Abstract
A dibenzothiophene (DBT)-desulfurizing bacterial strain was isolated and identified as Gordona strain CYKS1. Strain CYKS1 was found to transform DBT to 2-hydroxybiphenyl via the 4S pathway and to be able to also use organic sulfur compounds other than DBT as a sole sulfur source. Its desulfurization activity was susceptible to sulfate repression. Active resting cells for desulfurization could be prepared only in the early growth phase. When two types of diesel oils, middle distillate unit feed (MDUF) and light gas oil (LGO) containing various organic sulfur compounds including DBT, were treated with resting cells of strain CYKS1 for 12 h, the total sulfur content significantly decreased, from 0.15% (wt/wt) to 0.06% (wt/wt) for MDUF and from 0.3% (wt/wt) to 0.25% (wt/wt) for LGO. The newly isolated strain CYKS1 is considered to have good potential for application in the biodesulfurization of fossil fuels.
All fossil fuels contain organic sulfur compounds. When fossil fuels are combusted, sulfur dioxide generated from organic sulfur compounds is released into the atmosphere, causing air pollution. To remove sulfur from fossil fuels, refiners now rely on a hydrodesulfurization technique, which is costly and energy-intensive and not effective at removing polycyclic sulfur compounds (4). Therefore, microbial desulfurization has attracted attention for its application to the desulfurization of coal and petroleum.
A variety of sulfur-containing heterocyclic organic compounds in fossil fuels have been characterized. Since dibenzothiophene (DBT) is a typical recalcitrant organic sulfur compound in fossil fuels, desulfurization of DBT has been a model reaction in the treatment of fossil fuels. There have been several reports on the isolation of DBT-desulfurizing bacteria. Species of Brevibacterium and Pseudomonas utilize DBT as the sole source of carbon, sulfur, and energy (14, 23). Under anaerobic conditions, biphenyl was formed from DBT by Desulfovibrio desulfuricans M6 (26). Microbial systems have also been reported to selectively abstract the sulfur heteroatom from this model heterocycle. Rhodococcus rhodochrous IGTS8, Rhodococcus erythropolis D-1, and Corynebacterium sp. strain SY1 were observed to remove only sulfur from DBT, converting DBT to 2-hydroxybiphenyl (2-HBP) (6, 8, 17). The selective removal of organic sulfur by these strains looks promising for the treatment of fuel oils using an aqueous cell suspension, since valuable combustible compounds are retained in this reaction without being broken down into water-soluble compounds. However, there are only a few reports on the treatment of diesel oils with these isolated strains.
In this article, we describe the characteristics of DBT desulfurization by a newly isolated bacterial strain, CYKS1, and demonstrate the possibility of its application for the biocatalytic desulfurization of diesel oils.
Enrichment and isolation of DBT-desulfurizing microorganisms.
Deionized water (resistance = 20 MΩ · cm) was used to prepare all sulfur-free minimal salt medium (MSM) and stock solutions. The MSM contained, per liter of deionized water, 5.0 g of glucose, 5.0 g of K2HPO4, 1.0 g of NaH2PO4, 1.0 g of NH4Cl, 0.2 g of MgCl2, 0.01 g of CaCl2 · 2H2O, 1 ml of sulfur-free trace element solution dissolved with EDTA (21), and 1 ml of vitamin solution (25). DBT was dissolved in ethanol to a concentration of 100 mM and added to a sterilized basal MSM. Enrichment cultures were initiated by inoculating MSM containing 1 mM DBT with 10% (vol/vol) oil-contaminated wastewater discharged from the dye industry complex in Taegu, Korea. The cultures were performed in 250-ml Erlenmeyer flasks with 50 ml of MSM at 30°C on a gyratory shaker. All the data shown in this work are the mean values of data from duplicate or triplicate experiments. Determination of DBT and its metabolites was carried out by the method described previously (8). The culture broth or reaction mixture was acidified to pH 2.0 with 1 N HCl and extracted with 1 volume of ethyl acetate. A portion of the ethyl acetate layer was removed, and 10 μl of the supernatant was injected into a reverse-phase high-performance liquid chromatograph (Hitachi model L-6200, L-4200H UV-VIS detector) equipped with a μBondapak phenyl column (3.9 by 300 mm) (Waters, Milford, Mass.) and detected at 280 nm. The mobile phase was 65% (vol/vol) methanol in water. In all experiments involving extraction with ethyl acetate, carbazole was used as the internal standard. The range of concentrations effective for the quantification of DBT-related compounds was 0.02 to 12 mM.
