Abstract
Background
Peak oxygen pulse (O2pulse=oxygen consumption/heart rate) is calculated by the product of stroke volume (SV) and oxygen extraction. It has been shown to be reduced in patients with a Fontan circulation. However, in the Fontan population, it may be a poor marker of SV. We propose that the slope of the O2 pulse curve may be more reflective of SV during exercise.
Methods
We analysed cardiopulmonary exercise test data in 22 subjects with a Fontan circulation (cohort A) and examined the association between peak SV during exercise (aortic flow measured on exercise cardiac MRI), and O2 pulse parameters (absolute O2 pulse and O2 pulse slopes up to anaerobic threshold (AT) and peak exercise). In a separate Fontan cohort (cohort B, n=131), associations between clinical characteristics and O2 pulse kinetics were examined.
Results
In cohort A, peak aortic flow was moderately and significantly associated with O2pulseslopePEAK (r=0.47, p=0.02). However, neither absolute O2pulseAT nor O2pulsePEAK was significantly associated with peak aortic flow. In cohort B, O2pulseslopePEAK and O2pulseslopeAT were not significantly associated with clinical parameters, apart from a weak association with forced vital capacity.
Conclusion
The slope of the O2 pulse curve to peak exercise may be more reflective of peak SV in the Fontan population than a single peak O2 pulse value.
Keywords: Fontan Procedure; Heart Failure, Systolic; Heart Defects, Congenital
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with a Fontan circulation have reduced peak oxygen pulse, a marker of stroke volume (SV) in the healthy population. However, with the potential of impaired oxygen extraction by exercising muscles, peak O2 pulse may be a poor marker for SV in this cohort.
WHAT THIS STUDY ADDS
Peak O2 pulse slope may be more reflective of peak SV in the Fontan population than a single peak O2 pulse value.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The O2 pulse slope may be a useful submaximal marker of SV in the Fontan cohort.
Introduction
Cardiopulmonary exercise testing (CPET) is an important tool in assessing the haemodynamic response to exercise in patients with a Fontan circulation, and certain CPET parameters are emerging as useful in the prognostic assessment of these patients.1 2 However, maximal exercise is not met in a substantial proportion of Fontan patients3 and hence there is significant interest in submaximal exercise parameters, their determinants and association with clinically relevant outcomes.4 5
One maximal parameter, peak oxygen (O2) pulse, has been shown to be reduced in patients with a Fontan circulation.3 6 O2 pulse represents the relationship between oxygen consumption (VO2) and heart rate (HR) (O2 pulse=VO2/HR) and is determined by the product of stroke volume (SV) and oxygen extraction (arteriovenous oxygen difference (a-vO2)).7
In healthy populations, the change in a-vO2 between rest and peak exercise is predictable, such that peak O2 pulse has been used as a non-invasive surrogate measure of SV.8 9 However, as SV plateaus from mid-range exercise onward,10 O2 pulse from mid-exercise is mainly determined by a-vO2. As skeletal muscle abnormalities are highly prevalent and variable in patients with a Fontan circulation,11 12 with the potential of impaired oxygen extraction by exercising muscles,13 peak O2 pulse may be a poor marker for SV in this cohort.14
O2 pulse can be evaluated across the whole of exercise and O2 pulse kinetics, including the slope of the O2 pulse curve, have been examined in patients with a Fontan circulation.15 Fontan patients have been shown to have an altered O2 pulse slope compared with healthy controls. Given that O2 pulse slope takes into account both submaximal and maximal responses to exercise, we postulated that it, rather than peak O2 pulse, may be more strongly associated with SV.
The relationship between SV during exercise and O2 pulse slope in Fontan patients has not been determined. Thus, the primary aim of this study in patients with a Fontan circulation was to examine the association between SV (assessed as ascending aortic flow measured by exercise cardiac MR (exCMR)) obtained during exercise, and peak O2 pulse and O2 pulse slope during CPET. As a secondary aim, we explored associations between clinical characteristics and both peak O2 pulse and O2 pulse slope in a larger cohort of patients with a Fontan circulation.
