Highlights
-
•
Ethinyl estradiol was associated with increased alcohol use in women using oral contraceptives.
-
•
Progestin levels were not associated with alcohol consumption.
-
•
Oral contraceptive pill phase did not impact alcohol use.
Keywords: Birth control, Synthetic hormones, Binge drinking, Alcohol use
Abstract
Alcohol use is highly prevalent in young adult women and rates of alcohol use disorder are rising rapidly in this population. Further, emerging evidence suggests that circulating levels of ovarian hormones influence alcohol consumption, with increased consumption associated with higher estradiol and lower progesterone levels. However, less is known about the influence of synthetic hormones (contained in oral contraceptive (OC) pills) on alcohol use. The current study examined the influence of OC pill phase, ethinyl estradiol (EE) levels, and progestin levels on self-reported alcohol consumption in healthy female drinkers. Young adult female drinkers using OCs (N = 21) reported alcohol use across one OC pill pack using the Timeline Followback and provided blood samples during both pill phases to measure synthetic hormone levels. We compared alcohol use between OC pill phases (active vs. inactive) using linear mixed effects models for repeated measures and examined correlations between alcohol use and EE and progestin levels. Results showed that women with higher EE levels reported increased alcohol consumption (r = 0.56, p = 0.01) and binge drinking (r = 0.45, p = 0.04) in the active pill phase. Progestin levels and pill phase were not significantly associated with alcohol consumption. These findings provide preliminary data suggesting increased levels of EE from OC pills are associated with excessive alcohol consumption in women. Further research is needed to determine if EE plays a causal role in increased alcohol consumption. This line of research could inform female-specific AUD prevention and treatment strategies among the large subpopulation of women using hormonal contraceptives.
1. Introduction
Alcohol use is highly prevalent among young adults (National Institute on Alcohol Abuse and Alcoholism, 2023), and young adult women may be at particularly high risk for problematic alcohol use. Over the last two decades, problematic drinking and alcohol use disorder (AUD) prevalence have been rising sharply in women, largely driven by young adult women between the ages of 18–31 (Balodis et al., 2009; Dawson et al., 2015; Keyes et al., 2008). Additionally, women accelerate from alcohol use onset to AUD onset more quickly, are more vulnerable to medical consequences from alcohol consumption, and are less likely to seek treatment for AUD compared to men (Agabio et al., 2017; Gilbert et al., 2019). These findings highlight the need for research on female-specific vulnerability to alcohol use.
One important sex-specific, biological factor impacting addiction vulnerability in women is the influence of ovarian hormones, including estradiol (generally associated with increased risk for addiction) and progesterone (generally associated with decreased risk) (Moran-Santa Maria et al., 2014; Carroll and Anker, 2010). Recent studies have investigated the associations between alcohol use and ovarian hormone levels via blood, saliva, or urine samples. The majority of studies show that individuals with higher overall estradiol levels tend to drink more (Frydenberg et al., 2015; Hartman et al., 2016; Hirko et al., 2014;, although null results have also been reported (Endogenous Hormones and Breast Cancer Collaborative Group et al., 2013; Rinaldi et al., 2006; Tsuji et al., 2012). One study to date has correlated within-person changes in hormone levels with daily alcohol consumption, and found that periods with increased salivary estradiol levels and lower progesterone levels were associated with greater binge drinking (Martel et al., 2017). Taken together, these findings are in line with the overall addiction literature, such that greater alcohol consumption is linked to higher estradiol and lower progesterone levels.
Given that endogenous ovarian hormones impact alcohol consumption, it is also important to consider the large population of women using oral contraceptives (OCs), which dramatically alter ovarian hormone levels. The majority of OC users (94 %) are prescribed combined OC pills containing a synthetic estrogen and a synthetic progesterone (progestin). While ethinyl estradiol (EE) is the most commonly used synthetic estrogen, progestins vary widely based on brand and formulation and can be derived from either testosterone, progesterone, or spironolactone (Royer and Jones, 2014). OC use can be divided into distinct phases: the active pill phase, when synthetic hormones are delivered, and the inactive pill phase, when no hormones are delivered. The lack of hormone delivery in the inactive pill phase reduces hormone levels and triggers breakthrough bleeding similar to menses (Hampson, 2020). OCs suppress natural production of estradiol and progesterone and reduce fluctuations in these hormones typically seen in naturally-cycling women (Fleischman et al., 2010) while delivering high levels of synthetic hormones that enter the central nervous system (Fishman and Norton, 1977). These synthetic hormones are more potent than their endogenous forms (Benagiano et al., 2004), and could therefore serve to increase female vulnerability for alcohol use. Importantly, the dosages of EE and progestins vary between OC brands, potentially leading some OC users to have more exposure to synthetic hormones than others.