Evidence of sulfur-specific desulfurization was obtained by the high-performance liquid chromatography analysis of samples taken from the broth of stationary-phase enrichment cultures. Only trace concentrations (about 0.05 mM) of dibenzothiophene sulfone (DBTO2) and 2-HBP were detected as transformation products of DBT in primary enrichment culture. Sulfur-containing contaminants in the bacterial sources might prevent the enrichment of DBT-desulfurizing bacteria. After several transfers of enrichment cultures, the concentration of 2-HBP produced increased to about 0.3 mM in the stationary phase of cultures. It seemed that 2-HBP was the dead-end product of DBT desulfurization, since the concentration of 2-HBP did not significantly decrease in the stationary phase.
Transfer cultures were plated on minimal solid medium spread with DBT. For preparation of the medium, 200 μl of DBT stock solution (100 mM) was spread onto a solid medium containing a sulfur-free MSM with agar. After 2 weeks of incubation, individual colonies showing clear zones were selected and tested in a DBT-containing MSM for the ability to transform DBT to 2-HBP. Although clear zones might be indirect evidence of DBT desulfurization, we could easily screen DBT-desulfurizing bacteria by this method.
Identification of the isolated strain, CYKS1.
Isolated strains were identified on the basis of their morphological, physiological, and chemotaxonomical properties. A mucoid isolate, CYKS1, from the colonies was an aerobic, nonmotile, nonspore-forming, and gram-positive bacterium. CYKS1 bacteria were rod shaped, as observed by a light microscope. However, when CYKS1 cells were stained, four to five coccoid cells surrounded by a slimy material were found. The isolate was catalase positive and oxidase positive. Preparation of peptidoglycan and analysis of diaminopimelic acid isomer, isoprenoid menaquinone, and cell wall sugar were carried out by previously described methods (10). Strain CYKS1 had mycolic acid and the meso form of diaminopimelic acid in the cell wall. The major cell wall sugars were arabinose and galactose. This strain had MK-9(H2) as the sole menaquinone. By comparing these results with the reported chemotaxonomic characteristics of coryneform bacteria (1, 22), we identified this strain as Gordona strain CYKS1.
Characterization of DBT desulfurization by strain CYKS1.
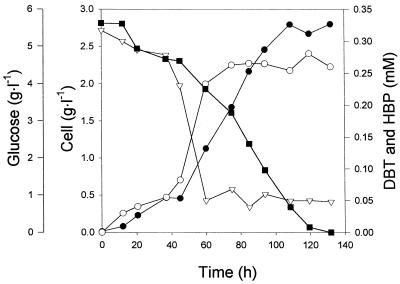
Figure 1 shows the time course of DBT utilization by strain CYKS1 in an MSM supplemented with 0.3 mM DBT as a sole sulfur source. Cell growth was monitored by measuring the absorbance of culture broth samples at 600 nm (A600). Dry cell weight (DCW) was determined by the method described previously (12). DBT and its metabolites were determined by use of a gas chromatograph (GC) equipped with a flame ionization detector (model 5890; Hewlett-Packard, Wilmington, Del.) and by mass spectrometry (MS; Fisons, Altrincham, England) as described by Denome et al. (3). An ethyl acetate-extracted sample was dried over anhydrous sodium sulfate and concentrated in a rotary evaporator, and the sample was then analyzed by GC-MS. The GC analysis was conducted with an HP5 column (30 m by 0.32 mm [inside diameter]; 1.0-μm phase thickness). Because sulfone was thermally unstable, the injector temperature maintained was rather low, at 250°C.
