insignificant. This value of incubation time has also been selected in other optimized methods and allows shorter analysis time [2,13]. Finally, a 10-min sampling time was selected. In Fig. S3, the effects of equilibration time, equilibration temperature, and sampling time on the extraction of alcohols and aldehydes are illustrated.
3.2. Selection of the IS
All four IS were applied for the normalization of all target analytes' peak areas for the determination of correlation coefficient of standard addition curve (R2), RSD %, Recovery %, LOD and LOQ. An example of the IS optimization methodology is presented in Table S7 regarding the normalization of octanal, while the selected IS for all the investigated analytes are presented in Table S8. The chemical similarity of the IS to the analytes, as well as their retention times, appeared to be the two most important parameters affecting mainly the repeatability of the signals’ ratio “analyte peak area/IS peak area” since the higher the similarity between analyte and IS the higher the similarity in extraction and chromatographic behavior.
It is noted that the method's performance on the last eluting analytes (RT > 20 min) was substantially improved when the analytes' peak areas were normalized with benzophenone peak area (RT = 27.8 min), and the same behavior was observed for organic acids, which are compounds with lower volatility. Benzophenone was also found to normalize benzaldehyde better, maybe due to their similar chemical structure consisting of a benzene ring. For the correction of the other analytes, 2-pentanol was found to be the most effective IS for alcohols, while 2-pentanone-4-methyl was the most suitable one for aldehydes and ketones. Regarding other analytes, such as esters and alkanes, for which no IS of the same chemical class was available, the best results were obtained with chlorobenzene.
To the best of our knowledge, this is the first time that such a thorough investigation regarding the suitability of the IS used in honey volatiles is reported. A single IS has been used for the correction of volatiles in the vast majority of studies [16,19]. Some researchers corrected all the identified volatiles with benzophenone [15,17] and also 2-pentanol has been used as a single IS [21]. However, this study clearly indicates that the application of methods using only one IS for the determination of volatile compounds from different classes is not the most appropriate analytical approach.
3.3. Method validation
Table 2 summarizes the validation data of the developed HS-SPME GC-MS method for 6 indicative analytes (isophorone, 1-nonanol, ethyl octanoate, octanal, nonane and nonanoic acid), belonging to different chemical classes. The results of the full validation of the method are presented in the supplementary material (Tables S9–S10). Regarding the linearity of the HS-SPME method, satisfactory linearity with correlation coefficients higher than 0.98 was achieved for all the analytes apart from the organic acids, dodecanoic and tridecanoic. Moreover, 25 out of 36 compounds achieved R2 > 0.99. Considering the evaluation of the matrix effect, the majority of volatiles showed a positive effect. Furthermore, 20 volatiles showed a matrix effect % > +20, indicating ion enhancement, while 5 compounds showed a matrix effect% < −20, indicating ion suppression. The fact that a large number of compounds showed a matrix effect more than ±20 % makes the preparation of matrix-matched calibration curves necessary for the adequate quantification of volatiles. Moreover, regarding recoveries, repeatability and intermediate precision, all analytes in all concentration levels conformed to the guidance of AOAC [29] achieving recoveries between 73 and 114 %, repeatability ranging between 1.2 and 18 RSD %, and intermediate precision 2.9–24 RSD %. Finally, LODs ranged from 2.12 μg kg−1 (decane) to 11.5 μg kg−1 (tridecanoic acid).
Table 2.
Validation data of HS-SPME method. (LL: Low Level: 10 μg kg−1, ML: Medium Level: 50 μg kg−1, HL: High Level: 200 μg kg−1).
| Isophorone | 1-Νonanol | Ethyl Octanoate | Octanal | Nonane | Nonanoic acid | ||
|---|---|---|---|---|---|---|---|
| Corelation coefficient (R2) | 0.9985 | 0.9985 | 0.9981 | 0.9992 | 0.9991 | 0.9900 | |
| LOD (μg kg−1) | 3.2 | 3.0 | 2.3 | 2.7 | 2.9 | 8.0 | |
| LOQ (μg kg−1) | 9.6 | 9.0 | 6.9 | 8.0 | 8.6 | 24 | |
| Repeatability RSD% | HL | 2.3 | 5.3 | 3.5 | 2.5 | 5.1 | 6.3 |
| ML | 6.5 | 7.4 | 13 | 6.8 | 7.6 | 13 | |
| LL | 8.1 | 11 | 14 | 12 | 9.2 | 11 | |
|
Intermediate Precision RSD% |
HL | 4.7 | 9.8 | 8.4 | 3.2 | 8.2 | 9.9 |
| ML | 7.8 | 8.7 | 12 | 7.7 | 8.3 | 14 | |
| LL | 6.3 | 11 | 19 | 9.0 | 9.8 | 14 | |
| Recovery% | HL | 95 | 99 | 99 | 97 | 97 | 98 |
| ML | 94 | 98 | 101 | 94 | 100 | 93 | |
| LL | 95 | 113 | 95 | 91 | 105 | 96 | |
In general, every developed method needs to be validated, and its performance needs to be evaluated by checking the quality parameters of analytical methods in terms of accreditation requirements and predefined acceptability limits. However, validated methods for the determination of volatiles in honey are limited. The validation procedure in this study not only verifies that the developed method provides satisfactory results and is suitable and efficient for the determination of honey volatiles but also provides validation data for a large number of compounds belonging to different chemical classes for which no previous reference exists in the literature.