There is initial evidence that OCs may influence alcohol use. The one study to date that has considered the effects of OCs on alcohol consumption found that hormonal contraceptive users (including OC users) report increased alcohol consumption compared to their naturally-cycling counterparts (Warren et al., 2021). Specifically, over 200 young adult women in the United Kingdom (ages 18–24) completed an online survey where they reported daily alcohol consumption over the previous two weeks using the Timeline Followback (TLFB). They found that women using hormonal contraceptives reported significantly greater weekly consumption of alcohol than naturally-cycling women. Given that 85 % of women in the hormonal contraceptive group used combined OCs, this result suggests that OC use may be associated with increased alcohol use. However, it is unclear how these results translate specifically to OC users and how these results may be influenced by synthetic hormone levels.
The current study examined how OC pills and synthetic hormones impact alcohol use among young adult women. Healthy OC users who regularly consume alcohol completed the Timeline Followback (TLFB; Sobell and Sobell, 1992) to assess alcohol consumption over one OC pill pack (28 days) and blood samples were obtained to measure synthetic hormone levels. We examined the influence of OC pill phase (active vs. inactive) as well as EE and progestin levels on alcohol consumption. We hypothesized that delivery of synthetic hormones would result in increased alcohol consumption in the active pill phase compared to the inactive pill phase. Further, we hypothesized that there would be a positive relationship between EE levels and alcohol consumption (i.e., higher EE levels would be related to more alcohol consumption) and that there would be an inverse relationship between progestin levels and alcohol consumption (i.e., higher progestin levels would be related to less alcohol consumption).
2. Method
2.1. Design
Healthy female volunteers using OC pills completed measures of alcohol use as part of a larger study investigating the influence of the synthetic hormones in OC pills on cognition and behavior related to motivation for alcohol and nicotine use (data not yet published). Here, we tested the degree to which alcohol use differed across OC pill phase and the degree to which circulating levels of synthetic hormones from OC pills were associated with alcohol use. This study was approved by the University of Kentucky Institutional Review Board and was carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent for participation.
2.2. Participants
Young adult female drinkers (n = 21) who reported current OC use were recruited through targeted social media advertisements and printed fliers posted in public areas of a large university campus and in local businesses in the surrounding area. Volunteers were eligible to participate if they used a 28-day combined EE-progestin OC pill and consumed alcohol on a weekly basis. Additional inclusion criteria included age between 18 and 35, English language fluency, at least a high school education, and use of current OC for at least 3 months. Participants were excluded if they used an OC without an inactive pill phase (e.g., Seasonale); used an OC with an extended-cycle (i.e., longer than 28-days); used other types of hormonal contraceptives; were currently pregnant or breastfeeding; or reported serious medical or psychiatric conditions, including severe drug or alcohol use disorder. As part of the larger parent study, all participants were required to report daily or near daily use of cigarettes or e-cigarettes.
2.3. Procedure
All participants completed an online screening form to assess study eligibility and provided informed consent prior to study procedures. Eligible participants attended two laboratory sessions (one during the active phase and one during the inactive phase of their OC pill pack) as part of a larger study. Participants were informed that the purpose of the study was to investigate the influence of OC pills on motivation for alcohol and nicotine. Participants were instructed to abstain from alcohol and recreational drugs for 24 h prior to each session, as verified by self-report, breath alcohol, and urine screens. Participants were familiarized with the laboratory procedures and study protocol. They then completed the TLFB followed by additional questionnaires and tasks (data not reported here). Blood samples were taken at each session to measure circulating levels of synthetic hormones. Upon completion of both sessions, participants were debriefed and compensated $75 for their time.
2.4. Measures
2.4.1. Timeline Followback (TLFB)
Daily patterns of alcohol consumption were measured using the self-administered TLFB (Sobell and Sobell, 1992). Participants were given detailed instructions on how to retrospectively self-report their alcohol consumption for the past 28 days, including information about standard alcohol beverage sizes. Participants estimated the total number of standard alcoholic beverages consumed each day and the amount of time spent drinking each day. Using this information, we calculated our drinking measures of interest, including number of drinking days, drinks per drinking day, and binge drinking episodes (defined as drinking days in which 4 or more alcoholic beverages were consumed; National Institute on Alcohol Abuse and Alcoholism, 2004).
