Abstract
Background. The concentration of pharmacologically active tetrahydrocannabinol (THC) in cannabis products has been increasing over the past decade. Concerns about potential harmful health effects of using these increasingly higher-concentration products have led some states to consider regulation of cannabis product THC concentration. We conducted a scoping review of health effects of high-concentration cannabis products to inform policy on whether the THC concentrations of cannabis product should be regulated or limited.
Objectives. We conducted a scoping review to (1) identify and describe human studies that explore the relationship of high-concentration cannabis products with any health outcomes in the literature and (2) create an interactive evidence map of the included studies to facilitate further analyses.
Search Methods. An experienced medical information specialist designed a comprehensive search strategy of 7 electronic databases.
Selection Criteria. We included human studies of any epidemiological design with no restrictions by age, sex, health status, country, or outcome measured that reported THC concentration or included a known high-concentration cannabis product.
Data Collection and Analysis. We imported search results into Distiller SR, and trained coders conducted artificial intelligence‒assisted screening. We developed, piloted, and revised data abstraction forms. One person performed data abstraction, and a senior reviewer verified a subset. We provide a tabular description of study characteristics, including exposures and outcomes measured, for each included study. We interrogated the evidence map published in Tableau to answer specific questions and provide the results as text and visual displays.
Main Results. We included 452 studies in the scoping review and evidence map. There was incomplete reporting of exposure characteristics including THC concentration, duration and frequency of use, and products used. The evidence map shows considerable heterogeneity among studies in exposures, outcomes, and populations studied. A limited number of reports provided data that would facilitate further quantitative synthesis of the results across studies.
Conclusions. This scoping review and evidence map support strong conclusions concerning the utility of the literature for characterizing risks and benefits of the current cannabis marketplace and the research approaches followed in the studies identified. Relevance of the studies to today’s products is limited.
Public Health Implications. High-quality evidence to address the policy question of whether the THC concentration of cannabis products should be regulated is scarce. The publicly available interactive evidence map is a timely resource for other entities concerned with burgeoning access to high-concentration cannabis. (Am J Public Health. 2023;113(12):1332–1342. https://doi.org/10.2105/AJPH.2023.307414)
PLAIN-LANGUAGE SUMMARY
The potency or tetrahydrocannabinol (THC) concentration in cannabis products has been increasing over the past decade. Policymakers have become interested in whether THC concentration should be regulated. To inform these discussions, we conducted a scoping review and created an evidence map of human studies that explore the relationship of high-concentration cannabis products with any health outcomes. The evidence map including 452 studies shows considerable variability in exposures, outcomes, and populations studied. A limited number of reports provided data that would facilitate synthesis of the results across studies. High-quality evidence to address the policy question of whether the THC concentration of cannabis products should be regulated is scarce. The publicly available interactive evidence map is a timely resource for policymakers and researchers concerned about the increasing access to high-concentration cannabis.
The concentration of pharmacologically active tetrahydrocannabinol (THC) in cannabis products has been increasing over the past decade. While smoking of cannabis products has been declining, routes of administration that use higher-concentration THC products, such as vaping and dabbing, have been increasing.1,2 Concerns about potential harmful health effects of using these high-concentration products and these routes of administration have also been on the rise.3–6
As of February 2023, 37 states allowed medical cannabis, and 19 states had legalized recreational cannabis, providing access to high-concentration products.7,8 Because of growing use of high-concentration products and related health concerns, several states including Connecticut, Illinois, New York, and Washington, have begun to regulate cannabis product “potency,” defined as THC concentration. The rationale for such regulation is that high-concentration cannabis products may pose a greater risk of harmful health effects than lower-concentration products. Colorado House Bill 21-1317 (HB 1317; Concerning the Regulation of Marijuana for Safe Consumption, and, in Connection Therewith, Making an Appropriation) required the Colorado School of Public Health to “do a systematic review of the scientific research related to the physical and mental health effects of high-potency THC marijuana and concentrates.”9 The review has high public health relevancy because the Colorado state legislature commissioned it to inform policy on whether the THC concentration of cannabis product should be regulated or limited. The review team was tasked to cover both harmful and beneficial health outcomes, but the completed review is not focused on clinical uses of cannabis.
The broad question posed by the Colorado state legislature was ideally suited to a scoping review approach. A scoping review is performed to map key concepts, types of evidence, and gaps in research related to a defined area or field.10 Given the heterogeneity in how concentration of cannabis is defined, the broad range of outcomes of interest, and the variety of study designs used to study the health effects of high-concentration cannabis products, the scoping review aimed to clarify the key concepts related to how high concentration is defined, examine how research on harms and benefits of cannabis is conducted, describe the key characteristics associated with these studies, and identify gaps in the evidence.11 Because scoping reviews use systematic review methods, we also aimed to identify subsets of homogeneous studies potentially eligible for future synthesis.
