Abstract
Objectives
Systemic inflammation is increasingly being recognised as a possible mechanism for acute arterial thrombotic events, including acute coronary syndrome (ACS). Despite this, there is conflicting data on the risk of ACS in patients with inflammatory bowel disease (IBD). We performed a contemporary systematic review and meta-analysis to identify the risk of ACS in patients with IBD.
Methods
PubMed, MEDLINE, EMBASE, CENTRAL and Web of Science were searched up to 27 October 2022. Multivariable-adjusted or propensity matched studies with a non-IBD control cohort were included. HRs were pooled using a random-effects model. Subgroup and sensitivity analyses were conducted in order to explore sources of heterogeneity.
Results
Twelve retrospective cohort studies were included (225 248 IBD patients). Patients with IBD were associated with an increased risk of ACS in both adjusted (HR 1.23; 95% CI 1.08 to 1.41) and unadjusted analyses (HR 1.50; 95% CI 1.16 to 1.92). Substantial heterogeneity was observed (i2=88, p=0.002 and i2=98%, p=0.002, respectively). Subgroup analysis of age revealed a greater association of ACS in IBD patients <40 years of age (relative HR 1.50; 95 CI 1.15 to 1.96).
Conclusion
Patients with IBD demonstrated an independently increased risk of ACS. Prospective studies are required to explore the relationship with disease activity and duration, concomitant medication use and angiographic characteristics and outcomes.
PROSPERO registration number
CRD42022367846.
Keywords: Acute Coronary Syndrome, Epidemiology, Coronary Artery Disease
WHAT IS ALREADY KNOWN ON THIS TOPIC
Systemic inflammation is increasingly being recognised as a possible mechanism for acute arterial thrombotic events, including acute coronary syndrome (ACS). Despite this, there is conflicting data on the risk of ACS in patients with inflammatory bowel disease (IBD).
WHAT THIS STUDY ADDS
In this largest to date systematic review and meta-analysis, patients with IBD demonstrated an independently increased risk of ACS after adjusting for traditional cardiovascular risk factors.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study demonstrated an increased risk of ACS in IBD patients. Determining this risk is important given the younger age of these patients, potential for treatment with available systemic therapies and primary preventative strategies.
Introduction
Acute coronary syndrome (ACS) and inflammatory bowel disease (IBD) are major causes of morbidity and mortality worldwide.1 2 In recent years, systemic inflammation is increasingly being recognised as a possible risk factor for venous and arterial thrombotic events, including ACS.3–7 The role of proinflammatory cytokines such as IL-1B, IL-6, IL-8, IL-12, serum amyloid A, C reactive protein in addition to nitric oxide production and chronic endothelial dysfunction have all been associated with accelerated atherogenesis.4 8 Atherosclerosis is a chronic inflammatory state of arterial walls; the anti-inflammatory properties of statin therapy have been well described with robust evidence of clinical use associated with decreased vascular events in the general population.9 As such, several chronic immune-mediated diseases have been linked to the risk of acute arterial events including ACS.10 11
Despite this, the risk of ACS in patients with ulcerative colitis (UC) and Crohn’s disease (CD) is ambiguous.12–20 Challenges in characterising this risk include the presence of concurrent ‘traditional’ cardiovascular risk factors and targeting studies specifically towards ACS. Previous meta-analyses that have identified an increased risk of ‘ischaemic heart disease’ (IHD) did not assess ACS specifically, which may be beneficial in excluding certain confounders such as the inclusion of those patients with pre-existing known coronary disease or unrelated coronary events.12 18–20 No definitive management guidelines have been recommended for these patients.8 21 Thus, we performed an updated systematic review and meta-analysis with the aim of evaluating the risk of ACS in patients with IBD.
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-analysis of Observational Studies in Epidemiology statement guidelines.22 23 This review is registered with PROSPERO and did not require institutional board review.
Study eligibility
Studies were eligible for inclusion if they satisfied the following criteria: retrospective cohort studies that are multivariable-adjusted or propensity matched with a non-IBD control cohort; primary exposure of IBD based on international classification of diseases codes or endoscopic/histopathological findings; ACS reported as outcome of interest. There was no restriction on language and our search included articles in all languages.
The exclusion criteria included: case–control and cross-sectional studies, case reports, editorials and previous systematic reviews; studies without a distinct IBD population (ie, overlapping with other immune-mediated inflammatory diseases); ACS data not clearly demarcated (ie, overlapping with coronary artery disease or other acute arterial events); studies including patients with a history of ACS prior to IBD diagnosis; data were derived from the same cohort or insufficient data to calculate risk estimates.