The 28-day TLFB timeframe encompassed one complete contraceptive pill pack for each participant. Each combined OC pill pack can be split into two distinct phases: the active pill phase when synthetic hormones are delivered consistently for 21–24 days (depending on brand) and the inactive pill phase where placebo pills containing no hormones are used for 4–7 days. Individual OC pill brands and pill days were verified using participants’ current pill pack. Each drinking measure was calculated for the total 28-day cycle, for the active pill phase, and for the inactive pill phase.
2.4.2. Synthetic hormone levels
Blood samples (approximately 5 mL) were taken by nursing staff at the University of Kentucky Center for Clinical and Translational Science (CCTS) Clinical Research Unit. Samples were centrifuged and serum was separated into approximately 0.75 mL aliquots and frozen until analysis. All samples were measured for EE and the specific progestin or progestin prodrug used by each participant. Serum concentrations of EE, norethindrone (NET), norelgestromin (NGMN), etonogestrel (ENG), levonorgestrel (LNG), and drospirenone (DRSP) were simultaneously determined in the Endocrine Technologies Core (ETC) at Oregon National Primate Research Center (ONPRC) using liquid chromatography-tandem triple quadrupole mass spectrometry (LC-MS/MS). The method for analysis of EE, NET, ENG, and LNG was previously described in Blue et al., (2018). Addition of NGMN and DRSP in the analytical method is described in Jensen et al., (2023). Intra-assay variation for all assays was <8 %. As all samples were analyzed in a single assay, no specific inter-assay variation was calculated for this sample set. Overall inter-assay variation for these assays in the ETC is <15 %.
To compare progestin levels across participants using different progestin types, progestational potency values were used. All serum progestin levels were converted to their progestin potency relative to that of NET (Dickey and Stone, 1976; Ness et al., 2000). For example, since 1 mg of NET has the same progestational activity as 1.5 mg of DRSP, measurements of DRSP were multiplied by 1.5 to be able to directly compare progestin levels.
2.5. Data analyses
We conducted a series of linear mixed effects models for repeated measures (Hedeker and Gibbons, 2006) to determine the influence of OC pill phase (active vs. inactive) on alcohol consumption (drinking days per week, drinks per drinking day, and binge episodes per week). We calculated drinking days per week and binge episodes per week to standardize comparisons across the longer active pill phase (typically 3 weeks) and the shorter inactive pill phase (typically 1 week). The models accounted for the correlation between repeated measures and were built by treating observations as nested within subjects and including a random intercept to allow for individual differences in pill phase effects on drinking. We then tested for associations between synthetic hormone levels (EE and progestins) in the active pill phase on alcohol consumption measures in the active pill phase and for associations between synthetic hormone levels in the inactive pill phase on alcohol consumption measures in the inactive pill phase using bivariate Pearson correlations.
3. Results
3.1. Sample characteristics
Of the 22 subjects who enrolled in the study, 1 was removed from analyses as no drinking was reported over the past 28 days on the TLFB and no hormone assays were measurable due to insufficient blood sample volume), leaving a final sample size of n = 21. One subject only attended a laboratory session in the inactive pill phase so only partial data were used (i.e., no hormone measures were available for the active pill phase correlational analyses). One subject had an inactive pill phase EE level greater than 3 SDs above the mean due to inaccurate session scheduling on the first inactive pill day, and this outlier was removed from the dataset for correlational analyses related to inactive EE levels (partial active phase data from the participant were still used). No other outliers (i.e., greater than 3 SDs above the mean) were detected for synthetic hormone levels or alcohol consumption.
Demographic, alcohol use, and lifetime substance use data are presented in Table 1. Nicotine use was common in the sample as all participants were required to report daily or near daily use of nicotine to be eligible. To address the possibility that nicotine use may have influenced alcohol use in the sample, we conducted bivariate correlation analyses between nicotine and alcohol use measures. We did not find any significant associations between alcohol consumption measures (total drinks, drinking days, and drinks per day) and nicotine consumption measures (total nicotine use sessions, nicotine use days, and nicotine use sessions per nicotine use day) (rs < 0.24, ps > 0.32). As such, it is not likely that nicotine use had a substantial impact on alcohol use in this sample.
Table 1.
