Abstract
Standard-of-care first-line treatment for metastatic urothelial carcinoma (mUC) is platinum-based chemotherapy (CTx). Maintenance immunotherapy is a treatment option for patients without progressive disease (PD) after induction CTx. IMvigor130 was a randomised, phase 3 study evaluating atezolizumab plus platinum-based CTx (arm A), atezolizumab monotherapy (arm B), or placebo plus platinum-based CTx (arm C) as first-line treatment for mUC. The primary progression-free survival (PFS) analysis showed a statistically significant PFS benefit favouring arm A versus arm C, which did not translate into overall survival (OS) benefit at the final OS analysis. We report exploratory analyses based on response to combination induction treatment (arm A vs arm C) using final OS data. Post-induction OS was analysed for patients without PD during induction (4–6 CTx cycles) who received at least one dose of single-agent atezolizumab/placebo maintenance treatment. Post-progression OS was analysed for patients with PD during induction CTx. Addition of atezolizumab to CTx did not impact OS outcomes, regardless of response to induction CTx, with hazard ratios of 0.84 (95% confidence interval [CI] 0.63–1.10) for patients without PD and 0.75 (95% CI 0.54–1.05) for those with PD during induction CTx. Treatment effects appeared to be greatest for patients treated with cisplatin and for those with PD-L1–high tumours.
Patient summary
The IMvigor130 trial showed that addition of atezolizumab to chemotherapy (CTx) did not improve survival over CTx alone in patients with bladder cancer. Overall, patients whose cancer did not progress during initial treatment tended to live longer than patients whose cancer did progress, but addition of atezolizumab to CTx did not help either group live longer in comparison to CTx alone. However, the results suggest that patients who received a certain CTx drug (cisplatin) or who had high levels of a marker called PD-L1 in their tumour may get the most improvement from addition of atezolizumab to CTx.
The IMvigor130 trial is registered on ClinicalTrials.gov as NCT02807636.
Keywords: Atezolizumab, Cancer immunotherapy, First line, IMvigor130, Induction chemotherapy, Maintenance treatment, Metastatic urothelial carcinoma, Overall survival final analysis, Platinum/gemcitabine, Randomised phase 3 study
First-line treatment for chemotherapy (CTx)-eligible patients with metastatic urothelial carcinoma (mUC) consists of cisplatin-based CTx. For patients deemed ineligible for cisplatin, treatment alternatives include carboplatin-based CTx or immune checkpoint inhibitors (ICIs), according to eligibility factors including PD-L1 expression [1], [2]. Additionally, the combination of enfortumab vedotin plus pembrolizumab received accelerated approval in the USA in early 2023 [3], [4]. For patients without progression during CTx, maintenance ICIs represent a therapeutic option [1], [2], [5]. However, approximately 20–30% of patients may be ineligible for first-line maintenance treatment with ICIs because of progression [6].
IMvigor130 was a global, randomised phase 3 study evaluating first-line atezolizumab (anti–PD-L1) plus platinum-based CTx (gemcitabine plus either cisplatin or carboplatin) (arm A), atezolizumab monotherapy (arm B), or placebo plus platinum-based CTx (arm C) in patients with mUC [7]. IMvigor130 met its co-primary endpoint of progression-free survival (PFS) in the intention-to-treat (ITT) population with addition of atezolizumab to CTx (arm A vs arm C) [7]. The final analysis of overall survival (OS; co-primary endpoint) suggested better OS with atezolizumab, but the results did not cross the efficacy boundary for statistical significance [8]. Here we report an updated exploratory analysis of OS by response to induction CTx with or without atezolizumab.
The study design and primary results for IMvigor130 were reported previously [7]. The study was conducted according to principles outlined in the Declaration of Helsinki, and enrolled patients provided written informed consent. The protocol was approved by the institutional review board at each site.
IMvigor130 allowed patients in arms A and C to continue single-agent atezolizumab or placebo as maintenance treatment following completion or discontinuation of induction CTx [7]. In this analysis, induction treatment was defined as four to six cycles of platinum/gemcitabine, combined with atezolizumab or placebo. Maintenance therapy was defined as at least one dose of atezolizumab or placebo monotherapy.