3.4. Comparison of HS-SPME with static headspace
The obtained validation results from the SHS-GC-MS method are found in Tables S11–S12. Comparison of the two methods reveals that the organic acids could not be determined by SHS in contrast to the HS-SPME, probably due to their lower volatility [30]. A number of 28 common volatile compounds belonging to the groups of alkanes, esters, ketones, aldehydes, and alcohols were included in the validation and comparison of the two techniques, while in the SPME validation set, 8 organic acids were also included (Table S13). Regarding linearity, the superiority of the HS-SPME method is highlighted by the fact that 25 out of 30 compounds presented higher R2 values with HS-SPME than with the SHS method. Moreover, in SHS-GC-MS, R2 values ranged between 0.9645 for ethyl nonanoate and 0.9990 for benzaldehyde whereas the corresponding R2 values range for HS-SPME ranged between 0.9832 (octadecane) and 0.9993 (nonanal). Among the 28 common volatiles, 20 of them in HS-SPME and 25 in SHS showed a matrix effect higher than ±20 %. Moreover, in HS-SPME, all analytes at all concentration levels conformed to the guidance of the AOAC regarding both their repeatability and their intermediate precision [29]. On the contrary, the repeatability for some of the analytes prepared under SHS was not acceptable. An example is ethyl octanoate, whose RSD % value ranged from 21 to 29, failing to meet the requirements of the evaluated concentration levels. In general, even the volatiles whose RSD % values were acceptable with both methods presented better precision with HS-SPME (e.g., isophorone). Recovery results also proved to be more satisfactory when the analysis was performed using HS-SPME in comparison to SHS. In HS-SPME, all analytes met the requirements of the AOAC, but regarding SHS, there were analytes that presented either lower or higher recovery % values (octanal: 76 %, β-damascenone: 138% at the low concentration level).
Finally, using HS-SPME, LODs ranged from 2.12 μg kg−1 (decane) to 11.5 μg kg−1 (tridecanoic acid) whereas the lowest LOD achieved with SHS, was that of benzaldehyde (11.2 μg kg−1) and the highest that of decanal (24.4 μg kg−1).
All in all, the HS-SPME method showed better method performance at all the evaluated validation parameters justifying that it is a more efficient technique regarding the determination of volatiles, not only considering its increased sensitivity and its wider range of chemical analytes but also due to its improved validation results. The comparison of HS-SPME and HS methods is illustrated in terms of linearity (Fig. 1A), accuracy (Fig. 1B), repeatability (Fig. 1C), intermediate precision (Fig. 1D) and LOD (Fig. 1E). Organic acids were not detected at all using the SHS methodology, so they were not included in the comparison.
Fig. 1.
Comparison of method performance parameters between SHS and HS-SPME methodology in terms of A) Linearity, B) Accuracy, C) Repeatability, D) Intermediate precision, E) LOD.
3.5. Analysis of honey samples using HS-SPME-GC-MS
3.5.1. Target screening
A total of 30 honey samples were analysed using the final HS-SPME method, and 22 compounds were detected and quantified. The results are presented in Table 3. It was observed that octane was more abundant in honeydew honey samples and especially in fir variety, with an average concentration equal to 0.57 mg kg−1. The fir variety also had the highest amount of nonanal, with a mean concentration equal to 0.19 mg kg−1. These results are in accordance with published literature, according to which increased concentration of these analytes in the fir variety is reported [31,32]. Another dominant aldehyde identified in all varieties was benzaldehyde. This volatile was more abundant in the thyme variety, as it has been reported before [25]. Oak was the richest variety regarding alcohol content, and particularly 1-hexanol, 1-octanol, and 1-nonanol. Ethyl octanoate, and nonanoate were esters that were identified in traces in some samples, but their average concentrations were below the LOQ in almost all varieties. Similarly, hexanoic, octanoic, nonanoic, and decanoic organic acids were detected in minor concentrations and only in pine and chestnut honeys. The norisoprenoid β-damascenone appeared to be characteristic for carob honey quantified at 0.086 mg kg−1, similar to another study of Portuguese honey [32]. In addition, another norisoprenoid, α-isophorone, was determined at the high levels of 13 mg kg−1 in arbutus honey samples of the present study. α-Isophorone has been determined as indicative of Greek arbutus variety [15]. These two compounds, β-damascenone and α-isophorone, are among the dominant volatile compounds determined in several varieties of honey from different countries [32].
Table 3.
Average concentrations (mg kg−1) of the target analytes.