Evidence maps refer to a wide range of practices that visually display evidence synthesis products. Evidence maps are increasingly used, particularly in environmental health, to display the results of scoping reviews of animal and human evidence.12,13 The aim of an evidence map is to catalog and describe evidence rather than to synthesize findings. An evidence map provides an interactive, user-friendly searchable database or visual display of systematically identified literature on a given topic.14,15 By giving a picture of the scope of evidence available, it is a public health good for a broad range of users. An evidence map can be used to identify studies with certain common characteristics, such as outcomes and exposures studied. Users can also interrogate the map to identify studies that can answer a particular policy question and possibly conduct a full systematic review and meta-analysis.12
We describe the scoping review on high-concentration cannabis with the dual goals of documenting the utility of this approach to evidence identification and introducing the evidence map to the public health community. The objectives of the scoping review were to (1) identify and describe human studies that explore the relationship of high-concentration cannabis products with any health outcomes and (2) create an interactive evidence map of included studies to facilitate further analysis.
METHODS
Details on the methods can be found in the published protocol for this scoping review.16 R. L., J.-P. O., and T. W. K. were added as authors because of their contributions following publication of the protocol. We used Joanna Briggs Institute17 and Cochrane18 methodologies for conducting scoping reviews.
Study Selection Criteria
We included research conducted in any country on recreational (nonprescription) cannabis use, medicinal cannabis use, or both. We included studies conducted in humans of any age and excluded animal studies, as well as laboratory or simulation-based mechanistic studies. We included studies of any epidemiological design.
THC concentration of products
We included studies that reported THC concentration for a cannabis product taken by any route or that reported a product description (e.g., “high-potency concentrate,” “dab,” and other names for concentrates) from which a high concentration could be inferred.
THC concentration is not the same as dose or level of exposure. Dose refers to the potential amount of THC available to the consumer of the product. The physiologic effect or health outcome experienced is influenced by THC concentration, the specific type of cannabis product, route of administration, duration of use, frequency of use, experience or tolerance of the user and their ability to self-titrate. Therefore, we included studies that assessed a dose‒response relationship or supported reaching a conclusion about dose.
We included reports that measured THC concentration in different ways (e.g., percentage THC, mg THC).
Some analyses of cannabis health effects use a THC:cannabidiol (CBD) ratio for medicinal use. Products with a high THC:CBD ratio may have a relatively low concentration of THC. Thus, we excluded studies that reported a THC:CBD ratio only and no THC concentration.
Types of products
We included exposures to the following types of cannabis products: plant (dried or undried), edibles, oral capsule or pill preparation, concentrated extract, oils, tinctures, marijuana e-cigarettes, and other or unknown preparations. We excluded CBD or cannabinol-only products and studies of dronabinol, nabilone, and other orally administered medicinal synthetic cannabinoid products.
Health outcomes
We included any health outcomes studied regardless of whether classified as beneficial or adverse. We extracted the verbatim text for each outcome and categorized each according to previous authoritative reports on cannabis.3–5 Categories were mental health, neurologic, pain, cardiometabolic, gastrointestinal, psychosocial, sleep, substance use or dependence, respiratory, cancer, ocular, injury and death, immunity, sexual and reproductive health, pregnancy-related outcomes (mother), and pre-, peri-, and neonatal outcomes.
Data Sources and Searches
A medical information specialist (C. P.) designed and conducted a comprehensive search for the concepts of marijuana or THC. Relevant publications were identified by searching 7 databases with a combination of controlled vocabulary and keywords. We limited the searches to English language and human studies. We excluded comments, editorials, interviews, news articles, and letters as publication types. We did not apply any date limitation. The search strategy was peer-reviewed by another medical information specialist before execution using the PRESS checklist10 (see “Search Strategy and Number of Records Identified” in the Appendix, available as a supplement to the online version of this article at https://ajph.org). We conducted the initial search in October 2021 and updated it in July 2022. We exported all results to DistillerSR19 where duplicates were identified and removed automatically.
Study Selection
Title and abstract screening
We used the artificial intelligence (AI) text-mining features available in DistillerSR to assist in screening.19 We trained the AI screening prioritization algorithm using 1000 randomly selected records. These records were screened and labeled by 2 senior screeners (L. L., T. R.) coding independently, with discrepancies decided by discussion (T. L., L. B., L. L., T. R.).
We used the “trained” DistillerSR’s AI algorithm to rank the remaining unreviewed titles and abstracts. This set of references used continuous AI prioritization; with every 200 records screened, the AI algorithm ranks and reorders records so those scored highly for inclusion are screened sooner.
