Abstract
BACKGROUND:
Emergency patients with sepsis or septic shock are at high risk of death. Despite increasing attention to microhemodynamics, the clinical use of advanced microcirculatory assessment is limited due to its shortcomings. Since blood gas analysis is a widely used technique reflecting global oxygen supply and consumption, it may serve as a surrogate for microcirculation monitoring in septic treatment.
METHODS:
We performed a search using PubMed, Web of Science, and Google scholar. The studies and reviews that were most relevant to septic microcirculatory dysfunctions and blood gas parameters were identified and included.
RESULTS:
Based on the pathophysiology of oxygen metabolism, the included articles provided a general overview of employing blood gas analysis and its derived set of indicators for microhemodynamic monitoring in septic care. Notwithstanding flaws, several parameters are linked to changes in the microcirculation. A comprehensive interpretation of blood gas parameters can be used in order to achieve hemodynamic optimization in septic patients.
CONCLUSION:
Blood gas analysis in combination with clinical performance is a reliable alternative for microcirculatory assessments. A deep understanding of oxygen metabolism in septic settings may help emergency physicians to better use blood gas analysis in the evaluation and treatment of sepsis and septic shock.
Keywords: Sepsis, Microcirculation, Blood gas analysis, Emergency service
INTRODUCTION
Sepsis is defined as life-threatening tissue hypoperfusion and organ dysfunction caused by a dysregulated host response to infection.[1] Sepsis and septic shock are major health problems in the emergency department (ED), and early recognition and interventions are essential for improving patient outcomes. Hemodynamics, comprising macrocirculation and microcirculation, is the foundation of septic resuscitation. Although ED physicians routinely depend on macrocirculation, microcirculation has recently been proposed to play a key role in the pathogenic mechanisms of sepsis-induced organ dysfunction.[2,3] Previous studies suggested that the occurrence of microcirculatory abnormalities in early sepsis, despite well-preserved macrocirculation, might result in multi-organ failure and poor outcomes.[2,3] Microcirculation perfusion can be measured by parameters including perfused vessel density (PVD), the proportion of perfused vessels (PPV), microcirculatory flow index (MFI), and heterogeneity index. PVD, PPV, and MFI comprehensively describe the functional perfusion of microvessels, while the heterogeneity index quantifies the heterogeneity of perfusion.[4] However, the availability of these advanced microcirculatory measures in clinical settings is limited because the techniques are difficult to use and lack uniformly defined endpoints.[5] Viable practical clinical surrogates are therefore needed, particularly for rapid assessment and decision-making in the ED. Blood gas analysis and its derived indicators, linked to systemic oxygen (O2) metabolism, may reflect the relationship between tissue O2 delivery (DO2) and consumption (VO2), and may thus provide alternative tools for microcirculatory assessment and for hemodynamics optimization during septic resuscitation.
Based on the pathophysiological mechanisms and the current findings, this review provides an overview of the use of blood gas analysis to assess microcirculatory status, detect early tissue hypoxia, and optimize hemodynamic treatment in sepsis.
METHODS
We performed a search using PubMed, Web of Science, and Google scholar. The studies and reviews that were most relevant to septic microcirculatory dysfunctions and blood gas parameters were identified and included. Based on the pathophysiology of oxygen metabolism, the included articles provided a general overview of employing blood gas analysis and its derived set of indicators for microhemodynamic monitoring in septic care.
RESULTS
Circulatory pathophysiology of sepsis
The principal role of the circulation is to deliver O2 and nutrients to peripheral tissues and remove metabolites. Circulatory dysfunction is common in sepsis, and its current management mainly aims to improve macrohemodynamic indicators, such as the mean arterial pressure, central venous pressure, and cardiac index.[1,6] However, abnormal microcirculation might persist despite improved or restored macrohemodynamic indicators,[7-9] highlighting a loss of coherence between the macrocirculation and microcirculation.[10] The microcirculation comprises a network of O2 exchange between peripheral tissues and blood circulation, and it is the main site for tissue oxygenation. When sepsis occurs, the microcirculatory network is disrupted by factors such as endothelial glycocalyx dysfunction, activation of microthrombosis and leukocytes, increased microvascular rigidity, and pathologic formation of arteriovenous shunts,[11,12] leading to impaired blood flow and deteriorated heterogeneity of tissue perfusion (Figure 1). Consistently sluggish or absent blood flow will compromise DO2 in related tissues or disrupt the balance between DO2 and VO2.
Figure 1.