FIG. 1.
Biodesulfurization of DBT by strain CYKS1 in a batch culture. Symbols: •, cell growth; ▪, glucose concentration; ▿, DBT concentration; ○, 2-HBP concentration.
The strain showed maximum growth (2.7 g [DCW]/liter) at 100 h of cultivation. The pH decreased from 7.4 to 5.9. Desulfurization activity and cell growth decreased about 23% according to this change in pH. The major product accumulated was identified as 2-HBP by GC-MS. Approximately 95% of the DBT was converted to 2-HBP, and the 2-HBP concentration did not decrease during stationary phase. 2-HBP could accumulate up to a concentration of 0.25 mM in the culture medium and was not used as a carbon source for cell growth. When the effect of 2-HBP on the growth of strain CYKS1 was studied, the generation time of strain CYKS1 was found to increase by about 50% in the presence of 0.2 mM 2-HBP. The strain could grow even at 0.30 mM 2-HBP but only with a very long generation time. Considering the report that the inhibitory effect of 2-HBP was the main limiting factor of the desulfurization of DBT (17), the resistance of strain CYKS1 to inhibition by 2-HBP was similar to that of any other reported strain (9, 17, 19). No other metabolite except 2-HBP was detected during the batch cultivation.
Although no desulfurization of DBT occurred after 70 h, cell growth was sustained to 100 h as shown in Fig. 1. We tried to determine the concentrations of sulfate and sulfite by using a 2000i/sp ion chromatograph (Dionex, Osaka, Japan), an IonPac AS4A anion-exchange column with a cation suppressor, and a conductivity detector as described by Omori et al. (17). The minimum detection limit for this method was about 0.01 mM. However, no detectable level of sulfate or sulfite accumulation in the broth was observed during the cultivation of strain CYKS1. The amount of sulfur assimilated per gram of DCW was about 0.11 mmol. If we consider that the sulfur content of Escherichia coli, for example, is about 0.14 mmol/g of DCW (2), it seems that all of the sulfur released from DBT was assimilated by the cells. After about 70 h, cells might be sufficiently supplied with sulfur and sulfur would no longer be a growth-limiting factor, making further DBT desulfurization unnecessary after this period of cultivation.
When sulfate was added to the MSM, DBT was not desulfurized, indicating that sulfate completely repressed the expression of DBT-desulfurizing activities as previously reported by Omori et al. (18). Therefore, for the efficient desulfurization of fossil fuels, it would be advantageous to develop new strains that are not susceptible to sulfate repression (20).
Metabolic pathway and characteristics of DBT desulfurization.
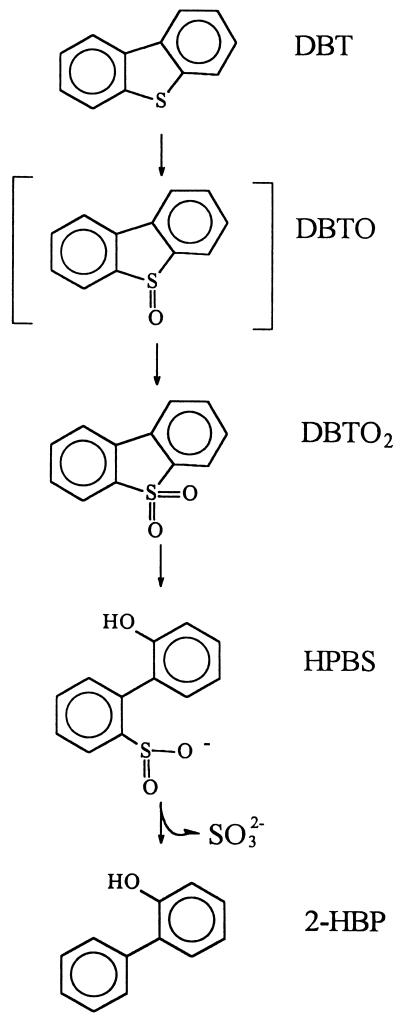
When cells were induced with DBT, DBTO2 could be desulfurized to 2-HBP with no lag time. This result implied that DBTO2 could be an intermediate of DBT desulfurization. The specific rates of desulfurization of DBT and DBTO2 by the resting cells were 8.9 and 17.9 μmol of S · g (DCW)−1 · h−1, respectively. Because DBTO2-desulfurizing activity was about two times higher than DBT-desulfurizing activity, DBTO2 could not be accumulated during the DBT desulfurization. When resting cells were prepared in a minimal medium with a modified trace element solution (13), a hydrophilic intermediate was accumulated during the DBT or DBTO2 desulfurization. For the characterization of the sulfur-containing