Abstract
Objective
To examine factors influencing the kinetics of monosodium urate (MSU) crystal dissolution measured with dual-energy computed tomography (DECT) during follow-up of patients with gout.
Methods
Patients with a diagnosis of gout with baseline knees and feet DECT scans exhibiting MSU crystal volumes ≥0.1 cm3 and at least one follow-up DECT were included. Spearman’s correlation coefficient was used to search for association between change from baseline MSU crystal volume at 6, 12, 18 and 24 months and serum urate (SU) level. Associations between percentage change from the baseline volume of MSU crystal deposits and explanatory variables were assessed using linear mixed models.
Results
Sixty-two patients (age 67.3±12.8 years; 53 (85%) males) cumulating 104 follow-up DECT scans were included. Overall, SU target levels (<6.0 and <5.0 mg/dL) were achieved by 48 (77%) and 36 (58%) patients, respectively. There was a good correlation (r=0.66; p<0.0001) observed between SU level and percentage change in MSU crystal volume. The median decrease from baseline MSU crystal volume was greater in patients reaching the <5.0 mg/dL SU target than in those reaching ≥5.0 SU <6.0 mg/dL: −85% (95% CI: −94% to −72%) versus −40% (−57% to −22%; p<0.05) at 12 months. In multivariable analysis, time (in days) with a multilevel coefficient of −0.06 (95% CI: −0.08 to −0.03, p<0.001), hypertension (coefficient: 41.87, 95% CI: 16.38 to 67.18, p<0.01) and SU level <5.0 mg/dL (coefficient: −39.46, 95% CI: −70.93 to −8.34, p=0.02) were the only variables significantly associated with MSU crystal volume change.
Conclusion
In patients with DECT-measured MSU crystal deposition, reaching the <5.0 mg/dL SU target provides more extensive and rapid crystal dissolution than reaching the <6.0 mg/dL SU target.
Keywords: gout, recurrence, crystal arthropathies
WHAT IS ALREADY KNOWN ON THIS TOPIC
Achieving clinical remission in gout requires monosodium urate (MSU) crystal dissolution. Dual-energy computed tomography (DECT) is useful to monitor MSU crystal depletion, but previous studies demonstrated disappointingly incomplete crystal dissolution even with treat-to-target urate-lowering therapy (ULT). Factors driving the kinetics of crystal dissolution are still poorly understood.
WHAT THIS STUDY ADDS
When considering patients with volumes of MSU crystals ≥0.1 cm3 on DECT scans limiting the weight of artefacts, near-complete crystal dissolution is achieved in most patients receiving T2T ULT at 2 years. The main driver of the achievement of crystal dissolution is reaching serum urate (SU) levels <5.0 mg/dL, while levels <6.0 mg/dL are associated with uneven and incomplete crystal dissolution at 2 years. Hypertension seems to slow down the kinetics of crystal dissolution.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In patients with detectable MSU crystals on DECT, the SU level target during ULT should be <5.0 mg/dL to ascertain crystal dissolution. Patients with hypertension exhibit slower crystal dissolution and may require lower SU targets.
Introduction
Gout is caused by the deposition of monosodium urate (MSU) crystals after prolonged hyperuricaemia.1 Gout flares and chronic arthritis are caused by the immune response to these MSU crystal deposits. The volume of MSU crystals deposited is associated with the risk of subsequent flares.2 The aim of gout management is to obtain complete dissolution of MSU crystals using urate-lowering therapy (ULT) to achieve sustained decrease of serum urate (SU) level.3 4
Dual-energy computed tomography (DECT) is a useful tool for the discrimination of urate, bone and soft-tissue, and provides direct measurement of significant of MSU crystal deposits.5 The technique is fully recognised in the diagnostic setting6 7 and the prognostic value of DECT in predicting the risk of flares and comorbidity onset is being actively explored.2 8
The first studies measuring MSU crystal depletion by DECT in patients taking conventional ULT produced disappointing results with only very partial crystal depletion at 18 and 24 months and over 50% persistence of baseline deposits.9 10 However, these studies may have actually involved substantial volumes of artefacts not expected to disappear as overall volumes were very small (<0.01 cm3) with a difficult-to-measure sensitivity to change.10 In groups of patients with more significant deposits at baseline, the change in MSU crystal volume measured with DECT was more substantial, and reached around 80% of depletion, on average, in quantitative11 and semiquantitative12 assessments of patients managed with a treat-to-target ULT approach. The kinetics of MSU crystal depletion assessed with DECT remain unpredictable, do not seem to be entirely dependent on SU levels, and may involve other biological factors.