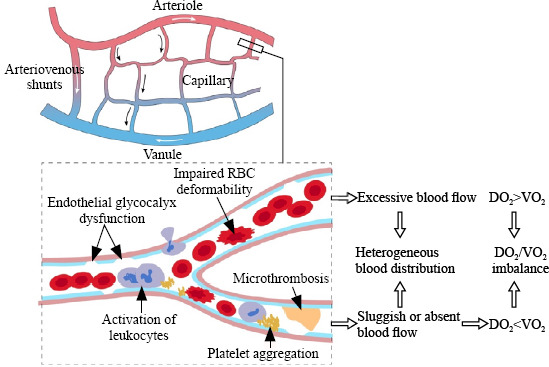
Microcirculatory alterations in sepsis. RBC: red blood cell; DO2: O2 delivery; VO2: O2 consumption.
The DO2/VO2 balance is the cornerstone of hemodynamic stability. DO2 represents the arterial supply of O2 to tissues (DO2=Q × CaO2, where Q is blood flow and CaO2 is arterial O2 content), while VO2 represents O2 removed from arterial blood for use by the tissues (VO2=Q × [CaO2−CvO2], where CvO2 is venous O2 content [Supplementary Figure S1]). Both DO2 and VO2 are affected by multiple factors, resulting in a dynamic balance (Figure 2 and Supplementary Table S1). Under normal circumstances, aerobic cell metabolism only requires a small fraction of DO2 (Figure 2A). Under conditions of an inadequate DO2/VO2 (decreased DO2, and/or increased VO2), VO2 initially remains satisfied as O2 extraction increases adaptively, known as DO2/VO2 independency. At this stage, aerobic metabolism is preserved, but CvO2 decreases (Figure 2B). However, if the inadequacy persists, the compensatory O2 extraction process approaches its maximum capacity, and DO2 fails to meet the demand of tissue oxygenation, leading to DO2/VO2 dependency and O2 debt.[13] At this point, aerobic metabolism switches to anaerobic metabolism (Figure 2C), resulting in further reduction of CvO2, elevated lactate levels, and abnormal carbon dioxide (CO2)-related parameters (Figure 2D).
Figure 2.
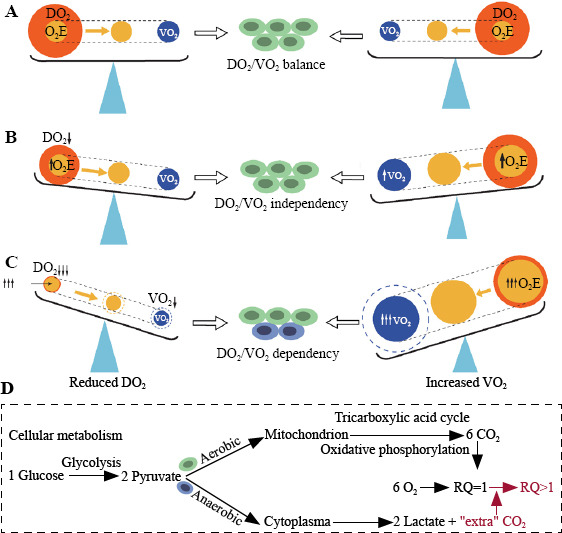
The pathophysiological determinants of the DO2/VO2. A: DO2/VO2 balance; B: DO2/VO2 independency; C: DO2/VO2 dependency; D: cellular metabolism. DO2: O2 delivery; VO2: O2 consumption; O2E: O2 extraction; RQ: respiratory quotient.
As the post-metabolism part of the circulation, venous blood includes the O2 left after cellular metabolism, thus providing valuable information on tissue oxygenation. “Venous blood” in this review refers exclusively to mixed or central venous blood. Mixed venous blood is the blood that travels through all the systemic capillary beds and returns to the right ventricle and is acknowledged to reflect global O2 metabolism by combining venous blood from the superior vena cava (SVC) and inferior vena cava. However, measurement of mixed venous parameters requires a pulmonary artery catheter, which is invasive and not feasible in all patients.[14] Central venous blood includes blood that returns via the SVC and thus only represents the O2 metabolism of the upper body; [15] however, it is easier to obtain via a central venous catheter (CVC) and may thus be used as a substitute for mixed venous blood. Notably, despite controversy regarding the ability to substitute mixed and central venous blood (e.g., venous O2 saturation [SvO2]);[16-19] when the CVC tip is close to the right atrium, central SvO2 (ScvO2) was an excellent estimate of mixed SvO2 (SmvO2), with a difference between the two parameters of about 1%.[19] Central venous blood is thus an acceptable substitute for mixed venous blood when the placement of the CVC is appropriate.
Parameters of DO2/VO2 independency
Despite a shortage of DO2 relative to VO2 during DO2/VO2 independency, tissue oxygenation is nevertheless satisfactory due to improved O2 extraction. Early signs of hypoxia include a decrease in venous blood O2 content and an increase in O2 extraction capability.