metabolite, a GC-sulfurchemiluminescence detector (SCD) (model 350; Sievers Instruments, Boulder, Colo.) was used. GC-SCD data indicated that the hydrophobic intermediate was a sulfur-containing compound. Since its mass ions (at m/z 216) corresponded to those of the dibenz[c,e][1,2]oxanthiin 6-oxide that was formed from the esterification of 2-(2-hydroxyphenyl) benzenesulfinate (HPBS) at acidic pH during its analysis as described by Gallagher et al. (6), our data were consistent with it being HPBS. HPBS is known as an intermediate detected during the desulfurization of DBT by the Rhodococcus sp. strain IGTS8 (6, 7). These results suggest that DBT was oxidized to DBTO2 and then cleaved hydrolytically to form HPBS by strain CYKS1. Therefore, the newly isolated strain, Gordona strain CYKS1, was considered to desulfurize DBT to 2-HBP via the 4S pathway (7, 17) as shown in Fig. 2. Considering that 2-HBP itself has valuable combustible compounds, strain CYKS1 could be a much more promising biocatalyst for the treatment of fuel oils than other strains that use DBT as a carbon and energy source and thus cause a fuel value loss (14, 23).
FIG. 2.
Proposed metabolic pathway for the degradation of DBT by strain CYKS1. DBTO, DBT sulfoxide.
As previously suggested by the batch culture results, desulfurizing activity was sensitive to the growth phase. To determine the desulfurization activity of cells in different growth phases, we cultivated strain CYKS1 under DBT-limited conditions of 0.1 mM DBT. Cell growth was sustained for 10 h after the complete depletion of DBT. However, during this late growth phase without DBT, the cell showed significantly decreased specific desulfurization activity (about 0.3 μmol of S · g [DCW]−1 · h−1) relative to that of early growth phase. As described above, this result indicated that the sulfur accumulated in the cells during DBT desulfurization might be used for late growth. Furthermore, we could not detect DBT desulfurization activity after the early growth phase even in the presence of sufficient DBT, as shown in the batch culture. From these results, we concluded that resting cells of the wild-type strain of CYKS1 must be prepared at the early growth phase when they are used for the desulfurization of fossil fuels.
Desulfurization of diesel oils.
There are some reports concerning the microbial desulfurization of DBT as described above. However, the treatment of fuel oils by desulfurizing microbial strains is in its infant stage, with only a few articles published in this area (11, 16, 24). Petroleum-based fuel oils including diesel oils contain an enormous number of organic sulfur compounds, including DBT. We investigated the capability of strain CYKS1 to (i) use organic sulfur compounds other than DBT as a sole sulfur source, (ii) desulfurize DBT in the organic rather than the aqueous phase, and (iii) be used in treatment of two types of diesel fuels, middle distillate unit feed (MDUF) and light gas oil (LGO).
We observed growth of strain CYKS1 on the organic sulfur compounds listed in Table 1. The results showed that strain CYKS1 could utilize various organic sulfur compounds which are believed to exist in fuel oils, although the list in Table 1 did not represent the full spectrum of sulfur compounds in fuel oils. Without glucose in the medium, strain CYKS1 was unable to grow on any of the compounds listed in Table 1 as a sole carbon source.
