Abstract
Rationale
Recent studies have shown that sleep apnea–specific intermittent hypoxemia quantified by the hypoxic burden (HB) predicted cardiovascular disease (CVD)–related mortality in community-based and clinical cohorts. Calculation of HB is based on manual scoring of hypopneas and apneas, which is time-consuming and prone to interscorer variability.
Objective
To validate a novel method to quantify the HB that is based on automatically scored desaturations.
Methods
The sample included 5,655 middle-aged or older adults from the Sleep Heart Health Study (52.8% women; age, 63.2 ± 11.3 yr). The original HB method was based on a subject-specific search window obtained from an ensemble average of oxygen saturation signals (as measured by pulse oximetry) and synchronized with respect to the termination of scored respiratory events. In this study, however, the search window was obtained from ensemble average of oxygen saturation signals that synchronized with respect to the minimum of all automatically identified desaturations (⩾2% and other thresholds, including 3% and 4%, in sensitivity analyses). The time interval between the two maxima around the minimum saturation was defined as the search window. The oximetry-derived HB (HBOxi) was defined as the total area under all desaturation curves (restricted by the search window) divided by the total sleep time. Logistic and Cox regression models assessed the adjusted odds ratio (aOR)/hazard ratio of excessive daytime sleepiness (EDS), hypertension (HTN), and CVD mortality per 1–standard deviation increase in HBOxi after adjusting for several covariates and confounders.
Results
The Spearman’s rank correlation between HB (median [interquartile range], 34.4 [18.4–59.8] % min/h) and HBOxi (median [interquartile range], 34.5 [21.6–53.8] % min/h) was 0.81 (P < 0.001). Similar to HB, HBOxi was significantly associated with EDS (aOR [95% confidence interval (CI)], 1.17 [1.09–1.26] per standard deviation), HTN (aOR [95% CI], 1.13 [1.05–1.21]), and CVD mortality (adjusted hazard ratio [95% CI], 1.15 [1.01–1.30]) in fully adjusted models.
Conclusions
The HBOxi was highly correlated with the HB based on manually scored apneas and hypopneas and was associated with EDS, HTN, and CVD mortality with similar effect sizes as previously reported. This method could be incorporated into wearable technology that accurately records oxygen saturation signals.
Keywords: sleep apnea, mortality, HB, hypertension, excessive daytime sleepiness
Obstructive sleep apnea (OSA) is a common disorder in the adult population with an estimated global prevalence as high as 1 billion (1) and an underrecognized comorbidity for cardiac patients (2). The apnea–hypopnea index (AHI) is a commonly used measure to diagnose OSA and assess its severity. However, a well-known limitation of the AHI is its inability to accurately quantify the severity of individual respiratory events by assigning a similar importance to all events that meet thresholds such as minimal duration (⩾10 s) and a linked desaturation (e.g., ⩾3% or 4%) or the presence of an arousal (3). The use of metrics that do not fully characterize an OSA-related physiological disturbance as inclusion criteria for clinical trials is a possible reason for the failure of those trials to detect a benefit of continuous positive airway pressure (4–8).
Recent studies have suggested that physiologically driven measures of OSA severity may help improve and better identify individuals with OSA at high risk who may benefit from continuous positive airway pressure (9–12). Among these measures, sleep apnea–specific hypoxic burden (HB) has been shown to predict cardiovascular disease (CVD) outcomes in observational and clinical-based cohorts (12–15). The HB is easily obtained from polysomnography (PSG) that includes manually scored respiratory events from respiratory channels (e.g., nasal cannula, thermistor, or abdomen/chest movement) and oxygen saturation (measured by pulse oximetry, i.e., SpO2) (12). The dependence of HB on manually scored events, however, makes it less applicable to simplified sleep studies with no respiratory channels (e.g., type IV sleep tests) and wearable devices that collect pulse oximetry (SpO2) but no other respiratory data (16). Therefore, streamlining the approach to measuring HB using automatically detected desaturations (oximetry-derived HB, i.e., HBOxi) could broaden its applicability to these simpler diagnostic modalities. This would have several important clinical implications. First, it could provide a more readily scalable approach, which would potentially be useful in underserved communities. Second, when integrated into wearable devices, it would enable multiple-night monitoring of OSA-related cardiovascular risk (12–14). Third, the calculation of HB eliminates any misclassification related to manual variability of annotating respiratory events and arousals (17, 18).
Therefore, in this study, we sought to calculate HBOxi, defined as the total area under the automatically identified desaturation curve divided by sleep time. Its convergent validity was evaluated through the correlation with HB calculated using the published method (12). To assess its predictive validity, its association with excessive daytime sleepiness (EDS) (defined as an Epworth Sleepiness Scale score >10), hypertension (HTN), and CVD-related mortality were assessed in the Sleep Heart Health Study (SHHS) (19–21).
