Abstract
Background & Aims
Concurrent fatty liver disease represents an emerging challenge in the care of individuals with autoimmune liver diseases (AILD). Therefore, we aimed to validate the ultrasound-based method of controlled-attenuation parameter (CAP) as a non-invasive tool to detect hepatic steatosis in individuals with AILD.
Methods
The diagnostic performance of CAP to determine biopsy-proven hepatic steatosis (>5%) was assessed in individuals with AILD (autoimmune hepatitis [AIH], primary biliary cholangitis [PBC], primary biliary cholangitis [PSC], or variant syndromes) who underwent liver biopsy at the University Medical Center Hamburg-Eppendorf between 2015-2020 by calculating the area under the receiver operating characteristic (AUROC) curves. In AIH, the impact of disease activity was evaluated by assessment of CAP upon resolution of hepatic inflammation during follow-up.
Results
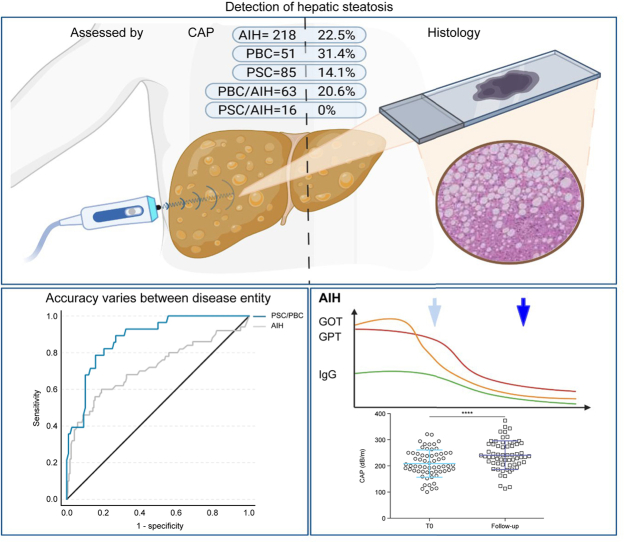
Overall, 433 individuals with AILD (AIH: 218, PBC: 51, PSC: 85, PBC/AIH: 63, PSC/AIH: 16) were included. Histologically proven steatosis was present in 90 individuals (20.8%). Steatosis was less frequently observed in people with PSC (14%) than in other AILD. CAP values correlated positively with grade of steatosis (ρ = 0.39) and the BMI (ρ = 0.53). In PBC and PSC, the ROC curves defined an AUROC of 0.81 and 0.93 for detecting steatosis at an optimal cut-off of 276 dB/m (sensitivity: 0.71; specificity: 0.82) and 254 dB/m (sensitivity: 0.91, specificity: 0.85), respectively. In AIH, the diagnostic performance of CAP was significantly lower (AUROC = 0.72, p = 0.009). However, resolution of hepatic inflammation under treatment was associated with a significant increase in CAP levels (median [IQR]: +38.0 [6-81] dB/m) and considerably improved diagnostic accuracy (AUROC = 0.85; cut-off: 288 dB/m; sensitivity: 0.67, specificity: 0.90).
Conclusions
In PBC and PSC, hepatic steatosis can be reliably detected by applying disease-specific thresholds of CAP. In AIH, the diagnostic accuracy of CAP is moderate at diagnosis, but improves after acute hepatitis has resolved.
Impact and implications
Non-invasive estimation of fat content in the liver can be performed with the ultrasound-based method of controlled-attenuation parameter (CAP). Here, we showed that the presence of a concomitant fatty liver is frequent in people with autoimmune liver diseases and we determined disease-specific thresholds of CAP to best predict the presence of a fatty liver. CAP measurement was shown to be a valid tool to detect fatty liver in individuals with PSC and PBC; however, in AIH, CAP had limited accuracy especially when significant inflammatory activity was present in the liver. In the context of substantial liver inflammation, therefore, CAP values should be interpreted with caution, and measurements should be repeated after acute hepatitis has resolved.
Keywords: Autoimmune liver disease, Autoimmune hepatitis, Primary biliary cholangitis, Primary sclerosing cholangitis, Controlled attenuation parameter, Steatosis hepatis, Non-invasive steatosis measurement, Inflammation
Graphical abstract
Highlights
-
•
Presence of hepatic steatosis significantly differs between AILD entities with lower rates in PSC and PSC variants.
-
•
CAP measurement is a reliable tool to determine hepatic steatosis in cholestatic liver diseases such as PSC and PBC.
-
•
In people with AIH and inflammatory activity, accuracy of CAP measurement is significantly reduced and should be interpreted with caution.
-
•
After resolution of acute hepatitis, CAP values and its diagnostic performance increased in AIH.
Introduction
The increasing prevalence of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) represents an increasing health burden in the western world.1 Consequently, concomitance of hepatic steatosis in individuals with autoimmune liver diseases (AILD) is frequent in western countries2,3 and represents an emerging challenge for health care.[4], [5], [6] Moreover, individuals with autoimmune hepatitis (AIH) frequently require treatment with corticosteroids7,8 setting the stage for de novo development of NAFLD and NASH or potentially aggravating pre-existing fatty liver disease.9,10 Liver biopsy is the gold standard for the evaluation of hepatic steatosis, which is accompanied by risks of an invasive procedure such as bleeding and sampling bias.[11], [12], [13] As a non-invasive tool to estimate hepatic steatosis, the ultrasound wave-based measurement of controlled attenuation parameter (CAP) has been integrated with the transient elastography (TE) measurement (FibroScan®, EchoSens, Paris, France).14,15 CAP displays the attenuation of an ultrasound beam and therefore correlates with the sonographic properties of a tissue, which are influenced by the quantity of fat droplets within hepatocytes.14
The accuracy of CAP to determine hepatic steatosis has been broadly evaluated in individuals with NAFLD and NASH[16], [17], [18] as well as in viral hepatitis and compensated liver diseases of different etiologies.19,20 However, comprehensive evaluation of the diagnostic reliability of CAP is lacking for people with AILD. As hepatic steatosis is a known factor for fibrosis progression and development of hepatocellular carcinoma,[21], [22], [23] concomitant steatosis also displays an important risk factor for disease progression in AILD.3 Thus, the detection and monitoring of hepatic steatosis by non-invasive means are of great clinical relevance for the care of people with AILD. Therefore, our aim was to evaluate the reliability of CAP measurements in individuals with AILD and to determine disease-specific cut-off values for detecting hepatic steatosis.
Patients and methods
Study cohort
People who underwent liver biopsy for diagnostic reasons at the University Medical Center Hamburg-Eppendorf between January 2015 and September 2020 (n = 1159) were screened for the presence of an underlying AILD, including autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and variant syndromes of AIH and PBC (PBC/AIH) or PSC (PSC/AIH), respectively. The diagnoses were based on clinical, biochemical, serological, histopathological and imaging findings in accordance with the respective EASL-Guidelines.[24], [25], [26] Individuals with AILD, who had undergone elastographic measurements close to liver biopsy (6 months before to 6 months after liver biopsy), where included in the final analysis. A total of 433 individuals were analysed in our study cohort. The process of participant selection for this study is illustrated in Fig. S1.
We assessed demographic, clinical and elastographic characteristics of the study cohort at the time of initial CAP assessment and during the disease course. Available elastography follow-up measurements after initial CAP assessment were available for 243 individuals (AIH: n = 153, PBC: n = 34; PSC: n = 56).