TABLE 1.
Growth of strain CYKS1 on various organic sulfur compoundsa
| Organic sulfur compound | Growthb |
|---|---|
| Methyl sulfide | ++ |
| Benzyl sulfide | ++ |
| Benzyl sulfone | ++ |
| 4,4′-Thiophenol | − |
| Phenyl sulfide | + |
| Phenyl sulfone | − |
| Thiophene | ++ |
| 2-Methyl thiophene | ++ |
| 3-Methyl thiophene | + |
| Thianaphthene | + |
| DBT | ++ |
| DBT-O2 | ++ |
| Methyl disulfide | − |
| Benzyl disulfide | ++ |
| p-Tolyl disulfide | − |
| Thiazole | ++ |
| 4,5-Dimethylthiazole | ++ |
| 2-Methyl-β-naphthiothiazole | − |
| Thianthrene | − |
| Trithiane | ++ |
Strain CYKS1 was grown aerobically in 50-ml test tubes containing 5 ml of MSM with various organic sulfur compounds (0.3 mM) as the sole sulfur source. Butyl rubber stoppers were used. Cell growth was determined by measuring optical density of culture broth samples at 600 nm. Sulfur-free MSM was used as a negative control.
++, good growth (more than 2 U of A600) within approximately 3 days or less; +, growth time longer than 3 days; −, no growth.
To study the capability of strain CYKS1 to desulfurize DBT in the organic phase, we employed a two-phase system: cells in DBT-free MSM and DBT in hexadecane, one of the major hydrocarbons in diesel oils. Two milliliters of hexadecane containing 5 mM DBT was mixed with 18 ml of inoculated MSM in 100-ml Erlenmeyer flasks and incubated in a gyratory shaker at 30°C. Strain CYKS1 grew well in this two-phase medium. The DBT concentration in hexadecane decreased from 5.0 to approximately 2.2 mM in 48 h, and a stoichiometric amount of 2-HBP was detected in the oil phase.
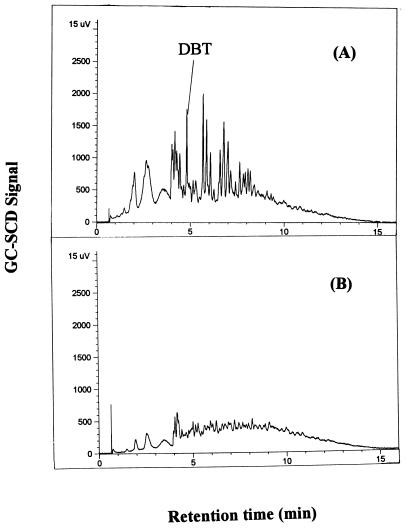
In the treatment of diesel oils, 1 ml of diesel oil was mixed with 9 ml of a resting cell suspension in 25 mM phosphate buffer and added to 100-ml Erlenmeyer flasks. The cultures were incubated in a shaker at 170 rpm and 30°C for 12 h. After sampling, the separation of the oil and cell suspension was done by centrifugation (15,000 × g, 20 min). To determine the total sulfur content of the oil, the oil phase was injected directly into a GC-SCD (without extraction) as described previously (5, 11). The lower limit of detection of total sulfur by this method was about 50 ppm. These data were confirmed by using a 7000S total sulfur analyzer (Antek Instruments, Inc.) as described previously (11). Figure 3A shows GC-SCD peaks for all of the sulfur compounds in the MDUF (with about 0.15% [wt/wt] sulfur initially) before treatment. After treatment of the MDUF, all of the peaks, including the one for DBT, significantly decreased in height as shown in Fig. 3B. After treatment, only a small amount of sulfate, equivalent to less than 30% of the sulfur removed from the oil, was detected in the reaction mixtures. It is important to note that the sulfur compounds with retention times longer than about 5 min nearly disappeared. Such characteristics of desulfurization by strain CYKS1 are opposite or complementary to those of hydrodesulfurization, in which sulfur compounds with a shorter residence time are more easily desulfurized (4). Based on these results, DBT-induced resting cells of strain CYKS1 are considered to have a sufficiently broad substrate specificity to desulfurize major organic sulfur compounds contained in diesel oils. Bacterial strains specifically cleaving the carbon-sulfur bonds like strain CYKS1 are more effective in the treatment of fuel oils than microorganisms using a carbon-destructive pathway such as Brevibacterium (23) or Pseudomonas (14), which might have a limited range of substrates to be desulfurized.