Venous O2 saturation (SvO2)
SvO2 reflects the O2 content in venous blood after cellular metabolism. SvO2 varies among organs, from up to 90% in the kidney to 40% in the myocardium, depending on the O2 demands of the tissues.[15] Under normal conditions, SmvO2 is about 75%, while ScvO2 is usually 2%–3% lower, because the lower body consumes less O2 than the upper body.[15] In contrast, ScvO2 will exceed SmvO2 during shock as blood is redistributed from the hepatosplanchnic region to support coronary or cerebral circulation.[20] Despite the lack of consensus regarding the best cutoff value for SvO2 in resuscitation, SmvO2 ≥65% or ScvO2 ≥70% is still advocated in clinical practice.[21,22]
Several studies have investigated the relationship between SvO2 and microcirculatory perfusion. In septic patients, an increase in SmvO2 following norepinephrine treatment was paralleled by improvements in PVD and MFI.[23] A recent meta-analysis showed that initiation of blood cell transfusion by lower SmvO2 rather than lower hemoglobin level maximized the benefit to microcirculatory blood flow in septic patients,[24] suggesting that SmvO2 may be a reliable index of microcirculatory perfusion. However, SvO2 reflects cellular oxygenation indirectly, by measuring the remaining O2 content in venous blood. It thus provides little information on the complex O2 metabolism within peripheral tissues. Several studies showed that higher ScvO2 was also linked to increased mortality.[25,26] High SvΟ2 is probably caused by impaired O2 utilization or pathological shunting in sepsis.[25,27,28] Aggressive resuscitation with fluids, vasopressors, and oxygen therapies may also result in a prompt rise in DO2 and a high SvΟ2.[29,30] Hence, SvO2 should be interpreted with caution, especially for high values.
O2 extraction rate (O2ER)
An increased O2ER, calculated as [CaO2−CvO2]/CaO2), is typically a marker of reduced DO2 during early-stage shock, with a normal value of 25%–30%.[31] O2ER increases in hypoxic settings to match the metabolic demand, with an upper limit of 40%–50%.[32]
O2ER is almost equal to 1−SvO2 when arterial O2 saturation (SaO2) is 100%. However, because of the non-negligible effect of SaO2, SvO2 is not considered a good estimator of O2ER in hypoxic individuals. Measuring O2ER thus compensates for SvO2 deficits. Negative correlations between increased systemic O2ER and deteriorating microcirculatory parameters including PPV, PVD, and MFI were observed in an animal model of septic shock.[33] In contrast, another animal experiment produced the opposite conclusion, showing no correlation between systemic O2ER and these microcirculatory indicators, despite parallel variations in mesenteric O2ER and jejunal-villi PPV and mesenteric lactate. [33,34] This discrepancy was likely caused by differences in microcirculation perfusion between the two studies.[34] Further research is required to determine the reliability of using O2ER to identify microcirculatory abnormalities.
Parameters of DO2/VO2 dependency
Anaerobic metabolism manifests at the point of DO2/VO2 dependency, when DO2 is severely compromised but O2ER has reached its upper limit. Anaerobic metabolism products, including lactate and abnormal respiratory quotient (RQ), serve as indicators of persistent hypoxia.
Lactate
Lactate is the end-product of anaerobic metabolism. The generation and clearance of lactate are equal under normal circumstances, maintaining a serum lactate level of around 2 mmol/L.[35] Excess lactate generated by increased anaerobic glycolysis is the primary cause of hyperlactatemia in hypoxic environments, while sepsis-induced aerobic glycolysis is also a significant source of lactate, independent of tissue hypoxia.[36] The underlying mechanisms include epinephrine-dependent activation of Na+-K+-adenosine triphosphatase and inflammatory cytokine-dependent stimulation of cellular glucose uptake, both of which stimulate lactate production in aerobic glycolysis.[37,38] Hyperlactatemia in sepsis is therefore assumed to arise from various sources, not solely due to hypoxia/hypoperfusion.