Data sources and search strategy
A comprehensive search strategy was designed and a thorough computer-based search was performed using PubMed, Ovid MEDLINE, EMBASE, CENTRAL and Web of Science databases. No time limit to start date was applied, and the search was conducted up to 27 October 2022. We manually searched the references cited in the previous reviews and other important studies related to this subject. We did not need to contact the corresponding authors of the studies, as the relevant information was easily accessible from the original studies. The search strategy, search terms used, inclusion and exclusion criteria are provided in online supplemental materials.
openhrt-2023-002483supp001.pdf (555.5KB, pdf)
Study selection and data extraction
Two reviewers (AZ and DS) screened all the titles and abstracts independently. This was performed with a free-to-use web application (Rayyan, Qatar Computing Research Institute, Ar-Rayyan, Qatar). Conflicts were resolved by inclusion of a third reviewer (NM). This was followed by the full-text review of the selected articles by the two independent reviewers (AZ and NM). We then extracted the data from selected studies using a standardised, pilot-tested extraction template. The following data were extracted: study characteristics (author, year of publication, country, study design, study population, number of participants and follow-up measures), clinical characteristics, baseline demographics, incidence and HRs ACS (adjusted and unadjusted), information on mortality (all-cause mortality and cardiac mortality), stroke, bleeding and angiographic outcomes if available.
Quality assessment
Two reviewers (AZ and DS) assessed quality of included studies by using the Down and Black checklist.24 Downs and Black score ranges were given corresponding quality levels as previously reported; excellent25–27; good20–24 28; fair15–19 and poor (≤14). We evaluated potential biases using classifications of ‘low risk of bias’ when data for the criterion were described, ‘high risk of bias’ when data were not stated and ‘unclear risk of bias’ when the criterion was not relevant to the study design. Any conflicting classification was resolved by discussion with a third reviewer (NM). We assessed publication bias by visual inspection of funnel plots. Eggers’ intercept was not performed if no asymmetry discerned.
Outcomes
The primary outcome was ACS, defined according to the criteria used in the original studies. Additional outcomes and subgroup analysis are detailed in online supplemental material.
Statistical analysis
Associations between IBD and ACS are reported using HRs with 95% CI and meta-analysed in adjusted form using a random effects model after pooling reported HRs. We also pooled unadjusted estimates if the studies provided sufficient data. Heterogeneity between studies was assessed by combination of I2 statistic, Cochran’s Q test and χ2 test. Subgroup analyses based on age and gender were performed in the studies that reported adjusted associations.28 Further subgroup analysis for follow-up duration and smoking were performed based on study-level variables. All calculations were performed using Review Manager V.5.4 Cochrane Collaboration and Stata V.16.1 for Windows using the DerSimonian and Laird random effects model to account for the variation in study design between studies.