The aim of this study was to examine factors influencing the kinetics of MSU crystal dissolution measured with DECT during follow-up of patients with gout treated with ULT.
Methods
Study population
The CRYSTALILLE cohort is an inception cohort that included patients newly referred with a clinical suspicion or diagnosis of crystal-induced arthropathy. Patients with an established diagnosis of gout according to the 2015 American College of Rheumatology/European Alliance of Associations for Rheumatology (ACR/EULAR) criteria,6 with baseline knees and feet DECT scans exhibiting MSU crystal volume ≥0.1 cm3 and with at least one follow-up DECT were included. Patients could be receiving ULT at baseline. Patients with baseline MSU crystal volume <0.1 cm3 were excluded from the study.
The study was approved by the French ethical committee CPP Sud-Est III (EudraCT 2020-A01269-30) and patients provided informed consent.
Baseline and follow-up visits
Patients were included at a baseline visit and had a structured clinical assessment with data collection: demographic data, gout history (date of diagnosis, frequency of flares, ULT, flare prophylaxis), comorbidities, other treatments and laboratory data including SU level and kidney function. A DECT scan of the knees and ankles/feet was performed for each patient at maximum 2 weeks after the baseline visit.
Patients were managed using a conventional treat-to-target ULT strategy with an SU level target set according to French guidelines: SU below 6.0 mg/dL and, at best, below 5.0 mg/dL.3 ULT was initiated or optimised remotely by the treating physician receiving SU level measurement reports and adjusting ULT doses accordingly .
Follow-up visits were planned every 6 months and follow-up scans were performed as decided by the treating physician or systematically at 6, 12 and 24 months for patients participating in a follow-up study (DECTUS).
Imaging protocol
DECT scans of the knees and feet were performed using a single-source CT system (Somatom Definition Edge; Siemens Healthineers, Erlangen, Germany) with a previously described protocol.8 DECT images were postprocessed and analysed using the ‘gout’ software (syngo.via VB10B; Siemens Healthineers): volumes of MSU crystal deposition were noted after careful retrieval of artefacts including submillimetric lesions. A first reading had been performed by local musculoskeletal radiologists, and a second reading was performed by a junior rheumatologist (VL) specifically trained to DECT readings validated on a random sample of 30 scans by expert DECT readers (TP and J-FB). All scans were read consecutively for each single patient and sizes of the boxes of the region of interests were adjusted in the post-treatment software to be consistent with the size of the box of the baseline scan.
Outcomes
The primary outcome measure was the percentage of the baseline MSU crystal deposition volume remaining on follow-up DECT scans at 6, 12, 18 and 24 months.
Secondary outcomes were the number of patients achieving MSU crystal depletion >50% of baseline crystal deposition at 6 months, >70% at 12 months, >90% at 18 months and >98% at 24 months. Secondary outcomes included the number of flares occurring during the past 6 months prior to follow-up DECT scans in patients achieving or not these thresholds of change in MSU crystal deposition volume.
Variables considered to be potentially explanatory of change in MSU crystal volume were time since the baseline DECT scans, patient age, estimated glomerular filtration rate (eGFR) ≤ or >60 mL/min/1.73 m2, chronic heart disease, hypertension, SU level at the time of the follow-up DECT scan ≥6.0 mg/dL, <6.0 mg/dL and ≥5.0 mg/dL and<5.0 mg/dL.13
Statistical analysis
All statistical analysis were performed using R software V.3.6.1.