Methods
Participants
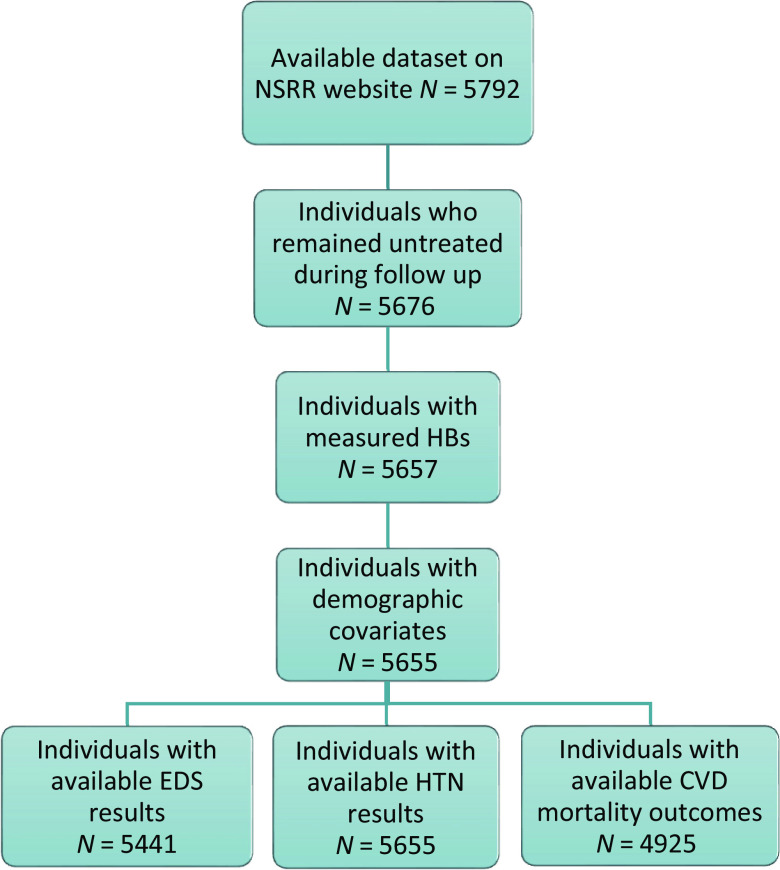
The study sample included individuals from the SHHS with publicly available data in the National Sleep Research Resource (NSRR) (https://sleepdata.org/). The SHHS is a prospective, community-based cohort of middle-aged or older adults designed to determine the impact of sleep-disordered breathing on CVD. The baseline examination (1995–1998), which included a standardized sleep habits questionnaire, anthropometric data, and a nocturnal unattended PSG, was performed in 6,441 men and women who were ⩾40 years of age, 5,792 of whom were in the NSRR (Figure 1). Each participating institution acquired institutional review board permission, and each participant signed an informed consent form (22).
Figure 1.
Ascertained study sample. CVD = cardiovascular disease; EDS = excessive daytime sleepiness; HTN = hypertension; NSRR = National Sleep Research Resource.
PSG
All participants completed a baseline unattended type 2 PSG study (1995–1998) and a standardized questionnaire. All PSGs were scored at a central sleep reading center (Case Western Reserve University) using methods detailed previously (20, 21, 23). Briefly, respiratory events were identified on the basis of amplitude reduction on the thermistor or respiratory inductance channels. The AHI was calculated using all apneas and hypopneas associated with desaturations of ⩾3% or arousal. SpO2 signals were captured by fingertip pulse oximetry (Nonin) and digitally sampled at 1 Hz (resolution of 1% and accuracy of ±2% in the range of 70–100% [24]).
Calculation of HBOxi
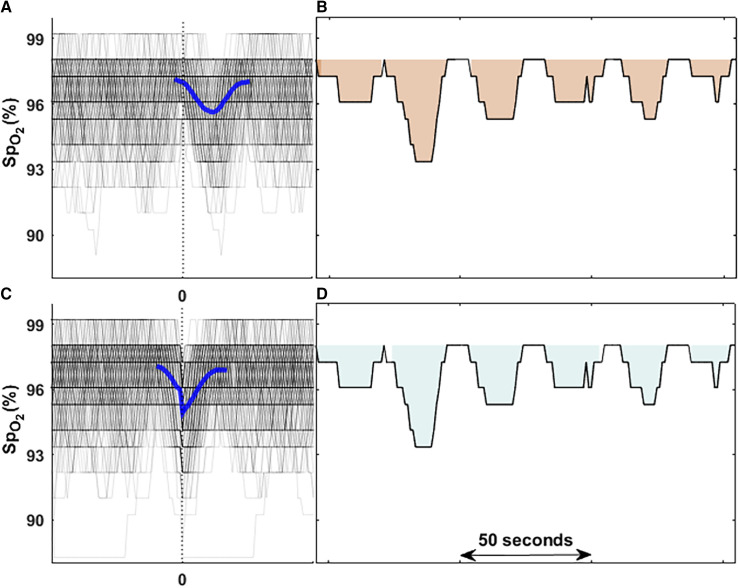
Calculation of HBOxi is based on our previously developed sleep apnea–associated HB. Briefly, HB measures the total OSA-related oxygen desaturation area during sleep. For each individually identified apnea or hypopnea, the maximum SpO2 during the 100 seconds before the end of the event is considered as the preevent baseline oxygen saturation. For each event, the area under this baseline value was calculated over a subject-specific search window that was determined from the ensemble average of time-aligned SpO2 curves (aligned with respect to the end of events; Figures 2A and 2B). The individual-level HB was defined as the sum of all areas divided by total sleep time (% min/h). In the original HB method, we included all respiratory events (based on >30% reduction in airflow signal) regardless of the level of desaturation or arousal (12), some of which were associated with 2% desaturations. In a similar approach, any desaturation that exceeded the 2% threshold was included. In sensitivity analyses, the results were compared when higher thresholds, including 3% or 4%, were used to detect desaturations.
Figure 2.
