Poor sleep is a frequent occurrence in the intensive care unit (ICU) setting, and despite decades of research describing poor sleep, relatively few gains in the care of ICU patients have been made in improving sleep in the ICU. Inertia to change clinical practice may be impeded by a lack of research regarding the efficacy of sleep-promoting interventions in the ICU; however, sleep and circadian disruption (SCD) appears to be an important potential target for improving important patient outcomes, such as delirium. Determining the impact of sleep-promoting interventions is challenging both in the feasibility of measure performance as well as in its interpretation, because of seemingly unavoidable confounders. Although multicomponent ICU SCD interventions have had mixed successes in decreasing environmental disturbance for ICU patients, the heterogenous nature of these studies and variable incorporation of objective measures of sleep or circadian function limit any firm conclusions (1).
Sleep measurement in critically ill patients remains an important barrier to large studies on ICU SCD. Interpretation of polysomnography, the gold standard, is difficult because commonly used medications, complex illness such as sepsis, various organ failures (e.g., hepatic encephalopathy), etc. in the ICU are associated with altered electroencephalography patterns (2, 3). Although advances in portable, wearable devices have improved measures of sleep and circadian function in the ICU, numerous challenges remain (4, 5). To be of utility at the bedside, sleep and circadian measures must have acceptable cost, feasibility, tolerance, and interpretability for longitudinal and around-the-clock monitoring without influencing the provision of patient care. Such advances, however, would facilitate meaningful ICU SCD definitions, guide the timing of interventions, and support rigorous outcome evaluation (1).
In this issue of the AnnalsATS, Georgopoulos and colleagues (pp. 1624–1632) use the automated electroencephalography odds ratio product (ORP), an automated means of measuring 10 different deciles of differing stages of alertness from deep sleep (decile 1–2) to full wakefulness (decile 10) in addition to spindle density over time (6). The ORP seems to be a feasible and tolerable measure that affords the possibility for longitudinal monitoring, although it has been criticized for having limited accuracy identifying sleep and sleep stages. The authors have used an established dataset from a multicenter longitudinal cohort from the National Heart, Lung, and Blood Institute that examined the cardiovascular consequences of sleep-disordered breathing in community dwelling adults as control subjects (7). A key strength of this study is that it compares the frequency of common ORP types that are associated with clinical phenotypes, furthering our understanding of underlying mechanisms of poor sleep in the ICU and in a limited number of ICU survivors through inference.
Results of this study show a marked difference, albeit not surprising, between sleep in ICU patients and community-dwelling adults. All ICU patients experienced one of four ORP types, characterized by low depth of sleep and variable (low to high) full wakefulness (types 1,1; 1,2; and 1,3; respectively) or average sleep depth with little time spent in full wakefulness (2,1). This is compared with the most common ORP architecture types in community-dwelling adults: high sleep depth with variable levels of full wakefulness (types 3,1; 3,2; and 3,3; respectively) and average sleep depth with either moderate or high full wakefulness (types 2,2 and 2,3). What is interesting is that these clinical phenotypes seen commonly in ICU patients, such as type 1,1, are akin to community-dwelling adults with very severe obstructive sleep apnea; arousal occurs upon falling asleep, and the individual fails to progress to deep sleep secondary to likely environmental stimuli or perceived need for hypervigilance (i.e., fear of injury if sleeping) (8). Furthermore, type 1,3 is seen most in community-dwelling adults with insomnia, as they experience low sleep pressure, as is seen with circadian dysrhythmia or hyperarousal states where sleep might occur at any time over a 24-hour cycle. Interestingly, in patients with 24-hour recordings, similarities existed between day and night recordings suggesting limited time needed. Over time, little deep sleep continued to dominate in an ICU survivor population.