Qualitative variables were described as numbers and percentages, quantitative variables as mean (SD) or medians (Q1–Q3 IQR). The association between percentage of change from baseline MSU crystal volume and, SU level change at the time the follow-up DECT scan was performed and the number of flares in the 6 months preceding the follow-up scan, respectively, were assessed using the non-parametric Spearman correlation coefficient. Percentage of remaining MSU crystal volume at each time point was compared between SU-level groups with the Mann-Whitney-Wilcoxon test, and achievement of MSU volume decrease targets with Fisher’s exact test. Linear associations between percentage change from baseline MSU crystal deposit volume and candidate explanatory variables were assessed using linear mixed models taking time and candidate variables as fixed effects, and patients as random effects to account for repeated measures. Candidate explanatory variables with coefficients associated with a p value <0.2 were included in a multivariable linear mixed model. Statistical significance was set at a p value<0.05.
Results
Study participants
Among the 334 patients with gout who were included in the CRYSTALILLE cohort between January 2016 and June 2022, there were 62 patients with a minimal 0.1 cm3 volume of MSU crystal deposition at baseline who cumulated 104 follow-up knees and feet DECT scans performed 2 months to 5 years after the baseline scan (online supplemental figure S1). Patient characteristics are detailed in table 1.
Table 1.
Characteristics of the study population
| Characteristics | Total population (n=62) |
| Demographics | |
| Age (years) (mean (SD)) | 67.3 (12.8) |
| Male (n (%)) | 53 (85.5) |
| Body mass index (kg/m2) (median (IQR)) | 27.1 (26.1 to 30.9) |
| Daily alcohol >30 g (n (%)) | 9 (15.8) |
| Excessive sugar-sweetened beverage intake (n (%)) | 1 (1.6) |
| Purine-rich diet (n (%)) | 10 (17.9) |
| Gout characteristics | |
| Symptom duration (years) (median (IQR)) | 10 (3–16) |
| Subcutaneous tophi (n (%)) | 26 (41.9) |
| Number of flares in past 6 months (median (IQR)) | 2 (1–3) |
| Urate-lowering therapy naive (n (%)) | 41 (66.1) |
| Comorbidities | |
| History of urolithiasis (n (%)) | 12 (19.7) |
| Hypertension (n (%)) | 45 (72.6) |
| Chronic heart failure (n (%)) | 18 (29.0) |
| History of myocardial infarction (n (%)) | 11 (17.7) |
| Coronary heart disease (n (%)) | 13 (21.0) |
| Cardiovascular disease | 26 (41.9) |
| History of stroke (n (%)) | 2 (3.2) |
| Dyslipidaemia (n (%)) | 29 (46.8) |
| Diabetes mellitus (n (%)) | 18 (29.0) |
| Obstructive sleep apnoea (n (%)) | 7 (11.3) |
| Treatments | |
| Diuretics (n (%)) | 19 (30.6) |
| Lipid-lowering drugs (n (%)) | 30 (48.4) |
| Antihypertensive drugs (n (%)) | 44 (71.0) |
| Antiplatelet/anticoagulants | 30 (48.4) |
| Laboratory findings | |
| Serum urate level (mg/dL) (median (IQR)) | 8.4 (6.6–9.9) |
| Estimated glomerular filtration rate <60 mL/min/1.73 m2 (n (%)) | 14 (24.6) |
| Dual-energy CT volumes of MSU crystal deposits | |
| Feet (cm3) (median (IQR)) | 0.5 (0.2–1.9) |
| Knees (cm3) (median (IQR)) | 0.4 (0.1–1.2) |
| Total (cm3) (median (IQR)) | 1 (0.4–2.7) |
MSU, monosodium urate.
rmdopen-2023-003725supp001.pdf (242.2KB, pdf)
Time course of SU level and MSU crystal deposition
Overall, SU target levels (<6.0 and <5.0 mg/dL) were achieved by 48 (77%) and 36 (58%) patients respectively.