Full-text screening
We retrieved full-text reports of potentially relevant citations. Two screeners (combinations of L. L., J.-P. O., T. R., T. W. Y., and trained graduate students) reviewed the full text against the eligibility criteria independently with disagreements decided by a senior review team member (L. B. or T. L.). Reasons for excluding full-text reports were recorded.
Quality control and quality assurance
Two reviewers (L. L. and T. R.) checked 2% of all screening decisions at both titles and abstracts and full text screening stages, discussed problems at routine group meetings, and retrained screeners as needed. We also ran the DistillerSRs “Check for Screening Errors” tool to check the human screening decisions against the AI rankings.19 A senior reviewer (L. L. or T. R.) reevaluated flagged references for inclusion.
We report the search and selection according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews (PRISMA-ScR).20 No reviewers involved in screening have published research on cannabis that could be eligible for inclusion and, therefore, they did not have an a priori basis to introduce bias in the selection of studies.
Data Collection
We developed and pilot tested a data extraction form in DistillerSR to manually extract study details from full-text reports. One reviewer extracted data into the data extraction form, which was checked by senior reviewers (L. L., J.-P. O., T. R., T. W. Y.). We extracted data on the following:
-
•
publication information, including authors, type of report, journal, year, type of publication, funding source, country;
-
•
study topic and objectives;
-
•
study design, including location, setting, and inclusion and exclusion criteria;
-
•
characteristics of population, including age, developmental stage, sex, race/ethnicity, indicators of health equity, pregnancy status, and comorbidities;
-
•
details of exposure, including type of cannabis product, route of administration, duration, frequency of intake, experience or tolerance of user, self-titration, and concentration;
-
•
details of comparison exposure, if applicable; and
-
•
outcomes, including outcome domain, outcome descriptor, measurement method, metric, method of aggregation, and time point.
The complete list of data extraction items can be found at our Open Science Framework Project page: https://osf.io/9kndw/?view_only=b6f472d680af41bc84e8a6aa337fd04b.
As per scoping review methods, we did not assess risk of bias for primary studies because of heterogeneity of study designs included.21
Presentation and Analysis of Included Studies
To facilitate exploration of the extracted information from the scoping review and to provide a resource to other researchers and the public, we created a publicly available evidence map. All extracted data were exported from DistillerSR to R Studio and reformatted into multiple data sets for the evidence map in Tableau. We used study ID to link all evidence map components, enabling cross-filtering. The evidence map is published to the University of Colorado public Tableau server:
https://viz-public.cu.edu/#/site/Anschutz/views/EvidenceMap/Home?:iid=1.
We provide a tabular description of study characteristics, including exposures and outcomes measured, for each included study.
We interrogated the evidence map to address the following questions, relevant to current policy discussions, and provide the results as text and visual displays:
-
•
Of the different types of cannabis products studied, how many have reported THC concentration, frequency, or duration?
-
•
What THC concentrations have been reported in the literature?
-
•
What types of outcomes have been examined for studies that reported THC concentration?
-
•
What types of outcomes have been studied for the different types of cannabis products?
-
•
What THC concentrations have been studied by outcome?
RESULTS
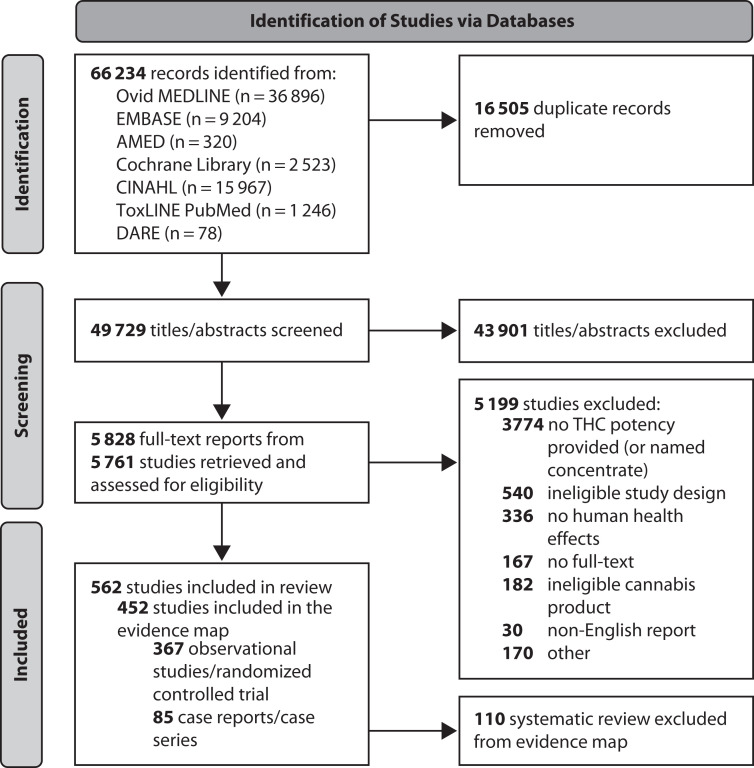
Database searches identified 49 729 unique titles and abstracts for screening, resulting in 5828 full text reports. We included 452 studies in the scoping review (367 observational studies or randomized trials and 85 case reports or case series) and evidence map (Figure 1). The earliest publication date of an included study was 1971, and 60% (n = 269) of the studies were published between 2017 and 2022.