Although those findings confirm the preexisting knowledge that sleep disturbances are common in critically ill patients and provides some evidence for phenotypes, there are important methodological limitations that should be considered. First, the ICU cohort is composed of a very specific population (ventilated patients, not sedated, with a Glasgow Coma Scale of 11 or greater, no delirium, etc.) that was culled from four previous cohorts. Those specific patient features do not represent most critically ill patients, warranting caution for generalizing of results. The high duration of mechanical ventilation in this population suggests that it was mostly composed of patients who would fulfill criteria for chronic critical illness, which is especially true for patients who require tracheostomy (>65%). The ICU survivor cohort (which is not fully contained in the ICU group) also has specific features that may not represent most ICU survivors; specifically, they represent a subgroup of ICU survivors who are fit enough to return for ambulatory testing, which represents an important source of selection bias. Finally, age and sex matching were not performed using robust techniques such as exact matching, as it appears that males and the eldest females were arbitrarily selected; this type of matching also ignores other important features, in particular comorbidities, which may hamper the conclusions.
Understanding the complex interplay between sleep in the ICU, survivors’ sleep, and post-ICU syndrome is no easy task, and causal inference models from broad populations of critically ill patients with sequential measurements are necessary. Many causal assumptions are still unclear. Does poor sleep in the ICU cause short-term complications, such as prolonged hospitalization or death? Does poor sleep in the ICU contribute to post–intensive care syndrome? Is poor sleep quality after ICU discharge a result of poor sleep in the ICU? These issues are graphically discussed in Figure 1.
Figure 1.
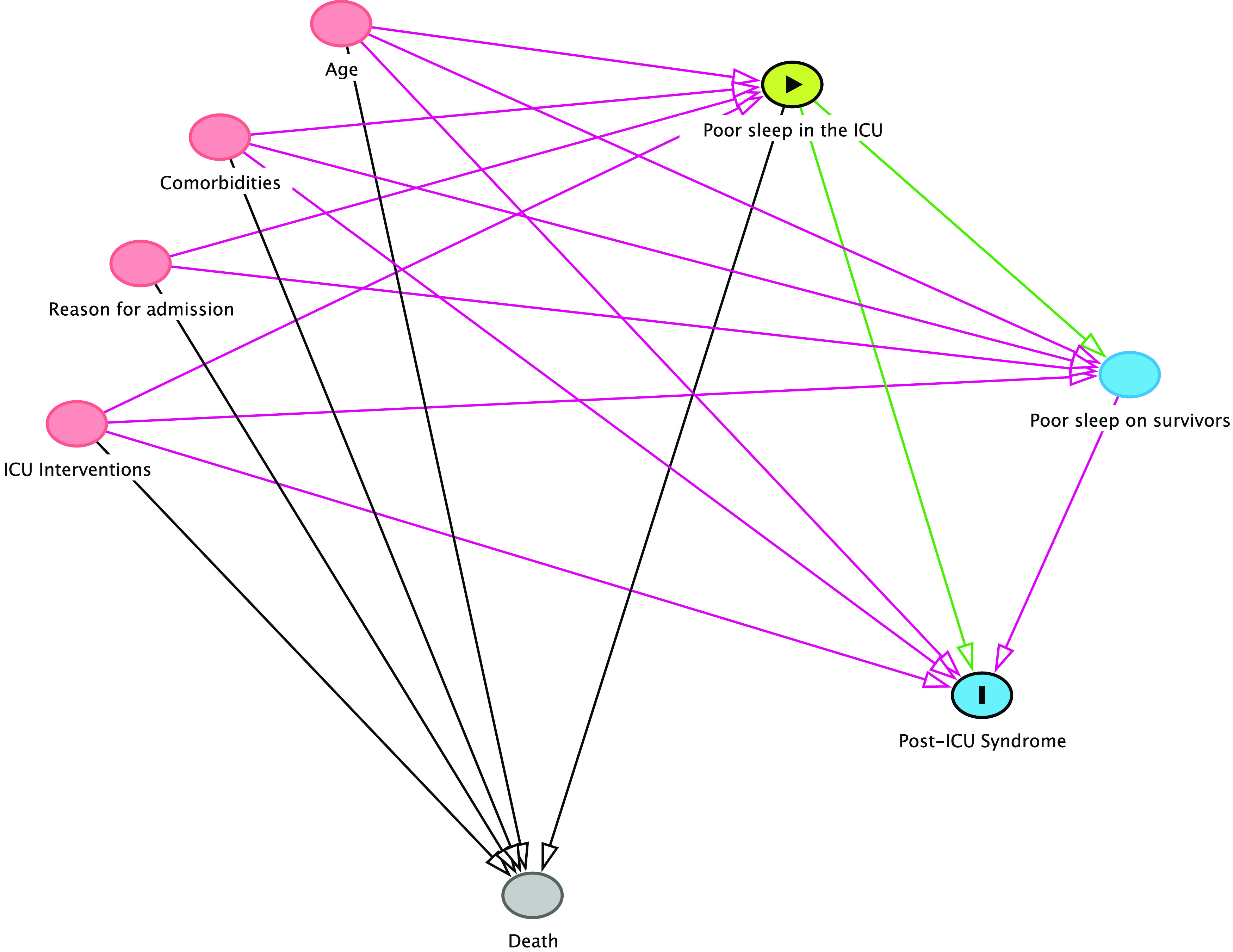
The complex association between intensive care unit (ICU) sleep and outcomes. Key factors that may influence ICU sleep are shown in red. Notoriously, all are also known contributors to mortality, generating a competing risk scenario (death competes with poor sleep). This becomes even more cumbersome as further follow-up evaluations of sleep are performed: only survivors can be referred to post-ICU sleep studies. The arrow in green represents the assumption that poor sleep in the ICU may cause post-ICU syndrome. No simple adjustment can remove all open pathways for causal inference using a simple regression or competing risk model.