Despite its complicated production processes, lactate is widely applied in clinical research and practice. Ospina-Tascon et al[39] discovered that decreased lactate following fluid delivery was consistent with improvements in PVD and PPV, with no corresponding changes in cardiac index or mean arterial pressure. A similar correlation between lactate and microcirculation perfusion was reported in septic patients without hypotension.[40] However, clinicians should remain aware that several distinct factors contribute to hyperlactatemia, and lactate only accurately reflects changes in the microcirculation for hyperlactatemia mainly caused by hypoperfusion. [41]
Respiratory quotient (RQ)
RQ is the volume of CO2 (VCO2) released divided by the volume of O2 (VO2) consumed during metabolism (normal range 0.7–1.0, depending on the types of substrates oxidized).[42] Under hypoxic conditions, RQ is >1.0 because of the anaerobic CO2 production. The underlying processes include increased buffering between bicarbonate and H+ as a result of lactate accumulation and accelerated high-energy phosphate hydrolysis, and extra CO2 produced by decarboxylation of intermediate substrates such as α-ketoglutarate during incomplete oxidation.[43]
RQ measurement currently requires indirect calorimetry, which is costly and vulnerable to patient and environmental conditions, and equipment, thus limiting its practical utility in critical patients.[44,45] According to the Fick equation, VCO2 equals the product of blood flow and the difference between venous and arterial CO2 content (Cv-aCO2). Likewise, VO2 is the product of blood flow and the difference between arterial and venous O2 content (Ca-vO2). RQ can thus be determined using the Cv-aCO2/Ca-vO2 ratio. Moreover, given the quasi-linear curve between CO2 content and CO2 tension (PCO2) within a physiological range (Supplementary Figure S2),[46] the easily accessible Pv-aCO2/Ca-vO2 ratio is considered a surrogate for the Cv-aCO2/Ca-vO2 ratio.
The Cv-aCO2/Ca-vO2 (or Pv-aCO2/Ca-vO2) ratio has attracted increasing attention recently. Several studies indicated that an elevated ratio (>1.4) is associated with inadequate lactate clearance, progressive organ dysfunction, and a higher risk of mortality.[47-50] However, the Cv-aCO2/Ca-vO2 (or Pv-aCO2/Ca-vO2) ratio was not in excellent agreement with changes at the micro level. An early trial found only a weak correlation between an increased Cv-aCO2/Ca-vO2 ratio and a decreased PPV,[51] while another study found no association between the Pv-aCO2/Ca-vO2 ratio and peripheral perfusion.[52] However, they pointed out that, even in patients with a higher peripheral perfusion index, those with poor lactate clearance after resuscitation had an obviously elevated Pv-aCO2/Ca-vO2 ratio.[52] These results suggested that anaerobic metabolism persisted even as perfusion improved, possibly related to impaired O2 utilization. Taken together, the Cv-aCO2/Ca-vO2 (or Pv-aCO2/Ca-vO2) ratio is an indicator of tissue O2 metabolism, instead of merely microcirculatory perfusion. An increased ratio may be useful for discriminating the inconsistency between tissue perfusion and O2 utilization.
Parameters of tissue hypoxia mechanisms
Hypoxia develops when DO2 is inadequate for VO2. DO2 characterizes the mechanisms of hypoxia as hypoxic (deficient O2 supply), anemic (low hemoglobin level), and circulatory (reduced blood flow). Compared with hypoxic and anemic hypoxia, which can be directly identified by variables like SaO2, PaO2, and hemoglobin, the assessment of circulatory hypoxia is more challenging. Intriguingly, Pv-aCO2 has been suggested as a potential marker for changes in microcirculation.
Pv-aCO2 represents the difference between venous and arterial CO2 tensions, and has been confirmed as a valid indicator of cardiac output. [53-55] In physiological settings, sufficient blood flow carries CO2 to the alveoli where it is exhaled from the body. In contrast, pathologically reduced blood flow results in the accumulation of tissue CO2, widening the CO2 gap between arterial and venous blood, in accordance with the Fick equation: Pv-aCO2=(k×VCO2tissue)/blood flowtissue, where k is a constant determining the relationship between CO2 tension and content (Supplementary Figure S2), and VCO2tissue is the amount of CO2 generated by the tissue.
Because k increases with the decreased blood flow, the inverse relationship between Pv-aCO2 and blood flow is curvilinear; when blood flow is reduced to the lowest range, Pv-aCO2 will increase remarkably.[56] However, the curvilinear relationship between Pv-aCO2 and blood flow is easily disturbed in pathological processes, because k is affected by various factors.[48] The theory of CO2 stagnation during decreased blood flow compensates for the Fick equation.[56, 57] When both afferent and efferent blood flows in the capillary network are arrested, the increased anaerobic production of CO2 from hypoxic tissues leads to increased Pv-aCO2. In addition, a different theory stated that when blood flow was lowered, PaCO2 also fell to adjust to the increased ventilation-perfusion ratio, further raising the Pv-aCO2.[58] These theories may account for the negative correlation between blood flow and Pv-aCO2.