FIGURE 1—
PRISMA Diagram for Study Identification for Health Effects of High-Concentration Cannabis Products: Scoping Review and Evidence Map
Note. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses; THC = tetrahydrocannabinol.
Evidence Map
The interactive evidence map is available at https://viz-public.cu.edu/#/site/Anschutz/views/EvidenceMap/Home?:iid=1.
Bibliographic details for the 452 included studies are provided in the evidence map. The dashboard is organized so that studies can be sorted by study characteristics, population investigated, exposures to cannabis products, and health outcomes. The dashboard links to bibliographic information and the abstracts for all studies identified.
Characteristics of Included Studies
The characteristics of included studies are summarized in Appendix Table A. The 452 studies had variable objectives: harm of a product (n = 349; 77%) and efficacy for a therapeutic indication (n = 233; 52%). Cannabis products addressed in the studies were used for several reasons including medicinal use (n = 177; 39%), recreational use (n = 68; 15%), and unintentional use (n = 20; 4%). One hundred fifty-six studies reported some other purpose of cannabis use (35%), and 87 studies did not report the purpose of cannabis use (19%).
The studies were classified by study design: observational studies (n = 225; 50%), randomized control trials (n = 142; 31%), case reports (n = 51; 11%), and case series (n = 34; 8%).
The studies were conducted across multiple countries, primarily in the United States (n = 220; 49%), the United Kingdom (n = 46; 10%), and Canada (n = 45; 10%). There was at least 1 study from 27 other countries. Within the United States, studies were done primarily in California (n = 47; 10%), Colorado (n = 27; 6%), and New York (n = 18; 4%), but participants from all states other than Alabama, Delaware, and West Virginia were involved in at least 1 study.
Disclosures of study funding source, author affiliations, and conflicts of interest were often lacking: 24% (n = 109) of studies did not report funding source, and 32% (n = 143) of studies did not report if authors had conflicts of interest. A small proportion of studies (n = 25; 6%) were funded by the cannabis industry; 13% (n = 58) had at least 1 author who disclosed a financial tie with the industry.
Only 11% (n = 48) of studies included any analysis on a health equity measure. Less than one tenth of studies (n = 41; 9%) included analysis or stratification by health equity subgroups, 1% (n = 6) of studies focused exclusively on historically excluded populations, and no studies included specific analyses of structural racism or inequalities.
The study populations were variable, including ages from newborn to adults aged older than 65 years, with a range of racial and ethnic groups. Some studies also had restrictions on eligibility requirements, such as a preexisting disease or condition.
Exposures
The most common cannabis products studies were of generic cannabis types (n = 284; 63%). Products that are typically high concentration, such as oils, concentrates, hash, extracts, skunk, and resins, were examined in approximately 2% to 10% of all studies. Overall, 384 studies (85%) reported the frequency of intake, with the most common being daily (n = 177; 39%), and 371 (82%) reported the duration of intake. The route of administration was reported in 393 studies (87%) including inhalation (n = 279; 62%), ingestion (n = 174; 38%), sublingual (n = 39; 9%), and topical (n = 31; 7%).
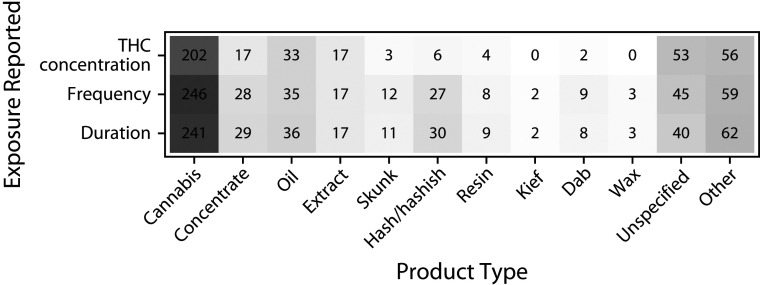
Studies did not consistently provide complete information on exposure characteristics such as THC concentration and frequency and duration of exposure. Figure 2 addresses the question, “Of the different types of cannabis products studied, how many have reported THC concentration, frequency, or duration?” Details of cannabis exposure were reported most often in studies that included generic cannabis (Figure 2).
