Summary
Background
Tisagenlecleucel was approved by the Food and Drug Administration (FDA) in 2017 for refractory B-cell acute lymphoblastic leukemia (B-ALL) and B-ALL in ≥2nd relapse. Outcomes of patients receiving commercial tisagenlecleucel upon 1st relapse have yet to be established. We aimed to report real-world tisagenlecleucel utilisation patterns and outcomes across indications, specifically including patients treated in 1st relapse, an indication omitted from formal FDA approval.
Methods
We conducted a retrospective analysis of real-world tisagenlecleucel utilisation patterns across 185 children and young adults treated between August 30, 2017 and March 6, 2020 from centres participating in the Pediatric Real-World CAR Consortium (PRWCC), within the United States. We described definitions of refractory B-ALL used in the real-world setting and categorised patients by reported Chimeric Antigen Receptor (CAR) T-cell indication, including refractory, 1st relapse and ≥2nd relapse B-ALL. We analysed baseline patient characteristics and post-tisagenlecleucel outcomes across defined cohorts.
Findings
Thirty-six percent (n = 67) of our cohort received tisagenlecleucel following 1st relapse. Of 66 evaluable patients, 56 (85%, 95% CI 74–92%) achieved morphologic complete response. Overall-survival (OS) and event-free survival (EFS) at 1-year were 69%, (95% CI 58–82%) and 49%, (95% CI 37–64%), respectively, with survival outcomes statistically comparable to remaining patients (OS; p = 0.14, EFS; p = 0.39). Notably, toxicity was increased in this cohort, warranting further study. Interestingly, of 30 patients treated for upfront refractory disease, 23 (77%, 95% CI 58–90%) had flow cytometry and/or next-generation sequencing (NGS) minimum residual disease (MRD)-only disease at the end of induction, not meeting the historic morphologic definition of refractory.
Interpretation
Our findings suggested that tisagenlecleucel response and survival rates overlap across patients treated with upfront refractory B-ALL, B-ALL ≥2nd relapse and B-ALL in 1st relapse. We additionally highlighted that definitions of refractory B-ALL are evolving beyond morphologic measures of residual disease.
Funding
St. Baldrick's/Stand Up 2 Cancer, Parker Institute for Cancer Immunotherapy, Virginia and D.K. Ludwig Fund for Cancer Research.
Keywords: Immunotherapy, CAR T cells, Tisagenlecleucel, First relapse, Pediatric oncology, Real-world analysis, Commercial CAR, CD19 CAR T cells
Research in context.
Evidence before this study
We searched PubMed for full-text clinical trials written in English published up to September 6 2023, to identify papers through search terms spanning outcomes for upfront refractory and relapsed B-ALL, prognostic implications of MRD in pediatric and young adult B-ALL, standard definitions for refractory disease and relapse in leukemia, and clinical trials using CD19 CAR T-cell therapy and eligibility criteria in registrational trials of tisagenlecleucel (CD19-specific CAR T-cell therapy). The search revealed both a scarcity of clinical reports of tisagenlecleucel use for patients with 1st relapse who are excluded from the approved indication unless considered refractory as well as lack of standardisation for definitions of refractory and relapse used to determine candidacy for tisagenlecleucel. Prior real-world retrospective analysis identified a patient cohort treated with commercial tisagenlecleucel upon first relapse.
Added value of this study
To the best of our knowledge, this is the first clinical report on response, toxicity and survival outcomes of patients treated with tisagenlecleucel upon 1st relapse, as compared to patients treated for primary refractory disease or second or greater relapse. We establish that survival of children and young adults with B-ALL treated upon 1st relapse are comparable to survival outcomes in patients treated for refractory B-ALL or ≥2nd relapse. To our knowledge, this is the first report distinctly reporting outcomes of this 1st relapse cohort. We additionally report that definitions of refractory and relapse in the real-world setting do not adhere to morphologic measures of disease.
Implications of all the available evidence
Our real-world evidence supports the tolerability and efficacy of tisagenlecleucel in patients with 1st B-ALL relapse that warrants further large and multi-centre outcomes research. Additionally, we highlight the need to refine the formal definitions of refractory and relapse B-ALL to incorporate the frequent use of flow cytometry and next-generation sequencing MRD to detect disease persistence or recrudescence early and alter therapy before patients meet classical definitions of relapsed or refractory disease. This information benefits clinicians and families evaluating CAR T-cell therapy as a treatment for refractory or relapsed B-ALL.
Introduction
In contrast to high survival rates approaching 90% in children and young adults treated for upfront B-cell acute lymphoblastic leukemia (B-ALL), survival declines profoundly for patients with relapsed or refractory disease.1, 2, 3 The five-year overall-survival (OS) rate upon first B-ALL relapse in children and young adults is 50%,4,5 with cumulative decline upon subsequent relapses.6,7 Studies have identified higher risk patient subsets, with inferior survival following first relapse, including patients experiencing early bone marrow relapse (<36 months from diagnosis),4 early isolated extramedullary relapse (<18 months from diagnosis),3 and infant B-ALL.8 The largest Children’s Oncology Group (COG) post-relapse OS analysis to date (N = 1967 patients with ALL relapse) highlights a 5-year OS rate of only 28% in patients with B-ALL bone marrow relapse <18 months from diagnosis.8 Patients with high-risk first-relapse present a critical gap ripe for novel therapies, yet despite established need, are excluded from the FDA-approved indication for CD19-chimeric antigen receptor (CAR) T-cells.
The five-year overall-survival (OS) rate for children and young adults with B-ALL declines to <40% when end of consolidation disease remains detectable at ≥0.01%.9 Efforts to overcome chemotherapy refractoriness have traditionally been approached through chemotherapy intensification and allogeneic stem cell transplantation.10, 11, 12 Despite traditional risk stratification systems and chemotherapy intensification, post-induction MRD status remains independently prognostic of poor survival,9 highlighting the need for mechanistically-distinct therapy, such as CAR T-cells, for chemotherapy resistant or refractory patients.
