Abstract
Two groups of nauplii from the brine shrimp (Artemia franciscana) were enriched with different bacteria, and the dynamics of bacterial uptake by the nauplii were observed. This study showed that the efficiency of Artemia nauplii in bioencapsulating bacteria strongly depends on the type of bacteria used, time of exposure, and status (live or dead) of the bacteria.
Live nauplii of the brine shrimp (Artemia spp.) have been used as vectors for delivering compounds of diverse nutritional (10, 26) and/or therapeutic (5, 6, 23, 27) value to larval stages of aquatic animals, a process known as bioencapsulation. Inoculating the digestive tracts of target organisms with probiotic bacteria through bioencapsulation and feeding is another alternative use for Artemia nauplii. Probionts can be defined as a live microbial feed supplement, which beneficially affects the host animal by improving its intestinal balance (12). Bioencapsulated lactic acid bacteria have been successfully introduced into turbot larvae with significant improvements in survival (13). Bacteria with various characteristics have been incorporated into Artemia nauplii to orally challenge turbot larvae with a pathogenic Vibrio anguillarum strain (7, 16). This route has also been used to vaccinate sea bass fry (8), juvenile carp (17), and fish fry (4).
It has been suggested that bacterial infections are initiated through the oral route in penaeid larvae and postlarvae (19). Therefore, an oral challenge should be a reliable method to reproduce actual infections and also to introduce probiotic strains. The aim of this study was to evaluate the bioencapsulation of (i) a potential pathogenic bacterium and (ii) a potential probiotic bacterium in Artemia nauplii.
Artemia cyst hatching and disinfection.
Artemia franciscana cysts from the Great Salt Lake, Utah, were employed for this study. The corion of the cysts was chemically removed by employing the methodology proposed by Sorgeloos et al. (25), a process known as decapsulation. Hatching of the decapsulated cysts was performed in a sealed flask with 200 ml of sterile seawater (3.5% salinity). The cysts were stocked at a density of 5.0 g liter−1 and incubated at 30°C with constant illumination and oxygenated through mechanical agitation to prevent bacterial contamination. To ensure a complete disinfection of the nauplii, chloramphenicol (Chloromycetin; Parke-Davis) at 30.0 mg liter−1 and trimethoprim-sulfamethoxaxole (Bactrim; Productos Roche) at 40.0 and 8.0 mg liter−1, respectively, were added to the hatching water. After 24 h, the recently hatched nauplii were collected aseptically in a 120-μm-pore-size sieve and washed thoroughly with sterile distilled water.
To evaluate if any antibiotic residue was left in the nauplii after rinsing, a modified disk diffusion test (2) which permitted the growth of marine bacteria was performed. Nauplii were rinsed and macerated with a tissue homogenizer, a Whatman no. 1 filter paper disc (7.0 mm in diameter; Whatman, Inc., Clifton, N.J.) was impregnated with 20 μl of the liquid supernatant. As control, other discs were also impregnated with samples from the hatching water and the antibiotic solution. Muller-Hinton agar (Bioxon) was prepared with 2.5% NaCl and dispensed in 10-mm-diameter petri dishes. The impregnated discs were placed in duplicate on the agar plates inoculated with reference bacteria as a lawn. The reference bacteria employed were Escherichia coli (ATCC 25922) from the American Type Culture Collection, Vibrio parahaemolyticus (LMG 2850T) from the Microbiology Laboratory, University of Gent, Vibrio harveyi (LMG 4044T), Vibrio damsela (LMG 7892T), and isolates C7b and HL57. After 48 h of incubation, the inhibition halos were measured.
Bacterial culture and preservation.
The bacterium HL57 was isolated from the hemolymph of a farm-grown, diseased juvenile shrimp caught in the state of Sinaloa, Mexico. The bacterium C7b was collected from unpolluted seawater in the same state. Thiosulfate-citrate-bile-sucrose agar (TCBS; Difco, Detroit, Mich.) was employed to isolate and partially purify the bacteria.