| Botanical origin of honey (number of samples) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Pine (N = 3) | Fir (N = 4) | Oak (N = 2) | Thyme (N = 4) | Heather (N = 3) | Orange (N = 3) | Sage (N = 3) | Chestnut (N = 2) | Arbutus (N = 4) | Carob (N = 2) |
| Heptane | 0.024 | 0.019 | 0.0072 | 0.014 | 0.013 | 0.013 | 0.011 | 0.021 | ND | 0.0092 |
| Octane | 0.49 | 0.57 | 0.35 | 0.057 | 0.18 | 0.15 | 0.29 | 0.32 | 0.06 | 0.32 |
| Nonane | 0.017 | 0.025 | 0.026 | 0.027 | 0.023 | 0.019 | 0.022 | 0.023 | 0.0095 | 0.017 |
| Decane | 0.016 | 0.0075 | 0.0098 | 0.0084 | 0.0087 | 0.011 | 0.0099 | 0.0068 | <LOQ | <LOQ |
| Undecane | ND | ND | 0.012 | 0.013 | ND | ND | ND | ND | ND | <LOQ |
| 1-Hexanol | 0.018 | <LOQ | 0.045 | <LOQ | <LOQ | ND | <LOQ | 0.021 | <LOQ | <LOQ |
| 1-Octanol | <LOQ | <LOQ | 0.021 | ND | ND | ND | ND | <LOQ | ND | <LOQ |
| 1-Nonanol | 0.030 | 0.018 | 0.032 | <LOQ | ND | <LOQ | <LOQ | 0.031 | <LOQ | ND |
| Hexanal | 0.010 | 0.012 | ND | 0.017 | 0.016 | ND | 0.027 | 0.016 | ND | <LOQ |
| Heptanal | ND | 0.009 | ND | ND | 0.014 | ND | 0.011 | <LOQ | ND | ND |
| Octanal | 0.015 | 0.022 | 0.012 | 0.021 | 0.018 | 0.011 | 0.0099 | 0.014 | 0.012 | 0.0096 |
| Nonanal | 0.17 | 0.19 | 0.022 | 0.034 | 0.027 | 0.032 | 0.034 | 0.028 | 0.023 | 0.041 |
| Decanal | 0.017 | 0.028 | 0.012 | 0.015 | 0.015 | 0.010 | 0.011 | 0.013 | 0.010 | 0.021 |
| Benzaldehyde | 0.10 | 0.095 | 0.052 | 0.42 | 0.28 | 0.093 | 0.20 | 0.19 | 0.040 | 0.048 |
| Ethyl octanoate | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ND | <LOQ | <LOQ | <LOQ | <LOQ |
| Ethyl nonanoate | <LOQ | 0.0089 | <LOQ | ND | ND | ND | ND | 0.0077 | <LOQ | ND |
| α-Isophorone | ND | 0.030 | 0.041 | ND | 0.36 | ND | ND | 0.010 | 13 | 0.073 |
| β-Damascenone | 0.012 | ND | ND | 0.012 | 0.009 | ND | ND | 0.010 | ND | 0.086 |
| Hexanoic acid | <LOQ | ND | ND | ND | ND | ND | ND | <LOQ | ND | ND |
| Octanoic acid | 0.028 | <LOQ | <LOQ | ND | ND | ND | ND | 0.023 | ND | <LOQ |
| Nonanoic acid | 0.024 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.028 |
| Decanoic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ND | ND | <LOQ | ND | <LOQ |
ND: Non-Detected (<LOD).
3.5.2. Suspect screening
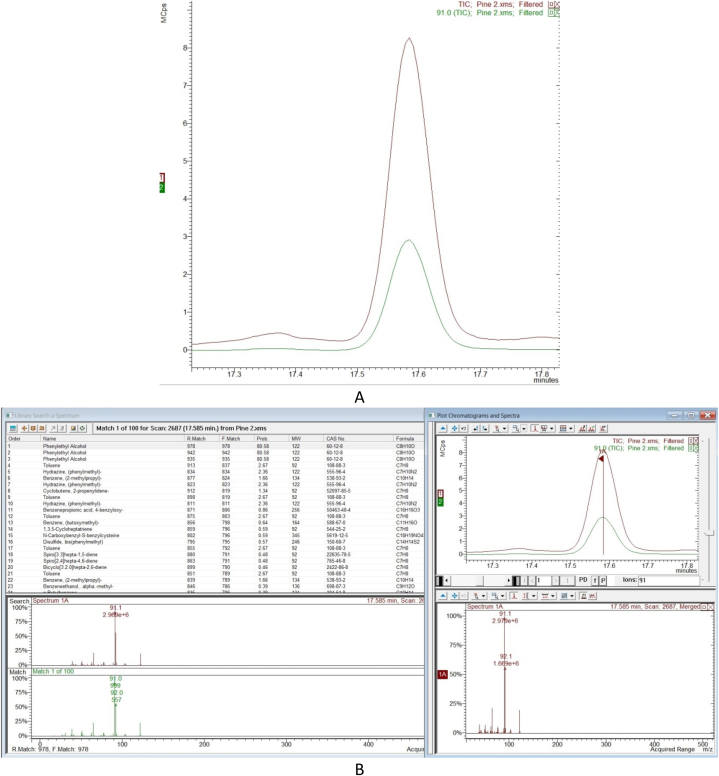
Following the suspect screening workflow presented in Section 2.9.2, all of the 31 volatiles from our suspect database were determined in at least one honey sample. An example of the identification of phenylethyl alcohol is presented in Fig. 2. Fig. 2A illustrates the total ion chromatogram and the extracted qualifier ion chromatogram of phenylethylalcohol (m/z = 91) at its retention time (tR = 17.60 min). The full scan spectrum at the same retention time is presented in Fig. 2B along with the reference spectrum and the similarity score of the NIST library which is greater than 80 %. In Table 4, the quantifier and qualifier ions of the suspect analytes, as well as their retention time, both experimental and derived from literature RIs, are included.
Fig. 2.
Phenylethyl alcohol identification workflow.
Table 4.