Participant characteristics (n = 21).
| Mean (SD) | |
|---|---|
| Age | 23.2 (3.7), range 19–31 |
| Education (years) | 14.9 (1.9) |
| Body Mass Index | 27.3 (6.8) |
| Race | |
| Caucasian | 19 |
| Multiracial | 1 |
| Other | 1 |
| Timeline Followback (28 days) | |
| Drinking Days | 8.9 (5.1) |
| Drinks per Drinking Day | 4.4 (1.7) |
| Binge Episodes* | 5.1 (3.9) |
| Nicotine Use Days | 22.6 (7.9) |
| Nicotine Use Sessions per Day** | 12.2 (14.1) |
| Lifetime Substance Use | |
| Alcohol | 100 % |
| Nicotine | 100 % |
| Cannabis | 80.9 % |
| Cocaine | 23.8 % |
| Amphetamines | 14.3 % |
| Sedatives | 4.8 % |
| Opiates | 4.8 % |
| Hallucinogens | 19.1 % |
Note. *Binge episode defined as 4+ drinks in one drinking episode. **Nicotine use sessions include cigarette and e-cigarette use.
All participants used a 28-day combined OC pill (see Table 2). Eighteen subjects used monophasic pills and two subjects used triphasic pills. Subjects used OC pills with either a 21-day active pill phase (n = 18) or a 24-day active pill phase (n = 3). Progestin types varied across the sample, with just over one-third of subjects using formulas containing norethindrone or norethindrone acetate (Table 2).
Table 2.
OC characteristics.
| Mean (SD) | |
|---|---|
| EE Levels (pg/ml) | |
| Active phase | 50.3 (38.7) |
| Inactive phase | 14.6 (23.2) |
| Progestin Levels (ng/ml)* | |
| Active phase | 14.6 (21.8) |
| Inactive phase | 9.9 (16.5) |
| Progestin Type | |
| Norethindrone | 7 |
| Norgestimate | 6 |
| Drospirenone | 4 |
| Levonorgestrel | 2 |
| Norgestrel | 1 |
| Desogestrel | 1 |
Note. *Progestin levels calculated relative to progestational activity of norethindrone.
3.2. Effects of OC pill phase on alcohol consumption
Mean alcohol consumption measures for each pill phase are presented in Table 3. Linear mixed effects models showed no differences in alcohol consumption on any measure between the active and inactive pill phases (ps > 0.87).
Table 3.
Mean alcohol consumption by pill phase.
| Active Pill Phase | Inactive Pill Phase | p | |
|---|---|---|---|
| Alcohol Consumption | |||
| Drinking Days per Week | 2.21 (1.26) | 2.24 (1.26) | 0.87 |
| Drinks per Drinking Day | 4.29 (1.78) | 4.36 (2.29) | 0.89 |
| Binge Episodes per Week | 1.27 (1.06) | 1.29 (1.19) | 0.95 |
3.3. Associations between EE levels and alcohol consumption
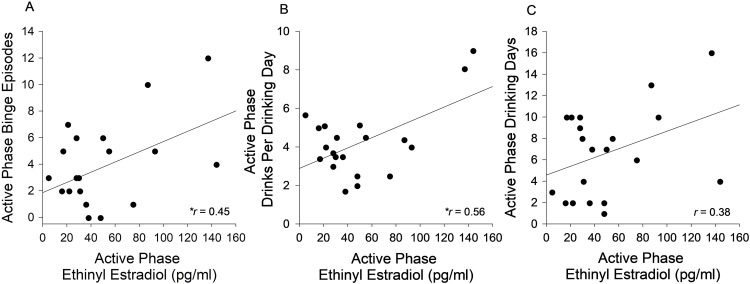
Scatterplots depicting associations between active phase mean alcohol consumption measures and EE levels are presented in Fig. 1. The figure shows that during the active pill phase, individuals with higher EE levels consumed greater amounts of alcohol. Specifically, there was a significant, positive association between EE levels in the active pill phase and binge episodes in the active pill phase, such that individuals with higher EE levels reported more binge drinking episodes (r = 0.45, p = 0.045). Additionally, there was a significant, positive association between EE levels in the active pill phase and drinks per drinking day in the active pill phase, such that individuals with higher EE levels reported consuming more drinks per drinking day (r = 0.56, p = 0.011). EE levels were not significantly associated with the number of drinking days in the active pill phase (r = 0.38, p = 0.096).
Fig. 1.
Associations Between Ethinyl Estradiol and Alcohol Consumption in the Active Pill Phase. There was a significant, positive correlation between ethinyl estradiol (EE) levels in the active pill phase and binge episodes in the active pill phase (A) and drinks per drinking day in the active pill phase (B). EE levels in the active pill phase were not significantly associated with the number of drinking days in the active pill phase (C). * indicates p < 0.05.