For further analyses, the bacteria were grown and purified in tryptic soy agar (TSA) or tryptic soy broth (TSB) (both from Bioxon) made with distilled water and 2.0% (wt/vol) NaCl to obtain a final concentration of 2.5%. Media and solutions were sterilized by autoclaving at 121°C for 20 min. The cultures were incubated at 30°C for 20 to 24 h or 48 h.
The isolates were preserved at −70°C in an ultralow mechanical freezer (Revco Scientific, Asheville, N.C.) in cryovials filled with glass beads (14). In order to recover strains from cryopreservation, a bead was obtained from the appropriate cryovial and placed in a test tube with TSB at 25 to 30°C and incubated overnight at 30°C with constant agitation. A sample was then taken from the test tube and streaked on TSA and incubated at 30°C for 20 to 24 h.
Bacterial inoculum.
Ten milliliters of a fresh bacterial culture were centrifuged at 5,000 rpm for 10 min at 10°C (Beckman, Instruments, Inc., Fullerton, Calif.), the liquid supernatant was then discarded, and the pellet was suspended in sterile saline solution. This process was repeated again, and the cell concentration in the suspension was adjusted to an optical density of 1.00 at 610 nm in a spectrophotometer (model DR-2000; Hach, Loveland, Colo.). To estimate the bacterial concentration achieved, the suspension was serially diluted in sterile saline and spread plated in TSA or Marine agar (Difco).
Bacterial characterization.
Tests were performed to characterize the isolates by following the recommendations of Baumann and Schubert (3) and Austin and Lee (1). The bacterial isolates were analyzed for their reaction to the following tests: Gram staining, oxidase production (Oxoid identification sticks; Oxoid, Basingstoke, England), motility, oxidative-fermentative metabolism of glucose, sensitivity to the vibriostatic agent O/129 (2,4-diamino-6,7-diisopropylpteridine phosphate; Oxoid), and swarming in solid media. The Biolog-GN microplates (Biolog, Hayward, Calif.) were employed to analyze the ability of the isolates to use different carbon sources. The API 20NE system (bioMérieux, Marcy l’Etoile, France) was also employed to further characterize the isolates. Both systems were inoculated according to the recommendations of the manufacturer, but the bacterial suspensions were prepared as described above.
Bioencapsulation of bacterial isolates in the Artemia nauplii.
Sterile nauplii were added to a 200-ml flask with the bacterial suspension (sterile seawater and the desired bacterium) at a density of 100 nauplii ml−1 and 107 bacterial cells ml−1. Controls were bacteria only and nauplii only. Four replicates were prepared for each treatment. Each experiment was repeated once for each isolate (HL57 and C7B).
Two 1.0-ml samples from each replicate were collected at the following intervals after the nauplii were placed in the flask: at 15, 30, and 45 min and at 1, 2, 4, 8, and 24 h. The nauplii from each sample (except from the “bacteria only” control) were collected in sterile conditions, thoroughly washed, and macerated in a tissue homogenizer. Serial dilutions of the supernatant fluid (or from the seawater in the “bacteria only” control) were prepared, and 0.1 ml was spread plated in Marine and TCBS agars. The petri dishes were incubated at 30°C for 24 h, and the CFU were counted.
To find out if the nauplii were digesting the bacteria, half of the nauplii were removed after 30 min from the bacterial broth under sterile conditions, thoroughly washed, and transferred to a flask with sterile seawater. The nauplii were sampled with a pipette and counted at the same intervals and in the same manner as the batch that remained in the bacterial suspension.