Identification features of the suspect analytes.
| Compound | Molecular Formula | Experimental tR (min) | Quantifier ion (m/z) | Qualifier ion (I) (m/z) | Qualifier ion (II) (m/z) | RI experimental | RI literature |
|---|---|---|---|---|---|---|---|
| 2,5-Dimethyl furan | C6H8O | 6.99 | 96 | 95 | 43 | 725 | – |
| Octene | C8H16 | 8.48 | 41 | 56 | 57 | 795 | – |
| 3-Hepten-2-one | C7H12O | 8.85 | 55 | 97 | 43 | 814 | – |
| Prenol | C5H10O | 9.13 | 71 | 41 | 86 | 828 | – |
| 2,3-Butanediol | C4H10O2 | 10.07 | 45 | 43 | 57 | 875 | 882 [22] |
| 3-Furaldehyde | C5H4O2 | 10.64 | 95 | 96 | 39 | 903 | 905 [22] |
| 3-methylbutanoic acid | C5H10O2 | 11.11 | 60 | 43 | 41 | 928 | 920 [22] |
| 2-Furanmethanol | C5H6O2 | 11.17 | 98 | 81 | 41 | 932 | – |
| α-Pinene | C10H16 | 11.54 | 93 | 92 | 77 | 952 | 948 [27] |
| Methyl hexanoate | C7H14O2 | 11.58 | 74 | 87 | 99 | 954 | – |
| Furan-2-pentyl | C9H14O | 12.66 | 81 | 82 | 138 | 1010 | 1006 [27] |
| 1-octen-3-ol | C8H16O | 12.90 | 57 | 72 | 43 | 1022 | 1025 [28] |
| 6-Methyl-5-hepten-2-one | C8H14O | 13.12 | 43 | 69 | 108 | 1032 | – |
| Limonene | C10H16 | 13.41 | 68 | 93 | 79 | 1046 | 1048 [27] |
| β-Isophorone | C9H14O | 14.65 | 81 | 96 | 95 | 1104 | – |
| Benzeneacetaldehyde | C8H8O | 15.06 | 91 | 92 | 120 | 1117 | 1123 [22] |
| 2-Nonanone | C9H18O | 15.66 | 43 | 58 | 57 | 1136 | 1140 [28] |
| Acetophenone | C8H8O | 15.71 | 105 | 77 | 51 | 1138 | – |
| Linalool | C10H18O | 15.81 | 71 | 43 | 41 | 1141 | 1151 [22] |
| Methyl octanoate | C9H18O2 | 16.02 | 74 | 87 | 127 | 1148 | – |
| Methyl benzoate | C8H8O2 | 16.07 | 105 | 77 | 136 | 1149 | – |
| Hotrienol | C10H16O | 16.19 | 71 | 43 | 82 | 1153 | 1161 [22] |
| Phenylethyl alcohol | C8H10O | 17.6 | 91 | 92 | 122 | 1198 | 1120 [22] |
| Lilac aldehyde (Isomer) | C10H16O2 | 18.23 | 55 | 43 | 67 | 1225 | – |
| Ethyl benzoate | C9H10O2 | 18.57 | 105 | 77 | 68 | 1239 | – |
| Dill ether | C10H16O | 18.87 | 137 | 109 | 67 | 1251 | – |
| Methyl nonanoate | C10H20O2 | 19.28 | 74 | 87 | 43 | 1268 | – |
| Myrtenol | C10H16O | 19.77 | 79 | 91 | 107 | 1289 | – |
| 4-Methoxybenzaldehyde | C8H8O2 | 21.75 | 135 | 136 | 77 | 1365 | – |
| 2-Aminoacetophenone | C8H9NO | 22.78 | 120 | 135 | 92 | 1407 | – |
| Methyl anthranilate | C8H9NO2 | 23.41 | 119 | 92 | 151 | 1443 | – |
Following a semi-quantification procedure, the average concentration of each suspect analyte of the investigated varieties was calculated. The results are found in Table 5. Interesting conclusions are derived from the results. In pine samples, a-pinene was more abundant (approx. 0.14 mg kg−1) in comparison to the other honey varieties. This is in accordance with other scientific studies about honey, which have presented increased concentrations of this analyte in pine honey samples [25,32]. Myrtenol is another compound that was detected in some pine samples, but it was absent from all the other varieties. The presence of myrtenol in pine samples from Turkey has also been reported [33]. However, this compound has never been detected in Greek pine samples in the past, according to the literature. Fir was the richest honey in 2-nonanone. The presence of this compound as a potential marker in fir honey has been proposed in the literature [25]. In addition, 2,3-butanediol was more abundant in oak honey samples. Although this compound seems to be indicative of this botanical origin, to the best of our knowledge, it has never been found in Greek oak honeys before, although it has been determined in Greek pine honeys [34]. Moreover, it has been proposed as an indicative compound of the oak variety from Slovakia [35] and, in general, of honeydew honey [36]. Thyme honey was characterized by the highest amounts of phenylethyl alcohol and furan-2-pentyl [3,25,31]. In the case of heather honey, the higher amounts of 4-methoxybenzaldehyde, 3-furaldehyde, ethyl, and methyl benzoate are distinguished [25,32,37]. Orange honey was the type of honey in which the compounds 3-hepten-2-one, lilac aldehyde, dill ether, and methyl anthranillate were most abundant. Especially the last two analytes were only identified in orange samples and were absent in the other varieties. Other scientific studies have also indicated the presence of these compounds in the orange variety [3,38,39]. Sage honey showed the highest amounts of benzeneacetaldehyde, a characteristic compound of this variety [40]. Acetophenone and aminoacetophenone were more abundant in the chestnut variety, also in accordance with previous studies on international honey varieties [11,32,41], while β-isophorone, and 2,5 dimethyl furan were indicative of the arbutus variety [32,42]. Finally, carob honey was characterized by increased concentrations of hotrienol, 3-methylbutanoic acid, linalool and methyl nonanoate [43].