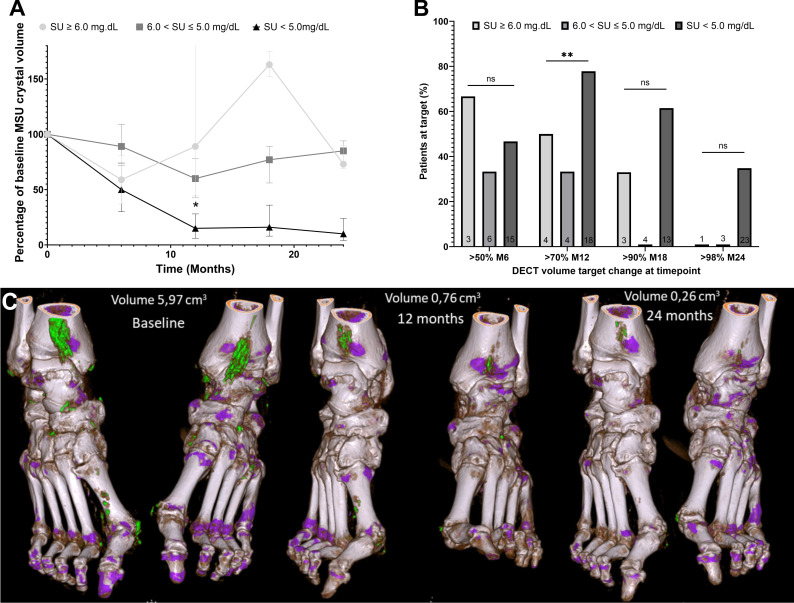
At 6 months, the median change from the baseline MSU crystal deposit volume was −43% (95% CI: −66% to −15%) and 11/24 (46%) patients had more than 50% decrease from baseline. At 12 months, the median change was −74% (95% CI: −90% to −40%) and 16/26 patients had more than 70% decrease from baseline. At 18 months, the median change was −54% (95% CI: −85% to 7%) and 9/20 (45%) patients had at least 90% decrease from baseline. At 24 months, the median change was −88% (95% CI: −94% to −72%) and 9/27 (33%) patients had more than 98% decrease from baseline. Changes in volume and achievement of targets of MSU volume percentage decrease are reported in figure 1 according to the achievement of SU-level targets.
Figure 1.
Percentage of change from the baseline volume of MSU crystal volume measured with DECT over time according to SU levels. (A) Median and IQR of remaining percentage of the baseline MSU crystal volume at 6, 12, 18 and 24 months according to SU level at follow-up DECT scans. (B) Proportion of patients obtaining targets of percentage MSU crystal volume decrease from baseline at months 6, 12, 18 and 24 months according to SU level at follow-up DECT scans. (C) Feet DECT scans at baseline, 12 months and 24 months of a 74-year-old male with a baseline SU at 7.5 mg/dL treated with 300 mg allopurinol daily whose SU during follow-up reached 4.5 mg/dL. DECT, dual-energy computed tomography; MSU, monosodium urate; SU, serum urate.
The correlation between the change in MSU crystal volume and the number of flares in the preceding 6 months of the follow-up scan was weak: Spearman’s correlation coefficient=0.32 (p<0.05).
Factors associated with the kinetics of MSU crystal dissolution
There was a good correlation between change in SU level and percentage change in MSU crystal volume: Spearman’s correlation coefficient=0.66 (p<0.0001).