Tisagenlecleucel, the sole CAR approved for pediatrics, is a CD19-specific autologous T cell product that was FDA approved for patients ≤25 years with refractory B-ALL or B-ALL in ≥2nd relapse in August 2017. Approval was based on an 81% complete response rate in the registrational trial (ELIANA), with long term follow-up data demonstrating 3-year relapse-free survival with and without censoring for subsequent therapy of 52% (95% CI, 37 to 66) and 48% (95% CI, 34 to 60), respectively.13 ELIANA inclusion criteria included ≥2nd bone marrow relapse, primary refractory disease, defined as not achieving complete response (CR) after 2 cycles of standard chemotherapy, chemorefractory B-ALL, defined as not achieving CR after 1 cycle of chemotherapy in the relapsed setting, as well as patients ineligible for hematopoietic stem cell transplant (HSCT) due to comorbidities or bone marrow relapse after HSCT.14
Whereas both refractory and relapsed disease are listed as indications for tisagenlecleucel by the Food and Drug Administration (FDA), methods and thresholds of disease detection defining refractory and relapse are left to physician discretion. Traditionally, primary refractory disease is defined as morphologic disease of ≥5% following two standard induction chemotherapy regimens, whereas relapsed B-ALL is defined as detection of morphologic disease after previously achieving complete response.15,16 Minimal residual disease (MRD) testing using flow-cytometry17,18 (sensitivity of 10−4 nucleated cells) and next-generation sequencing (NGS)19,20 (sensitivity of 10−6 nucleated cells) achieves B-ALL detection at lower levels compared to standard morphologic testing. Increasing data supports the prognostic value and clinical applications of detecting disease, and the rate of disease eradication, through higher sensitivity methods.9,11,17,20, 21, 22
We hypothesised that with CD19-CAR T cell commercialisation and increasing MRD data and access, real world use of CAR has diverged from traditional morphologic definitions of relapsed and refractory disease. We conducted a retrospective study analysing real-world utilisation patterns of tisagenlecleucel in children and young adults, and established associated outcomes stratified by indication. We explored the qualifying criteria used by physicians in the real-world setting to administer tisagenlecleucel and demonstrate that definitions of treatment refractoriness and relapse are not universally standard and continue to evolve across morphologic, flow-cytometric, and molecular measures of residual disease. We importantly establish outcomes across patients treated with CAR T cells in first relapse, an indication omitted from the tisagenlecleucel FDA approval, and a cohort with clinical need, where prior reporting remains limited.
Methods
Study design and participants
We conducted a retrospective study analysing clinical indications and outcomes across 185 children and young adults ≤26 years, treated with tisagenlecleucel between August 30, 2017 and March 6, 2020 (Median follow-up; 335 days, range 6–963 days).23 Data was collected from 15 sites within the United States of America participating in the Pediatric Real-World CAR Consortium (PRWCC). Retrospective, de-identified data was collected using a HIPAA compliant REDCap collection tool, with informed consent waived due to retrospective de-identified nature of data and ethical approval obtained from institutional review boards (IRBs). We analysed baseline characteristics, clinical response, relapse rates, survival, and toxicity outcomes, as stratified by indication for CAR T-cell therapy. We analysed 3 patient cohorts; patients receiving CAR upon determination of upfront refractory disease without relapse, patients treated following 1st relapse and patients treated following ≥2nd relapse (Table 1). We described indications for CAR T-cell therapy, as reported by primary oncology care team. As an exploratory aim, we descriptively reported end of upfront chemotherapy induction disease status by morphology and flow cytometry MRD (Table 2), to better understand real world definitions of refractory disease.
Table 1.
Baseline characteristics from CAR T-cell therapy of 185 patients treated across 15 Pediatric Real-World CAR Consortium (PRWCC) centres, stratified by CAR indication.
| Tisagenlecleucel indication | Refractory |
1st Relapse |
≥2nd Relapse |
p-value |
|---|---|---|---|---|
| (N = 30) | (N = 67) | (N = 87) | ||
| Baseline patient characteristics | ||||
| Age at Infusion (years) | ||||
| Median (Range) IQR | 13 (3–24) 10–18 | 10 (<1–26) 5–17 | 13 (1–25) 9–18 | 0.1197 |
| Age at diagnosis | ||||
| Median (Range) IQR | 12.5 (2–23) 9–18 | 7 (<1–25) 3–14 | 7 (<1–22) 3–13 | 0.0043 |
| Sex | ||||
| Male | 17 (57%) | 36 (54%) | 57 (66%) | 0.3169 |
| Female | 13 (43%) | 31 (46%) | 30 (34%) | |
| Race/Ethnicity | ||||
| Non-hispanic white | 15 (50%) | 35 (51%) | 40 (46%) | 0.1913 |
| Hispanic | 15 (50%) | 25 (37%) | 29 (33%) | |
| Black/African American | 0 | 0 | 7 (8%) | |
| Asian | 0 | 2 (3%) | 5 (6%) | |
| Other | 0 | 5 (7%) | 6 (7%) | |
| NCCN cytogenetic risk classification | ||||
| High risk | 21 (70%) | 29 (43%) | 14 (16%) | <0.0001 |
| Intermediate risk | 6 (20%) | 15 (22%) | 28 (32%) | |
| Low Risk | 2 (7%) | 8 (12%) | 15 (17%) | |
| Unknown | 1 (3%) | 15 (22%) | 30 (34%) | |
| Disease burden (4 unknown) | ||||
| High Burden (≥5% bone marrow lymphoblasts, CNS3, non-CNS extramedullary disease) | 11 (37%) | 38 (57%) | 45 (52%) | 0.2629 |
| Low burden (<5% bone marrow lymphoblasts) | 10 (33%) | 16 (24%) | 15 (17%) | |
| Undetectable Disease | 9 (30%) | 12 (18%) | 24 (28%) | |
| Prior HSCT | ||||
| Yes | 0 | 10 (15%) | 38 (44%) | <0.0001 |
| Prior CD19-targeted therapy | ||||
| Yes | 3 (10%) | 10 (15%) | 25 (29%) | 0.0371 |
Table 2.
Response and relapse rates, stratified by CAR indication.
| Refractory (N = 30) | 1st Relapse (N = 66) | ≥2nd Relapse (N = 87) | p-value | |
|---|---|---|---|---|
| Complete response rate | ||||
| Morphology | 28 (93%) | 56 (85%) | 71 (82%) | 0.3061 |
| MRD | ||||
| Flow cytometry | 28 (93%) | 52 (79%) | 67 (77%) | 0.1324 |
| Relapse rate∗ | ||||
| Overall | 7 (25%) | 17 (30%) | 28 (39%) | 0.3490 |
| CD19 status at relapse | ||||
| CD19+ | 6 (86%) | 10 (59%) | 14 (50%) | |
| CD19− | 1 (14%) | 7 (41%) | 14 (50%) | 0.2897 |
Relapse rate∗ amongst morphologic CR responders.
Outcomes
We described rates of morphologic complete response (CR) at day 28 post-CAR infusion and describe overall relapse rates and CD19+ and CD19-relapse rates across cohorts. For survival outcomes, the primary endpoint of interest was overall-survival (OS), and the secondary endpoints were event-free survival (EFS), duration of remission (DOR) and duration of B cell aplasia (DBA) across refractory, 1st relapse and ≥2nd relapse cohorts. Due to lack of standardisation in the field on BCA definition, timing of B cell recovery was captured as per institutional thresholds and reporting. Retrospective nature of data capture did not allow for harmonisation of BCA definition across all participating centres. As an exploratory analysis, we additionally compared OS, EFS, DOR and DBA in patients with refractory disease treated with CAR upfront, without relapse, to patients characterised as having refractory disease, yet only treated upon later relapse.