Table 5.
Average concentrations (mg kg−1) of the suspect screening analytes.
| Botanical origin of honey (number of samples) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Pine (N = 3) | Fir (N = 4) | Oak (N = 2) | Thyme (N = 4) | Heather (N = 3) | Orange (N = 3) | Sage (N = 3) | Chestnut (N = 2) | Arbutus (N = 4) | Carob (N = 2) |
| 2,5-Dimethyl furan | 0.043 | 0.11 | 0.17 | 0.099 | 0.027 | 0.014 | 0.015 | 0.18 | 1.5 | 0.066 |
| Octene | 0.14 | 0.17 | 0.11 | 0.074 | 0.025 | ND | ND | 0.21 | 0.016 | ND |
| 3-Hepten-2-one | ND | ND | ND | 0.0079 | ND | 0.29 | 0.052 | 0.0097 | ND | 0.014 |
| Prenol | 0.15 | 0.14 | 0.19 | 0.078 | 0.16 | 0.10 | 0.13 | 0.20 | 0.16 | 0.091 |
| 2,3-Butanediol | 1.1 | 0.19 | 9.2 | 0.13 | 0.099 | 0.12 | 0.41 | 4.4 | 1.0 | 0.23 |
| 3-Furaldehyde | 0.19 | 0.46 | 0.10 | 0.52 | 1.6 | 0.55 | 0.29 | 0.24 | 0.48 | 0.30 |
| 3-methylbutanoic acid | 0.69 | 0.17 | 0.38 | 0.89 | 0.32 | ND | 0.30 | 0.61 | 0.11 | 1.11 |
| 2-Furanmethanol | 0.12 | 0.12 | 0.36 | 0.038 | 0.084 | 0.024 | 0.039 | 0.22 | 0.19 | 0.092 |
| α-Pinene | 0.14 | 0.038 | 0.039 | 0.0031 | 0.071 | ND | 0.015 | 0.016 | 0.010 | 0.032 |
| Methyl hexanoate | 0.017 | 0.026 | 0.021 | 0.029 | 0.021 | 0.021 | 0.022 | 0.028 | 0.025 | 0.023 |
| Furan-2-pentyl | 0.32 | 0.34 | 0.71 | 1.5 | 0.27 | 0.15 | 0.65 | 0.57 | 0.92 | 0.51 |
| 1-octen-3-ol | 0.71 | 0.67 | 0.90 | 0.51 | 0.94 | ND | 0.92 | 0.60 | 0.48 | 1.2 |
| 6-Methyl-5-hepten-2-one | ND | 0.11 | 0.079 | 0.20 | 0.12 | 1.1 | ND | ND | ND | 0.074 |
| Limonene | 0.013 | 0.0061 | 0.0031 | 0.019 | 0.0039 | 0.011 | 0.0048 | 0.021 | 0.0061 | 0.011 |
| β-Isophorone | ND | ND | ND | ND | 0.19 | ND | ND | ND | 4.7 | ND |
| Benzeneacetaldehyde | 1.7 | 1.3 | 1.5 | 16 | 3.8 | 2.0 | 20 | 2.6 | 1.7 | 1.6 |
| 2-Nonanone | 0.052 | 0.091 | 0.044 | 0020 | ND | ND | ND | 0.057 | 0.029 | 0.018 |
| Acetophenone | 0.067 | 0.055 | 0.068 | 0.073 | 0.087 | ND | 0.10 | 0.94 | ND | 0.054 |
| Linalool | 0.32 | 0.71 | 1.6 | 0.29 | 0.27 | 1.7 | 1.7 | 0.76 | 0.16 | 2.5 |
| Methyl octanoate | 0.058 | 0.049 | 0.077 | 0.036 | 0.031 | 0.019 | 0.034 | 0.061 | 0.046 | 0.063 |
| Methyl benzoate | 0.035 | 0.021 | 0.090 | 0.015 | 0.49 | ND | 0.013 | 0.057 | 0.016 | ND |
| Hotrienol | 0.11 | 0.083 | 0.54 | 0.54 | 0.14 | 0.49 | 0.31 | 1.5 | 0.40 | 5.7 |
| Phenylethyl alcohol | 0.027 | 0.026 | 0.038 | 0.34 | 0.030 | 0.021 | 0.035 | 0.041 | 0.038 | 0.035 |
| Lilac aldehyde (Isomer) | ND | 0.25 | ND | 0.28 | ND | 2.4 | 0.19 | 0.31 | ND | 0.14 |
| Ethyl benzoate | ND | 0.069 | ND | ND | 0.87 | ND | ND | 0.31 | 0.56 | 0.12 |
| Dill ether | ND | ND | ND | ND | ND | 11 | ND | ND | ND | ND |
| Methyl nonanoate | 0.085 | 0.074 | 0.062 | 0.048 | 0.037 | 0.028 | 0.052 | 0.082 | 0.043 | 0.13 |
| Myrtenol | 0.24 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 4-Methoxybenzaldehyde | ND | ND | ND | 0.23 | 3.7 | ND | ND | ND | ND | ND |
| 2-Aminoacetophenone | ND | ND | 0.030 | ND | ND | ND | ND | 1.2 | ND | ND |
| Methyl anthranilate | ND | ND | ND | ND | ND | 1.7 | ND | ND | ND | ND |
ND: Non-Detected (<LOD).