In bivariable analysis of preselected candidate explanatory variables of the percentage of change from the baseline MSU crystal volume, those with multilevel coefficients with p values <0.2 were age (−0.70, 95% CI: −1.73 to 0.32, p=0.19), hypertension (22.59, 95% CI: −5.65 to 50.97, p=0.13), chronic heart disease (−20.48, 95% CI: −45.54 to 4.4, p=0.12) and SU level <5.0 mg/dL (−40.32, 95% CI: −73.95 to −6.5, p=0.03). eGFR ≥60 mL/min/1.73 m2 (13.43, 95% CI: −15.04 to 41.87, p=0.36) and SU level <6.0 mg/dL and ≥5.0 mg/dL (−6.40, 95% CI: −44.93 to 32.2, p=0.75) did not show any trend of association with MSU crystal dissolution in bivariable analysis.
Multivariable analysis showed that time from the baseline DECT scans, SU level <5.0 mg/dL and hypertension were the only explanatory variables significantly associated with the decrease of MSU crystal volume over time (table 2).
Table 2.
Multivariable analysis of variables explaining MSU crystal volume percentage change measured with DECT over time
| Explanatory variable | Percentage of remaining MSU crystal volume (cm3) (mean (SD)) | Multilevel coefficient (95% CI) | P value | |
| Time since baseline DECT scan (days) | (0.0, 822.0) | 43.5 (41.0) | −0.06 (−0.08 to −0.03) | <0.001 |
| Age (years) | (38.7, 88.6) | 43.5 (41.0) | −0.52 (−1.53 to 0.49) | 0.34 |
| Hypertension | No (reference) | 31.6 (33.3) | – | |
| Yes | 47.1 (42.6) | 41.87 (16.38 to 67.18) | <0.01 | |
| Chronic heart disease | No (reference) | 48.1 (44.2) | – | |
| Yes | 37.9 (36.4) | −19.64 (−44.5 to 5.4) | 0.15 | |
| Serum urate level at follow-up DECT scan (mg/dL) | ≥6.0 (reference) | 82.5 (67.7) | – | |
| <5.0 | 30.3 (32.1) | −39.46 (−70.93 to −8.34) | 0.02 | |
| 5.0 to <6.0 | 73.4 (29.8) | −4.62 (−39.54 to 30.27) | 0.80 |
DECT, dual-energy computed tomography; eGFR, estimated glomerular filtration rate (CKD-EPI formula); MSU, monosodium urate.
Discussion
Owing to a good correlation between decrease in SU level and MSU crystal dissolution, in patients with gout who have MSU crystal volumes >0.1 cm3 measured on DECT scans, obtaining SU levels below 5.0 mg/dL helps achieve far more substantial crystal dissolution than the <6.0 mg/dL target which has been associated with uneven and incomplete crystal disappearance. History of hypertension appears to be a factor contributing to decreased MSU crystal depletion under ULT.
MSU crystal volume is associated with the risk of flares and its decrease within an acceptable time frame should be a therapeutic objective. Previous studies have demonstrated an association between DECT-measured MSU crystal volume and recent or upcoming flares.2 14 It is now clear that gout flares are triggered by MSU crystals, and that risk of flares disappears with clearance of the crystal burden, which occurs far later than the achievement of SU-level objectives.15 Aiming for SU-level decrease below 5.0 mg/dL seems to be a reasonable and feasible option in routine practice, while aiming for even lower levels may be difficult to achieve clinically as shown by the failure of the trial aiming for SU levels below 3.3 mg/dL using intensive oral ULT.16
Our results suggest that the below 5.0 mg/dL SU target should be extended to all patients exhibiting significant MSU crystal volumes on DECT scans, even without tophaceous gout. Apart from French3 and British guidelines that recommend obtaining SU levels below 5.0 mg/dL, mainly based on data on tophus size reduction according to SU level,17 most national and international guidelines currently recommend to aim for the below 6.0 mg/dL target except for patients who were tophaceous.18–20 Given the uneven and relatively poor MSU crystal dissolution obtained in patients reaching only the below 6.0 mg/dL SU target, aiming for SU levels below 5.0 mg/dL may be warranted in patients with gout who have detectable crystal deposits, since reaching this objective seemed to provide a more secured and complete crystal dissolution after 24 months of ULT. Specific attention should be given to patients with hypertension as it was associated with slower crystal dissolution.