In conclusion, the paper by Georgopoulos and colleagues highlights the urgent need for more research on a broad population of critically ill patients to better understand the complex pathways and causal effects of poor sleep and outcomes in the ICU population.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Knauert MP, Ayas NT, Bosma KJ, Drouot X, Heavner MS, Owens RL, et al. Causes, consequences, and treatments of sleep and circadian disruption in the ICU: an official American Thoracic Society research statement. Am J Respir Crit Care Med . 2023;207:e49–e68. doi: 10.1164/rccm.202301-0184ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards KC, Wang YY, Jun J, Ye L. A systematic review of sleep measurement in critically ill patients. Front Neurol . 2020;11:542529. doi: 10.3389/fneur.2020.542529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watson PL, Pandharipande P, Gehlbach BK, Thompson JL, Shintani AK, Dittus BS, et al. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med . 2013;41:1958–1967. doi: 10.1097/CCM.0b013e31828a3f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Younes M, Ostrowski M, Soiferman M, Younes H, Younes M, Raneri J, et al. Odds ratio product of sleep EEG as a continuous measure of sleep state. Sleep (Basel) . 2015;38:641–654. doi: 10.5665/sleep.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kakar E, Priester M, Wessels P, Slooter AJC, Louter M, van der Jagt M. Sleep assessment in critically ill adults: a systematic review and meta-analysis. J Crit Care . 2022;71:154102. doi: 10.1016/j.jcrc.2022.154102. [DOI] [PubMed] [Google Scholar]
- 6. Georgopoulos D, Kondili E, Gerardy B, Alexopoulou C, Bolaki M, Younes M. Sleep architecture patterns in critically ill patients and survivors of critical illness: a retrospective study. Ann Am Thorac Soc . 2023;20:1624–1632. doi: 10.1513/AnnalsATS.202301-038OC. [DOI] [PubMed] [Google Scholar]
- 7. Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep . 1997;20:1077–1085. [PubMed] [Google Scholar]
- 8. Dres M, Younes M, Rittayamai N, Kendzerska T, Telias I, Grieco DL, et al. Sleep and pathological wakefulness at the time of liberation from mechanical ventilation (SLEEWE): a prospective multicenter physiological study. Am J Respir Crit Care Med . 2019;199:1106–1115. doi: 10.1164/rccm.201811-2119OC. [DOI] [PubMed] [Google Scholar]