In AIH, the impact of hepatic inflammation on CAP was investigated by grouping participants with AIH in subpopulations with high inflammatory activity, defined by serum transaminases levels of aspartate aminotransferase (AST) >2 × upper limit of normal (ULN), or low inflammatory activity (serum AST ≤2 × ULN) at the time of CAP measurement. In AIH, besides non-invasive steatosis measurement performed close to obtaining a liver biopsy sample, repeated CAP measurements after achievement of complete biochemical response (CBR) was evaluated for to determine accuracy of the degree of hepatic steatosis. CBR was defined as normalisation of transaminases and IgG, according to the current definitions.27 Two individuals with AIH were included in the analysis despite persistent IgG elevation due to multiple myeloma or monoclonal gammopathy of undetermined significance.
The study was approved by the local ethics committees (Ethikkommission der Ärztekammer Hamburg, Germany, project number PV4081) and informed consent was obtained from all participants.
CAP-measurement
Transient elastography and CAP measurements were performed using a FibroScan device (EchoSens, Paris, France) as reported previously.28 The target area of the right liver lobe was determined by ultrasonography to be 6 cm in depth without major vascular structures. The median values of liver stiffness and CAP measurements were recorded in kilopascal (kPa) and decibel per meter (dB/m), respectively. All elastographic measurements were performed by two experienced and well-trained investigators having performed more than 2000 measurements each (KF and IS). M and XL probes were used according to manufacturer’s recommendations.
Liver biopsy
Mini-laparoscopically guided liver biopsy is a standard at our centre and was conducted for people with AILD as described previously.29 In brief, mini-laparoscopy was performed using a 1.9 mm diameter end-viewing 0° optical instrument, which was inserted through a 2.75-mm trocar. Liver samples were obtained under view with a Tru-Cut 16 GAUGE needle. To minimize sampling bias, liver biopsies were routinely obtained from the left and right lobe, if not prohibited by intraprocedural issues, such as bleeding. In cases of contraindications for mini-laparoscopic-guided biopsy (e.g. because of pervious operation with scaring and high-risk of abdominal adhesions), liver biopsy was obtained via percutaneous biopsy using the Menghini technique with a 17 GAUGE × 90 mm needle (Epaset, M.D.L., Delebio, Italy).
Liver histology
Histopathological evaluation was based on haematoxylin and eosin and van Gieson-Elastin staining of paraffin-fixed liver tissue. Steatosis was graded based on the frequency of steatotic hepatocytes (S0: <5%, S1: 5–33%; S2: 33–66%; S3: >66%).30 Hepatic inflammation was graded using the modified hepatitis activity index (mHAI) by Ishak building a maximum score of 18 based on grade of inflammatory activity and necrosis.31 Fibrosis was staged on a scale of 0–4 as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis, in accordance to the classification of Batts and Ludwig.32 All histopathological evaluations were executed by two experienced pathologists with more than 5 years of specialist experience (TK or SW). In addition, inter-rater reliability for histological liver assessment was tested by calculating the intraclass correlation coefficient (ICC) of mHAI scoring between both pathologists. Previous ICC evaluation showed a very good strength of agreement (ICC1 = 0.78; 95%–confidence interval (CI): 0.72–0.83; p <0.001) with a mean difference of 1.19 points (95% CI: 0.87–1.48; p <0.001).33
In cases of differing results in histological steatosis assessment between left and right lobe biopsies, reported in about 5% of assessed individuals, results of the right lobe were taken as basis of this study, as non-invasive steatosis- and fibrosis assessment by FibroScan targets the right lobe.
Statistical analysis
Data are expressed as the mean and standard deviation (SD) or the median with range, as appropriate. Differences between two groups were compared using the Mann–Whitney U test. Differences of more than two groups were analysed by the Kruskal–Wallis test. For comparison of paired data, the Wilcoxon matched-pairs signed-rank test was used. Nominal characteristics were compared using the chi-square test or the Fisher‘s exact test, as appropriate. The correlation of ordinal and constant variables was estimated using the Spearman’s ρ coefficient. Relation of dichotomous variables was evaluated by binary logistic regression analysis; for constant variables uni- and multivariate regression analysis were performed.
The diagnostic accuracy of CAP was determined by the area under receiver operating characteristics (AUROC) curves. Optimal cut-off values for CAP measurements were determined by Youden’s index, displaying the maximum sum of sensitivity plus specificity, and further evaluated for sensitivity, specificity, and for positive and negative predictive values. Differences in the AUROC were calculated as proposed by Hanley and McNeil.34 For comparison of accuracy of CAP in paired groups (T0 and follow-up) McNemar’s test was used.35
P-values <0.05 were considered significant, and all p values were two-tailed. Statistical and graphical analyses were performed using IBM SPSS Statistics, v.27.0 (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Armonk, NY, USA) and GraphPad Prism Version 8.0.0 for Windows, (GraphPad Software, San Diego, CA, USA). The graphical abstract was created using BioRender.com (Science Suite Inc., Toronto, Canada).
Results
Hepatic steatosis is frequently found in AIH and PBC
A total of 433 individuals with AILD who underwent liver biopsy for diagnostic reasons were included in the study. CAP measurements and liver biopsies were 11 days (median) apart (IQR: 56 days before biopsy to 14 days after biopsy). The largest subgroup represented people with AIH (n = 218; 50.3%), followed by PSC (19.6%; n = 85), PBC/AIH (14.5%; n = 63), PBC (11.8%; n = 51), and PSC/AIH (3.7%; n = 16). The composition of the study cohort as well as the clinical, laboratory and elastographic characteristics at the time of CAP-measurement are summarised in Table 1 (Table S1; Fig. S2).
Table 1.
Clinical and demographic characteristics of liver-biopsied participants with AILD.
| Value | AIH (n = 218) | PBC (n = 51) | PSC (n = 85) | PBC/AIH (n = 63) | PSC/AIH (n = 16) | p value |
|---|---|---|---|---|---|---|
| Female, % (n) | 74.3% (162) | 82.4% (42) | 44.7% (38) | 84.1% (53) | 56.3% (9) | <0.0001 |
| Age, years | 53 (16–85) | 52 (26–71) | 46 (16–70) | 52 (2–78) | 19 (18–59) | <0.001 |
| BMI, kg/m² | 27.1 (12.2-46.5) | 26.0 (18.8-46.5) | 24.5 (15.9-33.9) | 25.5 (17.8-37.5) | 22.2 (20.0-35.5) | <0.001 |
| Haemoglobin, g/dl | 13.5 (±1.7) | 13.4 (±1.3) | 13.5 (±1.8) | 13.2 (±1.5) | 13.7 (±1.3) | 0.194 |
| Platelets, 109/L | 214.1(±90) | 249.4(±89) | 274.5 (±90) | 239.9 (±88) | 273.6 (±102) | <0.0001 |
| Albumin, g/L | 35.9 (±5.6) | 38.3 (±4.5) | 37.9 (±4.6) | 37.4 (±5.6) | 36.1 (±4.7) | 0.057 |
| Bilirubin, mg/dl | 0.8 (0.2-25.6) | 0.5 (0.2-2.2) | 0.7 (0.2-12.8) | 0.7 (0.3-15.8) | 0.8 (0.4-3.0) | <0.0001 |
| HbA1c, % | 5.4 (4.0-10.6) | 5.5 (3.8-13.4) | 5.4 (3.9-6.8) | 5.2 (4.4-6.3) | 5.3 (4.9-5.4) | 0.247 |
| Cholesterol, mg/dl | 190.0 (52-335) | 209.0 (134-314) | 200.0 (100-351) | 224.0 (103-391) | 214.5 (142-248) | 0.014 |
| Triglycerides, mg/dl | 102 (44-552) | 120 (50-617) | 86(41-694) | 95(42-317) | 126 (70-246) | 0.239 |
| AST, U/L | 71.0 (14-1262) | 40.0 (19-188) | 39.0 (14-399) | 52.0 (11-884) | 64.5 (20-382) | <0.0001 |
| ALT, U/L | 86.5 (14-2506) | 61.0 (24-375) | 70.0 (17-697) | 65.0 (17-898) | 83.0 (22-422) | 0.045 |
| GGT, U/L | 106 (15-1513) | 129 (20-1372) | 257 (15-1631) | 135 (20-1251) | 153 (18-662) | 0.004 |
| ALP, U/L | 112 (35-986) | 165 (53-887) | 204 (58-1011) | 139 (28-1022) | 145 (58-500) | <0.0001 |
| IgG, g/L | 15.3 (5.4-56.9) | 13.2 (5.9-26.8) | 13.7 (7.4-32.2) | 15.8 (4.2-47.7) | 20.1 (5.4-48.2) | 0.017 |
Nominal variables are presented as frequencies (and total numbers). Differences were calculated using the chi-square test. Continuous variables are presented as median (range) or mean (± standard deviation), as appropriate.