Methods
Recruitment
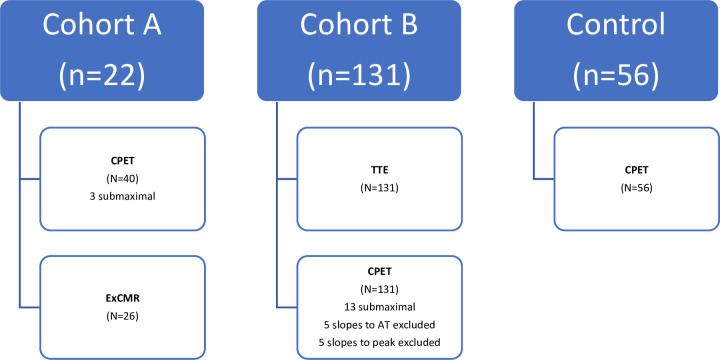
Two cohorts of Fontan participants were used in this study as follows (figure 1):
Figure 1.
Study participants. AT, anaerobic threshold; CPET, cardiopulmonary exercise testing; ExCMR, exercise cardiac magnetic resonance; n, number of subjects; N, number of tests; TTE, transthoracic echocardiogram.
In the first cohort (cohort A—22 subjects with 40 CPETs and 26 exCMR analysed), we examined the association between absolute O2 pulse, O2 pulse slope and SV during exercise. These participants were part of a study analysing the effects of inspiratory muscle training, as previously described.16 Cohort A was participants identified through the cardiology database at The Children’s Hospital at Westmead (CHW) in Sydney, Australia. Only participants with a non-fenestrated extracardiac conduit aged 12–20 years were included.
In the second cohort (cohort B—131 subjects with 131 CPETs and transthoracic echocardiograms (TTE)), we examined the association between clinical characteristics and O2 pulse parameters. These participants with a Fontan circulation were identified through the Australian and New Zealand Fontan Registry (ANZFR) and were part of the ANZFR Functional Outcomes after Fontan study conducted across Sydney, Melbourne and Auckland. Inclusion criteria for this study were age ≥13 years and ≥5 years since Fontan completion.
Exclusion criteria for both cohorts were those with severe heart failure, a history of significant exercise-induced arrhythmia, severe systemic outflow tract obstruction or severe systemic hypertension at rest, or significant cognitive impairment or intellectual disability. Clinical data for Fontan participants were retrospectively collected from the local cardiology database at CHW (cohort A) and the ANZFR REDCap database (cohort B, hosted by the Murdoch Children’s Research Institute).
Cohort A subjects underwent CPET at CHW. A subset of these patients underwent exCMR, also performed at CHW.16 Cohort B subjects underwent CPET and TTE at one of four sites—Sydney (CHW and The Royal Prince Alfred Hospital, RPAH), Melbourne (The Royal Children’s Hospital) and Auckland (Starship Children’s Hospital).
To compare O2 pulse parameters between Fontan subjects and healthy controls, data from healthy controls were obtained through the exercise labs at CHW, RPAH and the Women’s and Children’s Hospital in Adelaide, Australia (n=56). These subjects had no underlying cardiac, respiratory or neurological conditions and were undergoing exercise testing for non-specific symptoms.
Cardiopulmonary exercise testing
CPET was performed in all participants, in a standardised fashion on an upright cycle ergometer using a progressive ramp protocol, aiming to achieve peak oxygen consumption (VO2) within 7–10 min. Cohort A underwent CPET at baseline and at 6 weeks after inspiratory muscle training (n=40). HR, blood pressure and oxygen saturations were recorded from rest to recovery. Breath-by-breath VO2, carbon dioxide consumption (VCO2) and minute ventilation (VE) were measured. Anaerobic threshold (AT) was calculated using a combination of the V-slope, ventilatory equivalents and respiratory exchange ratio methods.