The ability of Pv-aCO2 to monitor microcirculatory dysregulation has been studied extensively. A Pv-aCO2 >6 mmHg was associated to microcirculatory hypoperfusion in septic patients, manifested by reduced PPV, lower functional capillary density, and higher heterogeneity of microvascular blood flow.[51] Despite a ScvO2 ≥70%, a Pv-aCO2 ≥8 mmHg in post-cardiac surgery patients was linked to hepatosplanchnic hypoperfusion, as evidenced by a significantly lower plasma clearance rate of indocyanine green.[59] In addition, in patients who achieved global hemodynamic goals, Pv-aCO2 still fluctuated with microcirculatory perfusion while there was no association between Pv-aCO2 and cardiac output, demonstrating that Pv-aCO2 may be a reliable indicator reflecting microcirculatory flow alterations. [51]
DISCUSSION
This review considers the application of blood gas parameters in microcirculatory monitoring based on the dynamic evolution of tissue hypoxia: (1) reduced SvO2 and increased O2ER indicate hypoxia; (2) hyperlactatemia and an elevated Cv-aCO2/Ca-vO2 (or Pv-aCO2/Ca-vO2) ratio are signs of deteriorating hypoxia and the emergence of anaerobic metabolism; and (3) parameters including hemoglobin, Pv-aCO2, SaO2, and PaO2 may help to distinguish between various hypoxia-causing processes.
Blood gas parameters have certain limitations. First, the parameters essentially reflect global O2 metabolism and cannot accurately follow the complex changes within the microvascular environment. Analysis of blood gas parameters must thus be combined with organ-perfusion performance. Second, the parameters are affected by multiple factors; a satisfactory value is never the ultimate target during septic treatment, and only a comprehensive assessment can reveal the tissue metabolism. Despite these limitations, blood gas analysis provides valuable information on tissue O2 metabolism, especially when advanced technologies for microcirculatory measurements are not available.
Taken together with medical practice, the recommended interpretation of blood gas analysis is presented in Figure 3. Lactate should be included in the initial clinical evaluation, given that it is acquired from arterial blood, which involves a relatively less-invasive procedure that is readily available in the ED. Notably, physicians need to be aware that interpretations should be combined with clinical judgments, and the ultimate goal of resuscitation is to improve clinical performance, rather than correcting the specific values.
Figure 3.
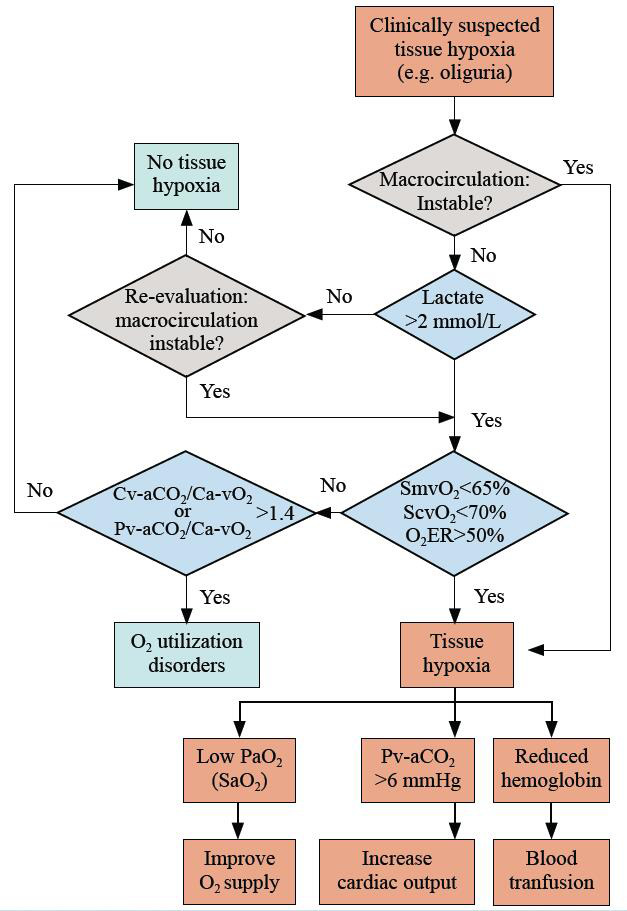
Integrated interpretation of blood gas analysis in hemodynamic monitoring. O2ER: O2 extraction rate; SvO2: venous O2 saturation; Pv-aCO2: venous to arterial CO2 tension; Ca-vCO2: arterial to venous CO2 content; Ca-vO2: arterial to venous O2 content; SaO2: arterial O2 saturation; ScvO2: central venous O2 saturation; SmvO2: mixed venous O2 saturation.