Differences between groups were compared using Kruskal–Wallis test.
Values in bold denote statistical significance.
AIH, autoimmune hepatitis; AILD, autoimmune/immune mediated liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma-glutamyl transferase; HbA1c, glycated hemoglobin; IgG, immunoglobulin G; PBC, primary biliary cholangitis; PBC/AIH, variant syndrome of PBC and AIH; PSC, primary sclerosing cholangitis; PSC/AIH, variant syndrome of PSC and AIH.
As expected, hepatic inflammation, as displayed by higher levels of serum AST and higher mHAI scores, was more pronounced in individuals with AIH (Table 1, Table 2). In addition, histologically proven cirrhosis was most frequent in the AIH subgroup, as well as in individuals with PSC/AIH (Table 2). With regard to risk factors for hepatic steatosis, individuals with AIH and PBC were significantly older, had higher BMI values, and received anti-diabetic treatment more frequently compared with individuals with PSC (Table 1, Table 3).
Table 2.
Histological characteristics of liver-biopsied participants with AILD.
| Value | AIH (n = 218) | PBC (n = 51) | PSC (n = 85) | PBC/AIH (n = 63) | PSC/AIH (n = 16) | p value |
|---|---|---|---|---|---|---|
| mHAI, /18 | 7.0 (±3.2) | 3.2 (±1.8) | 2.4 (±2.0) | 6.5 (±2.3) | 6.2 (±2.6) | <0.0001 |
| Treatment-naive participants, % (n) | 72.5% (158) | 58.8% (30) | 67.1% (57) | 42.9% (27) | 56.3% (9) | <0.001 |
| mHAI of treatment naive participants, /18 | 7.5 (±3.1) | 3.3 (±1.9) | 2.3 (±1.7) | 7.6 (±2.1) | 7.6 (±2.6) | <0.0001 |
| Presence of histological steatosis (>5%), % (n) | 22.5% (49) | 31.4% (16) | 14.1% (12) | 20.6% (13) | 0 | 0.025 |
| Steatosis grades, % (n) | ||||||
| S0 | 77.5% (169) | 68.6% (35) | 85.9% (73) | 79.4% (50) | 100% (16) | 0.027 |
| S1 | 21.1% (46) | 25.5% (13) | 9.4% (8) | 19.0% (12) | 0 | 0.017 |
| S2 | 1.4% (3) | 3.9% (2) | 4.7% (4) | 1.6% (1) | 0 | 0.329 |
| S3 | 0 | 2.0% (1) | 0 | 0 | 0 | 0.155 |
| Cirrhosis (F = 4), % (n) | 33.0% (72) | 17.6% (9) | 10.6% (9) | 25.4% (16) | 37.5% (6) | 0.001 |
mHAI is presented as mean (± standard deviation), differences between groups of ordinal values were compared using Kruskal-Wallis test.
Data on steatosis and cirrhosis (histological fibrosis grade = 4) are presented as frequencies (and total numbers). Steatosis was graded based on the frequency of steatotic hepatocytes: S0, <5%; S1, 5-33%; S2, 33-66%; S3, >66%.
Differences of nominale values were calculated using the chi-square test or Fisher’s exact-test as appropriate.
Values in bold denote statistical significance.
AIH, autoimmune hepatitis; AILD, autoimmune/immune mediated liver disease; mHAI, modified Histological Activity Index; PBC, primary biliary cholangitis; PBC/AIH, variant syndrome of PBC and AIH; PSC, primary sclerosing cholangitis; PSC/AIH, variant syndrome of PSC and AIH.
Table 3.
Treatment of liver-biopsied participants with AILD at time of CAP-measurement.
| Value | AIH (n = 218) | PBC (n = 51) | PSC (n = 85) | PBC/AIH (n = 63) | PSC/AIH (n = 16) | p value |
|---|---|---|---|---|---|---|
| Anti-diabetic treatment | 10.6% (23) | 17.6% (9) | 4.7% (4) | 6.3% (4) | 6.3% (1) | 0.128 |
| Immune-suppressive medication (any form) | 56.9% (124) | 3.9% (2) | 16.5% (14) | 44.4% (28) | 68.8% (11) | <0.0001 |
| Prednisolone | 48.2% (105) | 3.9% (2) | 9.4% (8) | 30.2% (19) | 25.0% (4) | <0.0001 |
| Budesonide | 2.3% (5) | 0 | 1.2% (1) | 6.3% (4) | 12.5% (2) | 0.038 |
| Azathioprine | 27.1% (59) | 0 | 3.5% (3) | 23.8% (15) | 37.5% (6) | <0.0001 |
| Second-line/third-line treatment for AIH | 7.8% (17) | 0 | 0 | 3.2% (2) | 12.3% (2) | 0.003 |
| UDCA | 3.2% (7) | 64.7% (33) | 47.1% (40) | 54.0% (34) | 68.8% (11) | <0.0001 |
| Second/third line treatment for PBC | 0 | 7.8% (4) | 0 | 7.9% (5) | 0 | <0.0001 |
| Biologicals | 0.9% (2) | 0 | 5.9% (5) | 0 | 6.3% (1) | 0.019 |
Data is presented as frequencies (and total numbers). Differences were calculated using Fisher’s exact-test as appropriate.
Values in bold denote statistical significance.
AIH, autoimmune hepatitis; AILD, autoimmune/immune mediated liver disease; CAP, controlled-attenuation parameter; PBC, primary biliary cholangitis; PBC/AIH, variant syndrome of PBC and AIH; PSC, primary sclerosing cholangitis; PSC/AIH, variant syndrome of PSC and AIH; UDCA, ursodeoxycholic acid.