The reason why a tophus becomes invisible to DECT in the course of ULT still needs to be clarified. DECT is limited by its spatial resolution and we had already shown that the technique could ‘miss’ tophi that had been detected with ultrasound, which also captures the soft tissue component of the tophus.21 Recently, another study showed that during T2T ULT, the ratio inside tophus between MSU crystals and the soft tissue content decreased over time,11 suggesting that the kinetics of crystal depletion were more rapid than the resolution of the soft tissue content of the tophi. It is not yet known if its the overall crystal concentration of the tophus which decreases (and therefore the whole tophus could not reach the detection threshold of DECT despite the persistence of substantial quantities of crystals) or the crystal core of the tophus which shrinks but should remain detectable for a longer period until the depletion of the vast majority of crystals, with potential different clinical implications in subsequent risk of flares.
This study has inherent limitations. First, without prior evidence of what extent of crystal dissolution should be targeted and when, thresholds of target percentage of MSU crystal volume decrease were chosen arbitrarily based on what a clinician could expect and may want to expose to patients as management objectives. Further studies are needed to explore what attaining these thresholds translates into in terms of clinical benefits. Second, this exploratory study was too underpowered at certain timepoints due to low numbers of patients in SU-level groups >5.0 mg/dL (eg, 24 months) to carry out statistical testing, explaining the lack of statistically significant differences despite obvious numerical differences. Nevertheless, all analyses suggest that, unlike the <5.0 mg/dL target, the<6.0 mg/dL target seems insufficient to achieve significant crystal dissolution in patients with positive DECT scans.
In patients with baseline crystal deposition ≥0.1 cm3, obtaining SU levels below 5.0 mg/dL is the best threshold to obtain subtotal crystal dissolution in 2 years, while the 6.0 mg/dL threshold allows uneven and incomplete crystal dissolution. Patients with hypertension exhibit slower crystal dissolution and may require lower SU targets.
Footnotes
Contributors: VL, TP, J-FB, LN, SV: study conception and design, development of study protocol, review of statistical analyses. VL, CJ, AP, VD, J-FB, JL,TP and JL: data collection. VL and TP: writing the first draft of the manuscript. CJ, AP, LN, VD, JL, SV and J-FB: critical revisions and submission of the manuscript, approval of the final manuscript version.
Funding: The DECTUS study has received funding from Horizon Pharmaceutical and the Lille Catholic Hospitals.
Competing interests: TP has received research support from Horizon Pharmaceuticals and Chugai; TP has also received personal speaker and advisory fees from Novartis and Menarini.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the French ethical committee CPP Sud-Est III (EudraCT 2020-A01269-30) and patients provided informed consent.