4. Conclusions
The present work describes the development and validation of a HS-SPME-GC/MS method for the determination of volatile compounds in honey samples. During the optimization of the method, it was demonstrated that the honey:water ratio and the incubation temperature consist the two most critical parameters for the efficient extraction of the volatiles. Special attention was drawn to the quantification of the target analytes using four different IS – based on structural similarity and method performance features of the individual analytes – as well as matrix matched calibration curves. The method was successfully validated in terms of linearity, recovery, repeatability, matrix effect, LODs and LOQs. Comparison of the novel and validated HS-SPME-GC/MS method to a validated static head space-GC/MS method was performed and highlighted the advantages of SPME over SHS as regards mainly sensitivity, analyte coverage, linearity and repeatability. The developed method can be applied for target and suspect screening of volatiles in honey and provides, for the first time, experimental values of the Retention Indexes for a number of compounds with the alternative medium polar Varian CP-Select 624 column as an additional identification feature for suspect analysis. The method was applied to 30 honey samples of 10 botanical origins and 53 compounds were identified following target and suspect screening workflows. Octane, nonanal, benzaldehyde, β-damascenone and α-isophorone were among the target analytes with the highest concentrations and frequency of appearance. Indicative examples of suspect compounds identified in the tested samples were myrtenol, 2,3-butanediol, linalool, and methyl anthranilate. It is noteworthy that it is the first time that myrtenol is detected in Greek pine honey and 2,3-butanediol in Greek oak honey. The proposed methodology is very promising for the thorough chemical characterization of honey volatile profile. The analysis of a larger number of samples, the extension of the target and suspect databases and the application of sophisticated chemometric approaches could be a valuable tool for the chemical interpretation of honey aroma and for establishment of honey authenticity protocols.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
CRediT authorship contribution statement
Panagiotis-Loukas P. Gialouris: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Data curation. Georgios A. Koulis: Methodology, Investigation. Eleni S. Nastou: Investigation. Marilena E. Dasenaki: Writing – review & editing, Validation, Supervision, Methodology. Niki C. Maragou: Writing – review & editing, Visualization. Nikolaos S. Thomaidis: Writing – review & editing, Supervision, Resources, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the Greek beekeepers who provided the honey samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21311.
Contributor Information
Panagiotis-Loukas P. Gialouris, Email: pgialour@chem.uoa.gr.
Georgios A. Koulis, Email: georgekoulis@chem.uoa.gr.
Eleni S. Nastou, Email: elenanastou@chem.uoa.gr.
Marilena E. Dasenaki, Email: mdasenaki@chem.uoa.gr.
Niki C. Maragou, Email: nmarag@agro.uoa.gr.
Nikolaos S. Thomaidis, Email: ntho@chem.uoa.gr.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C.O., Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 2.da Costa A.C.V., Sousa J.M.B., Bezerra T.K.A., da Silva F.L.H., Pastore G.M., da Silva M.A.A.P., Madruga M.S. Volatile profile of monofloral honeys produced in Brazilian semiarid region by stingless bees and key volatile compounds. Lwt. 2018;94:198–207. doi: 10.1016/j.lwt.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 3.Xagoraris M., Revelou P., Dedegkika S., Kanakis C.D., Papadopoulos G.K., Pappas C.S., Tarantilis P.A. SPME-GC-MS and FTIR-ATR spectroscopic study as a tool for unifloral common Greek honeys' botanical origin identification. Appl. Sci. 2021;11 doi: 10.3390/app11073159. [DOI] [Google Scholar]
- 4.Gliszczyńska-Świgło A., Chmielewski J. Electronic nose as a tool for monitoring the authenticity of food. A review. Food Anal. Methods. 2017;10:1800–1816. doi: 10.1007/s12161-016-0739-4. [DOI] [Google Scholar]
- 5.Koulis G.A., Tsagkaris A.S., Aalizadeh R., Dasenaki M.E., Panagopoulou E.I., Drivelos S., Halagarda M., Georgiou C.A., Proestos C., Thomaidis N.S. Honey phenolic compound profiling and authenticity assessment using hrms targeted and untargeted metabolomics. Molecules. 2021;26:1–21. doi: 10.3390/molecules26092769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koulis G.A., Tsagkaris A.S., Katsianou P.A., Gialouris P.P., Martakos I., Stergiou F., Fiore A., Panagopoulou E.I., Karabournioti S., Baessmann C., Van Der Borg N., Dasenaki M.E., Proestos C. Thorough investigation of the phenolic profile of reputable Greek honey varieties : varietal discrimination and floral and floral markers identification using liquid chromatography–high-resolution mass spectrometry. Molecules. 2022;27:1–17. doi: 10.3390/molecules27144444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsagkaris A.S., Koulis G.A., Danezis G.P., Martakos I., Dasenaki M., Georgiou C.A., Thomaidis N.S. Honey authenticity: analytical techniques, state of the art and challenges. RSC Adv. 2021;11:11273–11294. doi: 10.1039/d1ra00069a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayram N.E., Canli D., Gercek Y.C., Bayram S., Çelik S., Güzel F., Morgil H., Oz G.C. Macronutrient and micronutrient levels and phenolic compound characteristics of monofloral honey samples. J. Food Nutr. Res. 2020;59:311–322. [Google Scholar]