The analysis defined patients as having no progressive disease (PD) if they had a complete response, partial response, or stable disease, without PD at or before the week-18 tumour assessment. Patients in this subgroup had to have completed induction and maintenance treatment as defined above. Post-induction OS was examined starting at week 18, chosen because it corresponded to a maximum of six cycles of CTx. In the subgroup of patients who experienced PD at or before week 18, OS was examined starting at the time of PD.
OS was analysed using multivariable Cox proportional-hazards models. Results are presented descriptively, with no formal statistical testing performed for this post hoc analysis.
This analysis was based on final OS results (clinical cutoff date August 31, 2022); in the ITT population, time from the last patient randomised to the cutoff date was 49 mo (median survival follow-up, 13.4 mo). For both the no-PD (n = 318) and PD (n = 184) subgroups, imbalances in baseline characteristics occurred at a frequency of <10% between the treatment arms, with the following exceptions specific to the PD subgroup: PD-L1 tumour-infiltrating immune cell (IC)1 status: 52.7% in arm A versus 38.7% in arm C; male sex: 81.3% in arm A versus 71.0% in arm C; and Eastern Cooperative Oncology Group performance status 2: 17.6% in arm A versus 7.5% in arm C (Supplementary Table 1).
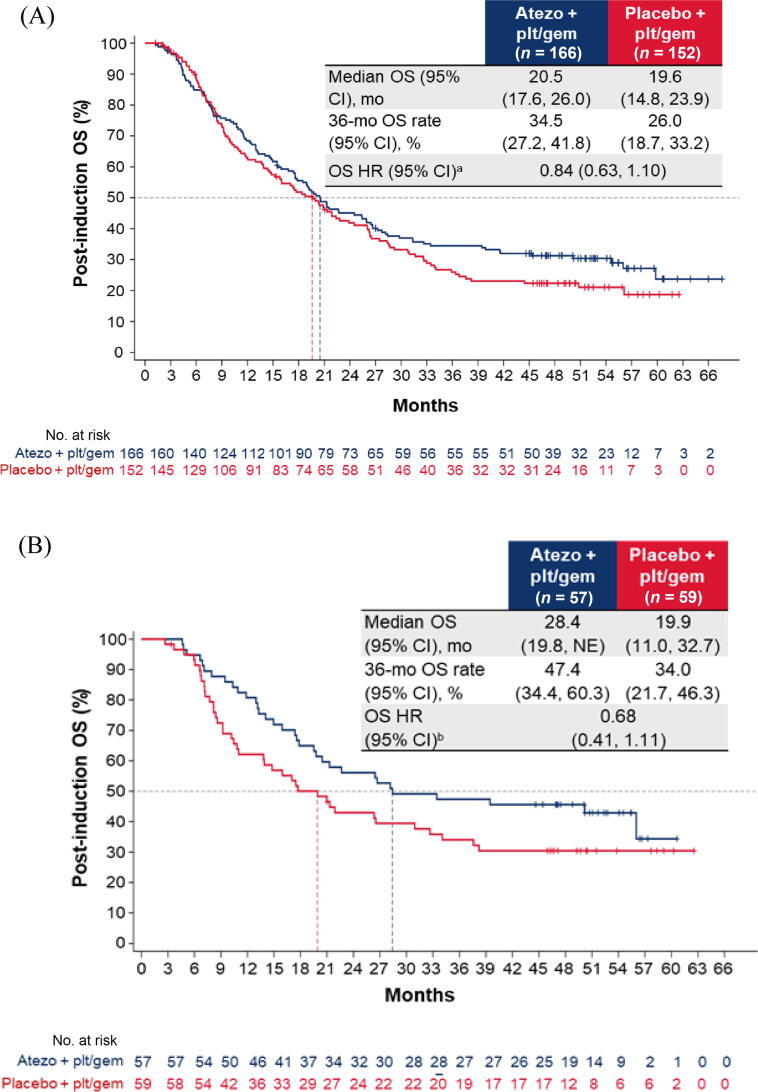
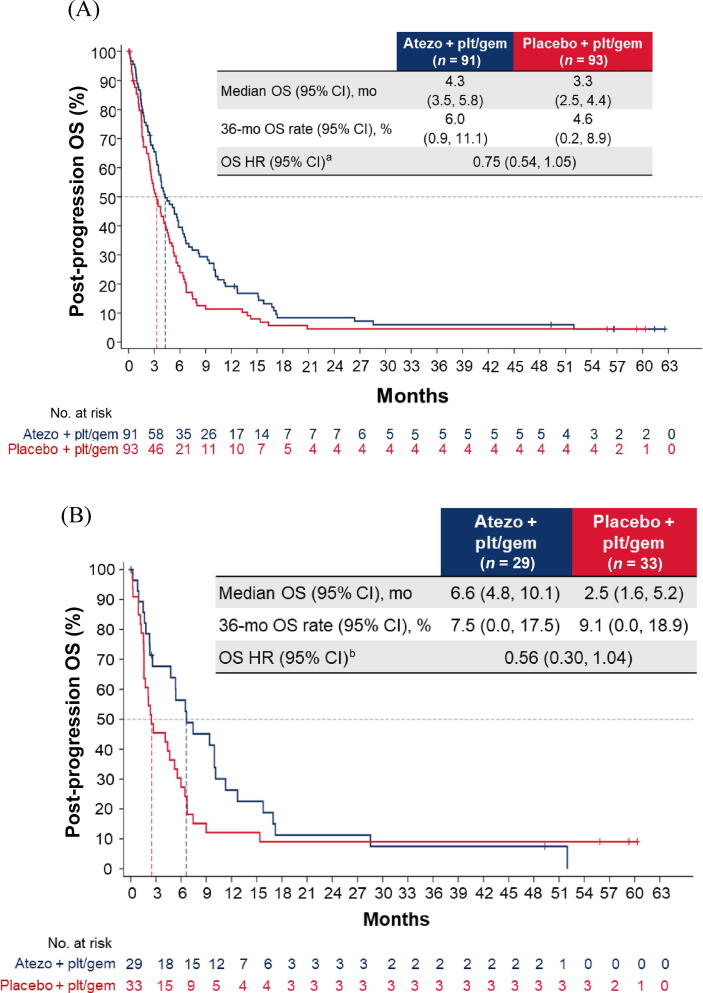
Addition of atezolizumab to CTx did not impact OS for patients without PD (hazard ratio [HR] 0.84, 95% confidence interval [CI] 0.63–1.10; Fig. 1A) or patients with PD (HR 0.75, 95% CI 0.54–1.05; Fig. 2A) on or after induction treatment. For both the no-PD and PD subgroups, a greater proportion of patients in arm C than in arm A received subsequent nonprotocol immunotherapy (Supplementary Table 2).
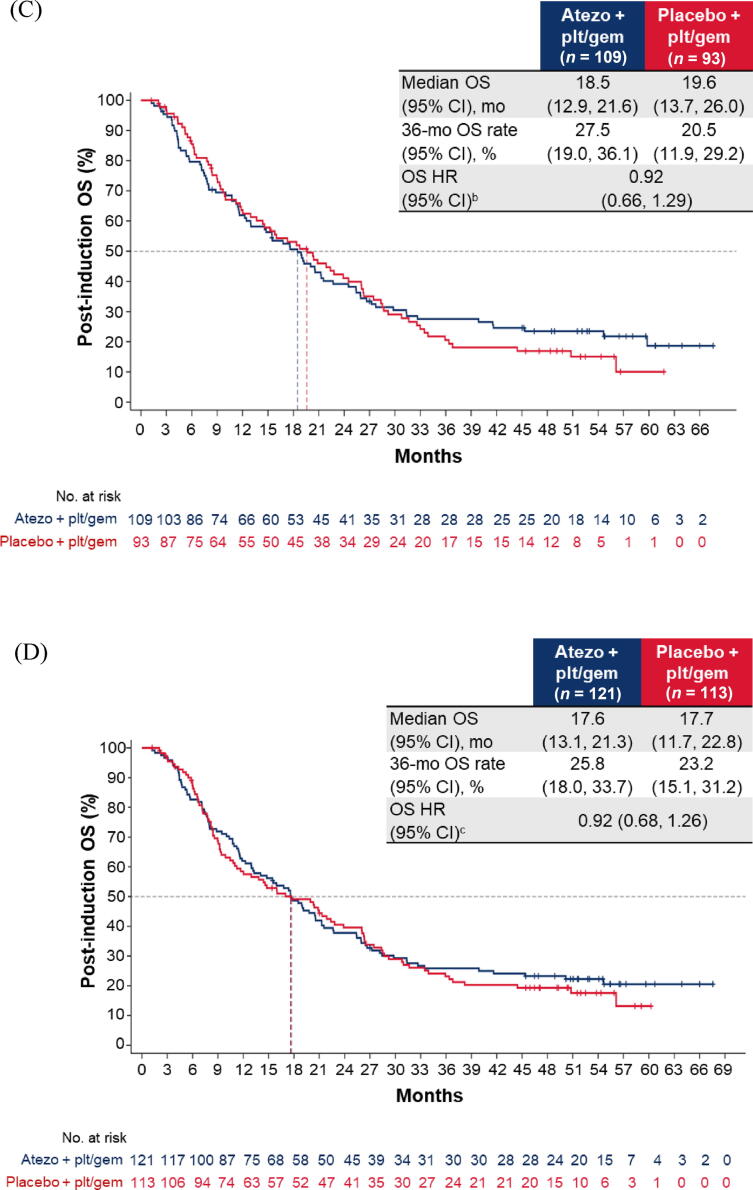
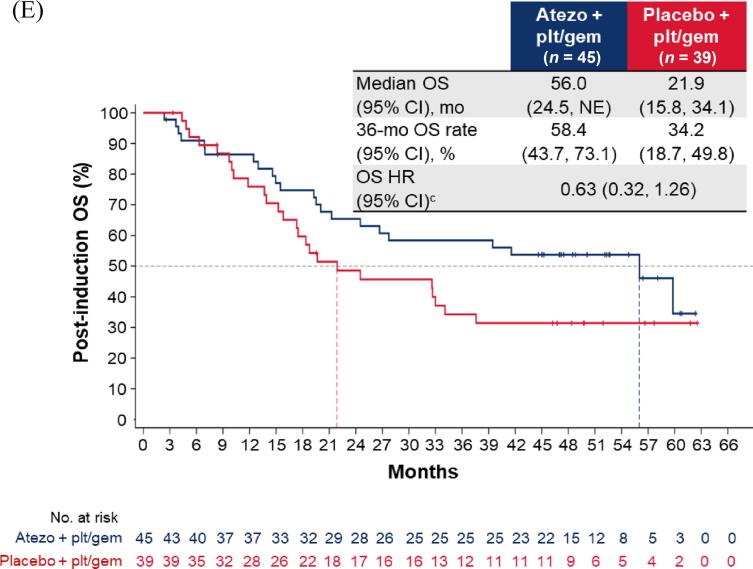
Fig. 1.
Post-induction OS in the cohort of patients without disease progression during induction chemotherapy: (A) all patients; (B) cisplatin-treated patients; (C) carboplatin-treated patients; (D) the IC0/1 subgroup; and (E) the IC2/3 subgroup. CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; gem = gemcitabine; GFR = glomerular filtration rate; HR = hazard ratio; IC = tumour-infiltrating immune cells; NE = not estimable; OS = overall survival; plt = platinum; ULN = upper limit of normal.aHR was stratified by enrolment stage and adjusted for age, baseline ECOG PS (0 vs 1 vs 2), cisplatin ineligibility (yes vs no), liver metastases (yes vs no), lymph node–only metastases (yes vs no), at least three metastatic sites (yes vs no), renal impairment (yes vs no), alkaline phosphatase ≥ ULN (yes vs no), GFR group (<60 vs ≥60 ml/min), Bajorin risk score (0 vs 1 vs 2 and/or liver metastases), PD-L1 status (IC0/1 vs IC2/3), investigator choice of chemotherapy (cisplatin vs carboplatin), and best response during induction. bHR was stratified by enrolment stage and adjusted for age, baseline ECOG PS, cisplatin ineligibility, liver metastases, lymph node only metastases, at least three metastatic sites, renal impairment, alkaline phosphatase ≥ ULN, GFR group, Bajorin risk score, PD-L1 status, and best response during induction. cHR was stratified by enrolment stage and adjusted for age, baseline ECOG PS, cisplatin ineligibility, liver metastases, lymph node–only metastases, at least three metastatic sites, renal impairment, alkaline phosphatase ≥ ULN, GFR group, Bajorin risk score, investigator choice of chemotherapy, and best response during induction.