CONCLUSIONS
This review contributes to our understanding of the use of blood gas analysis as a surrogate for visualizing microcirculation and tissue O2 metabolism. Despite its drawbacks, evidence suggests that blood gas analysis, combined with clinical performance, provides a feasible and reliable alternative for microcirculatory management. We believe that further insights into the DO2/VO2 ratio will help ED physicians utilize blood gas analysis effectively, leading to improved judgments and better decision-making in the treatment of sepsis.
Footnotes
Funding: This work was supported by the grants from Innovation Fund for Medical Sciences (CIFMS) from Chinese Academy of Medical Sciences (No. 2021-I2M-1-062); National Key R&D Program of China from Ministry of Science and Technology of the People’s Republic of China (No. 2022YFC2304601, 2021YFC2500801); National High Level Hospital Clinical Research Funding (2022-PUMCH-D-005, 2022-PUMCH-D-111,2022-PUMCH-B-126); National key clinical specialty construction projects from National Health Commission.
Ethical approval: Not needed.
Conflicts of interest: All authors declare that they do not have any potential conflict of interest in relation to this manuscript.
Author contribution: JYW and JX substantially contributed to the conception and design of the review; JYW was in charge of conducting the literature search, interpreting the results, and producing the initial draft; LW, JX and BD provided critical revisions to the manuscript. The final version of the manuscript was approved by all authors.
All the supplementary files in this paper are available at http://wjem.com.cn.
REFERENCES
- 1.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign:international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34(12):2210–7. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis:impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41(3):791–9. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 4.De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation:report of a round table conference. Crit Care. 2007;11(5):R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Backer D. Is microcirculatory assessment ready for regular use in clinical practice? Curr Opin Crit Care. 2019;25(3):280–4. doi: 10.1097/MCC.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 6.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock:relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49(1):88–98. doi: 10.1016/j.annemergmed.2006.08.021. 98.e1-2. [DOI] [PubMed] [Google Scholar]
- 8.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–31. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 9.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 10.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8. doi: 10.1186/cc14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yajnik V, Maarouf R. Sepsis and the microcirculation:the impact on outcomes. Curr Opin Anaesthesiol. 2022;35(2):230–5. doi: 10.1097/ACO.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 12.Arina P, Singer M. Pathophysiology of sepsis. Curr Opin Anaesthesiol. 2021;34(2):77–84. doi: 10.1097/ACO.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 13.Mallat J, Lemyze M, Meddour M, Pepy F, Gasan G, Barrailler S, et al. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care. 2016;6(1):10. doi: 10.1186/s13613-016-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CP, Bora V. In:StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Anesthesia monitoring of mixed venous saturation. [PubMed] [Google Scholar]
- 15.Hartog C, Bloos F. Venous oxygen saturation. Best Pract Res Clin Anaesthesiol. 2014;28(4):419–28. doi: 10.1016/j.bpa.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 16.van Beest PA, van Ingen J, Boerma EC, Holman ND, Groen H, Koopmans M, et al. No agreement of mixed venous and central venous saturation in sepsis, independent of sepsis origin. Crit Care. 2010;14(6):R219. doi: 10.1186/cc9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soussi MS, Jebali MA, Le Manach Y, Nasri M, Zouari B, Chenik S, et al. Central venous saturation is not an alternative to mixed venous saturation during cardiopulmonary bypass in coronary artery surgery patients. Perfusion. 2012;27(4):300–6. doi: 10.1177/0267659112442902. [DOI] [PubMed] [Google Scholar]
- 18.Lorentzen AG, Lindskov C, Sloth E, Jakobsen CJ. Central venous oxygen saturation cannot replace mixed venous saturation in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(6):853–7. doi: 10.1053/j.jvca.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Kopterides P, Bonovas S, Mavrou I, Kostadima E, Zakynthinos E, Armaganidis A. Venous oxygen saturation and lactate gradient from superior vena cava to pulmonary artery in patients with septic shock. Shock. 2009;31(6):561–7. doi: 10.1097/SHK.0b013e31818bb8d8. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004;30(8):1572–8. doi: 10.1007/s00134-004-2337-y. [DOI] [PubMed] [Google Scholar]