Results
Study characteristics
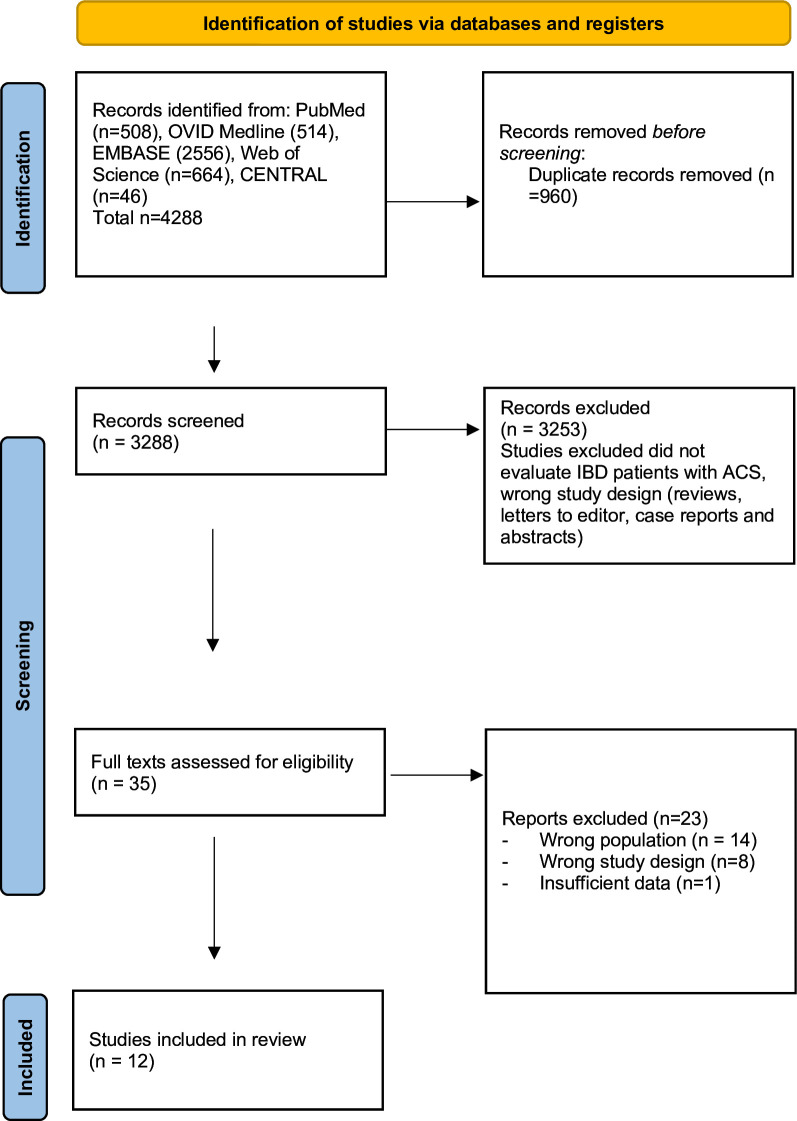
The literature search yielded 4288 citations. A total of 3328 records were identified after duplicates were removed. After excluding articles that did not evaluate IBD patients and non-primary research articles including reviews, letters as well as abstracts and conference abstracts, 35 articles were chosen for full-text review (figure 1). Of the 35 articles, 12 retrospective cohort studies met the inclusion and exclusion criteria. Detailed rationale for inclusion and exclusion after full-text review is provided in online supplemental materials. Complete study characteristics are shown in table 1.
Figure 1.
Flowchart. ACS, acute coronary syndrome; IBD, inflammatory bowel disease.
Table 1.
Characteristics of included studies
| Study | Data source | Study period | Number of IBD patients | Definition of IBD | Definition of ACS | Adjusted risk (95% CI) | Unadjusted risk (95% CI) | Covariates |
| Aniwan et al25 | Rochester Epidemiology Project | 1980–2010 | 746 | REP central diagnostic index; clinical, endoscopic, and histological findings | Rise of cardiac biomarker and with at least one of the new or presumed new significant ST-segment-T wave changes or new left bundle branch block (LBBB), development of pathological Q waves, r new regional wall motion abnormality or identification of intracoronary thrombus at cardiac catheterisation or autopsy | 1.98 (1.41 to 2.79) | 2.82 (1.97 to 201.03) | Age, sex, hypercholesterolaemia, diabetes mellitus, hypertension, familial coronary artery disease |
| Card et al26 | Clinical Practice Research Datalink | 1997–2017 | 31 175 | ICD unspecified | Based on diagnostic coding (unable to access appendix). Also codes from both primary care (primary care section of CPRD) and secondary care (HES) to identify the MI and stroke outcome events. | 0.92 (0.82 to 1.04) | 1.13 (1.03 to 1.24) | Age, gender, smoking, diabetes, hypertension, hypercholesterolaemia, alcohol use, 5-ASA use, BMI, hospitalisation |
| Kristensen et al35 | Danish National Patient Register | 1996–2009 | 20 795 | ICD-10 | ICD 10 121–122 | 1.17 (1.05 to 1.31) | 2.93 (2.64 to 3.25) | Age, gender, co-morbidity, cardiovascular medication and socioeconomic status |
| Rungoe16 2012 | Danish National Patient Register | 1997–2009 | 28 833 | ICD-10 | ICD 10 121–122 | CD 1.18 (0.92 to 1.51) UC 1.14 (1.00 to 1.29) |
CD 1,41 (1.21 to 1.64) UC 1.38 (1.27 to 1.49) |
Time-dependant use of comorbidity-related drugs (antidiabetic agents, antihypertensive drugs, cholesterol-lowering drugs, anticoagulant drugs and antiarrhythmic agents) |