O2 pulse was calculated throughout testing, from the start of exercise until the beginning of recovery. Absolute O2 pulse was determined at AT (O2pulseAT) and at peak exercise (O2pulsePEAK). O2 pulse against time curves were reviewed and the curves were determined to have an overall linear pattern, without clear inflection points at consistent time points. Due to this pattern, lines of best fit for O2 pulse against exercise time up to AT (O2pulseslopeAT) and peak (O2pulseslopePEAK) were determined by regression (GraphPad Prism V.9), and the slope of these regression lines were taken as the O2 pulse slope (see figure 2 as representative). Lines of best fit were visually checked by two independent observers (KL and JA) and poorly fitting lines were excluded by agreement (five slopes from cohort A and five slopes from cohort B were thus excluded). No slopes from the control cohort were excluded. For analysis, indexing of O2 pulse metrics to body surface area was not undertaken given that indexing of aortic flow was also not undertaken.
Figure 2.
O2 pulse. AT, anaerobic threshold.
Spirometry
Spirometry was performed in all participants using standardised techniques.17 Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were measured, and per cent predicted FVC and FEV1 were determined.18
ExCMR imaging
ExCMR was performed (1.5T, Phillips Intera, Best, The Netherlands) at baseline (n=14) and then 6 weeks after inspiratory muscle training (n=12).16 Exercise in the MRI scanner was undertaken using an MRI compatible stepper (Ergospect, Innsbruck, Austria). Testing was performed on the same day as the cardiopulmonary exercise test. The initial workload was set to 30W, and increased by 20W every 3 min until exhaustion. Flow and cine imaging was acquired from 50% of the participants’ maximum workload based on the prior CPET, and at every stage thereafter until fatigue. Resting and exercise non-gated real time phase contrast flow (200 consecutive dynamics, dynamic scan time 75 ms, corresponding to approximately 15 s of flow data) was measured. Ascending aortic flow was measured at rest (Aortic flowREST) and at the final stage prior to exhaustion (Aortic flowPEAK), as a measure of SV. Typical scan parameters for balanced steady-state free-precession cine imaging have been described previously.16 Ventricular volumes were determined by manual contouring of the endocardial border of each short axis slice, at end-diastole (EDV) and end-systole volume (ESV). Ejection fraction was calculated (EF=(EDV-ESV)/EDV×100%). To study the association between SV and O2 pulse metrics, all ExCMR studies and their corresponding CPET (n=26) were analysed.
Echocardiography
Participants in cohort B had TTEs performed and the following were obtained: anatomy, ventricular dominance, atrioventricular (AV)-valve regurgitation (none-trivial, or mild or more) and global systolic dysfunction as a qualitative measurement (normal or impaired).
Statistical analyses
Statistical analyses were performed using GraphPad Prism V.9. Non-parametric and parametric data are presented as median (range) or mean±SD, respectively. Categorical and continuous data were compared between healthy and Fontan groups using χ2 tests, t-tests or Mann-Whitney U tests, as appropriate. Correlations between clinical Fontan and O2 pulse parameters were assessed using Pearson or Spearman correlation and multiple linear regression models. A p<0.05 was considered statistically significant.
Results
The baseline characteristics of cohort A and cohort B are shown in table 1. Cohort B included a wider age range, with an older mean age than cohort A (23.0 vs 15.8 years). Due to the inclusion criteria of cohort A, more subjects in cohort B were fenestrated at the time of Fontan completion and had non-extracardiac conduits. Other baseline characteristics were similar between the two Fontan groups. Characteristics of the control subjects were: 33 (59%) males, age 29.5±12.0 years, height 169.8±11.8 cm, weight 77.5±22.2 kg and body mass index 22.7±6.0. Resting and peak parameters obtained during CPET and exCMR are compared in online supplemental table 1. Results from the CPET have previously been reported.16 19 Peak workload between CPET and exCMR positively correlated (r=0.64, p<0.001).
Table 1.