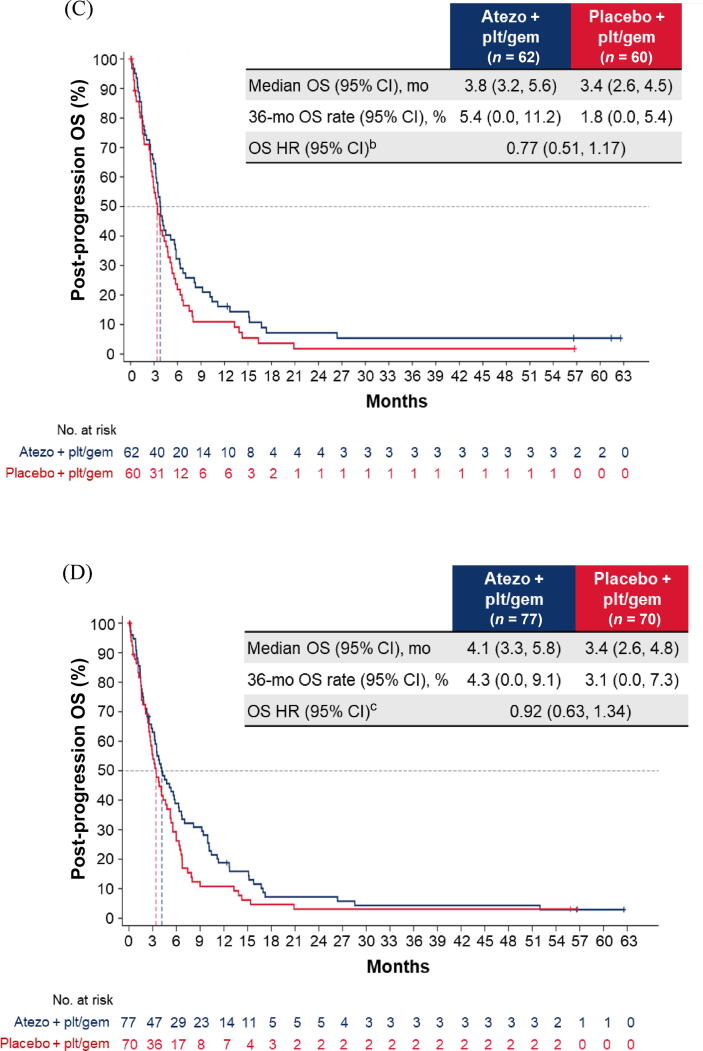
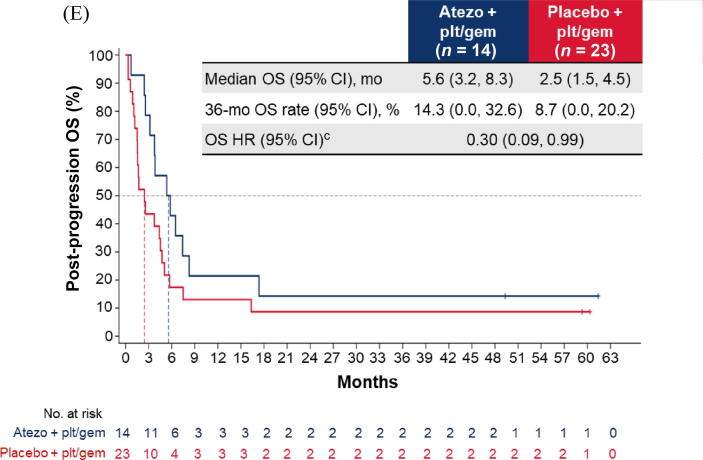
Fig. 2.
Post-progression OS in the cohort of patients with disease progression during induction chemotherapy: (A) all patients; (B) cisplatin-treated patients; (C) carboplatin-treated patients; (D) the IC0/1 subgroup; and (E) the IC2/3 subgroup. CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; gem = gemcitabine; GFR = glomerular filtration rate; HR = hazard ratio; IC = tumour-infiltrating immune cells; NE = not estimable; OS = overall survival; plt = platinum; ULN = upper limit of normal.aHR was stratified by enrolment stage and adjusted for age, baseline ECOG PS (0 vs 1 vs 2), cisplatin ineligibility (yes vs no), liver metastases (yes vs no), lymph node–only metastases (yes vs no), at least three metastatic sites (yes vs no), renal impairment (yes vs no), alkaline phosphatase ≥ ULN (yes vs no), GFR group (<60 vs ≥60 ml/min), Bajorin risk score (0 vs 1 vs 2 and/or liver metastases), PD-L1 status (IC0/1 vs IC2/3), and investigator choice of chemotherapy (cisplatin vs carboplatin). bHR was stratified by enrolment stage and adjusted for age, baseline ECOG PS, cisplatin ineligibility, liver metastases, lymph node–only metastases, at least three metastatic sites, renal impairment, alkaline phosphatase ≥ ULN, GFR group, Bajorin risk score, and PD-L1 status. cHR was stratified by enrolment stage and adjusted for age, baseline ECOG PS, lymph node–only metastases, at least three metastatic sites, renal impairment, alkaline phosphatase ≥ ULN, GFR group, Bajorin risk score, and investigator choice of chemotherapy.