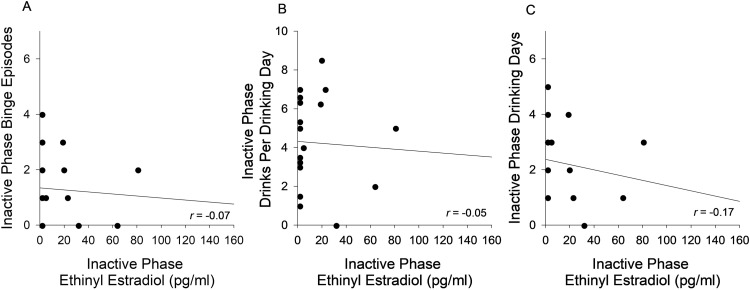
Scatterplots depicting associations between inactive phase mean alcohol consumption measures and EE levels are presented in Fig. 2. The figure shows that during the inactive pill phase, EE levels were not associated with alcohol consumption, include number of drinking days (r = -0.17, p = 0.50), drinks per drinking day (r = -0.05, p = 0.84), or binge episodes (r = -0.07, p = 0.79).
Fig. 2.
Associations Between Ethinyl Estradiol and Alcohol Consumption in the Inactive Pill phase. Ethinyl estradiol levels in the inactive pill phase were not associated with binge episodes in the inactive pill phase (A), drinks per drinking day in the inactive pill phase (B), or number of drinking days in the inactive pill phase (C).
3.4. Effects of progestins on alcohol consumption
Bivariate correlations between alcohol consumption and relative progestin levels in the active and inactive pill phases are shown in Table 4. There were no significant associations between relative progestin levels in the active pill phase and alcohol consumption (ps > 0.46) or between relative progestin levels in the inactive pill phase and alcohol consumption (ps > 0.08).
Table 4.
Bivariate correlations between synthetic hormone (EE and progestin) levels and alcohol consumption.
| Active Pill Phase | |||||
|---|---|---|---|---|---|
| EE Level | Progestin level | Drinking Days | Drinks per Drinking Day | Binge Episodes | |
| 1. EE level (pg/ml) | – | ||||
| 2. Progestin level (ng/ml) | 0.176 | – | |||
| 3. Drinking Days | 0.382 | 0.078 | – | ||
| 4. Drinks per Drinking Day | 0.557* | -0.118 | 0.239 | – | |
| 5. Binge Episodes | 0.454* | 0.176 | 0.850** | 0.592** | – |
| Inactive Pill Phase | |||||
|---|---|---|---|---|---|
| EE Level | Progestin Level | Drinking Days | Drinks per Drinking Day | Binge Episodes | |
| 1. EE level (pg/ml) | – | ||||
| 2. Progestin level (ng/ml) | 0.703** | – | |||
| 3. Drinking Days | -0.167 | -0.164 | – | ||
| 4. Drinks per Drinking Day | -0.049 | -0.400 | 0.294 | – | |
| 5. Binge Episodes | -0.066 | -0.337 | 0.752** | 0.681** | – |
*Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed).
4. Discussion
This study examined the influence of OCs and the synthetic hormones they contain on alcohol consumption in young adult females. In line with our hypothesis, higher EE levels were associated with greater alcohol consumption, particularly drinking quantity. Specifically, OC users with higher circulating EE levels in the active pill phase reported more binge drinking episodes and consuming more drinks per drinking day. This association was specific to the active pill phase, when synthetic hormones were actively delivered. Contrary to our hypothesis, alcohol use did not differ between the active and inactive OC pill phases and alcohol use was not associated with relative progestin levels.
Our findings related to EE levels and alcohol consumption align with previous findings linking estradiol with greater alcohol consumption in women. Multiple reports have shown that higher endogenous estradiol levels are related to increased alcohol intake (Frydenberg et al., 2015; Hartman et al., 2016; Hirko et al., 2014) and binge drinking (Martel et al., 2017). Importantly, our findings that OC users with higher EE levels reported increased alcohol consumption and binge drinking suggest that observed associations between endogenous estradiol and alcohol use may extend to synthetic EE as well. Moreover, these associations may be even more pronounced for synthetic estradiol, as the EE contained in OC pills delivers a considerably more potent version than the endogenous form (Benagiano et al., 2004).
The observed preliminary association between higher EE levels and increased drinking in OC users could have potentially important implications for AUD risk in young adult women. Although speculative at this point, it is possible that high circulating levels of EE from high dose OCs could be contributing to increased alcohol consumption in this already vulnerable population (Balodis et al., 2009; Dawson et al., 2015; Keyes et al., 2008). If so, it will be important for providers to consider the risk of elevated alcohol consumption when prescribing OCs. For instance, providers may consider reducing the prescription of OCs with high EE doses, particularly among young women with other risk factors for AUD, in favor of alternate contraceptive methods (e.g., progestin-only OCs, progestin-only intrauterine devices, or non-hormonal intrauterine devices). Additionally, it may be important to include information about the potential risks of increased drinking, including the development of AUD, in patient package inserts in OCs with high EE doses. Finally, it will be important to consider the impact of hormonal contraception methods that expose women to even higher levels of EE than OCs, particularly transdermal delivery systems (i.e., patches) (Agile Therapeutics, 2019).