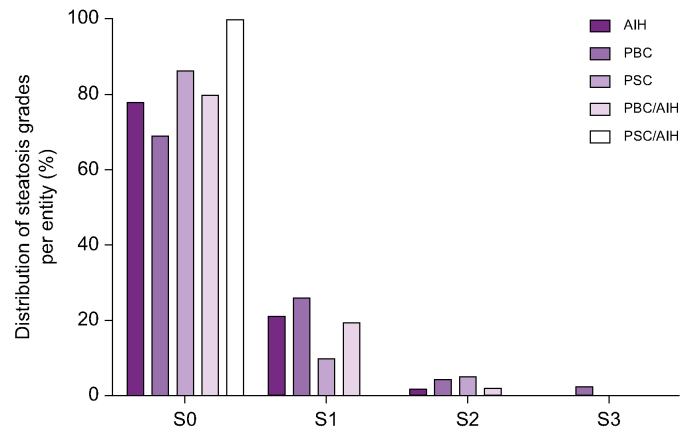
Of 433 participants with AILD, 90 individuals showed hepatic steatosis on histologic examination, which corresponds to 20.7% of the total cohort. In most cases, histological grade of hepatic steatosis was mild (S1) (87.8% of all cases with steatosis; Fig. 1, Table 2). Compared with non-steatotic individuals, people with AILD and concomitant presence of hepatic steatosis on liver histology showed higher BMI and triglyceride values, as well as increased rates of diabetes and were of older age (Table 4).
Fig. 1.
Steatosis grades in people with AILD.
Grading of steatosis in participants with AILD (n = 90) by disease entity. Steatosis was graded based on the frequency of steatotic hepatocytes: S0, <5%; S1, 5-33%; S2, 33-66%; S3, >66%. AIH, autoimmune hepatitis; AILD, autoimmune/immune mediated liver disease; PBC, primary biliary cholangitis; PBC/AIH, variant syndrome of PBC and AIH; PSC, primary sclerosing cholangitis; PSC/AIH, variant syndrome of PSC and AIH.
Table 4.
Clinical, demographical and laboratory characteristics of steatotic and non-steatotic subpopulations with AILD.
| Value | Non-steatotic (n = 343) | Steatotic (n = 90) | p value |
|---|---|---|---|
| Female, % (n) | 68.5% (235) | 76.7% (69) | 0.155 |
| Age, years | 49.0 (16–85) | 54.0 (24–80) | <0.001 |
| Cirrhosis, % (n) | 24.2% (83) | 31.1% (28) | 0.223 |
| BMI, kg/m² | 24.9 (12.1-42.5) | 30.0 (18.8-46.5) | <0.0001 |
| Haemoglobin, g/dl | 13.48 (±1.7) | 14.0 (±1.4) | <0.001 |
| Platelets, 109/L | 214.35 (±90.5) | 229.1 (±85.8) | 0.284 |
| Albumin, g/L | 36.0 (±5.5) | 37.4 (±4.4) | 0.205 |
| Bilirubin, mg/dl | 0.8 (0.2-25.6) | 0.6 (0.2-14.3) | <0.001 |
| HbA1c,% | 5.3 (3.8-10.4) | 5.7 (4.2-13.4) | <0.01 |
| Cholesterol, mg/dl | 196.0 (52.0-391.0) | 212.5 (80.0-335.0) | 0.091 |
| Triglycerides, mg/dl | 97 (41-401) | 169 (70-694) | <0.0001 |
| AST, U/L | 56 (11-1,262) | 41 (17-753) | 0.019 |
| ALT, U/L | 79 (14-2,506) | 62 (20-1,434) | <0.01 |
| GGT, U/L | 141 (15.0-1,631) | 105 (15-1,251) | 0.033 |
| AP, U/L | 146 (35-1,011) | 11 (28-1,022) | <0.0001 |
| IgG, g/L | 15.0 (4.2-56.6) | 14.8 (6.5-29.4) | 0.585 |
| Anti-diabetic treatment, % (n) | 7.6% (26) | 16.7% (15) | 0.014 |
| Immune-suppressive medication (any form), % (n) | 39.9% (137) | 46.7% (42) | 0.281 |
| Prednisolone, % (n) | 31.2% (107) | 37.8% (34) | 0.162 |
| UDCA, % (n) | 30.3% (104) | 21.1% (19) | 0.089 |
Nominal variables are presented as frequencies (and total numbers). Differences were calculated using the chi-square test or Fisher´s exact-test as appropriate. Continuous variables are presented as median (range) or mean (± standard deviation), as appropriate. Differences between groups were compared using Mann-Whitney-U-test.
Values in bold denote statistical significance.
AILD, autoimmune/immune mediated liver disease; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index, GGT, gamma glutamyl transferase; HbA1c, glycated hemoglobin; IgG, immunoglobulin G; UDCA, ursodeoxycholic acid.
Presence of concomitant hepatic steatosis significantly differed between AILD entities (p = 0.03). However, in the binary logistic regression analysis hepatic steatosis was not independently associated with the underlying disease entity, but with the BMI (OR: 1.285; 95% CI: 1.139-1.145, p <0.0001), triglyceride levels (OR: 1.022; 95%-CI:1.013-1.031, p <0.0001), and age (OR: 1.042; 95%-CI:1.013-1.031, p = 0.049). In line with higher BMI values in the AIH and PBC subpopulations, hepatic steatosis was significantly more frequent in these individuals than in those with PSC or PSC/AIH (AIH: 22.5%, n = 49; PBC: 31.4%, n = 16; PBC/AIH: 20.6%, n = 13; PSC: 14.1%, n = 12; PSC/AIH: 0%, p <0.05, Table 2). As histologically proven steatosis was not observed in individuals from the PSC/AIH group, no disease-specific analyses were performed in this subpopulation.
Validation of CAP to identify hepatic steatosis in AILD
Next, we explored the diagnostic performance of CAP as a non-invasive surrogate marker of hepatic steatosis in relation to liver histology. The overall elastographic characteristics of individuals with AILD are summarised in Table S1. In the overall cohort, CAP showed a significant positive correlation with hepatic fat content (grade of steatosis: ρ = 0.385, p <0.001, percentage of steatosis hepatocytes: ρ = 0.386, p <0.001) and BMI values (ρ = 0.527, p <0.001), but also within the respective subpopulations of AIH, PBC, PSC and PBC/AIH (not shown).
The optimal cut-off value to detect hepatic steatosis in people with AILD was determined according to the Youden’s index defined as the highest sum of sensitivity and specificity. Based on this, the optimal threshold in the overall cohort was determined to be 257 dB/m, which demonstrated an overall acceptable diagnostic performance (AUROC = 0.76), albeit with the limitation of moderate sensitivity (0.65) and low positive predictive value (0.47) (Table 5). Interestingly, using the uniform CAP threshold of 257 dB/m to discriminate individuals as either steatotic or non-steatotic, the disease entity was the only factor that was independently associated with incorrect allocation. In fact, AIH and PBC showed a significant increased risk for incorrect classification into the steatotic or non-steatotic group based on the universal cut-off value (PSC: OR = 0.891, p = 0.015; PBC: OR = 2.84, p = 0.023; AIH: OR = 2.35, p = 0.022).
Table 5.