Anthropometrics and clinical characteristics of Fontan subjects (cohorts A and B)
| Cohort A Fontan (n=22) | Cohort B Fontan (n=131) | P value | |
| Age (years) | 15.8±2.3 | 23.0±7.9 | <0.01 |
| Gender (male:female) | 11 (50%):11 (50%) | 67 (51%):64 (49%) | >0.9 |
| Height (cm) | 164.3±8.1 | 165±10.0 | 0.56 |
| Weight (kg) | 60.6±14.8 | 64.9±15.7 | 0.23 |
| Body mass index | 22.0±4.5 | 23.5±4.9 | 0.27 |
| Dominant ventricle (right:other) | 14 (64%):8 (32%) | 92 (70%):39 (30%) | 0.62 |
| Age of Fontan (years) | 5.2±2.0 | 6.0±4.3 | 0.40 |
| Type of Fontan (extracardiac conduit:other) | 22 (100%):0 (0%) | 82 (63%):49 (37%) | <0.01 |
| Fenestration (no:yes) | 22 (100%):0 (0%) | 89 (64%):41 (36%) one unknown |
<0.01 |
openhrt-2023-002324supp001.pdf (91KB, pdf)
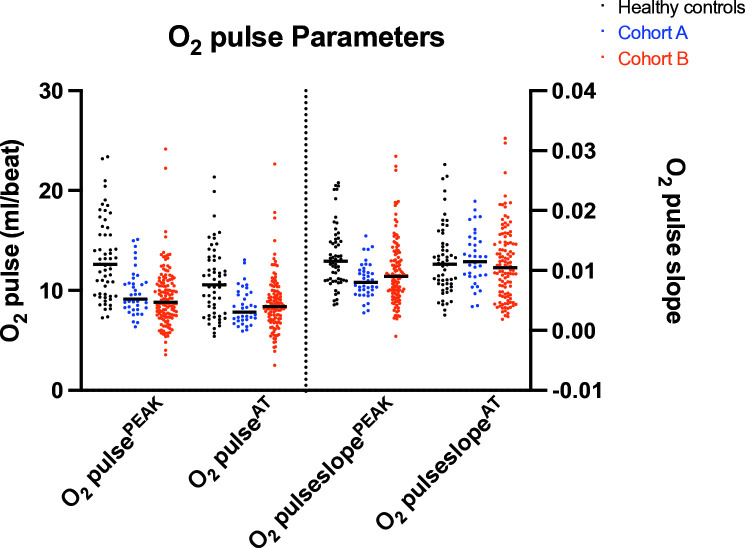
There was no significant difference in O2pulsePEAK, O2pulseAT, O2pulseslopePEAK or O2pulseslopeAT, between cohort A and B (figure 3). Both Fontan cohorts had significantly lower peak VO2, O2pulsePEAK, O2pulseAT and O2pulseslopePEAK compared with healthy controls. O2pulseslopeAT was significantly different between cohort A and healthy controls, but not between cohort B and healthy controls (table 2, figure 3).
Figure 3.
O2 pulse parameters. AT, anaerobic threshold.
Table 2.
O2 pulse parameters
| Parameter | Healthy controls | Cohort A Fontan | Cohort B Fontan |
| Peak VO2 (mL/kg/min) | 36.0±10.3 | 25.1±7.1* | 20.9±7.1* |
| O2 pulsePEAK (mL/beat) | 13.2±4.1 | 9.8±2.2* | 9.3±2.9* |
| O2 pulseAT (mL/beat) | 10.9±3.5 | 8.4±1.8* | 8.7±2.8* |
| O2 pulse slopePEAK | 0.01±0.005 | 0.008±0.003* | 0.009±0.005* |
| O2 pulse slopeAT | 0.01±0.006 | 0.01±0.004* | 0.01±0.0005† |
*Compared with healthy controls, p<0.01.
†Compared with healthy controls, not statistically significant (p>0.05).
AT, anaerobic threshold.
In cohort A, aortic flowPEAK was moderately and significantly associated with O2pulseslopePEAK (r=0.47, p=0.02) but was not significantly associated with O2pulseslopeAT (r=0.37, p=0.09). Neither O2pulsePEAK nor O2pulseAT correlated with aortic flowPEAK (r=−0.05, p=0.82; r=0.20, p=0.33, respectively). There was a weak negative association between EF measured at peak exercise on exCMR and O2 pulse at peak and rest (r=−0.40, p=0.03; r=−0.53, p<0.01, respectively). There were no significant associations between EF and other O2 pulse parameters.