OS and EFS were measured from time of CAR-infusion and data was censored at time of last follow-up. EFS events were defined as lack of Day 28 (d28) CR, relapse after achieving CR, myelodysplastic syndrome (MDS) or death. DOR was measured from time of response (d28 after infusion) in only patients achieving CR. The primary event for DOR analysis was defined as a composite of death and relapse, with censoring at HSCT, MDS or last follow-up. Exploratory competing risk analysis for DOR was also performed treating HSCT and MDS as competing risks and is included in supplemental data. DBA was measured in only patients achieving remission and BCA, from time of establishing BCA (d28). For DBA, relapse, death, HSCT and MDS were considered as competing risks, as these events eliminated our ability to subsequently attribute BCA specifically to tisagenlecleucel. Data was censored at time of last follow-up if no events occurred. No patient experienced death as an initial competing event in our competing risk analyses of DBA. We described select CAR-mediated toxicities, with CRS retrospectively graded according to the American Society for Transplantation and Cellular Therapy (ASTCT)24 for all patients. Neurotoxicity was graded per institutional standards, with ASTCT,24 CAR-related encephalopathy syndrome (CRES),25 as well as other institutional scales for grading neurotoxicity. Our reporting window predated the establishment of ASTCT guidelines and Immune Effector Cell-Associated Encephalopathy (ICE) scores were therefore not applied universally, precluding harmonised Immune Effector Cell-Associated Neurotoxicity syndrome (ICANS) reporting.
Statistical analysis
Baseline characteristics were summarised using descriptive statistics, such as median, range, and interquartile range (IQR) for continuous variables and frequency and percent for categorical variables. Difference in these characteristics were compared across 3 cohorts using Kruskal–Wallis tests for continuous variables and Fisher’s exact tests for categorical variables. Response and relapse rates among morphologic CR patients were compared across 3 cohorts using Fisher’s exact tests. Toxicities and their treatments were also compared across 3 cohorts using Fisher’s exact tests. For all the percentages, 95% exact confidence intervals (CIs) were calculated using the Clopper-Pearson method. For OS, EFS and DOR, Kaplan Meier (KM) curves were generated and compared across groups using log-rank tests. Survival estimates at 6 month and 1 year and associated 95% CIs were calculated through the KM method. For DBA and exploratory analysis of DOR, cumulative incidence curves (and 95% CIs) were generated and compared using Gray’s test. A two-sided p value < 0.05 was considered statistically significant. Software analyses were performed in R 4.2 and SAS 9.4.
Role of the funding source
This work was supported by the Lucille Packard Association of Auxiliaries for Children, St. Baldrick's, Stand Up 2 Cancer, Parker Institute for Cancer Immunotherapy, and Virginia and D.K. Ludwig Fund for Cancer Research, with the role of all funding sources in infrastructure support. Funding sources did not play a role in study design, data collection, analysis or interpretation, manuscript writing, or in decision to submit for publication. All authors have had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Results
Real-world CAR utilisation
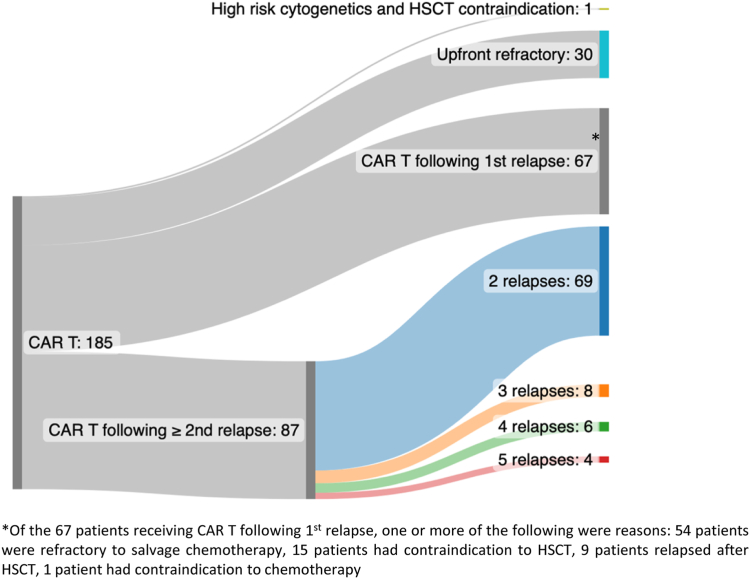
Across 185 tisagenlecleucel infused patients, 30 (16%) were treated due to upfront refractory disease, 67 (36%) were in first relapse, and 87 (47%) had ≥2 relapses at the time of treatment (Fig. 1). One patient was treated upfront due to high-risk disease and chemotherapy intolerance. Baseline characteristics across patients in refractory, 1st relapse and ≥2nd relapse cohorts were characterised (Table 1). We identified that high-risk cytogenetics (NCCN Cytogenetic Risk Classification) were more likely in patients treated with upfront refractory disease (70%) as compared to remaining cohorts (p < 0.0001) and in patients treated in 1st relapse (43%) as compared to ≥2nd relapse (16%) (p < 0.0001). Hispanic patients were more prevalent in the upfront refractory cohort, although not achieving statistical significance (50% vs. 37% (1st relapse) and 33% (≥2nd relapse); p = 0.19), aligning with established data describing higher rates of chemotherapy non-responsiveness amongst Hispanic patients.26,27 Median age at diagnosis was higher in patients treated with upfront refractory disease (12.5 years) as compared to patients treated following relapse (7 years) (p = 0.004). Prior HSCT and prior CD19-targeted therapy were expectedly lower in patients treated with tisagenlecleucel due to refractory disease, with increasing rates upon increasing number of relapses. Baseline characteristics were otherwise evenly distributed across cohorts. Disease burden was reported as measured at the final bone marrow assessment prior to CAR infusion. We interpret disease burden distribution across cohorts with caution, due to variability in duration between pre-CAR assessments and CAR T-cell infusions and use of bridging chemotherapy following disease burden assessment in patient subsets (Supplement Table S1).
Fig. 1.
Clinical indications and real-world utilisation of Tisagenlecleucel.