Comparison of two methods to calculate hypoxic burden (HB). (A and B) The original HB calculation based on scored respiratory events. In this method, all local oxygen saturation as measured by pulse oximetry (SpO2) curves were synchronized based on the endpoint of respiratory events (time zero; B) and then ensemble-averaged to obtain the search window for the area calculation from baseline that is the highest SpO2 value among 100 seconds before each event. (C and D) The HB calculation based on automatically detected oxygen desaturations. In this method, all local SpO2 curves were synchronized based on minimum saturation points of all identified desaturations (time zero; C) and then ensemble-averaged to obtain the search window used for area calculation of each local desaturation from baseline SpO2 value that is the start point of each local desaturation (D). The difference between the time zeros from two methods is the average lung-to-finger circulation time described previously (48).
After removing artifacts (i.e., SpO2 <40%), all the SpO2 desaturation/recovery episodes with a ⩾2% decrease or 2% increase in magnitude were automatically detected. Briefly, all SpO2 local minima are automatically identified. A desaturation is identified if the minimum point falls between two peaks (⩾2% in height). When all desaturations have been identified, an ensemble average of time-aligned SpO2 curves synchronized with respect to the minimum saturation is created. The interval between the two peaks around the minimum value is the search window used for the HBOxi calculation (Figure 2C). Therefore, the area calculation is guided by the duration of the search window. After identifying the search window and desaturation-specific baseline (SpO2 at the beginning of the desaturation), the individual areas for all desaturations were summed and normalized by the total sleep time (Figure 2D). In a sensitivity analysis, the total area was normalized by the total recording time and the associations with outcomes were examined separately. Normalization by the total recording time was to examine how associations changed if no electroencephalography recording or sleep scoring was available. Additionally, thresholds of 3% or 4% to identify desaturations were also tested. Figure E1 in the data supplement shows the algorithm for HBOxi calculation.
Outcomes
To compare HB and HBOxi, the associations of these two metrics with EDS, HTN, and CVD mortality were examined. EDS was defined as an Epworth Sleepiness Scale score >10 points. HTN was defined based on the average of the second and third sitting blood pressure readings (25) as an average systolic blood pressure of >140 mm Hg and diastolic blood pressure of >90 mm Hg, or as treatment with HTN medications. Preexisting CVD disease was identified by adjudicated surveillance data collected by the parent cohorts or by self-report as a history of angina, heart failure, myocardial infarction, stroke, or coronary revascularization that had been physician-diagnosed (24). CVD mortality was based on the underlying cause of death assessed by a study physician adjudicator (24). A CVD cause of death was broadly categorized by International Classification of Diseases, 9th Revision codes as CVD (codes 396.9–442, 966.71, 785.51). Participants’ demographic characteristics and history of smoking were all assessed using questionnaires.
Statistical Analysis
Descriptive statistics shown in Table 1 are presented as mean ± standard deviation, median and interquartile range, or relative frequencies (percentages). Spearman’s rank correlation was used to measure the degree of association between HB and HBOxi (based on 2%, 3%, or 4% desaturation thresholds). To test whether the association between HB and HBOxi depended on the baseline SpO2 levels (e.g., low baseline SpO2 due to cardiopulmonary disease), we compared the Spearman’s rank correlation in those with wakefulness SpO2 <90% and those with wakefulness SpO2 ⩾90%. Bland-Altman plots were used to visualize the agreement between HB and HBOxi (based on 2%, 3%, or 4% desaturation thresholds). Kaplan-Meier curves were used to assess the probability of CVD mortality per quartile of HB or HBOxi. Several multivariable logistic or Cox regression models were built to evaluate the associations of HBOxi with EDS, HTN, or CVD mortality; the observed associations were compared with those for HB. Covariates were chosen based on established clinical relationships (26). In the minimally adjusted model, covariates included age, race, and sex (all outcomes). For EDS, the fully adjusted model included body mass index, smoking status (never, former smoker, and current smoker), and wakefulness SpO2 (the average of SpO2 maxima during wakefulness before the start of the first sleep period). For the HTN analysis, the fully adjusted model also added diabetes as a covariate. Finally, for the CVD mortality models, we constructed three models: Model 1, a minimally adjusted model described above; Model 2, which included covariates in Model 1 plus body mass index, smoking status, and wakefulness SpO2; and Model 3, which added HTN, diabetes, myocardial infarction, angina, stroke, and heart failure to Model 2. Results are reported as adjusted odds ratios (ORs) with 95% confidence intervals (CIs) or hazard ratios with 95% CIs per 1–standard deviation increase in HBOxi or HB (both log-transformed, similar to previous studies [13, 27]). All signal analyses were performed in MATLAB (MathWorks), and all statistical analyses were performed in the R statistical package (https://www.R-project.org/).
Table 1.
Summary of participant characteristics
| Characteristic | Value | n |
|---|---|---|
| Age, yr | 63.2 ± 11.3 | 5,655 |
| Race | ||
| White | 4,781 (84.5%) | 5,655 |
| Black | 504 (8.9%) | |
| Other | 370 (6.6%) | |
| Sex | ||
| Male | 2,666 (47.1%) | 5,655 |
| Female | 2,989 (52.9%) | |
| Body mass index, kg/m2 | 28.1 ± 5.0 | 5,615 |
| Smoking | ||
| Never | 2,644 (46.7%) | 5,616 |
| Current | 549 (9.7%) | |
| Former | 2,423 (42.8%) | |
| Diabetes | 392 (6.9%) | 5,393 |
| History of COPD | 62 (1.1%) | 5,599 |
| EDS | 1,335 (24.5%) | 5,441 |
| Hypertension* | 2,384 (42.5%) | 5,655 |
| Apnea–Hypopnea Index† | 12.9 (6.7–23.2) | 5,655 |
| T90 | 0.18 (0.0–1.7) | 5,650 |
| Wakefulness SpO2‡ | 97.3 (96.1–98.0) | 5,655 |
| HB, % min/h | 34.4 (18.4–59.8) | 5,655 |
| HBOxi, % min/h | 34.5 (21.7–53.8) | 5,655 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; EDS = excessive daytime sleepiness (Epworth Sleepiness Scale score >10); HBOxi = oximetry-derived hypoxic burden; SpO2 = oxygen saturation as measured by pulse oximetry; T90 = total time arterial oxygen saturation is <90%.