In cohort B, neither resting nor peak oxygen saturation were correlated with any of the O2 pulse parameters (data not shown). O2pulseslopePEAK correlated with O2pulsePEAK (r=0.56, p<0.01) and O2pulseslopeAT with O2pulseAT (p<0.01, r=0.44). O2pulsePEAK and O2pulseAT were negatively associated with age of Fontan completion (r=−0.19, p=0.03;r=−0.20, p=0.03, respectively). O2pulseslopeAT was associated with severity of AV-valve regurgitation (U=847, p=0.03) with a lower slope in those with at least mild regurgitation. However, AV-valve regurgitation was not associated with O2pulseslopePEAK. O2pulseslopePEAK and O2pulseslopeAT were not significantly associated with other clinical variables including ventricular morphology or function, Fontan type, age of Fontan completion, presence of fenestration or pacemaker, presence of permanent pacemaker or use of HR-limiting medications (online supplemental table 2). Baseline FVC %predicted but not FEV1 was weakly associated with O2pulsePEAK O2pulseslopePEAK (r=0.2, p=0.03) and O2pulseslopeAT (r=0.21, p=0.03).
openhrt-2023-002324supp002.pdf (116.6KB, pdf)
Discussion
We have demonstrated a positive association between O2 pulse slope to peak exercise and SV, in patients with a Fontan circulation. We have also confirmed the lack of association between peak O2 pulse and SV, as previously demonstrated by other authors.14 Our data suggest that O2 pulse slope, which incorporates O2 pulse kinetics across exercise, may be a better surrogate marker than absolute peak O2 pulse of maximal SV during exercise in Fontan patients.
SV and the a-vO2 difference
O2 pulse is determined by SV and oxygen extraction (a-vO2 difference). In untrained healthy subjects, SV increases during exercise but then plateaus at a submaximal load.20 The a-vO2 difference, however, increases as a linear function during exercise in healthy subjects, and is the main determinant of O2 pulse from mid-exercise.10 SV and a-vO2 difference responses across exercise in patients with a Fontan circulation appear to be more heterogeneous.
SV has been shown to increase throughout exercise in some Fontan patients but plateau or decrease in others at maximum exertion.14 21–24 This variable SV response may relate to differences in underlying patient characteristics but also to differences in the way SV is measured across different studies. Although submaximal and maximal HR is lower in Fontan patients than healthy controls,6 24 25 the slope of the response of HR against workload or VO2 appears to be higher or equivalent.6 24 The exercise chronotropic response of patients with a Fontan circulation may thus be adaptive to preload insufficiency in order to maintain ventricular filling and cardiac output. In keeping with these data, we did not find a difference in the O2 pulse slope up to AT between Fontan patients and controls.
In a small study of 10 Fontan subjects before and after a single dose of sildenafil, Van De Bruaene et al showed decreasing SV (measured by ExCMR) and increasing a-vO2 difference (measured from direct arterial and venous blood sampling) from rest to peak exercise.23 In a study of 15 children with a Fontan circulation, Strömvall Larsson and Eriksson showed increasing a-vO2 difference across exercise but with considerable variability in the difference at peak exercise (median 16.8, range 10.5–21.5 mL/100 mL).22 Rosenthal et al demonstrated higher a-vO2 difference in 43 children with a Fontan circulation compared with healthy controls at rest and across all stages of exercise.26
The impact of chronic cyanosis on a-vO2 has been poorly studied. It is possible that cyanosis, related to the either the presence of a fenestration or systemic venous collateral, results in a reduced ability to augment oxygen extraction at peak exercise or impaired microcirculatory function. We found no association between O2 pulse parameters and either the presence of a fenestration or resting or peak oxygen saturation. This is in keeping with a study by Loomba et al showing similar exercise arterial-venous saturation difference (utilising regional near infrared spectroscopy) in fenestrated versus non-fenestrated Fontan patients27 and a study by Strieder et al showing similar tissue oxygenation during exercise in cyanotic versus repaired acyanotic CHD.28 We speculate that a more heterogeneous a-vO2 difference during exercise in patients with a Fontan circulation may explain the poor association of peak O2 pulse with SV. However, this would need to be examined in a future study where exercise SV, O2 pulse and a-vO2 difference are directly measured.