FIG. 3.
Desulfurization of MDUF by strain CYKS1. GC-SCD chromatograms for MDUF before (A) and after (B) treatment are shown.
Compared with those of DBT in the aqueous phase, the desulfurization rates of diesel oils were observed to be much lower. The specific desulfurization rates of MDUF and LGO were 5.3 and 4.7 μmol of S · g (DCW)−1 · h−1, respectively. The total sulfur content of MDUF decreased by about 70% (wt/wt) after treatment. For LGO, which had an initial sulfur content of approximately 0.3% (wt/wt), the total sulfur content decreased by about 50%. Organic sulfur compounds detected as the baseline by the GC-SCD could not be desulfurized by treatment with strain CYKS1.
As a conclusion, strain CYKS1 has a good potential for use in the biocatalytic desulfurization of fuel oils.
Acknowledgments
This work was supported by a grant from R & D Management Center for Energy and Resources, as a part of the Clean Energy Program.
REFERENCES
- 1.Bendinger B, Kroppenstedt R M, Klatte S, Altendorf K. Chemotaxonomic differentiation of coryneform bacteria isolated from biofilters. Int J Syst Bacteriol. 1992;42:474–486. doi: 10.1099/00207713-42-3-474. [DOI] [PubMed] [Google Scholar]
- 2.Chang C-F, Shuman H, Somlyo A P. Electron probe, X-ray mapping, and electron energy-loss spectroscopy of calcium, magnesium, and monovalent ions in log-phase and in dividing Escherichia coli B cells. J Bacteriol. 1986;167:935–939. doi: 10.1128/jb.167.3.935-939.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denome S A, Olson E S, Young K D. Identification and cloning of genes involved in specific desulfurization of dibenzothiophene by Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1993;59:2837–2843. doi: 10.1128/aem.59.9.2837-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dzidic I, Balicki M D, Rhodes I A L, Hart H V. Identification and quantification of nitrogen and sulfur compounds in catalytically cracked heavy oils by isobutane/CI GC/MS and GC using selective detectors. J Chromatogr Sci. 1988;26:236–240. [Google Scholar]
- 5.Finnerty W R. Organic sulfur biodesulfurization in non-aqueous media. Fuel. 1993;72:1631–1634. [Google Scholar]
- 6.Gallagher J R, Olson E S, Stanley D C. Microbial desulfurization of dibenzothiophene: a sulfur-specific pathway. FEMS Microbiol Lett. 1993;107:31–36. doi: 10.1016/0378-1097(93)90349-7. [DOI] [PubMed] [Google Scholar]
- 7.Gray K A, Pogrebinsky O S, Mrachko G T, Xi L, Monticello D J, Squires C H. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat Biotechnol. 1996;14:1705–1709. doi: 10.1038/nbt1296-1705. [DOI] [PubMed] [Google Scholar]
- 8.Izumi Y, Ohshiro T, Ogino H, Hine Y, Shimao M. Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Appl Environ Microbiol. 1994;60:223–226. doi: 10.1128/aem.60.1.223-226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayser K J, Bielaga-Jones B A, Jackowski K, Odusan O, Kilbane J J., II Utilization of organosulfur compounds by axenic and mixed cultures of Rhodococcus rhodochrous IGTS8. J Gen Microbiol. 1993;139:3123–3129. [Google Scholar]
- 10.Komagata K, Suzuki K I. Lipids and cell-wall analysis in bacterial systematics. In: Colwell R R, Grigorova G, editors. Methods in microbiology. Orlando, Fla: Academic Press; 1987. pp. 161–207. [Google Scholar]