Despite findings related to EE levels and alcohol consumption in the active pill phase, we did not find associations between EE levels and alcohol consumption in the inactive pill phase. This may suggest that EE is associated with alcohol consumption specifically when EE is being administered in the active pill phase, as opposed to when EE levels are quite low and being eliminated from the body in the inactive pill phase. In line with this, over half of participants displayed negligible EE levels in the inactive pill phase. Additionally, the half-life of EE is thought to be between 10 and 20 h (Stanczyk et al., 2013), further highlighting the steep drop off of EE levels during the inactive pill phase.
Contrary to hypothesis, we did not find a significant relationship between relative progestin levels and alcohol consumption in either pill phase. This may be due to the wide variety of progestins used across participants. The progestins contained in the various OC formulations differ based on their binding affinity for hormone receptors, making it somewhat difficult to conduct direct dose-related comparisons across OC formulations. Given that previous studies have shown different cognitive effects between progestin types (Gurvich et al., 2020; Hampson et al., 2022), it is possible that these effects may extend to different behavioral effects, such as alcohol use. This highlights a need for future research to investigate the effects of different classes of progestins on alcohol consumption in OC users.
This study has several limitations. First, the sample size was quite small and predominately Caucasian. Second, individual participants in our sample reported using a wide variety of OCs, including different progestin types and dosing schedules (i.e., monophasic and triphasic pills). Additionally, we did not account for duration of OC use despite evidence from previous studies suggesting that the influence of OC use on cognition may differ based on overall length of exposure (Egan and Gleason, 2012; Hidalgo-Lopez et al., 2023). It will be important to replicate these findings with larger samples that will be sufficiently powered to investigate differences in alcohol consumption based on different progestins, dosing schedules, or duration of use. Third, alcohol consumption measures relied on retrospective self-reports, which may not reflect accurate drinking levels or may be biased by the social desirability of the participants. Fourth, we did not collect data on quantity or frequency of recent cannabis use. Given that a large majority of the sample (80 %) reported lifetime cannabis use, as well as the high prevalence of alcohol and cannabis co-use (Hai et al., 2022; Yurasek et al., 2017), it will be important for future studies to look at associations between synthetic hormones and cannabis use as well. Finally, this study was correlational in nature, meaning that future, longitudinal studies will be needed to examine the casual effects of the delivery of EE on alcohol consumption.
In conclusion, this study provides preliminary evidence for a relationship between the synthetic hormone EE and alcohol use among young adult OC users. Specifically, we found that OC users with higher circulating EE levels reported increased alcohol consumption and binge drinking. It will be important for future studies to examine the degree to which EE exerts a causal influence on alcohol consumption, as well as potential mechanisms underlying the associations between EE and increased drinking. This line of research will likely have important implications for understanding risk for AUD in young adult women, as well as for the development of targeted prevention and treatment efforts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This research was supported by University of Kentucky Igniting Research Collaborations (IRC) Pilot Award Funds (MPIs JW and CDG), Lipman Foundation Fellowship (awarded to AS), National Institute on Alcohol Abuse and Alcoholism grants R01 AA028503 (JW) and K01 AA024519 (JW), and National Institute on Drug Abuse R21 DA033488 (EC). All authors declare no conflict of interest.
The University of Kentucky Clinical Research Unit is part of the Center for Clinical and Translational Science, which is supported by the NIH National Center for Advancing Translation Sciences (UL1TR001998).
The Endocrine Technologies Core (ETC) at Oregon National Primate Research Center (ONPRC) is supported (in part) by NIH grant P51 OD011092 for operation of the Oregon National Primate Research Center.
Role of Funding Sources
The funders were not involved in the design of the study, data collection, management, analysis, or interpretation of the results.
Contributors
JW and CDG obtained funding, designed the study, and wrote the protocol and methodology. AMS reviewed the literature, conducted data collection and analyses, and wrote the first draft. EC contributed to the analytic plan. All authors contributed to the interpretation of the results, reviewed and revised the manuscript, and approved the final version of the manuscript.