Patients treated in the upfront refractory setting
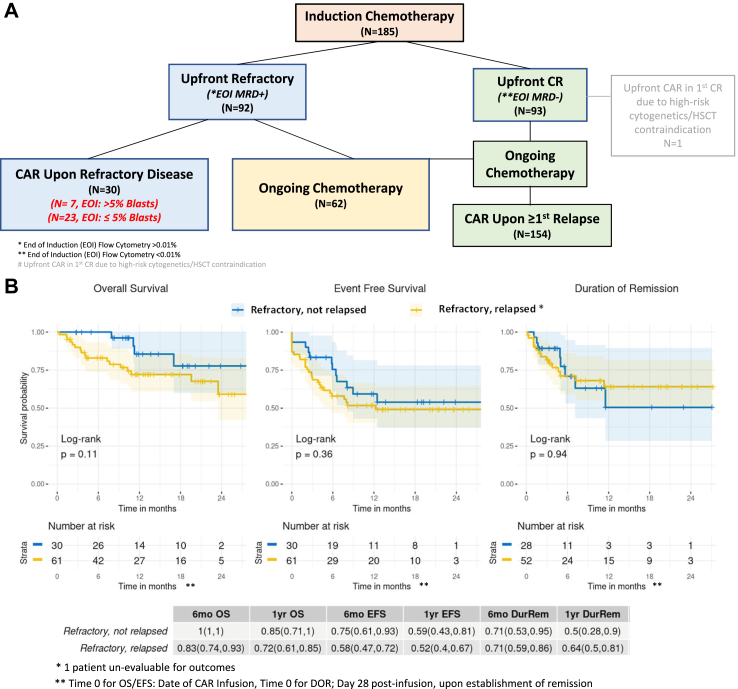
Of 30 patients treated due to upfront refractory disease, only 7 patients (23%, 95% CI 10–42%) had >5% morphologic bone marrow lymphoblasts at the end of induction (EOI). Twenty-three patients (77%, 95% CI 57–90%) had <5% lymphoblasts at EOI, as detected by morphology or flow cytometry, and did not meet the historical definition of primary refractory disease, yet were classified as refractory (Fig. 2A).
Fig. 2.
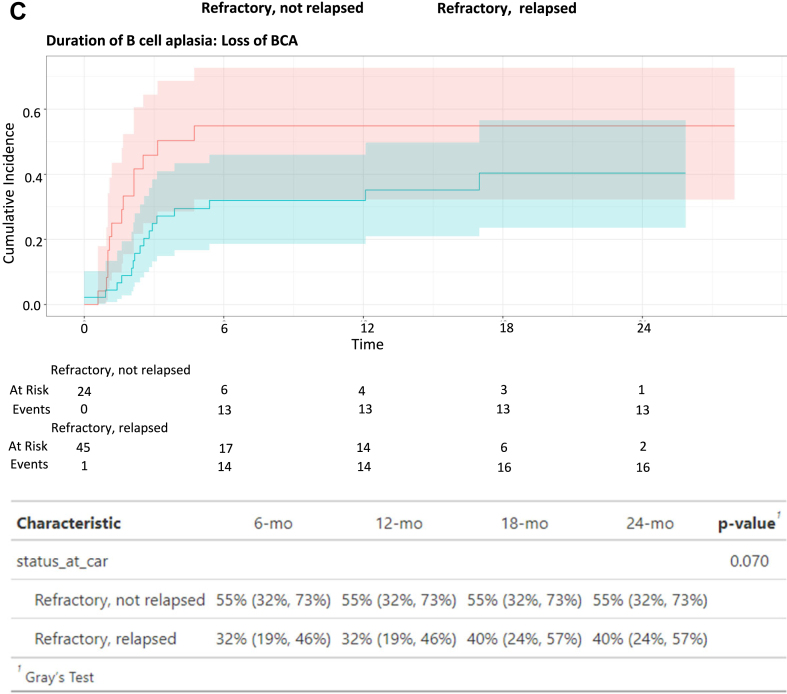
(A) Response to induction chemotherapy across infused patients. (B) Overall-survival (OS), Event-free survival (EFS) and Duration of remission (DOR) across patients with upfront refractory disease who were treated in the upfront refractory setting vs. patients with upfront refractory disease, who only received CAR T-cells upon subsequent relapse(s), using KM curves and log-rank tests. (C) Duration of B cell aplasia (DBA) across described cohorts, treating relapse, HSCT and MDS as competing risks, using cumulative incidence curves and Gray’s test. Shaded areas represent the 95% confidence intervals.
Of patients treated with upfront refractory disease, a morphologic complete response rate of 93% (95% CI 78–99%) was observed. Among the 28 responders, 7 patients (25%, 95% CI 11–45%) experienced disease relapse after tisagenlecleucel, with 6 (86%) CD19+ and 1 (14%) CD19-disease (Table 2). 1 year OS, EFS, DOR rates in the primary refractory cohort were 85% (95% CI 75–100%), 59% (95% CI 43–81%) and 50% (95% CI 28–90%), respectively (Fig. 3A). In the exploratory competing risk analysis of DOR with HSCT as a competing event, cumulative incidence of HSCT (before relapse) was higher in this refractory cohort than the remaining cohorts (Supplement Fig. S3B, p < 0.001). Cumulative incidence of B cell recovery is 55% (95% CI 32–73%) at both 6 and 12 months, which is increased, compared to remaining cohorts, yet without statistical significance (Fig. 3B, p = 0.055; see Supplement Fig. S4 for cumulative incidence of the competing events). Toxicity analysis of the 30 patients infused in the upfront refractory setting showed that 11 of 30 (37%, 95% CI 20–56%) patients experienced any grade cytokine release syndrome (CRS), with only 1 patient (3%, 95% CI 0.1–17%) having ≥ grade 3 CRS. Two of 30 (7%, 95% CI 1–22%) patients experienced any grade neurotoxicity and none were ≥ grade 3. One patient (3%, 95% CI 0.1–17%) required tocilizumab, no patients required steroids and 2 patients (6%, 95% CI 1–22%) required pediatric intensive care unit (PICU)-level care for management of hypotension for grade 2 and 3 CRS, respectively. As per previous clinical reporting,28 our toxicity analysis identifies patients treated in the upfront refractory setting to have less CRS/ICANS as compared to patients treated following relapse (p = 0.01) (Table 3), supporting CAR T-cell therapy in more upfront settings as a tolerable therapeutic option.
Fig. 3.
(A) Overall-Survival (OS), Event-free Survival (EFS) and Duration of Remission (DOR) across patients infused with refractory disease, 1 prior relapse or ≥2 relapses, using KM curves and log-rank tests. (B) Duration of B cell Aplasia (DBA) across described cohorts, treating relapse, HSCT and MDS as competing risks and using cumulative incidence curves and Gray’s test. Shaded areas represent the 95% confidence intervals.
Table 3.