For patients without PD during induction, the post-induction OS difference between arms A and C may have been greater for cisplatin-treated patients (HR 0.68, 95% CI 0.41–1.11) than for carboplatin-treated patients (HR 0.92, 95% CI 0.66–1.29; Fig. 1B,C). The OS improvement also appeared to be greater for patients with PD-L1 IC2/3 tumours (HR 0.63, 95% CI 0.32–1.26) than for those with PD-L1 IC0/1 tumours (HR 0.92, 95% CI 0.68–1.26; Fig. 1D, E).
Similarly, among patients with PD during induction, the post-progression OS difference between arms A and C may have been greater for cisplatin-treated patients (HR 0.56, 95% CI 0.30–1.04) than for carboplatin-treated patients (HR 0.77, 95% CI 0.51–1.17; Fig. 2B, C). The OS improvement also appeared to be greater for the PD-L1 IC2/3 subgroup (HR 0.30, 95% CI 0.09–0.99) than for the PD-L1 IC0/1 subgroup (HR 0.92, 95% CI 0.63–1.34; Fig. 2D, E).
Although 43% of patients in the control arm of the PD subgroup received at least one subsequent anticancer treatment, median OS was only 3.3 mo. The poor prognosis for this subgroup of patients with disease progression during induction treatment must be considered when determining subsequent management plans and highlights the need to find effective treatments for this clinical population.
This exploratory analysis based on final OS data from IMvigor130 showed that addition of atezolizumab to CTx did not impact OS outcomes, regardless of the initial response to induction CTx. These results are consistent with those in the ITT population showing a lack of OS benefit from addition of atezolizumab to CTx [7] and with analyses based on interim OS data [9]. Cisplatin-treated patients may have derived a greater benefit with the addition of atezolizumab than carboplatin-treated patients did, supportive of previous findings [8] and potentially related to underlying differences in immunomodulatory effects [10]. In addition, patients with PD-L1 IC2/3 tumours appeared to have a greater OS improvement with atezolizumab in comparison to patients with PD-L1 IC0/1 tumours; of note, those with no PD who had PD-L1 IC2/3 tumours had median OS of 56.0 mo in arm A and 21.9 mo in arm C. However, important limitations of this analysis are the small patient numbers and the retrospective nature, and the study was not powered to evaluate treatment effects in these subgroups.
Although these data cannot be directly compared with current benchmarks for maintenance immunotherapy because of differences in study design and small sample sizes, this analysis provides insights into the challenges associated with optimising treatment algorithms for patients with mUC.
Author contributions: Enrique Grande had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Grande, Davis, Rezazadeh Kalebasty, Bernhard, Telliez, De Santis.
Acquisition of data: Grande, Bamias, Galsky, Kikuchi, Davis, Arranz, Rezazadeh Kalebasty, Garcia del Muro, Park, De Giorgi, Alekseev, Mencinger, Izumi, Puente, Li, De Santis.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Grande, Rezazadeh Kalebasty, De Giorgi, Mencinger, Nicholas, Bernhard, Telliez.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Nicholas.
Obtaining funding: None.
Administrative, technical, or material support: Bamias.
Supervision: None.
Other (recruitment and treatment of patients): Arranz.