FIGURE 2—
Number of Studies With Reported THC Concentration, Frequency, or Duration of Cannabis Use by Cannabis Product Type in Health Effects of High-Concentration Cannabis Products: Scoping Review and Evidence Map
Note. THC = tetrahydrocannabinol. Counts indicate the number of studies with an included cannabis product type and cannabis exposure characteristic. Studies may include multiple product types and exposures. Color saturation indicates the number of studies with a reported product or exposure in relation to other product or exposures. Total n = 446 because 6 studies reported that they tested a high-concentration product but did not report numeric THC concentration, frequency, or duration.
There was substantial variability in reporting of THC concentration, including the units and indices used (e.g., range, threshold, exact values, mean). When THC concentration was reported, it was most commonly as percentage of THC (n = 172; 38%) or milligrams of THC (n = 113; 25%). We interrogated the evidence map to address the question, “What THC concentrations have been reported in the literature?” There were 172 studies that reported THC concentration with percentage of THC corresponding to 349 different exposures. For these studies, the median concentration was 12% (mean = 17.4%; range = 0%–100%; Q1 = 3.6%; Q3 = 24%). Of 113 studies with 143 exposures reporting concentration in milligrams of THC, the median concentration was 15 milligrams (mean = 37.4 mg; range = 0.3–500 mg; Q1 = 7. 5 mg; Q3 = 26 mg).
Outcomes
The most common outcome domain for the 452 included studies was mental health (n = 180; 40%), followed by neurologic (n = 134; 30%), pain (n = 133; 29%), cardiometabolic (n = 110; 24%), gastrointestinal (n = 101; 22%), psychosocial (n = 98; 22%), and sleep (n = 94; 21%). Outcome domains are broad. For example, the mental health outcome domain included depression, psychosis, memory, and cognition. Even a single outcome (such as depression) was measured in different ways in different studies (such as depression scales, clinical chart review, or self-report).
We interrogated the evidence map to address the question, “What types of outcomes have been studied for the different types of cannabis products?” We found that the outcome domains studied by product type showed a similar distribution to outcomes studied overall as shown in Appendix Table A.
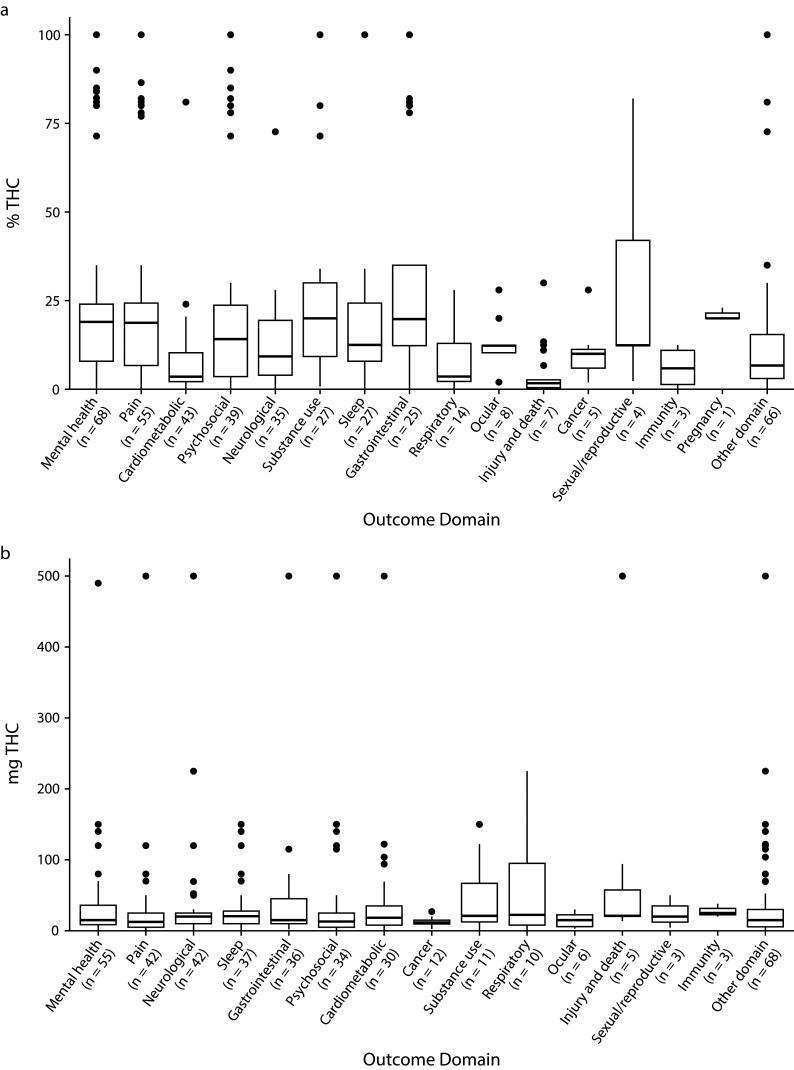
Studies across all outcome domains reported numeric THC concentrations. Figure 3 addresses the question “What THC concentrations have been studied for the different outcome domains?” Median THC concentrations reported were less than 50 milligrams or 25% THC for all outcome domains studied.