Data presented as mean ± standard deviation for continuous variables. Ranges in parentheses are interquartile ranges.
Hypertension status was defined based on the average of the second and third sitting blood pressure readings of >140 mm Hg in systolic blood pressure and >90 mm Hg in diastolic blood pressure or being treated with hypertension medications.
All apneas and hypopneas with ⩾30% nasal cannula reduction and ⩾3% oxygen desaturation or with arousal per hour of sleep.
Wakefulness SpO2 was the average of local SpO2 maxima during the wakefulness period before the start of the first sleep period.
Results
A total of 5,792 studies were available through the NSRR (Figure 1). After excluding individuals who received any treatment during follow-up, those who had inadequate SpO2 quality, and those with missing covariates for minimally adjusted models, a total of 5,655 were available for the analysis (24% had an Epworth Sleepiness Scale score >10 and 42% had HTN; Figure 1). Of these, 4,925 individuals had available CVD mortality status. A total of 351 CVD-related deaths were adjudicated over an average follow-up of 11.0 ± 3.1 years. A summary of the demographic and baseline characteristics of all participants is reported in Table 1. On average, 1.67% (0.53–6.8%) of total recording time (i.e., 8.5 [2.7–34.6] min) consisted of artifacts (i.e., SpO2 <40%).
Association between HB and HBOxi
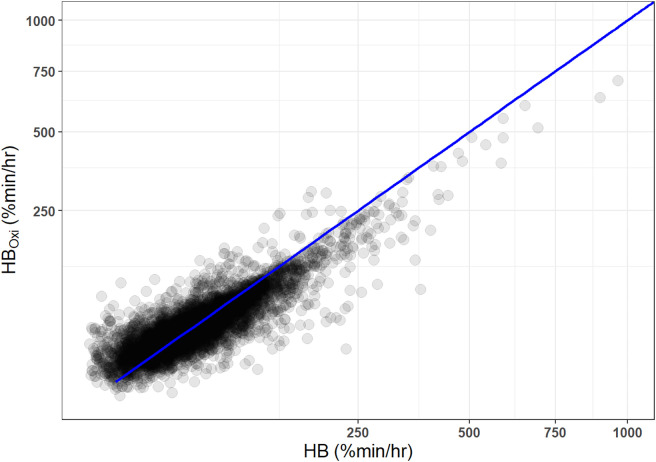
Figure 3 shows a scatter plot of HB (median [interquartile range], 34.4 [18.4–59.8] % min/h) and HBOxi (34.5 [21.7–53.8] % min/h), demonstrating a strong correlation between the two metrics with a Spearman’s rank correlation of 0.81 (P < 0.001; 2% threshold). The Spearman’s rank correlations between HB and HBOxi in those with wakefulness SpO2 ⩾90% and wakefulness SpO2 <90% were 0.81 (P < 0.001) and 0.79 (P < 0.001), respectively. Similarly, HB was significantly correlated (r = 0.82; P < 0.0001) with HBOxi based on a 3% threshold (23.0 [12.1–34.1] % min/h) and HBOxi based on a 4% threshold (15.2 [6.4–32.2] % min/h). Finally, HBOxi normalized by the total recording time instead of total sleep time had significant correlations with HB (r = 0.77; P < 0.0001).
Figure 3.
HB based on automatically identified desaturations and manually scored respiratory events. HBOxi = oximetry-derived hypoxic burden.
Figure E2 illustrates the Bland-Altman plots comparing the difference between HB and HBOxi across mean values of HB and HBOxi. The mean difference was near zero for HBOxi based on a 2% threshold (see Figure E2A), and it was highest for HBOxi based on ⩾4% desaturation (see Figure E2C). Finally, HBOxi based on a 2% threshold was higher than the HB at lower values, but the difference decreased as the HB increased (see Figure E2A; overall bias [95% CI], 3.9 [3.2–4.5] % min/h). Furthermore, oxygen desaturation indexes by manually and automatically detected desaturations were strongly correlated (Table E3).
Associations of HBOxi with EDS, HTN, and CVD Mortality
Similar to the HB, HBOxi was significantly associated with EDS, HTN, and CVD mortality in unadjusted and adjusted models. Every 1–standard deviation increase in HBOxi was associated with 23% increased odds of EDS (OR [95% CI], 1.23 [1.15–1.32]; Table 2) and 22% increased odds of HTN (OR [95% CI], 1.22 [1.15–1.30]; Table 3) in the minimally adjusted models. The ORs in the fully adjusted models for EDS and HTN were lower than in the minimally adjusted model (OR [95% CI], 1.17 [1.09–1.26] and 1.13 [1.05–1.21]; Tables 2 and 3).
Table 2.
Associations of HBs with EDS in minimally and fully adjusted models
| Model* | n | HB OR (95% CI) | HBOxi OR (95% CI) |
|---|---|---|---|
| Minimally adjusted model | 5,441 | 1.17 (1.09–1.25)† | 1.23 (1.15–1.32)† |
| Fully adjusted model | 5,376 | 1.12 (1.04–1.21)‡ | 1.17 (1.09–1.26)† |
Definition of abbreviations: CI = confidence interval; EDS = excessive daytime sleepiness (Epworth Sleepiness Scale score >10); HBOxi = oximetry-derived hypoxic burden; OR = odds ratio.