Financial disclosures: Enrique Grande certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Enrique Grande has received grants or contracts from Astellas, AstraZeneca, Ipsen, Lexicon, Merck KGaA, MTEM/Threshold/Tersera, Nanostring Technologies, Pfizer, and Roche; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Advanced Accelerator Applications, Amgen, Angelini, Astellas, AstraZeneca, Bayer, Blueprint, BMS, Caris Life Sciences, Celgene, Clovis Oncology, Dr. Reddy’s, Eisai, Esteve, EUSA Pharma, Genetracer, GSK, Guardant Health, HRA Pharma, Ipsen, ITM Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, OncoDNA (Biosequence), Palex, Pfizer, PharmaMar, Pierre Fabre, Raffo, Roche, Sanofi-Genzyme, Servier, Taiho, Tecnofarma, Thermo Fisher Scientific, and Zodiac; support for attending meetings and/or travel from Astellas, AstraZeneca, Ipsen, Janssen, Merck KGaA, MSD, Pfizer, and Roche; and has participated in a data safety monitoring board or advisory board for Faron and Roche. Aristotelis Bamias has received grants or contracts from AstraZeneca, BMS, and Pfizer; consulting fees from BMS, Roche, and Merck; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from BMS, MSD, and Pfizer; and has participated on a data safety monitoring board or advisory board with BMS and Excelya. Matthew D. Galsky reports grants or contracts from AstraZeneca, BMS, Dendreon, Genentech, Merck, and Novartis; and consulting fees from AbbVie, Alligator, Analog Devices, Asieris, AstraZeneca, Basilea, Bicycle, BMS, Curis, Dragaonfly, EMD Serono, Fujifilm, Genentech, Gilead, GlaxoSmithKline, Janssen, Merck, Numab, Pfizer, Rappta Therapeutics, SeaGen, Silverback, UroGen, and Veracyte. Eiji Kikuchi reports honoraria from Astellas, Bristol, Chugai, Janssen, Merck Bio Pharma, and MSD. Ian D. Davis has been a member of the IMvigor130 steering committee (uncompensated); served as director and chair of the ANZUP Cancer Trials Group (uncompensated); reports salaried employment with Eastern Health, Melbourne, Australia, and Monash University, Melbourne, Australia. José Ángel Arranz reports consulting or advisory fees from Astellas, Bayer, BMS, Merck, MSD, and Pfizer; and payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from BMS, Merck, and MSD; and has patents planned, issued, or pending with BMS and MSD. Arash Rezazadeh Kalebasty has stock and other ownership interests in ECOM Medical; has received fees for a consulting or advisory role from Amgen, Astellas/Medivation, AstraZeneca, AVEO, Bayer, BMS, Eisai, EMD Serono, Exelixis, Genentech/Roche, Gilead Sciences, Immunomedics, Janssen, Merck, Myovant Sciences, Novartis, Pfizer, Sanofi, and SeaGen/Astellas; research funding from Amgen, Arvinas, Astellas, AstraZeneca, Bavarian Nordic, Bayer, BeyondSpring, BioClin, BMS, Clovis, Eisai, Epizyme, Exelixis, Genentech, Immunomedics, Janssen, MacroGenics, Merck, Mirati, Novartis, POINT Biopharma, and SeaGen; and support for attending meetings and/or travel from Astellas/Medivation, AstraZeneca, Bayer, Eisai, Exelixis, Genentech, Janssen, Novartis, Pfizer, and Prometheus. Xavier Garcia del Muro reports consulting fees from BMS, Deciphera, Eisai, EusaPharma, GSK, Ipsen, Lilly, Merck, Pfizer, and Roche; payment or honoraria from Astellas Pharma, Eisai, and Pfizer; and support for attending meetings and/or travel from Merck and Pfizer. Se Hoon Park declares honoraria from Merck, Ono Pharmaceuticals and Pfizer; a consulting or advisory role with Janssen Oncology, and Research Funding from Ono Pharmaceutical and Sanofi. Ugo De Giorgi reports consulting fees from Amgen, Astellas Pharma, AstraZeneca, Bayer, BMS, Clovis Oncology, Dompé Farmaceutici, Eisai, Ipsen, Janssen, Merck KGaA, MSD, Novartis, Pfizer, and PharmaMar; and support for attending meetings and/or travel from AstraZeneca, Ipsen, and Pfizer. Boris Alekseev reports support for the present manuscript from Roche; consulting fees from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, MSD, Pfizer, and Roche; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, MSD, Pfizer, and Roche; and support for attending meetings and/or travel from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, MSD, Pfizer, and Roche. Marina Mencinger reports consulting or advisory fees from Astellas, and Roche; and speaker bureau participation for Astellas, Janssen, Merck, and Roche. Kouji Izumi reports financial interests, relationships, or affiliations with Chugai Pharmaceutical Co, Ltd. Javier Puente reports study funding from Roche; consulting fees from Pfizer and Roche; honoraria for lectures from AstraZeneca, BMS, Eisai, Janssen, Merck, MSD, and Pfizer; support for travel for attending meetings from Janssen, Merck, and Pfizer; and participated on an advisory board for AstraZeneca, BMS, Janssen, Merck, MSD, and Pfizer. Jian-Ri Li has nothing to disclose. Sandrine Bernhard is an employee of Roche Products Ltd. and holds stock options in F. Hoffmann-La Roche Ltd. Alan Nicholas reports employment and stock or other ownership in Genentech and Roche. Julie Telliez reports employment and stock or other ownership in Roche. Maria De Santis has received honoraria from AAA, Amgen, Astellas, AstraZeneca, Basilea, Bayer, Bioclin, BMS, Eisai, Ferring, Immunomedics/Gilead, Ipsen, Janssen, Merck, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, and SeaGen.