Toxicities from CAR T-cell therapy of 184 evaluable patients treated across 15 Pediatric Real-World CAR Consortium (PRWCC) centres, stratified by CAR indication.
| Toxicity | Refractory (N = 30) | 1st relapse (N = 66) | ≥2nd relapse (N = 87) | p-value |
|---|---|---|---|---|
| CRS | ||||
| None | 19 (63%) | 15 (23%) | 33 (38%) | |
| Any grade | 11 (37%) | 51 (77%) | 54 (62%) | 0.0006 |
| ≥Grade 3 | 1 (3%) | 27 (41%) | 11 (13%) | <0.0001 |
| Neurotoxicity | ||||
| None | 28 (93%) | 47 (71%) | 69 (79%) | |
| Any grade | 2 (7%) | 19 (29%) | 18 (21%) | 0.0413 |
| ≥Grade 3 | 0 | 9 (13%) | 3 (3%) | 0.0159 |
| Treatment | ||||
| Tocilizumab | ||||
| Yes; Median doses (range); IQR | 1 (3%) | 30 (45%); 2 (1–5); 1–2 | 15 (17%); 2 (1–3); 1–3 | <0.0001 |
| No | 29 (97%) | 36 (55%) | 72 (83%) | |
| Steroids | ||||
| Yes; Median days (range); IQR | 0 | 17 (26%); 7 (1–31); 5–9 | 9 (10%); 8 (2–18); 3–12 | 0.0009 |
| No | 30 (100%) | 49 (74%) | 78 (90%) | |
| PICU stay | ||||
| Yes, Duration (days); Median (range); IQR | 2 (7%); 5.5 (3–8); 3–8 | 29 (44%); 8 (1–24); 5–13 | 26 (30%); 5 (1–33); 2–8 | 0.0006 |
| No | 28 (93%) | 37 (56%) | 61 (70%) | |
Notably, although 30 patients received CAR T-cell therapy due to upfront refractory disease without relapse, of the remaining cohort treated for relapse, an additional 62 patients were reported to have upfront refractory disease (defined as any detectable disease following standard induction chemotherapy), yet did not proceed to tisagenlecleucel until later in the disease course (Fig. 2A). Within the 92 patients who were categorised with upfront refractory disease, we contrasted outcomes of patients receiving tisagenlecleucel in the upfront refractory setting (N = 30), and patients receiving continued chemotherapy and treated with tisagenlecleucel only upon ≥1st relapse (N = 61 evaluable). Survival outcomes from the time of tisagenlecleucel infusion in patients categorised with upfront refractory disease did not differ between patients treated with tisagenlecleucel upfront compared to patients infused later, upon relapse (Fig. 2B, OS; p = 0.11, EFS; p = 0.36). DOR was also similar between cohorts (Fig. 2B, p = 0.94). In the exploratory competing risk analysis of DOR with HSCT as a competing event, cumulative incidence of HSCT (before relapse) was higher in patients treated in the upfront refractory setting (Supplement Fig. S1B, p = 0.01). Increased cumulative incidence of BCA loss was observed in patients treated in the upfront refractory setting, yet not achieving statistical significance (Fig. 2C, p = 0.07; see Supplement Fig. S2 for cumulative incidence of the competing events).
Patients treated in 1st relapse
In our reported cohort, 36% of infused patients (N = 67) received tisagenlecleucel in first relapse, justified by one or more criteria spanning chemo-refractory disease (N = 54, 81%), contraindication to HSCT (N = 15, 22%), relapse after HSCT (N = 9, 13%) and contraindication to chemotherapy (N = 1, 1.5%) (Fig. 1). Notably, 9 of 15 (60%) patients contraindicated for HSCT had trisomy 21.
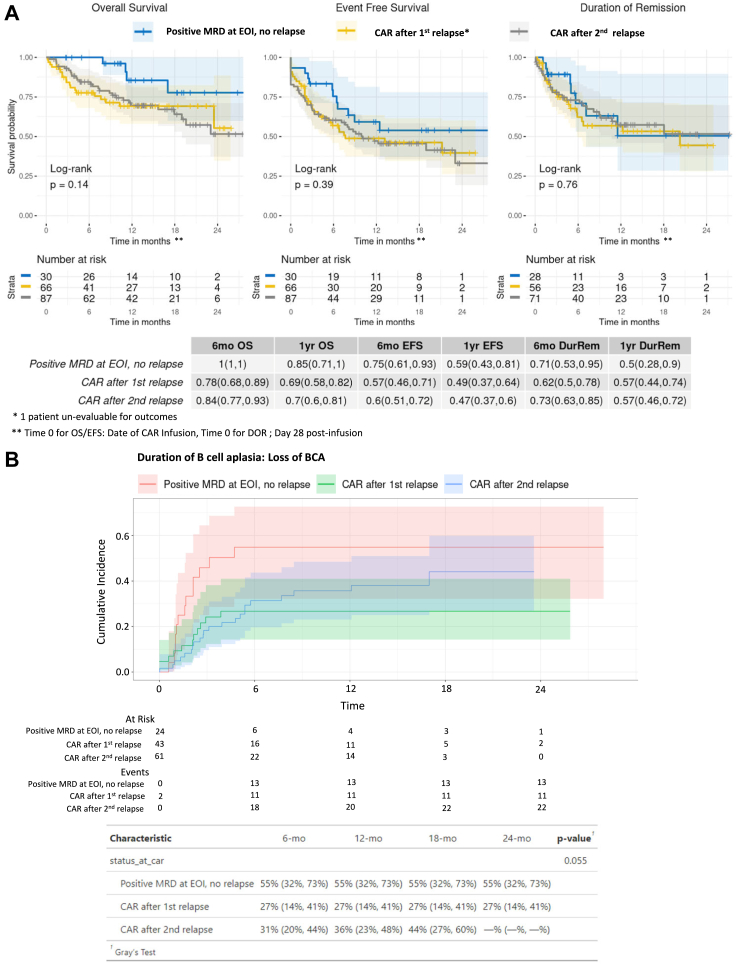
Sixty-six of 67 patients infused upon 1st relapse were evaluable for outcomes, with 56 of 66 (85%, 95% CI 74–92%), achieving morphologic CR. Among the 56 responders, 17 patients (30%, 95% CI 19–44%) experienced relapse, with 10 (59%) and 7 (41%) experiencing CD19+ and CD19-relapse, respectively. Across 10 patients with CD19+ relapse, 6 had documented B cell recovery prior to (n = 5), or at time of (n = 1) relapse and 4 patients did not have documentation of B cell recovery. OS, EFS and DOR rates at 1-year post-infusion were 69% (95% CI 58–82%), 49% (95% CI 37–64%), and 57% (95% CI 44–74%), respectively across patients treated after 1st relapse. Comparative analysis of OS, EFS and DOR demonstrate that outcomes of tisagenlecleucel recipients treated following 1st relapse were not significantly divergent compared to patients treated due to refractory B-ALL or disease in ≥2nd relapse (Fig. 3A, OS; p = 0.14, EFS; p = 0.39, DOR; p = 0.76). One-year cumulative incidence of B cell recovery was decreased in this cohort, though without statistical significance (1st relapse (27%, 95% CI 14–41%), refractory (55%, 95% C I32-73), ≥2nd relapse (36%, 95% CI 23–48) (Fig. 3B, p = 0.055; see Supplement Fig. S4 for cumulative incidence of the competing events)).