Performance of CAP measurements to determine any grade of steatosis (>S0).
| Value | All AILD | AIH | AIH (follow-up) in remission | PBC | PSC | PBC/AIH |
|---|---|---|---|---|---|---|
| Number of participants | 433 | 218 | 62 | 51 | 85 | 63 |
| AUROC | 0.76 | 0.72 | 0.85 | 0.81 | 0.93 | 0.70 |
| Optimal cuff-off (dB/m) | 257 | 256 | 288 | 276 | 254 | 255 |
| Sensitivity | 0.65 | 0.60 | 0.67 | 0.71 | 0.91 | 0.62 |
| Specificity | 0.81 | 0.80 | 0.90 | 0.82 | 0.85 | 0.76 |
| Positive predictive value | 0.47 | 0.48 | 0.61 | 0.63 | 0.55 | 0.40 |
| Negative predictive value | 0.90 | 0.81 | 0.92 | 0.88 | 0.98 | 0.88 |
AIH, autoimmune hepatitis; AILD, autoimmune/immune mediated liver disease; AUROC: area under the receiver operating characteristics CAP, controlled-attenuation parameter; PBC, primary biliary cholangitis; PBC/AIH, variant syndrome of PBC and AIH; PSC, primary sclerosing cholangitis.
Therefore, we investigated further whether the diagnostic accuracy could be improved by calculating disease-specific thresholds, and whether the diagnostic performance could differ between the respective autoimmune liver diseases.
Diagnostic accuracy of CAP differs between disease entities of AILD
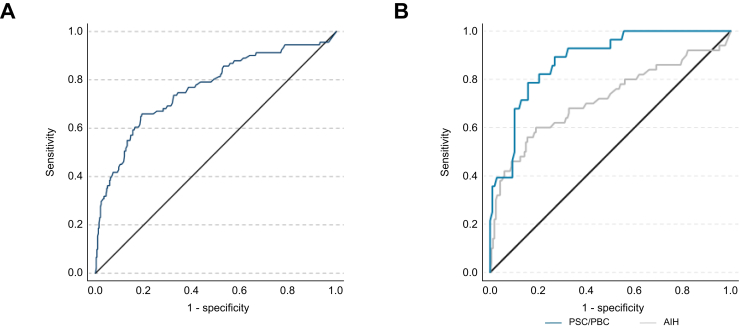
Calculating disease-specific cut-offs revealed that thresholds varied greatly depending on the underlying AILD (AIH: 256 dB/m, PBC: 276 dB/m, PSC: 254 dB/m) (Table 5). Moreover, applying disease-specific thresholds considerably improved the diagnostic accuracy in PBC (AUROC = 0.81, sensitivity: 0.71, specificity: 0.82) and PSC (AUROC = 0.93, sensitivity: 0.91, specificity: 0.85), but not in those with AIH (AUROC = 0.72, sensitivity: 0.60, specificity: 0.80) (Table 5, Fig. S3). These differences in the diagnostic performance between individuals with cholestatic liver disease (PBC, PSC) and AIH were statistically significant (p = 0.009) (Fig. 2).
Fig. 2.
Receiver operator characteristic curve of CAP measurement for diagnosis of hepatic steatosis (>S0).
(A) Participants with mixed aetiologies of AILD: area under the ROC (AUROC) = 0.76. (B) Comparison of ROC to detect hepatic steatosis in participants with AIH (grey) and cholestatic liver disease (blue), AUROC differed significantly (AUROC AIH = 0.72; AUROC PBC/PSC = 0.88), p = 0.009. Differences of AUROC were calculated as proposed by Hanley and McNeil. AIH, autoimmune hepatitis; AILD, autoimmune/immune mediated liver diseases; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; ROC, receiver operator characteristic curve.
Furthermore, also after the application of disease-specific cut-off values, AIH was associated with increased risk of incorrect assignment based on CAP (OR: 1.82, 95% CI:1.03–3.21, p = 0.04) compared with cholestatic liver diseases.
We also addressed other potential confounding factors that could have influenced our findings. As we evaluated the performance of CAP within a time frame 6 months of a biopsy, we validated the accuracy of CAP in individuals, who received liver biopsy within 30 days of elastography assessment (n = 256). In this sub-cohort CAP showed an even worse accuracy for AIH (AUROC: 0.64); however, the accuracy for PBC (AUROC: 0.76) and PSC (AUROC: 0.94) diagnosis was sufficient. We further addressed whether probe size of the elastography device influenced CAP measurements. Given that the probe size was chosen according to manufacturer’s recommendations based on patient morphology, the prevalence of steatosis in individuals with CAP measurements obtained using the XL probe was significantly higher (33.8%) compared with the cohort, in which CAP was obtained using the M probe (18.3%, p = 0.002). However, the probe size of the CAP measurement did not significantly influence the diagnostic accuracy of CAP (M probe: AUROC = 0.74; XL probe: AUROC = 0.79, p = 0.436).
Hence, the observed moderate accuracy for the detection of steatosis in the overall AILD cohort was mainly attributable to the moderate performance in people with AIH, who represented the largest subgroup (50.3%) of the total cohort.
Hepatic inflammation and BMI impact the diagnostic performance of CAP
In contrast to PBC and PSC, AIH is characterised by a higher degree of hepatic inflammation. Accordingly, individuals with AIH had significantly higher levels of serum transaminases and higher mHAI scores than those with cholestatic autoimmune liver diseases (Table 1, Table 3). As demonstrated previously, liver inflammation poses a potential confounder in liver stiffness measurements by transient elastography during the first months of AIH treatment.28 Therefore, we investigated whether hepatic inflammation may also have an impact on the diagnostic accuracy of CAP in AIH.
Of note, a weak but significant negative correlation was observed for CAP with serum transaminase levels (AST: ρ = -0.221, p = 0.001; alanine aminotransferase [ALT]: ρ = -0.194, p = 0.004) as well as bilirubin (ρ = -0.216, p = 0.001), whereas the time between biopsy and elastographic assessment was positively associated with CAP values (time in days: ρ = 0.173, p = 0.001). However, in multivariate regression analysis only the correlation between CAP with hepatic steatosis, BMI, and bilirubin maintained statistical significance (corrected R-square = 0.386, p <0.0001).
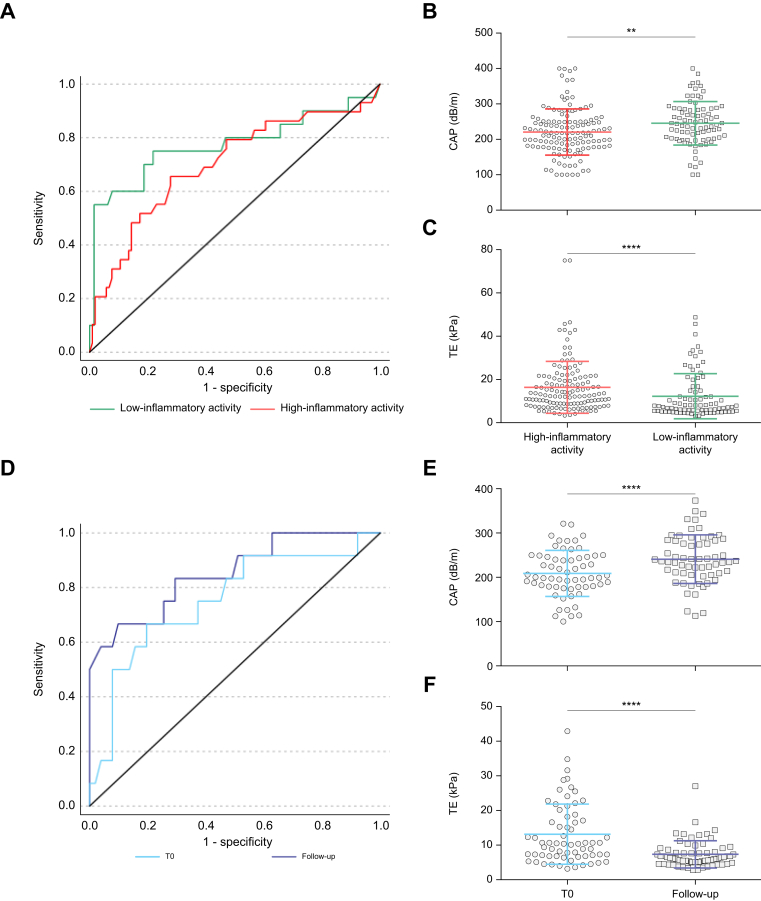
As liver biopsy was performed to establish AIH diagnosis, most individuals with AIH (72.5%, n = 158) did not receive immunosuppressive treatment at the time of biopsy. Consequently, also at time of first CAP measurement high inflammatory activity, displayed by increased levels of serum transaminases (AST >2 × ULN), was present in most participants (n = 134, 84.5% of the whole AIH cohort). In this subgroup characterised by high inflammatory AIH-activity the diagnostic accuracy of CAP to detect hepatic steatosis was worse (AUROC = 0.69) than in individuals with AIH and low inflammatory activity (AST ≤2 × ULN; n = 84) (AUROC = 0.77; Fig. 3A). Of note, mean CAP values in individuals with high inflammatory activity were significantly lower than those who had already cleared acute hepatitis (Fig. 3B).