HBs were based on manually scored events and automatically identified desaturations; HBOxi was based on ⩾2% desaturations. Odds ratios are expressed per 1–standard deviation increase in log-transformed HBs.
Minimally adjusted model was adjusted for age, sex, and race. Fully adjusted model was adjusted for age, sex, race, body mass index, smoking status, and wakefulness oxygen saturation as measured by pulse oximetry.
P < 0.001.
P < 0.01.
Table 3.
Associations of HBs with hypertension in minimally and fully adjusted models
| Model* | n | HB OR (95% CI) | HBOxi OR (95% CI) |
|---|---|---|---|
| Minimally adjusted model | 5,655 | 1.24 (1.16–1.32)† | 1.22 (1.15–1.30)† |
| Fully adjusted model | 5,355 | 1.16 (1.08–1.24)† | 1.13 (1.05–1.21)‡ |
Definition of abbreviations: CI = confidence interval; HBOxi = oximetry-derived hypoxic burden; OR = odds ratio; SpO2 = oxygen saturation as measured by pulse oximetry.
HBs were based on manually scored events and automatically identified desaturations; HBOxi was based on ⩾2% desaturations. Odds ratios are expressed per 1–standard deviation increase in log-transformed HBs. Hypertension status was defined based on the average of the second and third sitting blood pressure readings of >140 mm Hg in systolic blood pressure and >90 mm Hg in diastolic blood pressure or being treated with hypertension medications.
Minimally adjusted model was adjusted for age, sex, and race. Fully adjusted model was adjusted for age, sex, race, body mass index, smoking status, diabetes, and wakefulness SpO2.
P < 0.001.
P < 0.01.
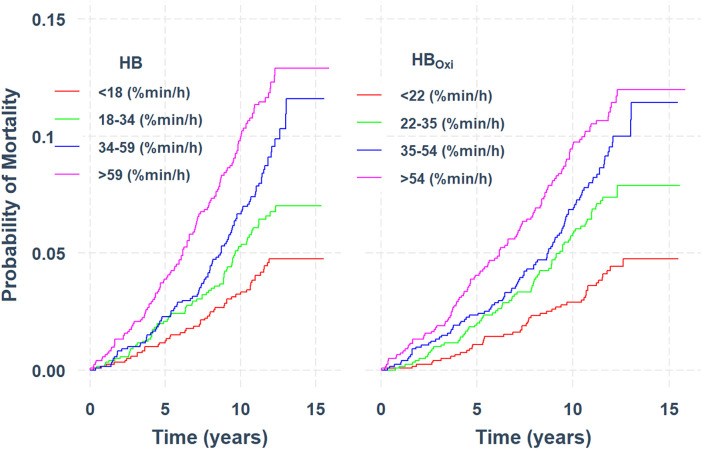
Finally, the associations of HBOxi with CVD mortality were similar to those of HBOxi with HB. Figure 4 displays similar unadjusted CVD-mortality survival curves per quartile of HB and HBOxi. Multivariable Cox regression analyses revealed hazard ratios (95% CI) of 1.15 (1.02–1.29), 1.18 (1.04–1.34), and 1.15 (1.01–1.30) for CVD mortality per 1–standard deviation increase in HBOxi in Models 1, 2, and 3, respectively (Table 4). In additional sensitivity analyses, thresholds of 3% and 4% were compared with the primary findings above. HBOxi for 3% and 4% desaturations was significantly associated with EDS and HTN. However, HBOxi based on 3% or 4% thresholds was not significantly associated with CVD mortality (Table E1). Finally, normalizing the HBOxi (based on the 2% threshold) by the total recording time provided similar associations with EDS, HTN, and CVD mortality (Table E2).
Figure 4.
Kaplan-Meier survival curves showing the probability of cardiovascular disease (CVD)–related mortality per quartile of hypoxic burden (left) and oxygen desaturation–based hypoxic burden (right). CVD was based on the underlying cause of death assessed by a study physician adjudicator. A CVD cause of death was broadly categorized by International Classification of Diseases, 9th Revision codes as CVD (codes 396.9–442, 966.71, and 785.51). HBOxi = oximetry-derived hypoxic burden.
Table 4.
Associations of HBs with CVD mortality in all adjusted models
| Model* | n | HB OR (95% CI) | HBOxi OR (95% CI) |
|---|---|---|---|
| Model 1 | 4,925 | 1.17 (1.03–1.32)† | 1.15 (1.02–1.29)† |
| Model 2 | 4,895 | 1.19 (1.04–1.36)‡ | 1.18 (1.04–1.34)‡ |
| Model 3 | 4,779 | 1.16 (1.01–1.32)† | 1.15 (1.01–1.30)† |
Definition of abbreviations: CI = confidence interval; CVD = cardiovascular disease; HBOxi = oximetry-derived hypoxic burden; OR = odds ratio; SpO2 = oxygen saturation as measured by pulse oximetry.
HBs were based on manually scored events and automatically identified desaturations; HBOxi was based on ⩾2% desaturations. Odds ratios are expressed per 1–standard deviation increase in log-transformed HBs. CVD mortality was based on the underlying cause of death assessed by a study physician adjudicator. A CVD cause of death was broadly categorized by International Classification of Diseases, 9th Revision codes as CVD (codes 396.9–442, 966.71, and 785.51).