Increased toxicity was observed in patients treated in first relapse as compared to remaining patients. Fifty-one of 66 (77%, 95% CI 65–87%) patients treated in 1st relapse experienced any grade CRS, with 27 patients (41%, 95% CI 29–54%) experiencing ≥ grade 3 CRS. 19 of 66 (29%, 95% CI 18–41%) patients experienced any grade neurotoxicity, with 9 (13%, 95% CI 6–24%) experiencing ≥ grade 3 neurotoxicity. Thirty (45%, 95% CI 33–58%) and 17 (26%, 95% CI 16–38%) patients required tocilizumab and steroid use, respectively, and 29 patients (44%, 95% CI 32–57%) required PICU stay (Table 3). All PICU admissions in this cohort had CRS, with 26/29 (90%) having ≥ grade 3 CRS, 26 (90%) patients received tocilizumab, 17 (59%) received steroids and 2 (7%) received anakinra. Fifteen (52%) PICU patients had neurotoxicity, 9 (31%) ≥ grade 3. Twenty-five patients (86%) had hypotension and received fluid boluses, 21 (72%) received vasopressors (9 high dose, 12 low dose). Eighteen (62%) experienced hypoxia, 10 (34%) received positive pressure ventilation (7; intubated, 3; BiPap only).
Patients treated in ≥2nd relapse
Of 87 patients in 2nd or greater relapse, 69 (79%) were treated in 2nd relapse and 8 (9%), 6 (7%) and 4 (5%) patients had 3, 4 and ≥ 5 relapses, respectively, prior to receiving tisagenlecleucel (Fig. 1). 71 patients (82%, 95% CI 72–89%) treated upon ≥2nd relapse achieved morphologic CR. Among the 71 responders, 28 patients (39%, 95% CI 28–52%) experienced relapse, with 14 (50%) and 14 (50%) patients experiencing CD19+ and CD19− relapse, respectively. 1-year OS, EFS and DOR rates were 70% (95% CI 60–81%), 47% (95% CI 37–60%), and 57% (95% CI 46–72%) respectively (Fig. 3A). One-year cumulative incidence of B cell recovery was 36% (95% CI 23–48%) (Fig. 3B). CRS occurred in 54 patients (62%, 95% CI 51–72%), with ≥grade 3 CRS in 11 (13%, 95% CI 6–21%) patients. 18 patients (21%, 95% CI 13–31%) experienced neurotoxicity and 3 patients (3%, 95% CI 1–10%) experienced ≥ grade 3 neurotoxicity. Fifteen (17%, 95% CI 10–27%) and 9 (10%, 95% CI 5–19%) patients required tocilizumab and steroid use, respectively and 26 patients (30%, 95% CI 21–41%) required PICU stay.
Sub-analysis of PICU indications demonstrated that 25/26 (96%) PICU patients had CRS, with 9/25 (36%) having ≥ grade 3 CRS. Thirteen (50%) patients received tocilizumab, 8 (31%) received steroids and one received anakinra. Eight of 26 (31%) had neutrotoxicity, 3 (12%) ≥ grade 3. Nineteen (73%) had hypotension, 17 (65%) had fluid boluses, 8 (31%) required vasopressors (4 high dose, 4 low dose). Eleven (42%) patients experienced hypoxia, 4 (15%) received positive pressure (3; intubated, 1; CPAP).
Discussion
Relapsed B-ALL or B-ALL that is refractory to chemotherapy portends poor survival.12,29 Disease detection at the end of upfront induction chemotherapy remains highly prognostic of B-ALL survival.9,17 Accordingly, clinical trials for upfront B-ALL include risk stratification systems designed to intensify therapy for this high-risk patient cohort.2,5,12,29, 30, 31, 32 Historically, the definition of primary refractory B-ALL was limited to detectable morphologic disease of ≥5% at the end of 2 cycles of chemotherapy. With expanded adoption of flow cytometry MRD, even low levels of MRD have been shown to correlate with prognosis9,10,17,20,33,34 such that current risk stratification systems integrate flow cytometry MRD to guide post-induction therapy. NGS of clonal B and T cell receptors further increases the sensitivity of ALL detection to levels of 1 × 10−6, which is 1–2 logs deeper than standard flow cytometry or PCR assays,35 yet prospective clinical trial data on treatment intensification using NGS-based MRD remains limited. Whereas CD19 CAR T-cell therapy has been approved for patients up to 25 years of age with B-ALL that is refractory or in second or later relapse (Tisagenlecleucel; Kymriah package insert), “refractory” remains without defining criteria and permissive of physician discretion not bound to traditional morphologic measures. We aimed to describe what measures of disease have been implemented in the real-world clinical CAR T-cell therapy setting to define refractory B-ALL and establish tolerability and survival.
In this real-world retrospective study, 23 of 30 (70%) upfront refractory patients treated with tisagenlecleucel had EOI lymphoblast levels of <5%, as detected by flow cytometry or morphology. We established that definitions of primary refractory disease in the real-world setting expand beyond morphologic detection of disease at levels of ≥5% and broadly include patients with persistent morphologic disease and/or disease detectable by MRD alone. One of the future most important questions in the B-ALL CAR T-cell field is how to advance immunotherapy to the upfront setting and thereby offset the prolonged toxicity and duration of standard chemotherapy. The first step, which is actively under investigation, is to study CAR T-cell therapy in the upfront refractory setting for high-risk patients where outcomes with chemotherapy alone remain poor. The Children’s Oncology Group phase II trial (AALLL1721; CASSIOPEIA) is specifically designed to prospectively study tisagenlecleucel outcomes in patients with high-risk B-ALL who are MRD positive by flow cytometry at the end of consolidation, a cohort where 5-year OS remains <40%.9 As ongoing efforts are pursued to advance CAR T-cell therapy to the upfront setting, it is notable that retrospective data is already existent using CAR T-cell therapy in the upfront MRD-positive setting,36,37 and continues to accumulate in the real-world.
In our real-world series, tisagenlecleucel was extremely well-tolerated in patients treated in the upfront refractory setting, with only 3% (1/30) ≥ grade 3 CRS and no ≥ grade 3 neurotoxicity. Patient response and survival outcomes are comparable to more heavily pre-treated patients. Notably, the incidence of CD19- relapse was numerically lower when treated in the upfront refractory setting, as compared to remaining patients, although this did not achieve statistical significance, perhaps due to small numbers (CD19- relapse rate: upfront refractory; 1/30 (14%), 1st relapse; 7/66 (41%), ≥2nd relapse; 14/87 (50%), p = 0.29). The impact of aggregate therapy, including chemotherapy and targeted agents, on CD19 antigen loss requires further exploration.
Children and young adults in first relapse comprise a unique cohort wherein a subset of patients, in the absence of high-risk features, can be re-salvaged with chemotherapy alone. Specifically, patients with late bone marrow relapse (≥36 months from diagnosis) and late isolated extramedullary (IEM) disease (≥18 months from diagnosis) represent a lower risk category, with likelihood of establishing durable remission further increasing if MRD at the end of block 1 of reinduction is <0.1%.4 Chemotherapy remains the standard of care for this cohort, with first relapse patients therefore commonly excluded from early phase clinical trials, which are intended for patients without standard salvage options.