| Osterman 201137 | General Practice Research Data Base | 1987–2003 | 25 327 | ICD-10 | ICD-10 121–122 | CD 1.09 (0.89 to 1.34) UC 1.11 (0.98 to 1.26) |
CD 1.15 (0.94 to 1.41) UC 1.18 (1.03 to 1.34) |
Age, sex, history of hypertension, diabetes mellitus, hypercholesterolemia, smoking status, body mass index and aspirin use |
| Setyawan et al27 | MarketScan Commercial and Medicare Supplemental Databases | 2014–2017 | 34 687 | ICD-9, ICD-10 | Based on previous subgroup analysis—ICD 9 code and ICD 10 codes. Patients with venous and arterial TEs were identified as those with ≥1 ICD-9-CM and/or ICD-10-CM code for DVT, PE, MI or IS during the study period. No further details provided | 0.62 (0.44 to 0.88) | 0.81 (0.73 to 0.90) | Age at index date, sex (female), baseline comorbidities including cancer, cardiovascular diseases, CKD, COPD, diabetes, PAD, hormonal therapies, baseline non-immune-mediating drugs and baseline thromboembolism of interest (yes/no) |
| Sinha et al29 | Northwestern Medicine Enterprise Data Warehouse | 2000–2019 | 1290 | ICD-9, ICD-10 | ICD 10 121–122 | 1.11 (0.62 to 2.00) | Not reported | Age, sex, race/ethnicity, insurance, baseline year, hypertension, diabetes, current smoking, total cholesterol, statin use and systemic steroid use. |
| Tsai 201438 | Taiwan National Health Insurance Research Database | 1998–2010 | 11 822 | ICD-9 | ICD9 410 | 1.73 (1.54 to 1.94) | 1.87 (1.77 to 1.97) | Age, sex, hypertension, diabetes, hyperlipidaemia, COPD and heart failure |
| Yarur 201139 | Jackson Memorial Hospital Medical Records | 1995–2009 | 356 | ICD-9 | ICD9 410 | 1.81 (0.83 to 3.97) | 1.81 (0.83 to 3.96) | Hypertension, diabetes mellitus, family history of coronary artery disease, CKD, BMI>30, WBC count, platelet count, anaemia |
| Choi et al30 | National Health Insurance Service South Korea | 2006–2009 | CD=10 708 UC=26 769 |
ICD-10 | ICD 10 121–122 | CD 1.80 (1.47 to 2.21) UC 11.11 (0.99 to 1.24) |
Not reported | Age, sex, residence type, income, hypertension, DM and dyslipidaemia |
| Ha 200940 | National Health Insurance Service South Korea | 2001–2003 | 17 448 | ICD-9 | Acute myocardial infarction defined as per ICD-9CM 410.x | CD 1.10 (0.92 to 1.32) | Not reported | Hypertension, hyperlipidaemia and diabetes |
| Gill 202031 | MedStar Health System | 1999–2011 | 15 292 | ICD-9, ICD-10 | Not defined | 1.31 (1.08 to 1.58) | Not reported | Age, gender, race hypertension, hyperlipidaemia, diabetes mellitus and smoking |
ACS, acute coronary syndrome; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; DM, diabetes mellitus; HES, Hospital episode statistics; IBD, inflammatory bowel disease; PAD, Peripheral arterial disease; REP, Rochester Epidiemiology Project; WBC, white blood cell.
Baseline characteristics
Twelve retrospective cohort studies included with 225 248 IBD patients, of which 51.1% (115,101) were men and 48.8% (110,147) were women. The mean age was 42.3±3.2 years. The average follow-up was 81.2±6.3 months. Detailed baseline characteristics for the IBD exposure cohort are provided in online supplemental materials.