References
- 1.Pascart T, Lioté F. Gout: state of the art after a decade of developments. Rheumatology (Oxford) 2019;58:27–44. 10.1093/rheumatology/key002 [DOI] [PubMed] [Google Scholar]
- 2.Pascart T, Grandjean A, Capon B, et al. Monosodium urate burden assessed with dual-energy computed tomography predicts the risk of flares in gout: a 12-month observational study: MSU burden and risk of gout flare. Arthritis Res Ther 2018;20:210. 10.1186/s13075-018-1714-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascart T, Latourte A, Flipo R-M, et al. 2020 recommendations from the French society of rheumatology for the management of gout: urate-lowering therapy. Joint Bone Spine 2020;87:395–404. 10.1016/j.jbspin.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Urquiaga M, Gaffo AL. Year in review: gout clinical research. GUCDD 2023;1:37–48. 10.3390/gucdd1010005 [DOI] [Google Scholar]
- 5.Filippou G, Pascart T, Iagnocco A. Utility of ultrasound and dual energy CT in crystal disease diagnosis and management. Curr Rheumatol Rep 2020;22:15. 10.1007/s11926-020-0890-1 [DOI] [PubMed] [Google Scholar]
- 6.Neogi T, Jansen TLTA, Dalbeth N, et al. Gout classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2015;74:1789–98. 10.1136/annrheumdis-2015-208237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richette P, Doherty M, Pascual E, et al. Updated European League against rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis 2020;79:31–8. 10.1136/annrheumdis-2019-215315 [DOI] [PubMed] [Google Scholar]
- 8.Marty-Ané A, Norberciak L, Andrès M, et al. Crystal deposition measured with dual-energy computed tomography: association with mortality and cardiovascular risks in gout. Rheumatology (Oxford) 2021;60:4855–60. 10.1093/rheumatology/keaa920 [DOI] [PubMed] [Google Scholar]
- 9.Dalbeth N, Billington K, Doyle A, et al. Effects of allopurinol dose escalation on bone erosion and urate volume in gout: a dual-energy computed tomography imaging study within a randomized, controlled trial. Arthritis Rheumatol 2019;71:1739–46. 10.1002/art.40929 [DOI] [PubMed] [Google Scholar]
- 10.Ellmann H, Bayat S, Araujo E, et al. Effects of conventional uric acid-lowering therapy on monosodium urate crystal deposits. Arthritis Rheumatol 2020;72:150–6. 10.1002/art.41063 [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Gamble GD, Horne A, et al. Changes in tophus composition during urate-lowering therapy: a dual-energy computed tomography study. Arthritis Care Res (Hoboken) 2023;75:1949–54. 10.1002/acr.25084 [DOI] [PubMed] [Google Scholar]
- 12.Uhlig T, Eskild T, Karoliussen LF, et al. Two-year reduction of dual-energy CT urate depositions during a treat-to-target strategy in gout in the NOR-gout longitudinal study. Rheumatology 2022;61(SI):SI81–5. 10.1093/rheumatology/keab533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascart T, Ramon A, Ottaviani S, et al. Association of specific comorbidities with monosodium urate crystal deposition in urate-lowering therapy-naive gout patients: a cross-sectional dual-energy computed tomography study. J Clin Med 2020;9:1295. 10.3390/jcm9051295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalbeth N, Nicolaou S, Baumgartner S, et al. Presence of monosodium urate crystal deposition by dual-energy CT in patients with gout treated with allopurinol. Ann Rheum Dis 2018;77:364–70. 10.1136/annrheumdis-2017-212046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty M, Jenkins W, Richardson H, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392:1403–12. 10.1016/S0140-6736(18)32158-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalbeth N, Doyle AJ, Billington K, et al. Intensive serum urate lowering with oral urate-lowering therapy for erosive gout: a randomized double-blind controlled trial. Arthritis Rheumatol 2022;74:1059–69. 10.1002/art.42055 [DOI] [PubMed] [Google Scholar]
- 17.Perez-Ruiz F, Calabozo M, Pijoan JI, et al. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum 2002;47:356–60. 10.1002/art.10511 [DOI] [PubMed] [Google Scholar]
- 18.FitzGerald JD, Dalbeth N, Mikuls T, et al. American college of rheumatology guideline for the management of gout. Arthritis Rheumatol 2020;72:879–95. 10.1002/art.41247 [DOI] [PubMed] [Google Scholar]
- 19.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. 10.1136/annrheumdis-2016-209707 [DOI] [PubMed] [Google Scholar]
- 20.Dahanayake C, Jordan KM, Roddy E. The 2022 NICE guideline for the diagnosis and management of gout. GUCDD 2023;1:7–10. 10.3390/gucdd1010002 [DOI] [Google Scholar]
- 21.Pascart T, Grandjean A, Norberciak L, et al. Ultrasonography and dual-energy computed tomography provide different quantification of urate burden in gout: results from a cross-sectional study. Arthritis Res Ther 2017;19:171. 10.1186/s13075-017-1381-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003725supp001.pdf (242.2KB, pdf)