The inverse of this cohort, patients with early bone marrow relapse (<36 months from diagnosis) and early IEM relapse (<18 months from diagnosis), represent a high-risk patient population with a pressing clinical need for approved alternatives to chemotherapy. Our outcomes analysis across patients treated with tisagenlecleucel following first relapse showed that survival outcomes were comparable to outcomes across patients treated for refractory B-ALL or ≥2nd relapse. Increased duration of B cell aplasia was seen in this cohort yet without statistical significance (Fig. 3B, p = 0.055). CRS and neurotoxicity incidence and severity were higher in the first relapse cohort. Across multiple studies, high pre-infusion disease burden has been shown to be associated with CRS risk and severity.23,38 While there were no significant differences in baseline disease burden distribution across cohorts in our analysis (Table 1; p = 0.26), this must be interpreted with caution due to variability in timing of baseline disease burden assessments and use of bridging therapy in patient subsets between disease assessment and CAR T-cell infusion. Formal prospective studies of CAR T-cell therapy outcomes in the first relapse setting will be critical, with a goal of identifying which patient subsets may benefit from an expanded CD19-CAR T-cell indication. Additional studies are also necessary to validate differences in toxicities across cohorts and determine causality. These studies should include an assessment of cumulative CD19 antigen load, which could also explain variable persistence and toxicity, and was not systematically measured in our dataset.
Expectedly, our upfront refractory cohort had higher risk cytogenetics, as previously defined by the NCCN Cytogenetic Risk Classification, supportive of known data that high-risk cytogenetics are associated with chemotherapy resistance.39 Our analysis did not capture treatment rationale for patients treated in the upfront refractory setting compared to those with upfront refractory disease who were only treated following successive lines of therapy, but perhaps the presence of MRD along with high-risk cytogenetics6,32,33,40 influenced the choice to move to tisagenlecleucel. Indeed, one limitation of our study was that reporting was limited to patients who received tisagenlecleucel without control data on a parallel cohort of patients with refractory disease or patients in 1st relapse who received ongoing chemotherapy and no CAR T-cell therapy. Another drawback of this study is that safety analysis was limited to select established post-CAR toxicities of interest, without complete capture and classification of additional adverse events such as infection, organ damage, and hematologic toxicities associated with conditioning regimens.
In summary, we highlight that in the real-world clinical pediatric and young adult B-ALL setting, definitions of relapse and refractory disease are evolving, and standard definitions of morphologic disease have been abandoned in exchange for more sensitive testing for residual disease. We highlight that in the real-world, MRD measures are being used to justify tisagenlecleucel use, generating a dataset of patients receiving CAR T-cells in the upfront MRD-setting. Treatment was very well-tolerated in this cohort and high response rates were seen, despite prior chemoresistance. This data can be used to complement formal prospective studies of CAR used in the upfront MRD-setting, such as COG AALL1721 (CASSIOPEIA). Outcome analysis across CAR T-cell therapy indications revealed that despite heterogeneity in real-world CAR T-cell utilisation, response, relapse, and survival rates overlap, supporting tisagenlecleucel therapy as an effective treatment across all indications, including refractory disease and importantly, 1st relapse, an indication omitted from the FDA approval.
Contributors
All authors were involved in conception and design and collection of patient data. Liora Schultz performed administrative duties. Christina Baggot and Liora Schultz designed the data collection tool. Valentin Barsan, Christina Baggott and Liora Schultz accessed and verified the underlying data reported in the manuscript. Valentin Barsan, Snehit Prabhu, Christina Baggott, YiMei Li and Liora Schultz performed statistical analysis. All authors have had full access to all the data in the study and accept responsibility for the decision to submit for publication. All authors were involved in data analysis and interpretation, manuscript writing and final manuscript approval and are accountable for all aspects of this work.
Data sharing statement
Researchers can request access to data by contacting the following authors directly: Christina Baggott (baggott@stanford.edu) or Liora Schultz (lioras@stanford.edu).
Declaration of interests
V.B. serves on the boards of ArsenalBio and Umoja Biopharma and consults or holds stock in Zafrens and Treeline Biosciences which are developing therapies for cancer treatment and Illumina, Invitae, Pacific Biosciences, and Guardant who are developing oncology NGS tests. C.L.M. is an inventor on several patents related to CAR T-cell therapies. C.L.M. is a cofounder of Lyell Immunopharma, CARGO Therapeutics and Link Cell Therapies, which are developing CAR-based therapies, and consults for Lyell, CARGO, Link, Ensoma, Mammoth, Immatics, Apricity, Glaxo Smith Klein, Nektar, Legend and Bristol Myers Squibb. C.L.M receives royalties for CD-22 CAR licensing from NIH, has had grant/contract funding from St. Baldrick’s Foundation, NIH, CIRM, Parker, Tune therapeutics, Lyell Immunopharma, Ludwig Institute, Emerson Collective, Department of Defense and Goldhirsh-Yellin Foundation. She is a member of the Board of Directors of CARGO Therapeutics and Link Cell Therapies and owns stocks in Lyell Immunopharma, CARGO Therapeutics, Link Cell Therapies, Ensoma, Mammoth and Apricity. T.W.L. served on advisory boards or consults for Novartis, Bayer, Aptitude Health, Jumo Health, Massive Bio, Medscape, AI Therapeutics, Jazz Pharmaceuticals, GentiBio, Menarini, Pyramid Biosciences, Targeted Oncology, Treeline Biosciences. He owns stocks/other ownership interest in advanced microbubbles. T.W.L. received research funding from Lily, Roche/Genentech, Taiho Oncology, Advanced Accelerator Applications/Novartis, Bristol-Myers Squibb, BioAtla, Pfizer, Bayer and Turning Point Therapeutics. G.D.M. received funding for medical writing from Novartis. C.L.P. served on an advisory board for Novartis. L.S. served on an advisory board for Novartis. H.S. served on an advisory board for Novartis. M.H. served on editorial advisory board for Novartis and Sobi Pharmaceuticals and is the Vice Chair for COG NHL committee and COG NHL Biology Committee. V.F. consulted for Adaptimmune. S.P. is supported by the UCSF-Stanford CERSI grant UOI FD005978 from the FDA. P.S. served on advisory board for Sobi Pharmaceuticals. A.K. received COG support for meeting attendance. K.J.C. received grant support for an investigator-initiated trial and sat on advisory boards for Novartis and Atara Biotherapeutics. M.R.V. consults for Novartis, Sanofi, Qihan, Forge, Takada and Equillium. M.R.V. has a provisional patent describing methods of producing and using immunotherapy for cancer. M.R.V.participates on the safety monitoring/advisory board for FBX-101 and owns stocks/options for Fate therapeutics.