- 11.Konishi J, Ishii Y, Onaka T, Okumura K, Suzuki M. Thermophilic carbon-sulfur-bond-targeted biodesulfurization. Appl Environ Microbiol. 1997;63:3164–3169. doi: 10.1128/aem.63.8.3164-3169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H-W, Pan J-G, Lebeault J M. Enhanced l-lysine production in threonine-limited continuous culture of Corynebacterium glutamicum by using gluconate as a secondary carbon source with glucose. Appl Microbiol Biotechnol. 1998;49:9–15. [Google Scholar]
- 13.Lee S T, Lee S B, Park Y H. Characterization of a pyridine-degrading branched gram-positive bacterium isolated from the anoxic zone of an oil shale column. Appl Microbiol Biotechnol. 1991;35:824–829. [Google Scholar]
- 14.Monticello D J, Bakker D T, Finnerty W R. Plasmid-mediated degradation of dibenzothiophene by Pseudomonas species. Appl Environ Microbiol. 1985;49:756–760. doi: 10.1128/aem.49.4.756-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monticello D J, Finnerty W R. Microbial desulfurization of fossil fuels. Annu Rev Microbiol. 1985;39:371–389. doi: 10.1146/annurev.mi.39.100185.002103. [DOI] [PubMed] [Google Scholar]
- 16.Monticello D J. Biological desulfurization (BDS) of middle distillates. Presented at the Annual Meeting of the Natonal Petroleum Refiners Association, San Antonia, Tex., 21 to 23 March 1993. 1993. [Google Scholar]
- 17.Omori T, Monna L, Saiki Y, Kodama T. Desulfurization of dibenzothiophene by Corynebacterium sp. strain SY1. Appl Environ Microbiol. 1992;58:911–915. doi: 10.1128/aem.58.3.911-915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omori T, Saiki Y, Kasuga K, Kodama T. Desulfurization of alkyl and aromatic sulfides and sulfonates by dibenzothiophene desulfurizing Rhodococcus sp. strain SY1. Biosci Biotechnol Biochem. 1995;59:1195–1198. [Google Scholar]
- 19.Ohshiro T, Suzuki K, Izumi Y. Regulation of dibenzothiophene degrading enzyme activity of Rhodococcus erythropolis D-1. J Ferment Bioeng. 1996;81:121–124. [Google Scholar]
- 20.Piddington C S, Kovacevich B R, Rambosek T. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1995;61:468–475. doi: 10.1128/aem.61.2.468-475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee S K, Lee G M, Yoon J H, Park Y H, Bae H S, Lee S T. Anaerobic and aerobic degradation of pyridine by a newly isolated denitrifying bacterium. Appl Environ Microbiol. 1997;63:2578–2585. doi: 10.1128/aem.63.7.2578-2585.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stackebrandt E, Smida J, Collins M D. Evidence of phylogenetic heterogeneity within the genus Rhodococcus: revival of the genus Gordona (Tsukamura) J Gen Appl Microbiol. 1988;34:341–348. [Google Scholar]
- 23.van Afferden M, Schacht S, Klein J, Trüper H G. Degradation of dibenzothiophene by Brevibacterium sp. DO. Arch Microbiol. 1990;153:324–328. [Google Scholar]
- 24.van Afferden M, Tappe D, Beyer M, Trüper H G, Klein J. Biochemical mechanisms for the desulfurization of coal-relevant organic sulfur compounds. Fuel. 1993;72:1635–1643. [Google Scholar]
- 25.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harber W, Schleifer K H, editors. The procaryotes. Berlin, Germany: Springer-Verlag; 1992. pp. 3352–3378. [Google Scholar]
- 26.Yeong K H, Kim T S, Kim B H. Degradation of organic sulfur compounds and the reduction of dibenzothiophene to biphenyl and hydrogen sulfide. Biotechnol Lett. 1990;12:761–764. [Google Scholar]