Association between O2 pulse slope and clinical variables
In our exploratory study, we found no association between clinical variables and O2pulseslopePEAK or O2pulseslopeAT, apart from a weakly positive association with baseline FVC %predicted. There was no association with other spirometry parameters.
Fontan subjects have been shown to have abnormal spirometry, with an association between FVC and peak VO2.19 29 Its association with O2 pulse slope is beyond the scope of this study, however, may reflect a ventilation driven inability to augment pulmonary blood flow, and therefore, SV.
O2 pulse and long-term outcomes
Neither O2 pulse nor its change over serial testing have been shown to predict long-term adverse Fontan outcomes.30 We speculate that, in part, this relates to its poor correlation with SV in Fontan patients. It would be of interest to investigate the association between O2 pulse slope and its change and long-term Fontan outcomes.
Implications of study findings and study limitations
In a study of 411 Fontan subjects (age 12.4±3.2 years, 166 achieving a maximal exercise test) Paridon et al found, as expected, that per cent predicted peak O2 pulse was strongly associated with per cent predicted peak VO2 and moderately with per cent predicted VO2 at AT.3 Based on the strength of these associations, the authors inferred that SV limitation was solely responsible for the variation in aerobic performance. However, our data suggest caution in using peak O2 pulse as a surrogate marker for SV in Fontan patients. We speculate that this may relate to a greater variability in the a-vO2 difference at peak compared with submaximal exercise.
As this was a preliminary study, our study cohorts were small, and we did not have exCMR data available for cohort B. However, even based on our small cohort A, we were still able to demonstrate a moderately significant association between O2 pulse slope to peak exercise and peak SV.
Our exCMR protocol was performed using recumbent exercise, likely producing submaximal exercise testing; compared with our upright cycle ergometer CPET. The use of serial gas measurements and CMR-augmented CPET, including continuous oxygen saturation monitoring, would have allowed simultaneous assessment of gas exchange, and a-vO2 during exercise.
Our healthy controls were retrospectively obtained from subjects previously undergoing CPET, and therefore, we did not have a prospectively matched cohort. However, given the older age of the control subjects, we are more likely to have underestimated the significance of the difference between our Fontan and control cohorts.
Given our relatively small sample size and short-term follow-up, we were unable to examine whether O2 pulse slope predicted adverse long-term Fontan outcomes. In our exploratory analysis of cohort B, adjustments for multiple comparisons were not performed, increasing the risk of type I error. Further larger studies would be of interest to analyse the association between O2 pulse parameters and clinical variables.
Conclusion
Peak O2 pulse slope may be more reflective of peak SV in the Fontan population than a single peak O2 pulse value, taking into account changes in a-vO2 during exercise. The O2 pulse slope may be a useful submaximal marker of SV.
Acknowledgments
This study is based on one of the authors’ thesis—Laohachai, K (2023). Cardiopulmonary Interactions and Exercise Capacity in Patients with a Fontan Circulation. PhD thesis, University of Sydney, Sydney Australia.
Footnotes
Contributors: KL and JA designed the study, collected the data, drafted the manuscript and were responsible for analysis and interpretation of results. All authors provided substantial contribution to reviewing the results, providing critical revisions and approving the final version of the manuscript. JA is responsible for the overall content as the guarantor.
Funding: The ANZFR Functional Outcomes after Fontan study was funded by a National Health and Medical Research Council Project grant (APP1065794).
Competing interests: None declared.