Acknowledgements
We acknowledge the following individuals for their roles in supporting successful execution of this multi-institutional study. Regulatory support: Sharon Mavroukakis and Emily Egeler. Administrative support: Anika Dove. Legal Council and Contracting: Joshua Murphy. REDCap Data: The Stanford REDCap platform is developed and operated by Stanford Medicine Research IT team. The REDCap platform services at Stanford are subsidised by a) Stanford School of Medicine Research Office, and b) the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001085. This work was supported by a St Baldrick's/Stand Up 2 Cancer Pediatric Dream Team Translational Cancer Research Grant (C.L.M.). Stand Up 2 Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. C.L.M is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. The work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research. All funding sources enabled infrastructure support for this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102268.
Appendix ASupplementary data
References
- 1.Parker C., Krishnan S., Hamadeh L., et al. Outcomes of patients with childhood B-cell precursor acute lymphoblastic leukaemia with late bone marrow relapses: long-term follow-up of the ALLR3 open-label randomised trial. Lancet Haematol. 2019;6:e204–e216. doi: 10.1016/S2352-3026(19)30003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhojwani D., Pui C.H. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14:e205–e217. doi: 10.1016/S1470-2045(12)70580-6. [DOI] [PubMed] [Google Scholar]
- 3.Lew G., Chen Y., Lu X., et al. Outcomes after late bone marrow and very early central nervous system relapse of childhood B-acute lymphoblastic leukemia: a report from the Children's Oncology Group phase III study AALL0433. Haematologica. 2021;106:46–55. doi: 10.3324/haematol.2019.237230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunger S.P., Raetz E.A. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 2020;136:1803–1812. doi: 10.1182/blood.2019004043. [DOI] [PubMed] [Google Scholar]
- 5.Oskarsson T., Söderhäll S., Arvidson J., et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. Haematologica. 2016;101:68–76. doi: 10.3324/haematol.2015.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun W., Malvar J., Sposto R., et al. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia. 2018;32:2316–2325. doi: 10.1038/s41375-018-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conter V., Aricò M., Basso G., et al. Long-term results of the Italian association of pediatric hematology and oncology (AIEOP) studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–264. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 8.Rheingold S.R., Ji L., Xu X., et al. Prognostic factors for survival after relapsed acute lymphoblastic leukemia (ALL): a Children's Oncology Group (COG) study. J Clin Oncol. 2019;37:10008. [Google Scholar]
- 9.Borowitz M.J., Wood B.L., Devidas M., et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood. 2015;126:964–971. doi: 10.1182/blood-2015-03-633685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conter V., Bartram C.R., Valsecchi M.G., et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 11.Coustan-Smith E., Sancho J., Behm F.G., et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002;100:52–58. doi: 10.1182/blood-2002-01-0006. [DOI] [PubMed] [Google Scholar]
- 12.Pui C.H., Campana D., Pei D., et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laetsch T.W., Maude S.L., Rives S., et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J Clin Oncol. 2023;41:1664–1669. doi: 10.1200/JCO.22.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maude S.L., Laetsch T.W., Buechner J., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arber D.A., Orazi A., Hasserjian R.P., et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchmann S., Schrappe M., Baruchel A., et al. Remission, treatment failure, and relapse in pediatric ALL: an international consensus of the Ponte-di-Legno Consortium. Blood. 2022;139:1785–1793. doi: 10.1182/blood.2021012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borowitz M.J., Devidas M., Hunger S.P., et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dworzak M.N., Fröschl G., Printz D., et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99:1952–1958. doi: 10.1182/blood.v99.6.1952. [DOI] [PubMed] [Google Scholar]
- 19.Wood B., Wu D., Crossley B., et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood. 2018;131:1350–1359. doi: 10.1182/blood-2017-09-806521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulsipher M.A., Han X., Maude S.L., et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov. 2022;3:66–81. doi: 10.1158/2643-3230.BCD-21-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazzaniga G., Gaipa G., Rossi V., Biondi A. Minimal residual disease as a surrogate marker for risk assignment to ALL patients. Rev Clin Exp Hematol. 2003;7:292–323. [PubMed] [Google Scholar]
- 22.Panzer-Grümayer E.R., Schneider M., Panzer S., Fasching K., Gadner H. Rapid molecular response during early induction chemotherapy predicts a good outcome in childhood acute lymphoblastic leukemia. Blood. 2000;95:790–794. [PubMed] [Google Scholar]
- 23.Schultz L.M., Baggott C., Prabhu S., et al. Disease burden affects outcomes in pediatric and young adult b-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2021;40(9):945–955. doi: 10.1200/JCO.20.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee D.W., Santomasso B.D., Locke F.L., et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neelapu S.S., Tummala S., Kebriaei P., et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn J.M., Cole P.D., Blonquist T.M., et al. An investigation of toxicities and survival in Hispanic children and adolescents with ALL: results from the Dana-Farber Cancer Institute ALL Consortium protocol 05-001. Pediatr Blood Cancer. 2018;65 doi: 10.1002/pbc.26871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aristizabal P., Winestone L.E., Umaretiya P., Bona K. Disparities in pediatric oncology: the 21st century opportunity to improve outcomes for children and adolescents with cancer. Am Soc Clin Oncol Educ Book. 2021;41:e315–e326. doi: 10.1200/EDBK_320499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine J.E., Grupp S.A., Pulsipher M.A., et al. Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic leukemia. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunger S.P., Mullighan C.G. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 30.Heikamp E.B., Pui C.H. Next-generation evaluation and treatment of pediatric acute lymphoblastic leukemia. J Pediatr. 2018;203:14–24.e12. doi: 10.1016/j.jpeds.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Mullighan C.G., Harvey R.C., et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2011;118:3080–3087. doi: 10.1182/blood-2011-03-341412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irving J.A., Enshaei A., Parker C.A., et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128:911–922. doi: 10.1182/blood-2016-03-704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paganin M., Zecca M., Fabbri G., et al. Minimal residual disease is an important predictive factor of outcome in children with relapsed 'high-risk' acute lymphoblastic leukemia. Leukemia. 2008;22:2193–2200. doi: 10.1038/leu.2008.227. [DOI] [PubMed] [Google Scholar]
- 34.Berry D.A., Zhou S., Higley H., et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short N.J., Kantarjian H., Ravandi F., et al. High-sensitivity next-generation sequencing MRD assessment in ALL identifies patients at very low risk of relapse. Blood Adv. 2022;6:4006–4014. doi: 10.1182/bloodadvances.2022007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curran K.J., Margossian S.P., Kernan N.A., et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–2368. doi: 10.1182/blood.2019001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay K.A., Gauthier J., Hirayama A.V., et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133:1652–1663. doi: 10.1182/blood-2018-11-883710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers R.M., Taraseviciute A., Steinberg S.M., et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol. 2022;40:932–944. doi: 10.1200/JCO.21.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullighan C.G. How advanced are we in targeting novel subtypes of ALL? Best Pract Res Clin Haematol. 2019;32 doi: 10.1016/j.beha.2019.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pehlivan K.C., Duncan B.B., Lee D.W. CAR-T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13:396–406. doi: 10.1007/s11899-018-0470-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.