The Artemia nauplii were completely disinfected without apparent damage to the organisms, as observed by Gorospe (15). No antibiotic residue was microbiologically detected. Inhibition halos were observed in all tested bacteria for the antibiotic solution (15.0 to 30.0 mm) and for the hatching seawater (11.0 to 20.0 mm), but no halos could be seen in the macerated nauplii after they were washed. Mohney et al. (21) and Dixon et al. (11) performed similar disk diffusion tests, and their results are comparable to those of this study. Cappellaro et al. (5) proved that with Artemia cysts hatched in two antibiotic solutions, sulfadimethoxine sodium salt and enrofloxacin, the residual antibiotic detected with high-pressure liquid chromatography and UV was only 5.0 to 16.7% of the initial solutions. It is possible that the microbial bioassay was not sensitive enough to detect small amounts of residual antibiotics left in the nauplii. Even when a low concentration of residues of antibiotics remains, it might not be enough to cause any bacterial inhibition in the experiments performed with the nauplii.
The bacterial isolates were identified as belonging to the Vibrio genus, as they were gram-negative short rods, oxidase positive, and motile, had fermentative metabolism of glucose, and were sensitive to the vibriostatic agent O/129 (20). Isolate C7b was able to utilize sucrose, d-mannitol, and l-leucine but did not utilize cellobiose, lactose, l-arabinose, d-melibiose, l-rhamnose, β-hydroxybutyric acid or γ-aminobutyric acid; it was positive for β-galactosidase (o-nitrophenyl-β-d-galactopyranoside [ONPG]), nitrate reduction, and gelatinase; it swarmed in solid media and produced big smooth yellow colonies in TCBS agar. With these characteristics, it was identified as Vibrio alginolyticus. The Biolog Microlog 2.0 identification system also identified it as V. alginolyticus, with a similarity index of 0.897.
Isolate HL57 showed positive utilization of citrate, d-mannitol, and l-arabinose but negative utilization of sucrose. It was positive for β-galactosidase (ONPG), nitrate reduction, and gelatinase, no swarming in solid media was observed, and it grew as 3- to 4-mm blue-green colonies in TCBS agar. These characteristics permitted its identification as Vibrio parahaemolyticus. The Biolog Microlog 2.0 system also gave the same identification, with a similarity index of 0.589. A similarity index of 0.5 is the minimum value for a reliable identification computed with the Microlog 2.0 software.
Optical densities of the inocula and their corresponding CFU milliliter−1 for the experiments with the two species are presented in Table 1. Significant differences among the values of the bacterial suspension inoculated were observed (P = 0.004, Kruskal-Wallis one-way analysis of variance). At the same optical density, isolate HL57 gave a concentration almost 10-fold higher than C7b, 1.50 × 108 and 3.17 × 107 CFU ml−1, respectively. Even within the C7b isolate, differences were found at two similar optical densities (Table 1). In another study, V. anguillarum was found to have a concentration of 2.5 × 108 CFU ml−1 at an optical density at 610 nm of 1.0 (4), while Roque (24) found 4.5 × 107 CFU ml−1 for Vibrio vulnificus at the same optical density.
TABLE 1.
Inoculation concentration of bacteria and maximum concentration bioencapsulated
| Isolate and expt | Optical density at 610 nm | Inoculation concn of bacteria (CFU ml−1)a
|
Minimum enrichment time | Maximum concn of bacteria bioencapsulated (CFU nauplius−1)b
|
||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| C7b | 45 min | |||||
| Expt 1 | 1.00 | 3.170 × 107 A | 1.915 × 105 | 2.70 × 103 | 1.22 × 102 | |
| Expt 2 | 1.20 | 3.485 × 107 B | 1.323 × 105 | 2.42 × 103 | 4.79 × 101 | |
| HL57 | 2 h | |||||
| Expt 1 | 1.00 | 1.487 × 108 C | 4.789 × 105 | 4.55 × 103 | 9.57 × 101 | |
| Expt 2 | 1.00 | 1.512 × 108 C | 1.109 × 106 | 4.77 × 103 | 4.79 × 101 | |
Concentration of bacteria inoculated into 200 ml of seawater to enrich Artemia nauplii. Values with the same letter are not significantly different (P < 0.05 by the Student-Newman-Keuls test).
Maximum concentration of bacteria that were bioencapsulated by the nauplii in the minimum time.