Model 1 was adjusted for age, sex, and race. Model 2 was adjusted for age, sex, race, body mass index, smoking status, and wakefulness SpO2. Model 3 was adjusted for age, sex, race, body mass index, smoking status, wakefulness SpO2, diabetes, heart failure, angina, myocardial infarction, and stroke.
P < 0.05.
P < 0.01.
As shown in Table E4, adjusting by covariates used in the original publication (12) revealed similar findings. Furthermore, even though AHI was associated with HTN and EDS with lower ORs than for HBOxi, it was not significantly associated with CVD-related mortality in any of the models used above (Table E5).
Discussion
In this study, HB was found to be reliably estimated without the need for manually scored respiratory events. We developed a novel surrogate for HB (HBOxi) based on automatically identified oxygen desaturations that was highly correlated with HB (Figure 3). In addition, HBOxi provided similar associations with EDS, HTN, and CVD mortality (Figure 4 and Tables 2–4) after adjusting for multiple covariates and confounders. This method ultimately can be used in conjunction with sleep apnea testing devices and wearable systems that incorporate validated oxygen saturation technology to screen for and monitor sleep apnea severity over time and also to risk-stratify individuals with sleep apnea.
Previous research demonstrated that OSA, if left undiagnosed or untreated, is associated with excessive daytime sleepiness and diminished quality of life and can lead to neurocognitive impairment (28–30) as well as systematic HTN, cardiovascular and cerebrovascular disease, and metabolic dysfunction (25, 31–33). An increasing number of studies have implicated OSA-related intermittent hypoxemia as a key underlying mechanism for these adverse outcomes (28, 34). In OSA, intermittent hypoxemia is conventionally measured by frequency-based metrics, such as oxygen desaturation index, or non–OSA-specific metrics, such as sleep time spent with oxygen saturation <90%; however, these metrics have well-known limitations, including their inability to accurately quantify the severity of OSA-related intermittent hypoxemia (10, 35). Therefore, several methods have been proposed to better quantify intermittent hypoxemia that consider frequency and severity (10, 35). Among these metrics, HB has been shown to predict several health outcomes in population-based and clinical-based observational studies (12–15, 35, 36, 37). For example, HB was shown to be cross-sectionally associated with higher blood pressure (14) and increased prevalence of chronic kidney disease (37). In addition, HB has been shown to predict CVD-related mortality (27), incident heart failure (13), incident stroke (28), and cardiovascular disease (15). Despite these promising findings, HB relies on scored respiratory events that may not be available in many research and clinical settings. In addition, scoring of respiratory events depends on the recording of airflow during sleep, which has its own limitations, including mouth breathing and calibration issues that may impact the scoring of respiratory events (38–40). Furthermore, manual scoring of respiratory events is prone to interscorer and night-to-night variability (18). Our method supports the use of an oximetry-based approach to monitor HB with minimal burden, with the potential for use over multiple nights, thus minimizing misclassification due to night-to-night variability as well as identifying trends over time.
HBOxi identified at thresholds of ⩾2%, ⩾3%, or ⩾4% desaturations was associated with EDS and HTN. However, only the 2% threshold revealed a significant association with CVD mortality. A potential explanation for this may be related to the ability of HBOxi at 2% to better capture disease severity in milder and more severe cases of OSA than HBOxi at 3% or 4%. As described previously, HB considers all events based on airflow reduction (i.e., >30% from a preevent baseline) regardless of the level of desaturation or arousal (12). Therefore, in individuals with respiratory events associated with mild desaturations (<3%), HBOxi at 3% or 4% underestimates the total HB because none of those events will be incorporated in the calculation of HBOxi at 3% or 4%. In contrast, in individuals who have only respiratory events with ⩾4% desaturations, HBOxi at 2% and 4% will be identical because both metrics will be derived from the same desaturations, resulting in identical search windows and the total cumulative desaturation areas. Finally, the desaturation area captured by HBOxi at 2% but missed by HBOxi at 3% or 4% was significantly correlated with HB (r[HBOxi 2% – HBOxi 3% and HB] = 0.14; P < 0.0001; r[HBOxi 2% – HBOxi 4% and HB] = 0.31; P < 0.0001), supporting the notion that HBOxi at 2% may better captures the HB across different levels of OSA severity.
The original HB is calculated by normalizing the desaturation area by total sleep time. In this study, we tested the associations of HBOxi with outcomes by normalizing the desaturation area by total sleep time (obtained from manual scoring of electroencephalograms) and total recording time. Although HBOxi normalized by total sleep time appeared to be more precise, the hazard ratios were not meaningfully different (Tables 2–4 and see Table E2), providing additional support that HB can be estimated from systems that do not record sleep time. Measuring HB using a low-burden portable or even wearable device could potentially improve the diagnosis and management of OSA provided that the oximetry technology in these wearable systems is validated and their sensitivity is tested with respect to the placement of the oximeter and the color of skin (41–43). For example, a study in a primary-care setting demonstrated that approximately 38% of adult patients were at high risk of sleep apnea (44). Many of these individuals could potentially remain untreated as a result of limited access to sleep clinics. This problem may be exacerbated in underserved communities, leading to delayed OSA diagnosis and undertreatment in these communities (45, 46). Therefore, providing a more readily scalable system could potentially be useful in underserved communities.