References
- Agabio R., Pisanu C., Gessa G.L., Franconi F. Sex differences in alcohol use disorder. Curr. Med. Chem. 2017;24(24):2661–2670. doi: 10.2174/0929867323666161202092908. [DOI] [PubMed] [Google Scholar]
- Agile Therapeutics . Reproductive and Urologic Drugs Advisory Committee, Food and Drug Administration; Bone: 2019. The Agile Patch: a Low-Dose Levonorgestrel/Ethinyl Estradiol Transdermal Contraceptive Delivery System.https://www.fda.gov/media/132041/download [Google Scholar]
- Balodis I.M., Potenza M.N., Olmstead M.C. Binge drinking in undergraduates: relationships with sex, drinking behaviors, impulsivity, and the perceived effects of alcohol. Behav. Pharmacol. 2009;20(5–6):518–526. doi: 10.1097/FBP.0b013e328330c779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano G., Primiero F., Farris M. Clinical profile of contraceptive progestins. Eur. J. Contracept. Reprod. Health Care. 2004;9(3):182–193. doi: 10.1080/13625180400007736. [DOI] [PubMed] [Google Scholar]
- Blue S.W., Winchell A.J., Kaucher A.V., Lieberman R.A., Gilles C.T., Pyra M.N., Heffron R., Hou X., Coombs R.W., Nanda K., Davis N.L., Kourtis A.P., Herbeck J.T., Baeten J.M., Lingappa J.R., Erikson D.W. Simultaneous quantitation of multiple contraceptive hormones in human serum by LC–MS/MS. Contraception. 2018;97(4):363–369. doi: 10.1016/j.contraception.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M.E., Anker J.J. Sex differences and ovarian hormones in animal models of drug dependence. Horm. Behav. 2010;58(1):44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Dawson D.A., Goldstein R.B., Saha T.D., Grant B.F. Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug Alcohol Depend. 2015;148:56–61. doi: 10.1016/j.drugalcdep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey R.P., Stone S.C. Progestational potency of oral contraceptives. Obstet. Gynecol. 1976;47(1):106–112. [PubMed] [Google Scholar]
- Egan K.R., Gleason C.E. Longer duration of hormonal contraceptive use predicts better cognitive outcomes later in life. J. Women's Health. 2012;21(12):1259–1266. doi: 10.1089/jwh.2012.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endogenous Hormones and Breast Cancer Collaborative Group. Key T.J., Appleby P.N., Reeves G.K., Travis R.C., Alberg A.J., Barricarte A., Berrino F., Krogh V., Sieri S., Brinton L.A., Dorgan J.F., Dossus L., Dowsett M., Eliassen A.H., Fortner R.T., Hankinson S.E., Helzlsouer K.J., Hoff man-Bolton J., Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–1019. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J., Norton B. Pharmacology of Steroid Contraceptive Drugs. Raven Press; 1977. Relative transport of estrogens into the central nervous system; pp. 37–41. [Google Scholar]
- Fleischman D.S., Navarrete C.D., Fessler D.M.T. Oral contraceptives suppress ovarian hormone production. Psychol. Sci. 2010;21(5):750–752. doi: 10.1177/0956797610368062. [DOI] [PubMed] [Google Scholar]
- Frydenberg H., Flote V.G., Larsson I.M., Barrett E.S., Furberg A.S., Ursin G., Wilsgaard T., Ellison P.T., McTiernan A., Hjartåker A., Jasienska G., Thune I. Alcohol consumption, endogenous estrogen and mammographic density among premenopausal women. Breast Cancer Res. BCR. 2015;17(1):103. doi: 10.1186/s13058-015-0620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P.A., Pro G., Zemore S.E., Mulia N., Brown G. Gender differences in use of alcohol treatment services and reasons for nonuse in a national sample. Alcohol. Clin. Exp. Res. 2019;43(4):722–731. doi: 10.1111/acer.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich C., Warren A.M., Worsley R., Hudaib A.R., Thomas N., Kulkarni J. Effects of oral contraceptive androgenicity on visuospatial and social-emotional cognition: a prospective observational trial. Brain Sci. 2020;10(4):194. doi: 10.3390/brainsci10040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai A.H., Carey K.B., Vaughn M.G., Lee C.S., Franklin C., Salas-Wright C.P. Simultaneous alcohol and marijuana use among college students in the United States, 2006-2019. Addict. Behav. Rep. 2022;16 doi: 10.1016/j.abrep.2022.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E. A brief guide to the menstrual cycle and oral contraceptive use for researchers in behavioral endocrinology. Horm. Behav. 2020;119 doi: 10.1016/j.yhbeh.2019.104655. [DOI] [PubMed] [Google Scholar]