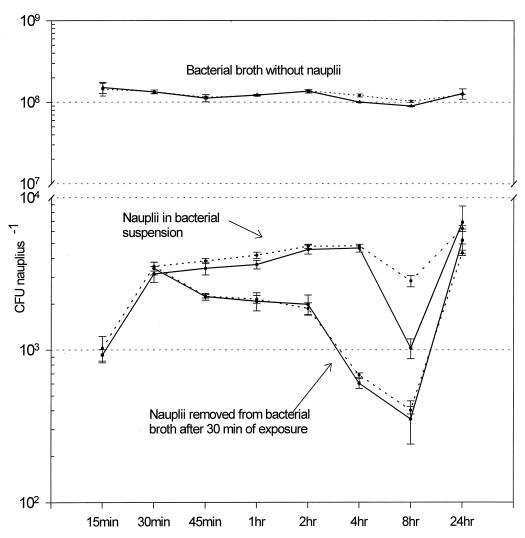
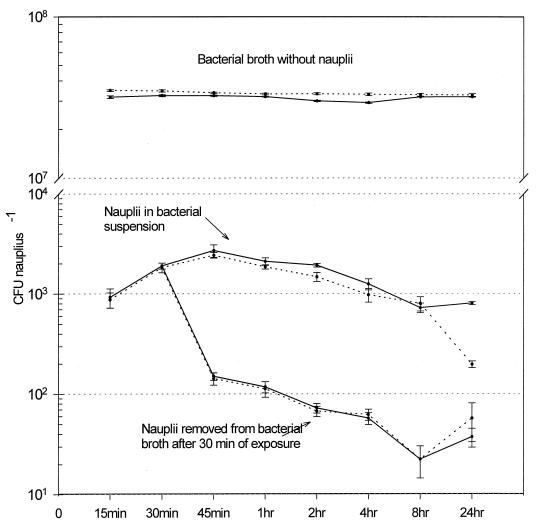
Bacterial isolates were successfully incorporated into the Artemia nauplii. In both experiments with isolate HL57, when nauplii were maintained in the bacterial broth, the quantity of bacteria bioencapsulated rapidly increased after 30 min (Fig. 1) with a sustained level above 2,000 CFU nauplius−1, followed by a slight decline after 8 h. At 24 h, the bacterial level increased again, but all the nauplii died. In the treatment where the nauplii were removed after 30 min from the bacterial broth and placed in sterile saline, the bacterial level in the nauplii started to decrease immediately, reaching a minimal level at 8 h. The concentrations increased again at 24 h, although all nauplii died. The experiments conducted with isolate C7b (Fig. 2) showed a different pattern from that of isolate HL57. In this set of experiments, the C7b isolate was rapidly bioencapsulated, reaching a peak at 45 min. The concentration of bacteria per nauplius decreased slowly to reach a minimum at 24 h. By the end of both experiments, no dead nauplii were observed. After the nauplii were removed from the bacterial broth (at 30 min), the bacterial concentration declined dramatically, 10-fold at the next measurement (45 min). They continued to decline to a minimum at 8 h, with a slight increment observed at 24 h (Fig. 2).
FIG. 1.
Concentration of isolate HL57 (V. parahaemolyticus) bioencapsulated in Artemia nauplii. The counts were done on Marine agar, and data are from two experiments. The mean ± 95% confidence interval (error bar) (n = 4) is shown for each point. Note the change of scale.
FIG. 2.
Concentration of isolate C7b (V. alginolyticus) bioencapsulated in Artemia nauplii. The counts were done on Marine agar, and data are from two experiments. The mean ± 95% confidence interval (error bar) (n = 4) is shown for each point. Note the change of scale.