Outcomes
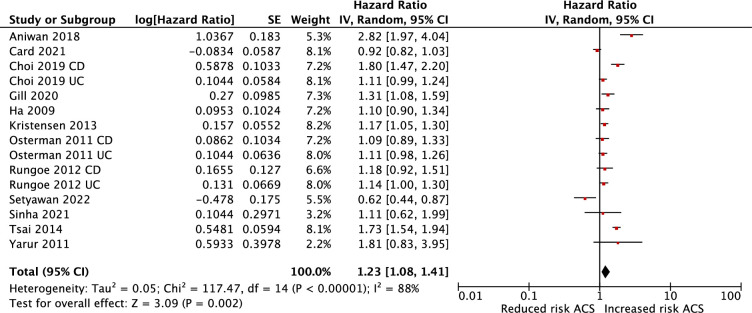
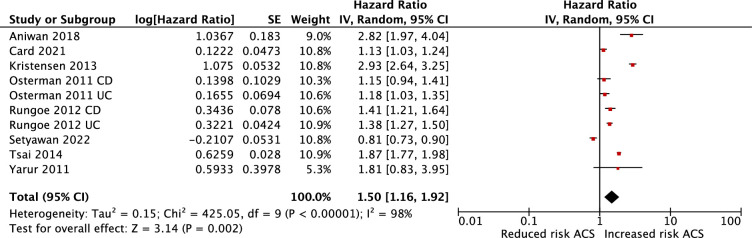
Patients with IBD were associated with an increased risk of ACS in both adjusted (HR 1.23; 95% CI 1.08 to 1.41) and unadjusted analyses (HR 1.50; 95% CI 1.16 to 1.92). Substantial heterogeneity was observed in the adjusted and unadjusted analysis (I2=88%, p=0.002 in adjusted and I2=98%, p=0.002 unadjusted) (figures 2 and 3, respectively). We also conducted a meta-analysis of studies that reported risk of ACS based on type of IBD. Patients with CD demonstrated an increased risk of ACS (adjusted HR 1.72; 95% CI 1.22 to 2.41), patients with UC also demonstrated an increased risk of ACS, though not as pronounced (adjusted HR 1.28; 95% CI 1.06 to 1.55). For studies with direct comparison, CD demonstrated greater association with ACS than UC (adjusted HR 1.24; 95% CI 0.98 to 1.56), but this was not statistically significant (p=0.07) (figure 10 in online supplemental material).
Figure 2.
Meta-analysis of the adjusted association between IBD and ACS. ACS, acute coronary syndrome; IBD, inflammatory bowel disease.
Figure 3.
Meta-analysis of the unadjusted association between IBD and ACS. ACS, acute coronary syndrome; IBD, inflammatory bowel disease.
We investigated sources of heterogeneity by performing a subgroup analysis based on age, gender, follow-up duration and smoking. A within-trial comparison of women and men was possible for the three studies that reported adjusted associations between IBD and ACS stratified by sex (see figure 6 in online supplemental material). Meta-analysis suggested the association of IBD and ACS is 28% higher for women compared with men (HR 1.28; 95% CI 1.11 to 1.48) with no significant heterogeneity. Furthermore, after within-trial comparison of the three studies that reported adjusted association between ACS and IBD stratified by age, there was a greater association between IBD and ACS in those with age <40 years compared with age >40 years (HR 1.50; 95 CI 1.15 to 1.96) with no significant heterogeneity (figure 7 in online supplemental material).
For the subgroup analysis of follow-up duration, six studies were stratified based on the average follow-up time (figure 8 in online supplemental material). Studies that did not report average follow-up duration were excluded from this subgroup analysis. Our analysis found that patients with longer duration of follow-up (greater than 5 years) were not associated with a higher risk of ACS than patients with shorter duration (HR 1.25, 95% CI 1.05 to 1.48 vs HR 1.12, 95% CI 1.00 to 1.24, p=0.26); the mean age of follow-up >5 years was 39.7 years and mean age follow-up <5 years was 46.7 years. Finally, we assessed whether the final risk estimates adjusted for smoking (figure 9 in online supplemental file 1). The group of studies that did not control for smoking demonstrated a greater risk of ACS (HR 1.31; 95% CI 1.06 to 1.62 vs HR 1.03; 95% CI 0.92 to 1.15, p=0.05).
Methodological quality and bias
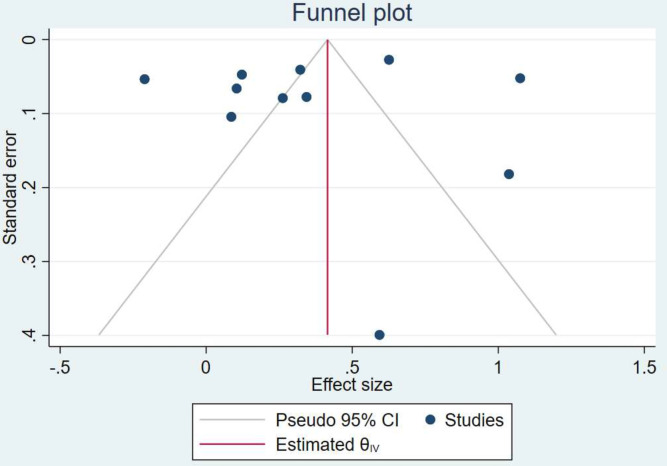
Funnel plots are provided for the unadjusted analysis (figure 4) and adjusted analysis (figure 5 in online supplemental material), which do not suggest publication bias. Based on the Down and Black checklist for risk of bias, the overall quality of the included studies was fair and no critical risk of bias was observed (see online supplemental materials).