Funding/Support and role of the sponsor: This study was sponsored by F. Hoffmann-La Roche Ltd. The sponsor was involved in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Data sharing statement: Qualified researchers may request access to individual patient-level clinical data via a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://go.roche.com/data_sharing. Anonymised records for individual patients across more than one data source external to Roche cannot, and should not, be linked owing to a potential increase in the risk of patient re-identification.
Acknowledgements: We thank the patients and their families. We also thank Qian Zhu for contributions to the statistical analyses. This study was sponsored by F. Hoffmann-La Roche. Medical writing assistance was provided by Marcia Gamboa, PhD, of Health Interactions and funded by F. Hoffmann-La Roche. Ian D. Davis is supported in part by an Australian National Health and Medical Research Council Investigator Grant (2016274).
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.10.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Witjes JA, Bruins HM, Carrión A, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer. Arnhem, The Netherlands: European Association of Urology; 2023. https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer.
- 2.Powles T., Bellmunt J., Comperat E., et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment, and follow-up. Ann Oncol. 2022;33:244–258. doi: 10.1016/j.annonc.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Friedlander T.W., Milowsky M.I., O’Donnell P.H., et al. Enfortumab vedotin (EV) with or without pembrolizumab (P) in patients (pts) who are cisplatin-ineligible with previously untreated locally advanced or metastatic urothelial cancer (la/mUC): additional 3-month follow-up on cohort K data. J Clin Oncol. 2023;41(16 Suppl):4568. doi: 10.1200/JCO.22.02887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merck. FDA approves Merck’s KEYTRUDA® (pembrolizumab) in combination with Padcev® (enfortumab vedotin-ejfv) for first-line treatment of certain patients with locally advanced or metastatic urothelial cancer. Rahway, NJ: Merck and Co. Inc.; April 3, 2023 https://www.merck.com/news/fda-approves-mercks-keytruda-pembrolizumab-in-combination-with-padcev-enfortumab-vedotin-ejfv-for-first-line-treatment-of-certain-patients-with-locally-advanced-or-metastatic/.
- 5.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 6.Cheeseman S., Thompson M., Sopwith W., et al. Current treatment and outcomes benchmark for locally advanced or metastatic urothelial cancer from a large UK-based single centre. Front Oncol. 2020;10:167. doi: 10.3389/fonc.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galsky M.D., Arija J.A., Bamias A., et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 8.Galsky M.D., Angel Arranz Arija J., De Santis M., et al. Atezolizumab (atezo) + platinum/gemcitabine (plt/gem) vs placebo + plt/gem for first-line (1L) treatment (tx) of locally advanced or metastatic urothelial carcinoma (mUC): final OS from the randomized phase 3 IMvigor130 study. J Clin Oncol. 2023;41(6 Suppl):LBA440. [Google Scholar]
- 9.Grande E., Bamias A., Galsky M.D., et al. Overall survival (OS) by response during “induction” from the global, randomized phase III IMvigor130 study of atezolizumab (atezo) + platinum/gemcitabine (plt/gem) vs placebo + plt/gem in patients (pts) with previously untreated metastatic urothelial carcinoma (mUC) Cancer Res. 2021;81(13 Suppl):CT187. [Google Scholar]
- 10.Galsky M.D., Guan X., Banchereau R., et al. Cisplatin (cis)-related immunomodulation and efficacy with atezolizumab (atezo) + cis- vs carboplatin (carbo)-based chemotherapy (chemo) in metastatic urothelial cancer (mUC) Ann Oncol. 2021;32(5 Suppl):S682–S683. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.