Patient and public involvement: Patients and the public were not directly involved in the study design or analyses. This study was endorsed by the ANZFR steering committee, which include patients and their representatives.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the study was approved by the Sydney Children’s Hospitals Network Human Research Ethics Committee. Reference numbers: HREC/15/SCHN/249 and HREC/14/SCHN/278. Participants gave informed consent to participate in the study before taking part.
References
- 1.Egbe AC, Driscoll DJ, Khan AR, et al. Cardiopulmonary exercise test in adults with prior Fontan operation: the prognostic value of serial testing. Int J Cardiol 2017;235:6–10. 10.1016/j.ijcard.2017.02.140 [DOI] [PubMed] [Google Scholar]
- 2.Diller G-P, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J 2010;31:3073–83. 10.1093/eurheartj/ehq356 [DOI] [PubMed] [Google Scholar]
- 3.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008;52:99–107. 10.1016/j.jacc.2008.02.081 [DOI] [PubMed] [Google Scholar]
- 4.Terol Espinosa de Los Monteros C, Harteveld LM, Kuipers IM, et al. Prognostic value of maximal and submaximal exercise performance in Fontan patients < 15 years of age. Am J Cardiol 2021;154:92–8. 10.1016/j.amjcard.2021.05.049 [DOI] [PubMed] [Google Scholar]
- 5.Chen C-A, Chen S-Y, Chiu H-H, et al. Prognostic value of submaximal exercise data for cardiac morbidity in Fontan patients. Med Sci Sports Exerc 2014;46:10–5. 10.1249/MSS.0b013e31829f8326 [DOI] [PubMed] [Google Scholar]
- 6.Hedlund ER, Söderström L, Lundell B. Appropriate heart rate during exercise in Fontan patients. Cardiol Young 2020;30:674–80. 10.1017/S1047951120000761 [DOI] [PubMed] [Google Scholar]
- 7.Mezzani A. Cardiopulmonary exercise testing: basics of methodology and measurements. Ann Am Thorac Soc 2017;14:S3–11. 10.1513/AnnalsATS.201612-997FR [DOI] [PubMed] [Google Scholar]
- 8.Unnithan V, Rowland TW. Use of oxygen pulse in predicting Doppler-derived maximal stroke volume in adolescents. Pediatr Exerc Sci 2015;27:412–8. 10.1123/pes.2014-0215 [DOI] [PubMed] [Google Scholar]
- 9.Crisafulli A, Piras F, Chiappori P, et al. Estimating stroke volume from oxygen pulse during exercise. Physiol Meas 2007;28:1201–12. 10.1088/0967-3334/28/10/006 [DOI] [PubMed] [Google Scholar]
- 10.Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physio 1997;82:908–12. 10.1152/jappl.1997.82.3.908 [DOI] [PubMed] [Google Scholar]
- 11.Cordina R, O’Meagher S, Gould H, et al. Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Heart 2013;99:1530–4. 10.1136/heartjnl-2013-304249 [DOI] [PubMed] [Google Scholar]
- 12.Brassard P, Poirier P, Martin J, et al. Impact of exercise training on muscle function and ergoreflex in Fontan patients: a pilot study. Int J Cardiol 2006;107:85–94. 10.1016/j.ijcard.2005.02.038 [DOI] [PubMed] [Google Scholar]
- 13.Wittekind S, Mays W, Gerdes Y, et al. A novel mechanism for improved exercise performance in pediatric Fontan patients after cardiac rehabilitation. Pediatr Cardiol 2018;39:1023–30. 10.1007/s00246-018-1854-3 [DOI] [PubMed] [Google Scholar]
- 14.Legendre A, Guillot A, Ladouceur M, et al. Usefulness of stroke volume monitoring during upright ramp incremental cycle exercise in young patients with Fontan circulation. Int J Cardiol 2017;227:625–30. 10.1016/j.ijcard.2016.10.087 [DOI] [PubMed] [Google Scholar]
- 15.Bansal M, Fiutem JJ, Hill JA, et al. Oxygen pulse Kinetics in Fontan patients during treadmill ramp protocol cardiopulmonary exercise testing. Pediatr Cardiol 2012;33:1301–6. 10.1007/s00246-012-0308-6 [DOI] [PubMed] [Google Scholar]