Results observed by Campbell et al. (4) with formalin-killed bacteria showed that maximum uptake of V. anguillarum occurred at 60 min in a bacterial concentration of 1.5 × 107 CFU ml−1, while at a lower concentration of 1.5 × 106, a peak was observed after 120 min. A similar pattern was observed with Brachionus plicantilis when challenged with a V. anguillarum vaccine (18). The Artemia nauplii ingested the maximum quantity of isolate HL57 cells after 2 h of contact with the bacterial broth (Fig. 1 and Table 1). No statistical difference was encountered between both experiments at 2 h of bioencapsulation (Student’s t test = 2.10, P = 0.080). The difference between the values at 1 and 2 h was highly statistically significant (t test = 7.61 and P = 0.0003 for experiment 1; t test = 7.59 and P = 0.0003 for experiment 2). There was no significant difference between the 2- and 4-h bacterial counts (t test = 0.77 and P = 0.4680 for experiment 1; t test = 0.40 and P = 0.7049 for experiment 2). With these data, it was concluded that 2 h is the optimum exposure time when the largest number of isolate HL57 can be bioencapsulated in the least time.
The nauplii bioencapsulated the maximum number of C7b bacteria after 45 min of contact with the bacterial broth in both experiments (Fig. 2 and Table 1). No difference was encountered between either experiment at 45 min (t test = 2.091, P = 0.0815). The difference between values at 30 and 45 min was highly significant (t test = 6.197 and P = 0.0008 for experiment 1; t test = 7.59 and P = 0.0003 for experiment 2). The difference between values at 45 min and 1 h was also significantly different, with a higher count at 45 min (t test = 4.431 and P = 0.0044 for experiment 1; t test = 10.29 and P < 0.0001 for experiment 2). Therefore, 45 min was the minimum time when the most cells of isolate C7b could be bioencapsulated.
In this study, Artemia nauplii could bioencapsulate from 2.42 × 103 to 4.77 × 103 CFU nauplii−1 of live bacteria (Table 1). Different results were found by Campbell et al. (4) (105 formalin-killed CFU nauplii−1) with dead V. anguillarum cells, but Chair et al. (7) obtained results similar to this study (8.0 × 103 CFU nauplii−1) with live V. anguillarum. Analysis of the results suggest the following: if the bacteria are dead, the concentration is an important factor in the uptake of bacteria by Artemia nauplii, but if the bacteria are alive, the species or strain is also a significant factor. Although the HL57 isolate was at a concentration almost 10-fold higher than that of the C7b isolate, the latter reached an intake peak in less than half the time. One explanation is that isolate C7b had the ability to more rapidly colonize the Artemia nauplii.
The control (bacteria without nauplii) maintained a constant level of bacteria (108 CFU ml−1) throughout the course of experiments with both isolates (Fig. 1 and 2). The bacterial levels in the controls and in the treatments did not show a positive correlation in either experiment with isolate HL57 (for experiment 1, Spearman correlation test, r = −0.180, P = 0.619, n = 8; for experiment 2, Pearson correlation test, r = −0.289, P = 0.487, n = 8). The same correlation was observed in experiments with isolate C7b (Pearson correlation test, r = 0.357, P = 0.385, n = 8 for experiment 1; and r = −0.321, P = 0.439, n = 8 for experiment 2). These negative correlations indicate that the change in the nauplius bacterial levels is not due to the quantity of bacteria available in the broth but to whether the bacteria were ingested or firmly attached to the nauplii. In the control where nauplii remained in sterile seawater only, no bacteria were registered during the course of all experiments.
Bacterial colonization of the nauplii could occur externally, via attachment to the body surfaces or internally by ingestion (16). After the nauplii were removed from the bacterial suspension, the bacterial content decreased rapidly. This decrease might be due to the removal of the external bacteria after the nauplii were washed and placed in sterile seawater. The bacteria still detected could be the ones colonizing the interior or firmly attached to the external surfaces. Similar trends were observed with rotifers after they were removed from a bacterial suspension (18). It should be emphasized that the bacterial counts in the nauplii placed in the bacterial solution had no correlation with the bacterial counts of the solution, which suggests active uptake by the nauplii.