FIGURE 3—
THC Concentration Reported as Percentage of THC and Milligrams of THC by Outcome Domain in Health Effects of High-Concentration Cannabis Products: Scoping Review and Evidence Map
Note. IQR = interquartile range; THC = tetrahydrocannabinol. Boxplot of highest reported THC concentration for concentrations reported in % THC (n = 172) or mg THC (n = 113) by outcome domain. We report data on THC concentration reported as an exact concentration (e.g., 10% THC), a range (e.g., 1–10 mg THC), a threshold (e.g., < 5% THC), or some other method of aggregation (e.g., mean % THC). THC concentration values were not standardized. The midline of the box is the median THC concentration, the top and bottom of the box are first and third quartile (Q1 and Q3), whiskers represent the Q1‒1.5(IQR) and Q3 + 1.5(IQR), and points beyond the whiskers are outliers beyond this range.
Potential for Evidence Synthesis
Few studies provided data that would facilitate further quantitative synthesis of the results across studies. Fifty-four studies (12%) examined a direct association between cannabis concentrates and a health outcome, whereas 189 (42%) examined a direct association between THC concentration and a health outcome. One hundred fourteen studies (25%) examined an indirect association between THC concentration and health outcomes. Sixty-two studies (14%) examined an indirect association between concentrates and health outcomes. Two hundred twelve studies (47%) included a control group. Outcomes were reported with effect estimates (n = 184; 41%), measures of precision (n = 274; 61%), significance tests (n = 335; 74%), sample size (n = 349; 77%), correlation coefficients (n = 71; 16%), raw data (n = 232; 51%), and parameter estimates (n = 91; 20%).
DISCUSSION
This scoping review and the related evidence map provide the most comprehensive look at the literature on high-concentration cannabis and health to date, to our knowledge. We developed this review at a time when the majority of states made cannabis available for medical purposes, and an increasing number were legalizing cannabis for recreational use. The frequency of use is increasing, raising critical questions for public health about risks associated with ready access, particularly to higher-concentration products. These questions were recognized by the Colorado General Assembly in its request to the Colorado School of Public Health. The scoping review depicts a heterogenous evidence base with important gaps regarding the health effects of high-concentration cannabis.
Concentrations Studied
There is a mismatch between the THC concentrations and types of cannabis products that are used now, and the types of cannabis products and concentrations studied in the literature identified. A 2018 survey of THC concentration in cannabis products sold in 7 states that allowed cannabis found that most products in all states contained between 15% and 30% THC.22 From more than 70% of products sampled in Maine to more than 91% of products sampled in Colorado contained greater than 15% THC. THC concentrations have also increased over time. For example, the concentration of THC in cannabis flowers assayed in Colorado has increased from an average of 14% to 19% from 2014 through 2020.23 Cannabis concentrate products have increased in strength from an average of 46% THC in 2014 to 68% THC in 2020.23 The range of products continues to expand, including not only flowers but also edible products and a variety of concentrate products.24
Our review documents a wide range of concentrations in cannabis products that have been studied, with a median of 12% THC concentration, well below what is currently available on the market. Sixty percent of the included studies were published in the past 6 years, from 2017 to 2022. The low concentrations of THC studied likely reflect the restriction of cannabis for research purposes in the United States to that available through the National Institute for Drug Abuse (NIDA) Drug Supply Program.25 The varieties of cannabis available to investigators through NIDA are limited in scope and lower in concentration than what people can obtain from their local dispensaries or the illegal market, and cannabis concentrates are not available to researchers.26 Epidemiological studies can address the products in use, but, inevitably, their findings will lag behind what is happening in today’s dynamic marketplace.
Exposure Assessment
The THC exposure dose, or amount of THC entering the body, depends not only on concentration in the product but also on route of administration, frequency of use, and characteristics of the individual using the product. We found a wide range of approaches to assessing exposure to cannabis products; most studies failed to capture all of the elements of cannabis use history needed to estimate exposure dose. Incomplete reporting of exposure characteristics makes it difficult to assess the association between exposure and likelihood of an adverse (or beneficial) health outcome.