Fig. 3.
Effects of liver inflammation on CAP measurement in AIH.
(A) ROC curves of CAP for the detection of steatosis in individuals with AIH: in the presence of high inflammatory activity (AST >2 × ULN, red) (n = 134, AUROC = 0.69) or low inflammatory activity (AST ≤2 × ULN, green) (n = 84 AUROC = 0.77). (B) CAP- and (C) TE-values differed significantly in AIH-subpopulations with low and high inflammatory activity. (D) ROC curves of people with AIH and high inflammatory activity at first CAP measurement (T0, light blue; AUROC = 0.75) and of the same individuals upon follow-up after CBR was achieved (follow-up, dark blue; AUROC = 0.85). After CBR was achieved mean CAP values significantly increased (E), whereas liver stiffness decreased (F). Mann-Whitney-U-test was used to test between participants with high and low inflammatory activity; Wilcoxon matched-pairs signed-rank test was used to test differences of individuals with AIH upon follow-up; ∗∗p <0.01; ∗∗∗∗p <0.0001. AIH, autoimmune hepatitis; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristics; CAP, controlled-attenuation parameter; CBR, complete biochemical response; ROC, receiver operating characteristic; TE, transient elastography; ULN, upper limit of normal.
However, only substantial inflammation appeared to influence the CAP as individuals who already achieved complete biochemical response (CBR, defined by normalization of serum transaminases and IgG) did not show any differences in CAP compared with those with mild disease activity (abnormal serum transaminases with AST≤2 × ULN) (Fig. S4A). As jaundice at the time of diagnosis is a marker of severe hepatitis, we further studied people with AIH and elevated serum bilirubin levels above 2 mg/dl at the time of elastographic assessment. In line with our previous results, we found that jaundice in individuals with AIH was associated with significantly lower CAP values and particularly low diagnostic accuracy (AUROC: 0.52, Fig. S4B and C).
Association of CAP development over time with changes in clinical parameters
To estimate the clinical factors that influence changes in CAP values over time regardless of the underlying disease and its respective activity, we analysed follow-up CAP measurements of individuals with AIH, PBC, and PSC after initial CAP measurement. Complete elastographic and clinical follow-up data was available for 243 individuals (AIH: n = 153, PBC: n = 34; PSC: n = 56) with a median follow-up time of 12 months (minimum: 1, maximum: 24 months). Clinical and elastrographic characteristics of the cohort are summarised in Table S2.
Changes in markers, expressed as their delta value, that significantly correlated with changes of CAP are displayed in Table S3. In the multivariate analysis we found that delta CAP was positively associated with delta of BMI (regression coefficient β = 4.327, p = 0.02), whereas the delta of bilirubin ((B) = -4.749, p = 0.007), and gamma-glutamyl transferase (GGT) ((B) = -0.042, p = 0.005) was independently and negatively associated with delta of CAP. However, the impact of those factors was not evenly distributed among the diseases. In cholestatic liver diseases predominantly delta of BMI showed positive correlation with CAP increases (PBC: ρ = 0.367, p = 0.0034; PSC: ρ = 0.367, p = 0.006), whereas in AIH, delta of CAP negatively correlated with delta of bilirubin (ρ = -0.244, p = 0.003), delta of GGT (ρ = -0.354, p <0.0001), and also delta of AST (ρ = -0.241, p = 0.003) and ALT (ρ = -0.265, p = 0.001) (Table S3).
Taken together, our data show that an increase of body weight and decrease of hepatic inflammation markers positively correlate with increases in CAP values in people with AILD.
The diagnostic performance of CAP improves in AIH after resolution of hepatic inflammation
To address the impact of hepatic inflammation on CAP values we assessed follow-up CAP measurements in individuals with high inflammatory AIH activity after a complete biochemical response was achieved.
In AIH, induction of remission usually requires treatment with corticosteroids,26 which may lead to weight gain (Fig. S5A), and thus represents a potential confounder by promoting hepatic steatosis development after liver biopsy was obtained. Additionally, as demonstrated earlier, weight gain may directly influence CAP values. Therefore, individuals with substantial weight gain (>10% of the initial body weight) during follow-up were excluded from this analysis.
In the AIH cohort with high inflammatory activity (serum transaminases >2xULN), a total of 78 participants achieved CBR during follow-up, of which 62 individuals did not show substantial weight gain (Fig. S5B) and had complete follow-up data available. A flowchart of participant recruitment for this analysis is given in Fig. S6; clinical characteristics are summarised in Table S4.
Upon achievement of CBR median CAP values significantly increased by a median of 38.0 dB/m from baseline (IQR: 6.0-81.0, p <0.0001, Fig. 3E). Importantly, resolution of hepatic inflammation during follow-up as indicated by normal serum transaminase levels was associated with an improvement of the diagnostic performance of CAP (T0: AUROC = 0.75 vs. follow-up: AUROC = 0.85). In line with the increased CAP values upon CBR, the optimal cut-off for the detection of hepatic steatosis increased from 241.5 dB/m at baseline (T0) (sensitivity: 0.64; specificity: 0.80) to 288.0 dB/m at follow-up (sensitivity: 0.67; specificity: 0.93). Consequently, the rate of correct assignment as steatotic or non-steatotic based on the respective cut-offs increased from 79.0% (T0) to 88.7% (follow-up) (p = 0.07) after CBR was achieved.
Of note, the increase of CAP values in individuals with biochemical response was reciprocal to changes of liver stiffness in this cohort. As expected, mean liver stiffness significantly declined after CBR was achieved (p <0.0001) (Fig. 3F) and was generally lower in individuals with AIH and low inflammatory activity (AST ≤2 × ULN) (p <0.0001) (Fig. 3C).
It should also be noted that the effects of disease activity on elastographic measurements were solely seen in individuals with AIH. Upon longitudinal follow-up of people with PSC and PBC, no significant changes in CAP, its diagnostic performance, or liver stiffness could be observed in a median follow-up time of 12 months (Fig. S7, Table S2).