- 9.Bodó A., Radványi L., K oszegi T., Csepregi R., Nagy D.U., Farkas Á., Kocsis M. Quality evaluation of light- and dark-colored Hungarian honeys, focusing on botanical origin. Antioxidant Capac. Mineral Content Mol. 2021;26 doi: 10.3390/molecules26092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maragou N.C., Strati I.F., Gialouris P.L., Dasenaki M., Sinanoglou V.J., Ačanski M., Švarc Gajić J., Pastor K. In: Emerging Food Authentication Methodologies Using GC/MS. Pastor K., editor. Springer; Cham: 2023. Honey and bee products. [DOI] [Google Scholar]
- 11.de Lima Morais da Silva P., de Lima L.S., Caetano Í.K., Torres Y.R. Comparative analysis of the volatile composition of honeys from Brazilian stingless bees by static headspace GC–MS. Food Res. Int. 2017;102:536–543. doi: 10.1016/j.foodres.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Robotti E., Campo F., Riviello M., Bobba M., Manfredi M., Mazzucco E., Gosetti F., Calabrese G., Sangiorgi E., Marengo E. Optimization of the extraction of the volatile fraction from honey samples by SPME-GC-MS, experimental design, and multivariate target functions. J. Chem. 2017 doi: 10.1155/2017/6437857. [DOI] [Google Scholar]
- 13.Xagoraris M., Skouria A., Revelou P.K., Alissandrakis E., Tarantilis P.A., Pappas C.S. Response surface methodology to optimize the isolation of dominant volatile compounds from monofloral Greek thyme honey using spme-gc-ms. Molecules. 2021;26 doi: 10.3390/molecules26123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva P., Freitas J., Silva C.L., Perestrelo R., Nunes F.M., Câmara J.S. Establishment of authenticity and typicality of sugarcane honey based on volatile profile and multivariate analysis. Food Control. 2017;73:1176–1188. doi: 10.1016/j.foodcont.2016.10.035. [DOI] [Google Scholar]
- 15.Karabagias I.K., Nikolaou C., Karabagias V.K. Volatile fingerprints of common and rare honeys produced in Greece: in search of PHVMs with implementation of the honey code. Eur. Food Res. Technol. 2019;245:23–39. doi: 10.1007/s00217-018-3137-x. [DOI] [Google Scholar]
- 16.Xagoraris M., Chrysoulaki F., Revelou P., Alissandrakis E., Tarantilis P., Papas C. Unifloral autumn heather honey from indigenous Greek erica manipuliflora salisb.: SPME/GC-MS characterization of the volatile fraction and optimization of the isolation parameters. Foods. 2021;10 doi: 10.3390/foods10102487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu M., Sun J., Zhao H., Wu F., Xue X., Wu L., Cao W. Volatile compounds of five types of unifloral honey in Northwest China: correlation with aroma and floral origin based on HS-SPME/GC–MS combined with chemometrics. Food Chem. 2022;384 doi: 10.1016/j.foodchem.2022.132461. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W., Paolini J., Bereau D., Battesti M.J., Yang Y., Jean-Marie É., Costa J., Robinson J.C. French Guiana honeys from the Amazon biome: first description of volatile fraction and antioxidant capacity. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e18526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karabagias I.K., Karabagias V.K., Nayik G.A., Gatzias I., Badeka A.V. A targeted chemometric evaluation of the volatile compounds of Quercus ilex honey in relation to its provenance. Lwt. 2022;154 doi: 10.1016/j.lwt.2021.112588. [DOI] [Google Scholar]
- 20.Escriche I., Sobrino-Gregorio L., Conchado A., Juan-Borrás M. Volatile profile in the accurate labelling of monofloral honey. The case of lavender and thyme honey. Food Chem. 2017;226:61–68. doi: 10.1016/j.foodchem.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Tanleque-Alberto F., Juan-Borrás M., Escriche I. Quality parameters, pollen and volatile profiles of honey from North and Central Mozambique. Food Chem. 2019;277:543–553. doi: 10.1016/j.foodchem.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Gianelli Barra M.P., Ponce-Díaz M.C., Venegas-Gallegos C. Volatile compounds in honey produced in the central valley of ñuble province, Chile. Chil. J. Agric. Res. 2010;70:75–84. doi: 10.4067/s0718-58392010000100008. [DOI] [Google Scholar]
- 23.Plutowska B., Chmiel T., Dymerski T., Wardencki W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011;126:1288–1298. doi: 10.1016/j.foodchem.2010.11.079. [DOI] [Google Scholar]