- 21.He HW, Long Y, Zhou X, Wang XT, Zhang HM, Chai WZ, et al. Oxygen-flow-pressure targets for resuscitation in critical hemodynamic therapy. Shock. 2018;49(1):15–23. doi: 10.1097/SHK.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 22.Squara P. Central venous oxygenation:when physiology explains apparent discrepancies. Crit Care. 2014;18(6):579. doi: 10.1186/s13054-014-0579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thooft A, Favory R, Salgado DR, Taccone FS, Donadello K, De Backer D, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15(5):R222. doi: 10.1186/cc10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arango-Granados MC, Umaña M, Sánchez ÁI, García AF, Granados M, Ospina-Tascón GA. Impact of red blood cell transfusion on oxygen transport and metabolism in patients with sepsis and septic shock:a systematic review and meta-analysis. Rev Bras Ter Intensiva. 2021;33(1):154–66. doi: 10.5935/0103-507X.20210017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Textoris J, Fouché L, Wiramus S, Antonini F, Tho S, Martin C, et al. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care. 2011;15(4):R176. doi: 10.1186/cc10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perz S, Uhlig T, Kohl M, Bredle DL, Reinhart K, Bauer M, et al. Low and “supranormal”central venous oxygen saturation and markers of tissue hypoxia in cardiac surgery patients:a prospective observational study. Intensive Care Med. 2011;37(1):52–9. doi: 10.1007/s00134-010-1980-8. [DOI] [PubMed] [Google Scholar]
- 27.Wittayachamnankul B, Apaijai N, Sutham K, Chenthanakij B, Liwsrisakun C, Jaiwongkam T, et al. High central venous oxygen saturation is associated with mitochondrial dysfunction in septic shock:a prospective observational study. J Cell Mol Med. 2020;24(11):6485–94. doi: 10.1111/jcmm.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber W, Zanner R, Schneider G, Schmid R, Lahmer T. Assessment of regional perfusion and organ function:less and non-invasive techniques. Front Med (Lausanne) 2019;6:50. doi: 10.3389/fmed.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haase N, Perner A. Central venous oxygen saturation in septic shock—a marker of cardiac output, microvascular shunting and/or dysoxia? Crit Care. 2011;15(4):184. doi: 10.1186/cc10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He HW, Liu DW, Long Y, Wang XT. Mind the influence of arterial oxygen tension on central venous oxygen saturation. Crit Care. 2014;18(5):569. doi: 10.1186/s13054-014-0569-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent JL, Theerawit P, Simion D. Alternatives to Blood Transfusion in Transfusion Medicine. Oxford: Wiley-Blackwell; 2010. Basic principles of oxygen transport and calculations; pp. 203–9. [Google Scholar]
- 32.Nasser B, Tageldein M, AlMesned A, Kabbani M. Effects of blood transfusion on oxygen extraction ratio and central venous saturation in children after cardiac surgery. Ann Saudi Med. 2017;37(1):31–7. doi: 10.5144/0256-4947.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An X, Zhang H, Sun YN, Ma XC. The microcirculatory failure could not weaken the increase of systematic oxygen extraction rate in septic shock:an observational study in canine models. Clin Hemorheol Microcirc. 2016;63(3):267–79. doi: 10.3233/CH-152022. [DOI] [PubMed] [Google Scholar]
- 34.Ospina-Tascón GA, García Marin AF, Echeverri GJ, Bermudez WF, Madriñán-Navia H, Valencia JD, et al. Effects of dobutamine on intestinal microvascular blood flow heterogeneity and O2 extraction during septic shock. J Appl Physiol (1985) 2017;122(6):1406–17. doi: 10.1152/japplphysiol.00886.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy B. Lactate and shock state:the metabolic view. Curr Opin Crit Care. 2006;12(4):315–21. doi: 10.1097/01.ccx.0000235208.77450.15. [DOI] [PubMed] [Google Scholar]
- 36.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371(24):2309–19. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 37.Levy B, Desebbe O, Montemont C, Gibot S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock. 2008;30(4):417–21. doi: 10.1097/SHK.0b013e318167378f. [DOI] [PubMed] [Google Scholar]
- 38.Taylor DJ, Faragher EB, Evanson JM. Inflammatory cytokines stimulate glucose uptake and glycolysis but reduce glucose oxidation in human dermal fibroblasts in vitro . Circ Shock. 1992;37(2):105–10. [PubMed] [Google Scholar]
- 39.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949–55. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 40.Filbin MR, Hou PC, Massey M, Barche A, Kao E, Bracey A, et al. The microcirculation is preserved in emergency department low-acuity sepsis patients without hypotension. Acad Emerg Med. 2014;21(2):154–62. doi: 10.1111/acem.12314. [DOI] [PubMed] [Google Scholar]