Strengths and Limitations
A major strength of this study is its validation of an SpO2-based metric with automatically detected desaturations that can be used to gauge the severity of OSA and predict its health outcomes, including excessive daytime sleepiness, HTN, and CVD mortality. This metric has the potential to be incorporated in wearable devices that could record HB over multiple nights to potentially improve risk stratification and management of OSA. Additionally, this method has been tested in a relatively large and well-characterized population study. Despite this, there are some limitations to our study, including underrepresentation of younger and more ethnically and racially diverse individuals. For example, recent studies have demonstrated that the accuracy of pulse oximetry may depend on skin color and may be lower in individuals with darker skin colors (41–43). Therefore, future studies are needed to examine the association of HB and HBOxi with health outcomes across racial/ethnic groups. Furthermore, the sample size limitation and limited number of events did not allow for an evaluation of the observed associations in women or younger individuals. These studies are currently ongoing in larger and more diverse samples. The SHHS study used only the aforementioned Nonin pulse oximeters. Therefore, more studies are needed to better understand the effect of recording settings (i.e., longer average time) and/or filtering characteristics on the associations of HBOxi and health outcomes. Finally, neither HB nor HBOxi is designed to distinguish shallow/deep versus short/long desaturations (47). However, in an exploratory analysis involving HB, giving higher weights to deeper desaturations did not reveal a stronger association with CVD mortality (12). Future studies are needed to better understand the contribution of desaturation duration versus depth to CVD outcomes.
Conclusions
In this study, we developed and validated a novel approach to measure HB from automatically detected oxygen desaturations. The findings revealed a strong correlation between this metric and the original method based on manually scored events. In addition, the associations of HBOxi with excessive daytime sleepiness, HTN, and CVD mortality were similar to those of HB. This method can be used in conjunction with validated wearable systems to monitor and risk-stratify individuals with sleep apnea.
Footnotes
Supported by National Institutes of Health grants R21 HL161766 and R01HL153874; American Academy of Sleep Medicine grant 254-FP-21 (G.L.), the CHEST Foundation (G.L.); National Institutes of Health grants R01HL153874, R01 HL158765, and R21 HL161766 (A.A.); American Heart Association grant 19CDA34660137 (A.A.); American Academy of Sleep Medicine Foundation grants 188-SR-17 and SR-2217 (A.A.); and National Heart, Lung, and Blood Institute (NHLBI) grant HL R35 135818 (S.R.). The Sleep Heart Health Study was supported by NHLBI cooperative agreements U01HL53916 (University of California, Davis), U01HL53931 (New York University), U01HL53934 (University of Minnesota), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL53938 (University of Arizona), U01HL53940 (University of Washington), U01HL53941 (Boston University), and U01HL63463 (Case Western Reserve University). The National Sleep Research Resource was supported by the NHLBI (R24 HL114473, 75N92019R002).
Author Contributions: N.E. and A.A. contributed to study design, analysis, interpretation of data, and drafting the manuscript. G.L. contributed to the analysis, interpretation of the data and critical revision of the manuscript. W.H., S.A.S., and D.V. contributed to the analysis and critical revision of the manuscript. S.R. contributed to the data collection, interpretation of the data and critical revision of the manuscript. M.H., L.M, L.G., L.T., A.W., and M.S. contributed to interpretation of the data and critical revision of the manuscript. All authors approved this manuscript in its final form.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jaffe LM, Kjekshus J, Gottlieb SS. Importance and management of chronic sleep apnoea in cardiology. Eur Heart J . 2013;34:809–815. doi: 10.1093/eurheartj/ehs046. [DOI] [PubMed] [Google Scholar]
- 3. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med . 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, et al. Spanish Sleep Network Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med . 2020;8:359–367. doi: 10.1016/S2213-2600(19)30271-1. [DOI] [PubMed] [Google Scholar]
- 5. Elbadawi A, Elgendy IY, Shnoda M, Abuzaid AS, Barssoum K, Gouda M, et al. Impact of continuous positive airway pressure ventilation on cardiovascular outcomes among patients with obstructive sleep apnea: a meta-analysis of randomized trials. Am Heart J Plus Cardiol Res Pract. . 2021;11:100056. doi: 10.1016/j.ahjo.2021.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med . 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 7. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med . 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 8. Labarca G, Dreyse J, Drake L, Jorquera J, Barbe F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: systematic review and meta-analysis. Sleep Med Rev . 2020;52:101312. doi: 10.1016/j.smrv.2020.101312. [DOI] [PubMed] [Google Scholar]
- 9. Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput . 2013;51:697–708. doi: 10.1007/s11517-013-1039-4. [DOI] [PubMed] [Google Scholar]
- 10. Hajipour M, Baumann B, Azarbarzin A, Allen AJH, Liu Y, Fels S, et al. Association of alternative polysomnographic features with patient outcomes in obstructive sleep apnea: a systematic review. J Clin Sleep Med . 2022;19:225–242. doi: 10.5664/jcsm.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azarbarzin A, Zinchuk A, Wellman A, Labarca G, Vena D, Gell L, et al. Cardiovascular benefit of continuous positive airway pressure in adults with coronary artery disease and obstructive sleep apnea without excessive sleepiness. Am J Respir Crit Care Med . 2022;206:767–774. doi: 10.1164/rccm.202111-2608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J . 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW, et al. The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest . 2020;158:739–750. doi: 10.1016/j.chest.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JS, Azarbarzin A, Wang R, Djonlagic IE, Punjabi NM, Zee PC, et al. Association of novel measures of sleep disturbances with blood pressure: the Multi-Ethnic Study of Atherosclerosis. Thorax . 2020;75:57–63. doi: 10.1136/thoraxjnl-2019-213533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trzepizur W, Blanchard M, Ganem T, Balusson F, Feuilloy M, Girault JM, et al. Sleep apnea-specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all-cause mortality. Am J Respir Crit Care Med . 2022;205:108–117. doi: 10.1164/rccm.202105-1274OC. [DOI] [PubMed] [Google Scholar]