The evidence map documents the broad scope of the exposure assessment problem, showing that THC concentration, route of administration, and frequency of use are not consistently reported, particularly in observational studies. The heterogeneity in how THC exposure was reported and measured, and in the units used also complicates evidence synthesis. The evidence map highlights a problem that needs to be addressed with urgency: attention should be given to developing systematic and standardized approaches for assessing use of cannabis products. At a minimum, studies should report exposure as THC concentration, product used, frequency of use, and route of administration, and use THC units that can be standardized. There have been significant steps in this direction, including the development of consensus standards for cannabis measurement and THC units, but these must take high-concentration exposures into account.27,28
Outcomes Studied
The evidence map shows that a wide array of health outcome domains, both harmful and beneficial, have been studied, as found in other recent reports.25 For some outcomes, such as depression and anxiety, standardized instruments are available, but they are used variably. Large heterogeneity in specific outcomes studied and how they were measured hinders the possibility of conducting quantitative evidence synthesis. Bringing some homogeneity to this aspect of research on cannabis may not be feasible, given the wide range of outcomes. Within the cannabis research community, perhaps agreement could be reached on standardizing approaches to some of the most critical outcomes, using methods similar to those used to develop core outcomes sets for clinical trials.29,30 Despite the heterogeneity of the outcome measures, the evidence map can be used to identify clusters of studies within outcomes domains that can be summarized by using narrative or visual methods.31
Populations Studied
Another key issue identified by our review is the range of populations studied and how well the characteristics of the study populations align with the characteristics of people who use cannabis products. Generalizability of findings is critical, but it cannot be readily gauged because we lack sufficiently specific information on the demographic characteristics of those who use different cannabis products. In addition, key populations may not be included among those studied, particularly racial and ethnic minority groups. The scoping review also revealed a major gap in use of health equity indicators. Most studies did not include any measures of income, education, poverty, employment, disability, structural racism, racial inequalities, or other indicators that would allow prespecified subgroup analysis of those who might experience high rates of adverse effects.
Limitations
To be comprehensive, our searches were designed to be sensitive rather than specific. Because of the broad search terms used, a large number of studies needed to be screened for the inclusion criteria for the scoping review. Although we used AI-assisted screening and trained graduate students to screen identified records, it is possible that relevant studies were not included. Some limitations are inherent to the nature of scoping reviews. Heterogeneity in the designs of the included studies did not allow for risk-of-bias assessment of individual studies. Such assessment could be conducted in the future if the identified studies are considered sufficient for a full systematic review. Lastly, incompleteness and inconsistencies in how studies were reported resulted in variability and gaps in data extracted.
Conclusions
This scoping review supports strong conclusions concerning the utility of the literature for characterizing risks and benefits of the current cannabis marketplace and the research approaches followed in the studies identified. The review suggests that major improvements are needed in how studies measure and report exposures and outcomes to facilitate future evidence synthesis. There is heterogeneity in approaches taken for describing products and for characterizing their use. We found serious limitations in generalizability of the studies to the current marketplace or user base.
Our scoping review and evidence map assessed all available evidence addressing the timely policy question of whether THC concentration of cannabis products should be regulated. Our review shows that high-quality evidence to address this question is scarce. However, the publicly available interactive evidence map enables researchers and other interested individuals to identify specific studies or groups of studies that address a particular question. As the evidence base expands and improves, updating of the evidence map will provide ready access to relevant studies.
With funding from the State of Colorado, we have developed a resource that we are using to address issues raised by the Colorado General Assembly as it seeks to protect public health in the state. The evidence map is a timely resource for other entities concerned with burgeoning access to cannabis. Ideally, it will be maintained as an “evergreen” resource, tracking the expanding literature.
ACKNOWLEDGMENTS
This review was funded by the State of Colorado as specified in House Bill 21-1317. The funder was not involved in the design, conduct, or publication of this paper.
We thank the scientific review committee for comments on the protocol for the scoping review and the following public health graduate students for help with screening studies and data extraction: Jamali Bilquees, Haley Burns, Hannah Craig, Madison Davenport, Austin Daw, Ashley Dehmlow, Karan Dhindsa, Emily Drewniany, Alexis Engelgau, Utibeabasi Ettah, Noah Featherman, Jackson C. Fox, Efrn Garcia, Sarah Gelinas, Andrew Jones, Colin Joseph, Deanna Kapitanec, Shania Lunna, Tenejah Mathis, Samuel Messenger, Devin E. Miller, Lydia Mudd, Natalia Rahman, Daphna Rubin, Sagun Sharma, Nicole Tabor, Kevin Wells, and Cydney Wood. We thank Neeloofar Soleimanpour and Meghan Buran for help with preparing the article.
CONFLICTS OF INTEREST
L. Bero is a paid consultant as conflict of interest advisor for Health Canada, including its cannabis committees. G. S. Wang is a paid author for UpToDate for a chapter on cannabis intoxication. A. Brooks-Russell has research funding from the Institute of Cannabis Research, Colorado State University, and serves on the Colorado Department of Public Health and Environment’s Marijuana Public Health Advisory Committee.