- 41.Puskarich MA, Shapiro NI, Massey MJ, Kline JA, Jones AE. Lactate clearance in septic shock is not a surrogate for improved microcirculatory flow. Acad Emerg Med. 2016;23(6):690–3. doi: 10.1111/acem.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel H, Kerndt CC, Bhardwaj A. In:StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Physiology, respiratory quotient. [PubMed] [Google Scholar]
- 43.Randall HM, Jr, Cohen JJ. Anaerobic CO2 production by dog kidney in vitro. Am J Physiol Leg Content. 1966;211(2):493–505. doi: 10.1152/ajplegacy.1966.211.2.493. [DOI] [PubMed] [Google Scholar]
- 44.da Rocha EE, Alves VG, da Fonseca RB. Indirect calorimetry:methodology, instruments and clinical application. Curr Opin Clin Nutr Metab Care. 2006;9(3):247–56. doi: 10.1097/01.mco.0000222107.15548.f5. [DOI] [PubMed] [Google Scholar]
- 45.De Waele E, Honore PM, Spapen HD. New generation indirect calorimeters for measuring energy expenditure in the critically ill:a rampant or reticent revolution? Crit Care. 2016;20(1):138. doi: 10.1186/s13054-016-1315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ltaief Z, Schneider AG, Liaudet L. Pathophysiology and clinical implications of the veno-arterial PCO2 gap. Crit Care. 2021;25(1):318. doi: 10.1186/s13054-021-03671-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002;28(3):272–7. doi: 10.1007/s00134-002-1215-8. [DOI] [PubMed] [Google Scholar]
- 48.Mesquida J, Saludes P, Gruartmoner G, Espinal C, Torrents E, Baigorri F, et al. Central venous-to-arterial carbon dioxide difference combined with arterial-to-venous oxygen content difference is associated with lactate evolution in the hemodynamic resuscitation process in early septic shock. Crit Care. 2015;19(1):126. doi: 10.1186/s13054-015-0858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He H, Long Y, Liu D, Wang X, Tang B. The prognostic value of central venous-to-arterial CO2 difference/arterial-central venous O2 difference ratio in septic shock patients with central venous O2 saturation ≥80. Shock. 2017;48(5):551–7. doi: 10.1097/SHK.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 50.Ospina-Tascón GA, Umaña M, Bermúdez W, Bautista-Rincón DF, Hernandez G, Bruhn A, et al. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med. 2015;41(5):796–805. doi: 10.1007/s00134-015-3720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ospina-Tascón GA, Umaña M, Bermúdez WF, Bautista-Rincón DF, Valencia JD, Madriñán HJ, et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med. 2016;42(2):211–21. doi: 10.1007/s00134-015-4133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He HW, Liu DW, Long Y, Wang XT, Yu C, Yao B, et al. Using peripheral perfusion index and venous-to-arterial CO(2) difference/arterial-central venous O(2) difference ratio to assess lactate clearance in septic patients after resuscitation. Zhonghua Nei Ke Za Zhi. 2018;57(12):917–21. doi: 10.3760/cma.j.issn.0578-1426.2018.12.008. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 53.Troskot R, Šimurina T, Žižak M, Majstorović K, Marinac I, Mrakovčić-Šutić I. Prognostic value of venoarterial carbon dioxide gradient in patients with severe sepsis and septic shock. Croat Med J. 2010;51(6):501–8. doi: 10.3325/cmj.2010.51.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mallat J, Pepy F, Lemyze M, Gasan G, Vangrunderbeeck N, Tronchon L, et al. Central venous-to-arterial carbon dioxide partial pressure difference in early resuscitation from septic shock:a prospective observational study. Eur J Anaesthesiol. 2014;31(7):371–80. doi: 10.1097/EJA.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 55.Guo ZQ, Yin M, Kong JC, Wang B, Dai KP, Zuo T, et al. Relationship analysis of central venous-to-arterial carbon dioxide difference and cardiac index for septic shock. Sci Rep. 2019;9(1):8822. doi: 10.1038/s41598-019-45252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dres M, Monnet X, Teboul JL. Hemodynamic management of cardiovascular failure by using PCO(2) venous-arterial difference. J Clin Monit Comput. 2012;26(5):367–74. doi: 10.1007/s10877-012-9381-x. [DOI] [PubMed] [Google Scholar]
- 57.Mallat J, Lemyze M, Tronchon L, Vallet B, Thevenin D. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med. 2016;5(1):47–56. doi: 10.5492/wjccm.v5.i1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durkin R, Gergits MA, Reed JF, 3rd, Fitzgibbons J. The relationship between the arteriovenous carbon dioxide gradient and cardiac index. J Crit Care. 1993;8(4):217–21. doi: 10.1016/0883-9441(93)90005-6. [DOI] [PubMed] [Google Scholar]
- 59.Habicher M, von Heymann C, Spies CD, Wernecke KD, Sander M. Central venous-arterial pCO2 difference identifies microcirculatory hypoperfusion in cardiac surgical patients with normal central venous oxygen saturation:a retrospective analysis. J Cardiothorac Vasc Anesth. 2015;29(3):646–55. doi: 10.1053/j.jvca.2014.09.006. [DOI] [PubMed] [Google Scholar]