Discussion
With the rising prevalence and incidence of obesity and associated hepatic steatosis, fatty liver disease is increasingly diagnosed as a comorbidity in individuals with AILD and affects treatment response as well as disease course.[3], [4], [5], [6] Thus, validation of non-invasive approaches to detect and monitor hepatic steatosis, such as CAP, is highly relevant for the clinical management of people with AILD. However, people with AILD have been underrepresented in studies evaluating CAP for the assessment of hepatic steatosis.20,36,37 Hence, in this study we comprehensively assessed the diagnostic performance of CAP in a large, well-characterised cohort of individuals with AIH, PBC, PSC and variant syndromes compromising a total of 433 individuals. The large cohort size allowed to determine disease-specific thresholds for CAP to detect hepatic steatosis, which revealed that optimum cut-offs vary substantially depending on the underlying AILD. This expands beyond findings of a previous report which determined a uniform cut-off values for AILD by analysing a cohort that included individuals with AIH and PBC.38 Our data clearly supports that disease-specific cut-offs should be applied, especially as PSC showed a considerably lower optimal cut-off threshold.
Disease-specific cut-offs demonstrated a good to excellent diagnostic accuracy for the identification of hepatic steatosis in PBC (AUROC = 0.81) and PSC (AUROC = 0.93). However, the diagnostic accuracy of CAP was significantly lower in newly diagnosed AIH. For people with AIH we identified hepatic inflammation as a potential confounder of CAP measurement: CAP values significantly increased in AIH after the presumed resolution of hepatic inflammation under treatment.
We have previously reported that liver inflammation leads to increased values for liver stiffness measured by transient elastography.28 However, contrary to the determination of liver stiffness, for CAP, we observed an opposing effect with significantly lower CAP values in the presence of high inflammatory activity in AIH (Fig. 3 A–C). Given that CAP estimates hepatic steatosis by changes of ultrasonic back-propagation in the liver tissue,14 it seems plausible that inflammation and concurrent tissue oedema could affect these physical characteristics. Hence, we suspect that decreased dispersion of the ultrasound wave in oedematous livers might explain the observed decrease in CAP values in individuals with ongoing liver inflammation. This postulation is further underlined by our finding that particularly high inflammatory activity was associated with lower CAP values and limited diagnostic accuracy in individuals with AIH (Fig. S4). Therefore, caution should be taken when interpreting CAP values in the setting of active AIH. Furthermore, the association of decreased CAP values in presence of substantial hepatic inflammation is of clinical relevance as low CAP values do not sufficiently indicate the absence of hepatic steatosis in AIH with high disease activity. Therefore, in order to reliably assess hepatic fat content at diagnosis of AIH or to distinguish AIH activity form NASH, liver biopsy is needed. Nonetheless, once biochemical response is achieved, our data showed that the reliability of CAP was substantially increased. For instance, the application of the cut-off adapted for the resolution of hepatic inflammation (288 dB/m) resulted in considerably improved diagnostic accuracy in individuals with AIH and biochemical response. Diagnostic confidence was similar to that observed in cholestatic liver diseases (PBC, PSC) (Table 5), which suggests that CAP can be a reliable instrument for monitoring hepatic steatosis in the clinical follow-up of people with AIH once CBR is achieved.
Our data underlines the high frequency of hepatic steatosis in individuals with AILD, as approximately 21% of our cohort showed steatosis on liver histology. This is in line with the expected prevalence of hepatic steatosis in adults in Europe.39 In addition, our data indicates that fatty liver disease seems to be not evenly distributed among people with AILD, as individuals with PSC and PSC/AIH had a lower prevalence NAFLD along with lower BMI values and lower frequency of diabetes and hyperlipidaemia (Table 1, Table 2, Table 3). The low frequency of concomitant hepatic steatosis in people with PSC is in line with other studies that report a low prevalence of steatosis in PSC40 or even suggest a protective role for PSC in steatosis development.41 In contrast, our data suggests that the differences in steatosis prevalence among AILD entities may be attributed to differential expression of risk factors in our cohort, namely obesity, hypertriglyceridemia, and age, rather than the disease entity itself.
This study has the inherent limitations of a retrospective, single-centre study design. We evaluated CAP measurements that were obtained over a period of 6 months around time of the liver biopsy. However, the majority of CAP measurements were performed within 35 days of the histological assessment. The time intervals did not differ between the respective AILD and we validated our findings of differences in performance of CAP in a cohort of individuals with AILD who had undergone elastographic assessment within 30 days to the liver biopsy. Nevertheless, the time interval between liver biopsy and CAP measurement varied and could have allowed for changes in liver tissue to occur that were not captured by histological evaluation. This would explain why we found negative correlations of CAP with transaminase and bilirubin levels, indirect markers of hepatic inflammation that were obtained on the day of elastography assessment, but did not observe a significant correlation of CAP values with the histological quantification of hepatitis by mHAI (not shown). As our centre does not perform routine follow-up biopsies, we were not able to correlate our follow-up data with histological data. Therefore, despite controlling for weight gain (>10% of the initial bodyweight) in AIH, it should be kept in mind that the onset of hepatic steatosis during the time of follow-up cannot be ruled out with certainty. A further consideration is that, in contrast to AIH, liver biopsy is not routinely performed as a procedure for the diagnosis of cholestatic liver diseases, but only when diagnosis is unclear or when concomitant liver diseases, such as variant-syndromes or NAFLD/NASH, are suspected. Therefore, a selection bias in our study might have led to an overestimation of the prevalence of steatosis in PBC and PSC. In addition, our cohort of people with AILD was imbalanced and dominated by individuals with AIH. Therefore, validation of our findings and of our cut-off values in larger cohorts of people with PBC and PSC is needed.
In summary, we here demonstrate that CAP is a reliable non-invasive surrogate biomarker of hepatic steatosis in PBC and PSC with a high accuracy in detecting hepatic steatosis. In AIH, ongoing hepatic inflammation may cause decreased CAP values, therefore CAP should be interpreted with caution during the first months of AIH treatment. However, once hepatic inflammation has declined, the measurement of CAP represents a reliable tool to monitor hepatic steatosis also in individuals with AIH.
Financial support
The project was supported by ‘‘Deutsche Forschungsgemeinschaft” (KFO306, project numbers: 290522633 and 426581255), ‘‘YAEL Stiftung”-Foundation for Liver Research and Medical Education, and the “Helmut and Hannelore Greve Foundation”. We acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf and DFG – German Research Foundation.
Authors’ contributions
Substantial contribution to the conception and design, data acquisition and analysis, interpretation of data and drafting of the article: SS. Interpretation of data, drafting of the article and critical revision for important intellectual content: JH. Histopathological examination and critical revision: SW. Data acquisition and critical revision: KF, CK. Substantial contribution to the design and critical revision: MS. Substantial contribution to the design and critical revision for important intellectual content: AWL. Substantial contribution to the design, interpretation of data, drafting of the article and critical revision for important intellectual content: CS. All authors reviewed and approved the final version of the manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author, SS and CS, upon reasonable request.
Conflicts of interest
The authors declare no conflicts of interest related to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100898.
Contributor Information
Silja Steinmann, Email: si.steinmann@uke.de.