- 24.Fortini M., Migliorini M., Cherubini C., Cecchi L., Calamai L. Multiple internal standard normalization for improving HS-SPME-GC-MS quantitation in virgin olive oil volatile organic compounds (VOO-VOCs) profile. Talanta. 2017;165:641–652. doi: 10.1016/j.talanta.2016.12.082. [DOI] [PubMed] [Google Scholar]
- 25.Tananaki C., Liolios V., Kanelis D., Rodopoulou M.A. Investigation of volatile compounds in combination with multivariate analysis for the characterization of monofloral honeys. Appl. Sci. 2022;12:264. doi: 10.3390/app12010264. [DOI] [Google Scholar]
- 26.Panseri S., Manzo A., Chiesa L.M., Giorgi A. Melissopalynological and volatile compounds analysis of buckwheat honey from different geographical origins and their role in botanical determination. J. Chem. 2013 doi: 10.1155/2013/904202. [DOI] [Google Scholar]
- 27.Kiralan M., Ozkan G., Koyluoglu F., Ugurlu H.A., Bayrak A., Kiritsakis A. Effect of cultivation area and climatic conditions on volatiles of virgin olive oil. Eur. J. Lipid Sci. Technol. 2012;114:552–557. doi: 10.1002/ejlt.201100289. [DOI] [Google Scholar]
- 28.Marco A., Navarro J.L., Flores M. Quantitation of selected odor-active constituents in dry fermented sausages prepared with different curing salts. J. Agric. Food Chem. 2007;55:3058–3065. doi: 10.1021/jf0631880. [DOI] [PubMed] [Google Scholar]
- 29.AOAC . AOAC International, Assoc. Off. Anal. Chem.; 2019. Official Methods of Analysis.https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ 2019. [Google Scholar]
- 30.Bugelyte B., Jonkute R., Vičkačkaite V. Determination of some short chain carboxylic acids in food by headspace gas chromatography. Chemija. 2018;29:193–197. doi: 10.6001/chemija.v29i3.3822. [DOI] [Google Scholar]
- 31.Karabagias I.K., Badeka A., Kontominas M.G. A decisive strategy for monofloral honey authentication using analysis of volatile compounds and pattern recognition techniques. Microchem. J. 2020;152 doi: 10.1016/j.microc.2019.104263. [DOI] [Google Scholar]
- 32.Machado A.M., Miguel M.G., Vilas-boas M., Figueiredo A.C. Honey volatiles as a fingerprint for botanical origin — a review on their occurrence on monofloral honeys. Molecules. 2020:1–32. doi: 10.3390/molecules25020374. https://doi:10.3390/molecules25020374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duru M.E., Taş M., Çayan F., Küçükaydın S., Tel G. Characterization of volatile compounds of Turkish pine honeys from different regions and classification with chemometric studies. Eur. Food Res. Technol. 2021 doi: 10.1007/s00217-021-03817-8. [DOI] [Google Scholar]
- 34.Karabagias I., Badeka Α., Kontakos S., Karabournioti S., Kontominas M. Characterisation and classification of Greek pine honeys according to their geographical origin based on volatiles, physicochemical parameters and chemometrics. Food Chem. 2014;146:548–557. doi: 10.1016/j.foodchem.2013.09.105. [DOI] [PubMed] [Google Scholar]
- 35.Jánošková N., Vyviurska O., Špánik I. Identification of volatile organic compounds in honeydew honeys using comprehensive gas chromatography. J. Food Nutr. Res. 2014;53:353–362. [Google Scholar]
- 36.Pita-Calvo C., Vázquez M. Honeydew honeys: a review on the characterization and authentication of botanical and geographical origins. J. Agric. Food Chem. 2018;66:2523–2537. doi: 10.1021/acs.jafc.7b05807. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-flores M.S., Falcão S.I., Escuredo O., Seijo M.C., Vilas-boas M. Description of the volatile fraction of Erica honey from the northwest of the Iberian Peninsula. Food Chem. 2021;336 doi: 10.1016/j.foodchem.2020.127758. [DOI] [PubMed] [Google Scholar]
- 38.Papotti G., Bertelli D., Plessi M. Use of HS-SPME-GC-MS for the classification of Italian lemon, orange and citrus spp. honeys. Int. J. Food Sci. Technol. 2012:2352–2358. doi: 10.1111/j.1365-2621.2012.03109.x. [DOI] [Google Scholar]
- 39.Verzera A., Tripodi G., Condurso C., Dima G., Marra A. Chiral volatile compounds for the determination of orange honey authenticity. Food Control. 2014;39:237–243. doi: 10.1016/j.foodcont.2013.11.012. [DOI] [Google Scholar]
- 40.Pažitná A., Džúrová J., Špánik I. Enantiomer distribution of major chiral volatile organic compounds in selected types of herbal honeys. Chirality. 2014;26:670–674. doi: 10.1002/chir. [DOI] [PubMed] [Google Scholar]
- 41.Demir Kanbur E., Yuksek T., Atamov V., Ozcelik A.E. A comparison of the physicochemical properties of chestnut and highland honey: the case of Senoz Valley in the Rize province of Turkey. Food Chem. 2021;345 doi: 10.1016/j.foodchem.2020.128864. [DOI] [PubMed] [Google Scholar]
- 42.Graikou K., Andreou A., Chinou I. Chemical profile οf Greek Arbutus unedo honey: biological properties. J. Apic. Res. 2022;61:100–106. doi: 10.1080/00218839.2021.1917860. [DOI] [Google Scholar]
- 43.Boi M., Llorens J.A., Cortés L., Lladó G., Llorens L. Palynological and chemical volatile components of typically autumnal honeys of the western Mediterranean. Grana. 2013;52:93–105. doi: 10.1080/00173134.2012.744774. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.