HUMAN PARTICIPANT PROTECTION
This study did not involve human participants.
REFERENCES
- 1.Patrick ME, Miech RA, Kloska DD, Wagner AC, Johnston LD. Trends in marijuana vaping and edible consumption from 2015 to 2018 among adolescents in the US. JAMA Pediatr. 2020;174(9):900–902. doi: 10.1001/jamapediatrics.2020.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boakye E, Obisesan OH, Uddin SMI, et al. Cannabis vaping among adults in the United States: prevalence, trends, and association with high-risk behaviors and adverse respiratory conditions Prev MedPrev Med. 2021153106800. 10.1016/j.ypmed.2021.106800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannabis concentration and health risks. Seattle, WA: Washington State Prevention Research Subcommittee; 2020. [Google Scholar]
- 4.Retail Marijuana Public Health Advisory Committee. Monitoring health concerns related to marijuana in Colorado. Denver, CO: Colorado Department of Public Health and Environment; 2020. [Google Scholar]
- 5.The health and social effects of nonmedical cannabis use. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 6.Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000–1010. doi: 10.1111/add.15253. [DOI] [PubMed] [Google Scholar]
- 7.ProCon/Encyclopedia Brittanica. https://medicalmarijuana.procon.org/legal-medical-marijuana-states-and-dc
- 8.National Conference of State Legislatures. 2022. https://www.ncsl.org/civil-and-criminal-justice/cannabis-overview
- 9.Regulating Marijuana Concentrates. 2021.
- 10.Colquhoun HL, Levac D, O’Brien KK, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67(12):1291–1294. doi: 10.1016/j.jclinepi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 12.Wolffe TAM, Whaley P, Halsall C, Rooney AA, Walker VR. Systematic evidence maps as a novel tool to support evidence-based decision-making in chemicals policy and risk management. Environ Int. 2019;130:104871. doi: 10.1016/j.envint.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saran A, White H. Evidence and gap maps: a comparison of different approaches. Campbell Syst Rev. 2018;14(1):1–38. doi: 10.4073/cmdp.2018.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5(1):28. doi: 10.1186/s13643-016-0204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelch KE, Bolden AL, Kwiatkowski CF. Environmental chemicals and autism: a scoping review of the human and animal research. Environ Health Perspect. 2019;127(4):046001. doi: 10.1289/EHP4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bero L, Li T, Rittiphairoj T, et al. Health effects of high-potency cannabis products: a scoping review protocol. OSF Registries. 2021. [DOI]
- 17.Peters M, McInerney P, Munn Z, Tricco A, Khalil H. JBI Manual for Evidence Synthesis. Philadelphia, PA: Wolters Kluwer; 2020. Scoping reviews. [Google Scholar]
- 18.2020. https://training.cochrane.org/resource/scoping-reviews-what-they-are-and-how-you-can-do-them
- 19.2021.
- 20.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 21.Clarke DE, Gonzalez M, Pereira A, Boyce-Gaudreau K, Waldman C, Demczuk L. The impact of knowledge on attitudes of emergency department staff towards patients with substance related presentations: a quantitative systematic review protocol. JBI Evid Synth. 2015;13(10):133–145. doi: 10.11124/jbisrir-2015-2203. [DOI] [PubMed] [Google Scholar]
- 22.Cash MC, Cunnane K, Fan C, Romero-Sandoval EA. Mapping cannabis potency in medical and recreational programs in the United States. PLoS One. 2020;15(3):e0230167. doi: 10.1371/journal.pone.0230167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.University of Colorado Leeds School of Business, MPG Consulting. 2020. https://sbg.colorado.gov/sites/sbg/files/2020-Regulated-Marijuana-Market-Update-Final.pdf
- 24.Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol. 2019;30:98–102. doi: 10.1016/j.copsyc.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda, Board on Population Health and Public Health Practice, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press; 2017. [DOI] [PubMed] [Google Scholar]
- 26.Stith SS, Vigil JM. Federal barriers to cannabis research. Science. 2016;352(6290):1182. doi: 10.1126/science.aaf7450. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzetti V, Hindocha C, Petrilli K, et al. The International Cannabis Toolkit (iCannToolkit): a multidisciplinary expert consensus on minimum standards for measuring cannabis use. Addiction. 2022;117(6):1510–1517. doi: 10.1111/add.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman TP, Lorenzetti V. A standard THC unit for reporting of health research on cannabis and cannabinoids. Lancet Psychiatry. 2021;8(11):944–946. doi: 10.1016/S2215-0366(21)00355-2. [DOI] [PubMed] [Google Scholar]
- 29.Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13(1):132. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinsen CAC, Vohra S, Rose MR, et al. Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a “core outcome set.”. Trials. 2014;15(1):247. doi: 10.1186/1745-6215-15-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]