Christoph Schramm, Email: c.schramm@uke.de.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Lazarus J.V., Mark H.E., Anstee Q.M., Arab J.P., Batterham R.L., Castera L., et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2021;19:60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani S., Mathur K., Shammas N., Orman E., Vuppalanchi R., Lammert C. Hepatic steatosis is highly prevalent but is not correlated with stiffness in autoimmune hepatitis. Medicine. 2020;99 doi: 10.1097/MD.0000000000022805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Luca-Johnson J., Wangensteen K.J., Hanson J., Krawitt E., Wilcox R. Natural history of patients presenting with autoimmune hepatitis and coincident nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61:2710–2720. doi: 10.1007/s10620-016-4213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalekos G.N., Gatselis N.K., Zachou K., Koukoulis G.K. NAFLD and autoimmune hepatitis: do not judge a book by its cover. Eur J Intern Med. 2020;75:1–9. doi: 10.1016/j.ejim.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Weiler-Normann C., Lohse A.W. Nonalcoholic fatty liver disease in patients with autoimmune hepatitis: further reason for teeth GNASHing? Dig Dis Sci. 2016;61:2462–2464. doi: 10.1007/s10620-016-4258-3. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi A., Arinaga-Hino T., Ohira H., Abe K., Torimura T., Zeniya M., et al. Non-alcoholic fatty liver disease in patients with autoimmune hepatitis. JGH Open. 2018;2:54. doi: 10.1002/jgh3.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyson J.K., Wong L.L., Bigirumurame T., Hirschfield G.M., Kendrick S., Oo Y.H., et al. Inequity of care provision and outcome disparity in autoimmune hepatitis in the United Kingdom. Aliment Pharmacol Ther. 2018;48:951–960. doi: 10.1111/apt.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi A., Arinaga-Hino T., Ohira H., Torimura T., Zeniya M., Abe M., et al. Autoimmune hepatitis in Japan: trends in a nationwide survey. J Gastroenterol. 2016;52:631–640. doi: 10.1007/s00535-016-1267-0. [DOI] [PubMed] [Google Scholar]
- 9.Woods C.P., Hazlehurst J.M., Tomlinson J.W. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94–103. doi: 10.1016/j.jsbmb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi L., Rajpal A., Ismail-Beigi F. Glucocorticoid-induced fatty liver disease. Diabetes Metab Syndr Obes. 2020;13:1133–1145. doi: 10.2147/DMSO.S247379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchesini G., Day C.P., Dufour J.F., Canbay A., Nobili V., Ratziu V., et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Ando Y., Jou J.H. Nonalcoholic fatty liver disease and recent guideline updates. Clin Liver Dis (Hoboken) 2021;17:23–28. doi: 10.1002/cld.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E., et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 14.Sasso M., Beaugrand M., de Ledinghen V., Douvin C., Marcellin P., Poupon R., et al. Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Myers R.P., Pollett A., Kirsch R., Pomier-Layrargues G., Beaton M., Levstik M., et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910. doi: 10.1111/j.1478-3231.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 16.Pu K., Wang Y., Bai S., Wei H., Zhou Y., Fan J., et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:51. doi: 10.1186/s12876-019-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y.T., Xiang L.L., Qi F., Zhang Y.J., Chen Y., Zhou X.Q. Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. EClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trowell J., Alukal J., Zhang T., Liu L., Maheshwari A., Yoo H.Y., et al. How good are controlled attenuation parameter scores from fibroscan to assess steatosis, NASH, and fibrosis? Dig Dis Sci. 2021;66:1297–1305. doi: 10.1007/s10620-020-06269-4. [DOI] [PubMed] [Google Scholar]
- 19.Kumar M., Rastogi A., Singh T., Behari C., Gupta E., Garg H., et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013;28:1194–1201. doi: 10.1111/jgh.12134. [DOI] [PubMed] [Google Scholar]
- 20.Piccinni R., Rodrigues S.G., Montani M., Murgia G., Delgado M.G., Casu S., et al. Controlled attenuation parameter reflects steatosis in compensated advanced chronic liver disease. Liver Int. 2020;40:1151–1158. doi: 10.1111/liv.14325. [DOI] [PubMed] [Google Scholar]
- 21.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654.e9. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenmeyer C.C., McCullough A.J. The Natural history of nonalcoholic fatty liver disease-an evolving view. Clin Liver Dis. 2018;22:11–21. doi: 10.1016/j.cld.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanwal F., Kramer J.R., Mapakshi S., Natarajan Y., Chayanupatkul M., Richardson P.A., et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–1837.e2. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chazouilleres O., Beuers U., Bergquist A., Karlsen T.H., Levy C., Samyn M., et al. EASL clinical practice guidelines on sclerosing cholangitis. J Hepatol. 2022;77:761–806. doi: 10.1016/j.jhep.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Hirschfield G.M., Beuers U., Corpechot C., Invernizzi P., Jones D., Marzioni M., et al. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Lohse A.W., Chazouillères O., Dalekos G., Drenth J., Heneghan M., Hofer H., et al. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Pape S., Snijders R.J.A.L.M., Gevers T.J.G., Chazouilleres O., Dalekos G.N., Hirschfield G.M., et al. Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group. J Hepatol. 2022;76:841–849. doi: 10.1016/j.jhep.2021.12.041. [DOI] [PubMed] [Google Scholar]
- 28.Hartl J., Denzer U., Ehlken H., Zenouzi R., Peiseler M., Sebode M., et al. Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65:769–775. doi: 10.1016/j.jhep.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Helmreich-Becker I., Schirmacher P., Denzer U., Henzel A., Meyer Zum Büschenfelde K.H., Lohse A.W. Minilaparoscopy in the diagnosis of cirrhosis: superiority in patients with Child-Pugh A and macronodular disease. Endoscopy. 2003;35:55–60. doi: 10.1055/s-2003-36419. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 31.Ishak K., Baptista A., Bianchi L., Callea F., de Groote J., Gudat F., et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 32.Batts K.P., Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Laschtowitz A., Zachou K., Lygoura V., Pape S., Derben F., Jaeckel E., et al. Histological activity despite normal ALT and IgG serum levels in patients with autoimmune hepatitis and cirrhosis. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2021.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley J.A., McNeil B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 35.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 36.Semmler G., Wöran K., Scheiner B., Unger L.W., Paternostro R., Stift J., et al. Novel reliability criteria for controlled attenuation parameter assessments for non-invasive evaluation of hepatic steatosis. United Eur Gastroenterol J. 2020;8:321. doi: 10.1177/2050640619900820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong V.W.S., Petta S., Hiriart J.B., Cammà C., Wong G.L.H., Marra F., et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67:577–584. doi: 10.1016/j.jhep.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Ni X.X., Lian M., Wu H.M., Li X.Y., Sheng L., Bao H., et al. Evaluation of controlled attenuation parameter in assessing hepatic steatosis in patients with autoimmune liver diseases. World J Gastroenterol. 2021;27:80. doi: 10.3748/wjg.v27.i1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 40.Doycheva I., Cox K., Haseeb A., Adler D.G. Prevalence and relevance of nonalcoholic fatty liver disease in patients with primary sclerosing cholangitis. Dig Dis Sci. 2014;59:1645–1646. doi: 10.1007/s10620-014-3051-4. [DOI] [PubMed] [Google Scholar]
- 41.Bosch D.E., Yeh M.M. Primary sclerosing cholangitis is protective against nonalcoholic fatty liver disease in inflammatory bowel disease. Hum Pathol. 2017;69:55–62. doi: 10.1016/j.humpath.2017.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, SS and CS, upon reasonable request.