Rico-Mora and Voltolina (22) challenged Artemia nauplii with V. alginolyticus and V. parahaemolyticus isolates and obtained almost 100% mortality after 24 h for the first species and 48 h for the second species. In our work, V. alginolyticus isolated from seawater caused no mortalities after 24 h, while the V. parahaemolyticus isolated from diseased shrimp caused almost 100% mortality. In peneid shrimp, differences in the pathogenicity of bacteria could depend on the species tested (28) and on the strain characteristics (9); a similar case could be presumed for Artemia nauplii.
Acknowledgments
We appreciate the kind cooperation of Jean Swings and Johan Vanderberghe, Microbiology Laboratory, University of Gent, for providing reference bacteria.
This work was supported in part by the International Foundation for Science grant A/2203-1.
REFERENCES
- 1.Austin B, Lee J V. Aeromonadaceae and Vibrionaceae. In: Board R G, Jones D, Skinner F A, editors. Identification methods in applied and environmental microbiology. Oxford, England: Blackwell Scientific Publications; 1992. pp. 163–182. [Google Scholar]
- 2.Bauer A W, Kirby W M M, Sherris J C, Turck M. Antibiotics susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 3.Baumann P, Schubert R H M. Vibrionaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1983. pp. 516–550. [Google Scholar]
- 4.Campbell R, Adams A, Tatner M F, Chair M, Sorgeloos P. Uptake of Vibrio anguillarum vaccine by Artemia salina as a potential oral delivery system to fish fry. Fish Shellfish Immunol. 1993;3:451–459. [Google Scholar]
- 5.Cappellaro H, Gennari L, Achene L, Brambilla G. Artemia salina as medicated feed for marine fry. Boll Soc Ital Patol. 1993;5:29. [Google Scholar]
- 6.Chair M, Romdhane M, Dehasque M, Nelis H, De Leenheer A P, Sorgeloos P. Live-food mediated drug delivery as a tool for disease treatment in larviculture. II. A case study with the European seabass. In: Lavens P, Sorgeloos P, Jaspers E, Ollevier F, editors. Larvi’91—Fish & Crustacean Larviculture Symposium. Special publication no. 15. Ghent, Belgium: European Aquaculture Society; 1991. pp. 412–414. [Google Scholar]
- 7.Chair M, Dehasque M, Van Poucke S, Nelis H, Sorgeloos P, De Leenheer A P. An oral challenge for turbot larvae with Vibrio anguillarum. Aquac Int. 1994;2:270–272. [Google Scholar]
- 8.Chair M, Gapasin R S J, Dehasque M, Sorgeloos P. Vaccination of European sea bass fry through bioencapsulation of Artemia nauplii. Aquacul Int. 1994;2:254–261. [Google Scholar]
- 9.de la Peña L D, Tamaki T, Momoyama K, Nakai T, Muroga K. Characteristics of the causative bacterium of vibriosis in the kuruma prawn, Penaeus japonicus. Aquaculture. 1993;115:1–12. [Google Scholar]
- 10.Dhert P, Lavens P, Duray M, Sorgeloos P. Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using omega 3-HUFA-enriched live food. Aquaculture. 1990;90:63–74. [Google Scholar]
- 11.Dixon B A, Van Poucke S O, Chair M, Dehasque M, Nelis H J, Sorgeloos P, De Leenheer A P. Bioencapsulation of the antibacterial drug sarafloxacin in nauplii of the brine shrimp Artemia franciscana. J Aquat Anim Health. 1995;7:42–45. [Google Scholar]
- 12.Fuller R. History and development of probionts. In: Fuller R, editor. Probiotics. The scientific basis. New York, N.Y: Chapman & Hall; 1992. pp. 1–8. [Google Scholar]