Figure 4.
Funnel plot of the unadjusted association between IBD and ACS. ACS, acute coronary syndrome; IBD, inflammatory bowel disease.
openhrt-2023-002483supp002.xlsx (35.9KB, xlsx)
Discussion
To our knowledge, this is the largest meta-analysis of studies performed to date evaluating the risk of ACS in patients with IBD. This contemporary study with streamlined methodology provides incremental value to previous literature by demonstrating that patients with IBD have approximately 23% increased risk of ACS. Adjusted analysis shows a diminished association between IBD and ACS compared with unadjusted analysis, suggesting the association can partly be explained by mediating variables, including traditional cardiovascular risk factors such as hypertension, diabetes, and hyperlipidaemia. Certainly, prospective studies would be beneficial to establish a temporal relationship between disease diagnosis, severity, treatment and incidence of ACS.
Our findings support previous data, while attempting to address some limitations of the previous studies.12–20 Many of these studies included patients with non-IBD immune-mediated diseases. Furthermore, the primary endpoint remained broad with ‘IHD’ as opposed to ‘ACS’; the retrospective nature of the included studies and strong reliance on diagnostic and billing codes raised the possibility of misclassification or ascertainment bias. Given the heterogeneous nature of these disease groups and the absence of prospective data, we sought to streamline the patient population by only including studies with the specific endpoint of ACS and excluded studies that overlapped with other immune-mediate inflammatory diseases. In order to maximise the power of our analysis and ensure robust subgroup associations, we opted for subgroup analysis instead of meta-regression.28 Finally, 6 out of the 12 adjusted studies included in this review were published after a previous meta-analysis by Feng et al.25–27 29–31
Systemic inflammation predisposes vascular endothelial dysfunction through atherosclerotic plaque initiation evolving into plaque rupture and subsequent thrombosis.4 5 8 An interesting finding in our study was that younger adults with IBD demonstrated a greater association with ACS. This result should be interpreted within the limits of the included studies, as only three studies provided adjusted HRs stratified by age. To minimise overlap with non-IBD immune-mediated diseases, we only included studies that defined a distinct IBD population. It has been postulated that earlier age of diagnosis leads to longer exposure to inflammatory dysregulation with an increased risk of ACS among younger patients.32–34 We further explored this relationship by only including risk estimates adjusted for traditional cardiovascular risk factors in multivariable models as well as performing subgroup analysis based on follow-up duration. Our analysis found that patients with longer duration of follow-up (greater than 5 years) was not associated with a greater risk of ACS than patients with shorter duration.
Limitations
As with any meta-analysis, however, our article shares the limitations of original studies. First, the included studies are commonly based on diagnostic coding, leaving results susceptible to inherent misclassification and detection bias. Second, the study design attempted to minimise selection bias, by including ‘ACS’ as opposed to broader categories of IHD. This inherently relies on the appropriate clinical diagnosis of ACS and does not account for variations in clinical contexts (such as type two myocardial injuries). Third, we did not explore the relationship with disease severity, presence or absence of disease flare during index ACS, duration of IBD diagnosis and systemic therapies due to paucity of data. Disease severity and duration of IBD are thought to exert an important influence on the risk of ACS.8 12 16 35 36 Some studies have suggested concomitant treatment for IBD such as systemic therapy might reduce the risk of ACS by reducing the inflammatory burden.5 Patients with IBD who present with ACS also pose the challenge of balancing risk of ischaemia and haemorrhage.4 Additionally, there is limited available data reporting bleeding and angiographic outcomes.4 25 Finally, substantial statistical heterogeneity was observed among studies, despite including only adjusted results in a random effects model and performing subgroup analyses. Despite adjusting for traditional cardiovascular risk factors, unmeasured covariates may have an unclear impact on the relation between IBD and risk of ACS, and this reflects inherent limitations of retrospective cohort studies. All of the included studies matched cases with controls at baseline by sex, age and index date. As such, our study demonstrates an independent association between IBD and ACS.