- 16.Laohachai K, Winlaw D, Selvadurai H, et al. Inspiratory muscle training is associated with improved Inspiratory muscle strength, resting cardiac output, and the ventilatory efficiency of exercise in patients with a Fontan circulation. J Am Heart Assoc 2017;6:e005750. 10.1161/JAHA.117.005750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. an official American Thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993;15:75–88. 10.1002/ppul.1950150204 [DOI] [PubMed] [Google Scholar]
- 19.Laohachai K, Badal T, Thamrin C, et al. Older age at Fontan completion is associated with reduced lung volumes and increased lung reactance. Int J Cardiol 2022;364:38–43. 10.1016/j.ijcard.2022.06.037 [DOI] [PubMed] [Google Scholar]
- 20.Stringer WW, Whipp BJ, Wasserman K, et al. Non-linear cardiac output dynamics during ramp-incremental cycle ergometry. Eur J Appl Physiol 2005;93:634–9. 10.1007/s00421-004-1258-3 [DOI] [PubMed] [Google Scholar]
- 21.Driscoll DJ, Danielson GK, Puga FJ, et al. Exercise tolerance and cardiorespiratory response to exercise after the Fontan operation for Tricuspid Atresia or functional single ventricle. J Am Coll Cardiol 1986;7:1087–94. 10.1016/s0735-1097(86)80227-3 [DOI] [PubMed] [Google Scholar]
- 22.Strömvall Larsson E, Eriksson BO. Haemodynamic adaptation during exercise in Fontan patients at a long-term follow-up. Scand Cardiovasc J 2003;37:107–12. 10.1080/4017430310001221 [DOI] [PubMed] [Google Scholar]
- 23.Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging 2014;7:265–73. 10.1161/CIRCIMAGING.113.001243 [DOI] [PubMed] [Google Scholar]
- 24.Claessen G, La Gerche A, Van De Bruaene A, et al. Heart rate reserve in Fontan patients: chronotropic incompetence or hemodynamic limitation J Am Heart Assoc 2019;8:e012008. 10.1161/JAHA.119.012008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diller G-P, Okonko DO, Uebing A, et al. Impaired heart rate response to exercise in adult patients with a systemic right ventricle or univentricular circulation: prevalence, relation to exercise, and potential therapeutic implications. Int J Cardiol 2009;134:59–66. 10.1016/j.ijcard.2008.01.032 [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal M, Bush A, Deanfield J, et al. Comparison of cardiopulmonary adaptation during exercise in children after the atriopulmonary and total cavopulmonary connection Fontan procedures. Circulation 1995;91:372–8. 10.1161/01.cir.91.2.372 [DOI] [PubMed] [Google Scholar]
- 27.Loomba RS, Danduran ME, Dixon JE, et al. Effect of Fontan fenestration on regional venous oxygen saturation during exercise: further insights into Fontan fenestration closure. Pediatr Cardiol 2014;35:514–20. 10.1007/s00246-013-0817-y [DOI] [PubMed] [Google Scholar]
- 28.Strieder DJ, Mesko ZG, Zaver AG, et al. Exercise tolerance in chronic hypoxemia due to right-to-left shunt. J Appl Physiol 1973;34:853–8. 10.1152/jappl.1973.34.6.853 [DOI] [PubMed] [Google Scholar]
- 29.Opotowsky AR, Landzberg MJ, Earing MG, et al. Abnormal spirometry after the Fontan procedure is common and associated with impaired aerobic capacity. Am J Physiol Heart Circ Physiol 2014;307:H110–7. 10.1152/ajpheart.00184.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udholm S, Aldweib N, Hjortdal VE, et al. Prognostic power of cardiopulmonary exercise testing in Fontan patients: a systematic review. Open Heart 2018;5:e000812. 10.1136/openhrt-2018-000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002324supp001.pdf (91KB, pdf)
openhrt-2023-002324supp002.pdf (116.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.