- 16. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med . 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loredo JS, Clausen JL, Ancoli-Israel S, Dimsdale JE. Night-to-night arousal variability and interscorer reliability of arousal measurements. Sleep . 1999;22:916–920. doi: 10.1093/sleep/22.7.916. [DOI] [PubMed] [Google Scholar]
- 18. Rosenberg RS, Van Hout S. The American Academy of Sleep Medicine inter-scorer reliability program: respiratory events. J Clin Sleep Med . 2014;10:447–454. doi: 10.5664/jcsm.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dean DA, II, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D, et al. Scaling up scientific discovery in sleep medicine: the national sleep research resource. Sleep (Basel) . 2016;39:1151–1164. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Sleep Heart Health Research Group Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep . 1998;21:759–767. [PubMed] [Google Scholar]
- 21. Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep . 1997;20:1077–1085. [PubMed] [Google Scholar]
- 22. Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation . 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med . 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casal R, Di Persia LE, Schlotthauer G. Sleep-wake stages classification using heart rate signals from pulse oximetry. Heliyon . 2019;5:e02529. doi: 10.1016/j.heliyon.2019.e02529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA . 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 26. Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA . 2017;318:156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Azarbarzin A, Sands SA, Younes M, Taranto-Montemurro L, Sofer T, Vena D, et al. The sleep apnea–specific pulse-rate response predicts cardiovascular morbidity and mortality. Am J Respir Crit Care Med . 2021;203:1546–1555. doi: 10.1164/rccm.202010-3900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White DP, Younes MK. Obstructive sleep apnea. Compr Physiol . 2012;2:2541–2594. doi: 10.1002/cphy.c110064. [DOI] [PubMed] [Google Scholar]
- 29. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res . 1997;6:199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- 30. Engleman HM, Douglas NJ. Sleep. 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax . 2004;59:618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koren D, Dumin M, Gozal D. Role of sleep quality in the metabolic syndrome. Diabetes Metab Syndr Obes.: Targets Ther. 2016;9:281–310. doi: 10.2147/DMSO.S95120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med . 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med . 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol . 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez-Garcia MA, Sánchez-de-la-Torre M, White DP, Azarbarzin A. Hypoxic burden in obstructive sleep apnea: present and future. Arch Bronconeumol . 2022;59:36–43. doi: 10.1016/j.arbres.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 36. Blanchard M, Gervès-Pinquié C, Feuilloy M, Le Vaillant M, Trzepizur W, Meslier N, et al. ERMES study group Hypoxic burden and heart rate variability predict stroke incidence in sleep apnoea. Eur Respir J . 2021;57:2004022. doi: 10.1183/13993003.04022-2020. [DOI] [PubMed] [Google Scholar]
- 37. Jackson CL, Umesi C, Gaston SA, Azarbarzin A, Lunyera J, McGrath JA, et al. Multiple, objectively measured sleep dimensions including hypoxic burden and chronic kidney disease: findings from the Multi-Ethnic Study of Atherosclerosis. Thorax . 2021;76:704–713. doi: 10.1136/thoraxjnl-2020-214713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madeiro F, Andrade RGS, Piccin VS, Pinheiro GDL, Moriya HT, Genta PR, et al. Transmission of oral pressure compromises oronasal CPAP efficacy in the treatment of OSA. Chest . 2019;156:1187–1194. doi: 10.1016/j.chest.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 39. Teichtahl H, Cunnington D, Cherry G, Wang D. Scoring polysomnography respiratory events: the utility of nasal pressure and oro-nasal thermal sensor recordings. Sleep Med . 2003;4:419–425. doi: 10.1016/s1389-9457(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 40. Budhiraja R, Goodwin JL, Parthasarathy S, Quan SF. Comparison of nasal pressure transducer and thermistor for detection of respiratory events during polysomnography in children. Sleep . 2005;28:1117–1121. doi: 10.1093/sleep/28.9.1117. [DOI] [PubMed] [Google Scholar]
- 41. Seitz KP, Wang L, Casey JD, Markus SA, Jackson KE, Qian ET, et al. Pulse oximetry and race in critically ill adults. Crit Care Explor . 2022;4:e0758. doi: 10.1097/CCE.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kupke A, Shachar C, Robertson C. Pulse oximeters and violation of federal antidiscrimination law. JAMA . 2023;329:365–366. doi: 10.1001/jama.2022.24976. [DOI] [PubMed] [Google Scholar]
- 43. Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med . 2020;383:2477–2478. doi: 10.1056/NEJMc2029240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med . 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 45. Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med . 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henry O, Brito A, Lloyd MC, Miller R, Weaver E, Upender R. A model for sleep apnea management in underserved patient populations. J Prim Care Community Health . 2022;13:21501319211068969. doi: 10.1177/21501319211068969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panza GS, Puri S, Lin HS, Badr MS, Mateika JH. Daily exposure to mild intermittent hypoxia reduces blood pressure in male patients with obstructive sleep apnea and hypertension. Am J Respir Crit Care Med . 2022;205:949–958. doi: 10.1164/rccm.202108-1808OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwon Y, Sands SA, Stone KL, Taranto-Montemurro L, Alex RM, White DP, et al. Prolonged circulation time is associated with mortality among older men with sleep-disordered breathing. Chest . 2021;159:1610–1620. doi: 10.1016/j.chest.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]