- 13.Garcia-de-la-Banda I, Chereguini O, Rasines I. Influence of lactic bacterial additives on turbot (Scophtalmus maximus L.) larvae culture. Bol Inst Esp Oceanogr. 1992;8:247–254. [Google Scholar]
- 14.Gherna L R. Culture preservation. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 278–292. [Google Scholar]
- 15.Gorospe J N, Nakamura K, Abe M, Higashi S. Nutritional contribution of Pseudomonas sp. in Artemia culture. Fish Sci. 1996;62:914–918. [Google Scholar]
- 16.Grisez L, Chair M, Sorgeloos P, Ollevier F. Mode of infection and spread of Vibrio anguillarum in turbot Scophthalmus maximus larvae after oral challenge through live feed. Dis Aquat Org. 1996;26:181–187. [Google Scholar]
- 17.Joosten P H M, Aviles-Trigueros M, Sorgeloos P, Rombout J H W M. Oral vaccination of the juvenile carp (Cyprinus carpio) and gilthead seabream (Sparus aurata) with bioencapsulated Vibrio anguillarum bacterin. Fish Shellfish Immunol. 1995;5:289–299. [Google Scholar]
- 18.Kawai K, Yamamoto S, Kusuda R. Plankton-mediated oral delivery of Vibrio anguillarum vaccine to juvenile ayu. Nippon Suisan Gakkaishi. 1989;55:35–40. [Google Scholar]
- 19.Lavilla-Pitogo C R, Baticados M C L, Cruz-Lacierda E R, de la Pena L D. Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture. 1990;91:1–13. [Google Scholar]
- 20.Lee J V, Hendrie M S, Shewan J M. Identification of Aeromonas, Vibrio and related organisms. In: Skinner F A, Lovelock D W, editors. Identification methods for microbiologist. London, England: Academic Press; 1979. pp. 151–166. [Google Scholar]
- 21.Mohney L L, Lightner D V, Williams R R. Bioencapsulation of therapeutic quantities of the antibacterial Romet-30 in nauplii of the brine shrimp Artemia and the nematode Panagrellus redivivus. J World Aquacult Soc. 1990;21:186–191. [Google Scholar]
- 22.Rico-Mora R, Voltolina D. Effects of bacterial isolates from Skeletonema costatum cultures on the survival of Artemia franciscana nauplii. J Invertebr Pathol. 1995;66:203–204. [Google Scholar]
- 23.Roque, A., J. F. Turnbull, and B. Gomez-Gil. Delivery of bioencapsulated oxytetracycline to the marine shrimp Penaeus monodon (Fabricius). J. World Aquacul. Soc., in press.
- 24.Roque A. The experimental induction of Vibrio spp. infection in Penaeus monodon (Fabricius). Ph.D. thesis. Stirling, Scotland: University of Stirling; 1995. [Google Scholar]
- 25.Sorgeloos P, Bossuyt E, Laviña E, Baeza-Mesa M, Persoone G. Decapsulation of Artemia cysts: a simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture. 1977;12:311. [Google Scholar]
- 26.Tackaert W, Camara M R, Sorgeloos P. The effect of dietary phosphatidylcholine in postlarval penaeid shrimp. 1. Diet preparation. In: Lavens P, Sorgeloos P, Jaspers E, Ollevier F, editors. Larvi’91—Fish & Crustacean Larviculture Symposium. Special publication no. 15. Ghent, Belgium: European Aquaculture Society; 1991. pp. 76–79. [Google Scholar]
- 27.Touraki M, Rigas P, Pergantas P, Abatzopoulos T, Kastritsis C. Optimizing bioencapsulation of the antibiotics trimethoprim and sulfamethoxazole in Artemia nauplii. In: Lavens P, Sorgeloos P, Jaspers E, Ollevier F, editors. Larvi’91—Fish & Crustacean Larviculture Symposium. Special publication no. 15. Ghent, Belgium: European Aquaculture Society; 1991. pp. 415–418. [Google Scholar]
- 28.Vera P, Navas J I, Quintero M C. Experimental study of the virulence of three species of Vibrio bacteria in Penaeus japonicus (Bate 1881) juveniles. Aquaculture. 1992;107:119–123. [Google Scholar]