Conclusion
Patients with IBD demonstrated an independently increased risk of ACS. Prospective studies are required to bridge existing evidence gaps, including characterising the relationship with disease severity, duration and interplay with IBD-modifying therapies in addition to angiographic characteristics and outcomes.
Footnotes
Contributors: All authors were involved in project conceptualisation, data analysis, manuscript editing and validation. There were no contributors to the study that are not acknowledged in the authorship. AZ accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The data underlying this article are available in the article and in its online supplementary material.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the global burden of disease 2010 study. Circulation 2014;129:1493–501. 10.1161/CIRCULATIONAHA.113.004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. The Lancet Gastroenterology & Hepatology 2020;5:17–30. 10.1016/S2468-1253(19)30333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 4.Pepe M, Carulli E, Forleo C, et al. Inflammatory bowel disease and acute coronary syndromes: from pathogenesis to the fine line between bleeding and ischemic risk. Inflamm Bowel Dis 2021;27:725–31. 10.1093/ibd/izaa160 [DOI] [PubMed] [Google Scholar]
- 5.Wu GC, Leng RX, Lu Q, et al. Subclinical atherosclerosis in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Angiology 2017;68:447–61. 10.1177/0003319716652031 [DOI] [PubMed] [Google Scholar]
- 6.Dagli N, Poyrazoglu OK, Dagli AF, et al. Is inflammatory bowel disease a risk factor for early atherosclerosis Angiology 2010;61:198–204. 10.1177/0003319709333869 [DOI] [PubMed] [Google Scholar]
- 7.Hatoum OA, Miura H, Binion DG. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am J Physiol Heart Circ Physiol 2003;285:H1791–6. 10.1152/ajpheart.00552.2003 [DOI] [PubMed] [Google Scholar]
- 8.Cainzos-Achirica M, Glassner K, Zawahir HS, et al. Inflammatory bowel disease and atherosclerotic cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol 2020;76:2895–905. 10.1016/j.jacc.2020.10.027 [DOI] [PubMed] [Google Scholar]
- 9.Kobiyama K, Ley K. Atherosclerosis. Circ Res 2018;123:1118–20. 10.1161/CIRCRESAHA.118.313816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer LM, Schlienger RG, Matter C, et al. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol 2004;93:198–200. 10.1016/j.amjcard.2003.09.037 [DOI] [PubMed] [Google Scholar]
- 11.Ajeganova S, Hafström I, Frostegård J. Patients with SLE have higher risk of cardiovascular events and mortality in comparison with controls with the same levels of traditional risk factors and intima-media measures, which is related to accumulated disease damage and antiphospholipid syndrome: a case-control study over 10 years. Lupus Sci Med 2021;8:e000454. 10.1136/lupus-2020-000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng W, Chen G, Cai D, et al. Inflammatory bowel disease and risk of ischemic heart disease: an updated meta-analysis of cohort studies. J Am Heart Assoc 2017;6:e005892. 10.1161/JAHA.117.005892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fumery M, Xiaocang C, Dauchet L, et al. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis 2014;8:469–79. 10.1016/j.crohns.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Singh H, Loftus EV, et al. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:382–93. 10.1016/j.cgh.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Hu T, Hao H, et al. Inflammatory bowel disease and cardiovascular diseases: a concise review. Eur Heart J Open 2022;2:oeab029. 10.1093/ehjopen/oeab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rungoe C, Basit S, Ranthe MF, et al. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut 2013;62:689–94. 10.1136/gutjnl-2012-303285 [DOI] [PubMed] [Google Scholar]
- 17.Barnes EL, Beery RM, Schulman AR, et al. Hospitalizations for acute myocardial infarction are decreased among patients with inflammatory bowel disease using a nationwide inpatient database. Inflammatory Bowel Diseases 2016;22:2229–37. 10.1097/MIB.0000000000000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Ascenzo F, Bruno F, Iannaccone M, et al. Patients with inflammatory bowel disease are at increased risk of Atherothrombotic disease: a systematic review with meta-analysis. Int J Cardiol 2023;378:96–104. 10.1016/j.ijcard.2023.02.042 [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Qiao L, Yun X, et al. Increased risk of ischemic heart disease and diabetes in inflammatory bowel disease. Z Gastroenterol 2021;59:117–24. 10.1055/a-1283-6966 [DOI] [PubMed] [Google Scholar]