- Hampson E., Morley E.E., Evans K.L., Fleury C. Effects of oral contraceptives on spatial cognition depend on pharmacological properties and phase of the contraceptive cycle. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.888510. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman T.J., Sisti J.S., Hankinson S.E., Xu X., Eliassen A.H., Ziegler R. Alcohol consumption and urinary estrogens and estrogen metabolites in premenopausal women. Horm. Cancer. 2016;7(1):65–74. doi: 10.1007/s12672-015-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D., Gibbons R.D. Wiley-Interscience; 2006. Longitudinal Data Analysis.https://www.wiley.com/en-us/Longitudinal+Data+Analysis-p-9780471420279 [Google Scholar]
- Hidalgo-Lopez E., Noachtar I., Pletzer B. Hormonal contraceptive exposure relates to changes in resting state functional connectivity of anterior cingulate cortex and amygdala. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1131995. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirko K.A., Spiegelman D., Willett W.C., Hankinson S.E., Eliassen A.H. Alcohol consumption in relation to plasma sex hormones, prolactin and sex hormonebinding globulin in premenopausal women. Cancer Epidemiol. Biomark. Prev. 2014;23(12):2943–2953. doi: 10.1158/1055-9965.EPI-14-0982. A Publication of the American Association for Cancer Research Cosponsored by the American Society of Preventive Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J., Waites B., Boniface E., McCrimmon S., Blue S., Erikson D. Contraception, in Press; 2023. Development and Validation of an Expanded Panel of Progestins Using Liquid Chromatography-Tandem Triple Quadrupole Mass Spectrometry (LC-MS/MS) to Monitor Protocol Compliance in Hormonal Contraceptive Pharmacokinetic/Pharmacodynamic Studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes K.M., Grant B.F., Hasin D.S. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol. Depend. 2008;93(1):21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M.M., Eisenlohr-Moul T., Roberts B. Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. J. Abnorm. Psychol. 2017;126(8):1104–1113. doi: 10.1037/abn0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria M.M., Flanagan J., Brady K. Ovarian hormones and drug abuse. Curr. Psychiatry Rep. 2014;16(11):511. doi: 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism NIAAA council approves definitoin of binge drinking. NIAAA Newsletter. 2004;3(3) https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism . Alcohol and Young Adults Ages 18 to 25; 2023. Alcohol and Young Adults Ages 18 to 25.https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-and-young-adults-ages-18-25 [Google Scholar]
- Ness R.B., Ann Grisso J., Klapper J., Schlesselman J.J., Silberzweig S., Vergona R., Morgan M., Wheeler J.E., the SHARE Study Group Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. Am. J. Epidemiol. 2000;152(3):233–241. doi: 10.1093/aje/152.3.233. [DOI] [PubMed] [Google Scholar]
- Rinaldi S., Peeters P.H.M., Bezemer I.D., Dossus L., Biessy C., Sacerdote C., Berrino F., Panico S., Palli D., Tumino R., Khaw K.T., Bingham S., Allen N.E., Key T., Jensen M.K., Overvad K., Olsen A., Tjonneland A., Amiano P., Kaaks R. Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2006;17(8):1033–1043. doi: 10.1007/s10552-006-0041-7. [DOI] [PubMed] [Google Scholar]
- Royer P.A., Jones K.P. Progestins for contraception: modern delivery systems and novel formulations. Clin. Obstet. Gynecol. 2014;57(4):644–658. doi: 10.1097/GRF.0000000000000072. [DOI] [PubMed] [Google Scholar]
- Sobell L.C., Sobell M.B. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; 1992. Timeline follow-back; pp. 41–72. R. Z. Litten & J. P. Allen (Eds. [DOI] [Google Scholar]
- Stanczyk F.Z., Archer D.F., Bhavnani B.R. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception. 2013;87(6):706–727. doi: 10.1016/j.contraception.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Tamai Y., Wada K., Nakamura K., Hayashi M., Takeda N., Yasuda K., Nagata C. Associations of intakes of fat, dietary fiber, soy isoflavones, and alcohol with levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Causes Control CCC. 2012;23(5):683–689. doi: 10.1007/s10552-012-9935-8. [DOI] [PubMed] [Google Scholar]
- Warren J.G., Goodwin L., Gage S.H., Rose A.K. The effects of menstrual cycle stage and hormonal contraception on alcohol consumption and craving: a pilot investigation. Compr. Psychoneuroendocrinol. 2021;5 doi: 10.1016/j.cpnec.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurasek A.M., Aston E.R., Metrik J. Co-use of alcohol and cannabis: a review. Curr. Addict. Rep. 2017;4(2):184–193. doi: 10.1007/s40429-017-0149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]