- 20.Sun HH, Tian F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: a meta-analysis. Eur J Prev Cardiol 2018;25:1623–31. 10.1177/2047487318792952 [DOI] [PubMed] [Google Scholar]
- 21.Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American college of cardiology/American heart Association task force on clinical practice guidelines. Circulation 2019;140:e596–646. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 24.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aniwan S, Pardi DS, Tremaine WJ, et al. Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1607–15. 10.1016/j.cgh.2018.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Card TR, Zittan E, Nguyen GC, et al. Disease activity in inflammatory bowel disease is associated with arterial vascular disease. Inflamm Bowel Dis 2021;27:629–38. 10.1093/ibd/izaa156 [DOI] [PubMed] [Google Scholar]
- 27.Setyawan J, Mu F, Zichlin ML, et al. Risk of thromboembolic events and associated healthcare costs in patients with inflammatory bowel disease. Adv Ther 2022;39:738–53. 10.1007/s12325-021-01973-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher DJ, Carpenter JR, Morris TP, et al. Meta-analytical methods to identify who benefits most from treatments: daft, deluded, or deft approach BMJ 2017:j573. 10.1136/bmj.j573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha A, Rivera AS, Chadha SA, et al. Comparative risk of incident coronary heart disease across chronic inflammatory diseases. Front Cardiovasc Med 2021;8:757738. 10.3389/fcvm.2021.757738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YJ, Lee DH, Shin DW, et al. Patients with inflammatory bowel disease have an increased risk of myocardial infarction: a nationwide study. Aliment Pharmacol Ther 2019;50:769–79. 10.1111/apt.15446 [DOI] [PubMed] [Google Scholar]
- 31.Gill GS, Abusnina W, Alla V. Risk of myocardial infarction among patients with inflammatory bowel disease. Journal of the American College of Cardiology 2021;77:18. 10.1016/S0735-1097(21)01552-733413936 [DOI] [Google Scholar]
- 32.Bruzzese V, Palermo G, Ridola L, et al. Preclinical atherosclerosis in patients with inflammatory bowel diseases: a case-control study. Ann Transl Med 2017;5:158. 10.21037/atm.2017.03.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pemmasani G, Elgendy I, Mamas MA, et al. Epidemiology and clinical outcomes of patients with inflammatory bowel disease presenting with acute coronary syndrome. Inflamm Bowel Dis 2021;27:1017–25. 10.1093/ibd/izaa237 [DOI] [PubMed] [Google Scholar]
- 34.Ruisi P, Makaryus JN, Ruisi M, et al. Inflammatory bowel disease as a risk factor for premature coronary artery disease. J Clin Med Res 2015;7:257–61. 10.14740/jocmr2102w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristensen SL, Ahlehoff O, Lindhardsen J, et al. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PLoS One 2013;8:e56944. 10.1371/journal.pone.0056944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kristensen SL, Ahlehoff O, Lindhardsen J, et al. Prognosis after first-time myocardial infarction in patients with inflammatory bowel disease according to disease activity: nationwide cohort study. Circ Cardiovasc Qual Outcomes 2014;7:857–62. 10.1161/CIRCOUTCOMES.114.000918 [DOI] [PubMed] [Google Scholar]
- 37.Osterman MT, Yang YX, Brensinger C, et al. No increased risk of myocardial infarction among patients with ulcerative colitis or Crohn’s disease. Clin Gastroenterol Hepatol 2011;9:875–80. 10.1016/j.cgh.2011.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai M-S, Lin C-L, Chen H-P, et al. Long-term risk of acute coronary syndrome in patients with inflammatory bowel disease: a 13-year nationwide cohort study in an Asian population. Inflamm Bowel Dis 2014;20:502–7. 10.1097/01.MIB.0000441200.10454.4f [DOI] [PubMed] [Google Scholar]
- 39.Yarur AJ, Deshpande AR, Pechman DM, et al. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol 2011;106:741–7. 10.1038/ajg.2011.63 [DOI] [PubMed] [Google Scholar]
- 40.Ha C, Magowan S, Accortt NA, et al. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol 2009;104:1445–51. 10.1038/ajg.2009.81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002483supp001.pdf (555.5KB, pdf)
openhrt-2023-002483supp002.xlsx (35.9KB, xlsx)
Data Availability Statement
Data are available in a public, open access repository. The data underlying this article are available in the article and in its online supplementary material.