Abstract
Objective The course of systemic sclerosis-associated interstitial lung disease (SSc-ILD) is highly variable and different from continuously progressive idiopathic pulmonary fibrosis (IPF). Most proposed definitions of progressive pulmonary fibrosis or SSc-ILD severity are based on the research data from patients with IPF and are not validated for patients with SSc-ILD. Our study aimed to gather the current evidence for severity, progression and outcomes of SSc-ILD.
Methods A systematic literature review to search for definitions of severity, progression and outcomes recorded for SSc-ILD was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines in Medline, Embase, Web of Science and Cochrane Library up to 1 August 2023.
Results A total of 9054 papers were reviewed and 342 were finally included. The most frequent tools used for the definition of SSc-ILD progression and severity were combined changes of carbon monoxide diffusing capacity (DLCO) and forced vital capacity (FVC), isolated FVC or DLCO changes, high-resolution CT (HRCT) extension and composite algorithms including pulmonary function test, clinical signs and HRCT data. Mortality was the most frequently reported long-term event, both from all causes or ILD related.
Conclusions The studies presenting definitions of SSc-ILD ‘progression’, ‘severity’ and ‘outcome’ show a large heterogeneity. These results emphasise the need for developing a standardised, consensus definition of severe SSc-ILD, to link a disease specific definition of progression as a surrogate outcome for clinical trials and clinical practice.
PROSPERO registration number CRD42022379254.Cite Now
Keywords: Systemic Sclerosis; Pulmonary Fibrosis; Outcome Assessment, Health Care; Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Progression of interstitial lung disease (ILD) is associated with the poorer outcome in systemic sclerosis (SSc).
Established and validated criteria of progressive and severe SSc-ILD are required to drive monitoring and intensification of treatment strategy.
Most proposed definitions are derived from idiopathic pulmonary fibrosis and are not fully validated for patients with SSc-ILD.
WHAT THIS STUDY ADDS
This systematic literature review summaries the current data of different definitions used in clinical trials and observational studies.
The large heterogeneity of presented definitions emphasises the need to develop standardised, consensus definitions.
Patients’ perspective need also to be taken into account, in particular when defining disease-related outcomes.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The definition of progression should be the surrogate outcome for long-term severe events, meaningful for both patients and physicians.
Our data are the basis to create a disease-specific definition of progression of SSc-associated interstitial lung disease.
This will further allow the identification of a patient phenotype at higher risk of progression, as possible candidate for treatment intensification.
Introduction
Systemic sclerosis (SSc) is a rare autoimmune disease characterised by tissue inflammation and vasculopathy, leading to skin and internal organs fibrosis.1 2 Mortality related to SSc is the highest among all rheumatic diseases,3 and the 10-year survival rate of patients with SSc is 65%–73% in Europe and 54%–82% in North America.4 Interstitial lung disease (ILD) is a prominent feature of SSc and is the leading cause of death according to 'European League Against Rheumatism (EULAR) Scleroderma Trials and Research (EUSTAR) cohort data.3 5 Nearly 50% of patients with SSc develop ILD during the disease course, with the higher risk in early phases, in particular for the diffuse cutaneous subset (dcSSc).6 7 Although the high-resolution CT (HRCT) of the chest is the gold standard imaging technique for ILD assessment, pulmonary function tests (PFTs) and clinical assessment of respiratory symptoms also play a significant role in the management of patients with SSc-ILD. Half of the patients with SSc have confirmed ILD on first chest HRCT assessment.8 Restrictive patterns on PFTs with reduced forced vital capacity (FVC) and diffusion capacity of the lung for carbon monoxide (DLCO) are detected more rarely.9
At SSc diagnosis, screening for ILD is recommended in every patient with SSc based on expert evidence-based European and American consensus documents.10 11 Despite this, still too many physicians tend to perform diagnostic HRCTs only when patients develop respiratory symptoms or to screen only in patients at higher risk (eg, dcSSc, positive for Scl-70 antibodies, African-American ethnicity, high modified Rodnan skin score (mRSS)).12–17
Compared with the progressive fibrosing disease course of idiopathic pulmonary fibrosis (IPF), the course of SSc-ILD may be highly heterogeneous,18 with stable and progressive periods. A recent EUSTAR database analysis showed that 67% of all patients with SSc-ILD may experience progression at any time over an average follow-up of 5 years, with most patients with progressive ILD showing a rather slow lung function deterioration afterwards or even no episode of further major decline.19
Currently, no established consensus is available on the definition of SSc-ILD progression among experts and in the literature. In observational studies and clinical trials, the most used definition of ILD progression is a ≥10% relative decline in FVC.20–29 An FVC relative decline ≥10% is a strong predictor of reduced survival in patients with SSc-ILD.29 Some studies prefer to use the CT fibrosis extent as a marker of ILD progression.30–32 The recent 2022 American Thoracic Society (ATS)/ European Respiratory Society (ERS)/ Japanese Respiratory Society (JRS)/ Latin American Thoracic Association (ALAT) Clinical Practice Guideline on Progressive Fibrosing ILDs defined progressive pulmonary fibrosis on the basis of the presence of at least two of the following three criteria occurring within 1 year: worsening respiratory symptoms, physiological evidence of disease progression (absolute decline in FVC of at least 5% predicted or/and absolute decline in DLCO at least 10% predicted) and radiological evidence of disease progression.33 Taking into account differences between SSc-ILD and IPF, post-hoc analysis of the Scleroderma Lung Study (SLS) I and II indicated a decline in FVC of 3.0% as the minimal change for a clinically important difference.34
The objective of this systematic literature review (SLR) is to collect the available scientific evidence about definitions of severity, progression and outcomes in SSc-ILD. The final aim is to inform the creation process of the definition of ‘long-term severe SSc-ILD’. This long-term outcome will then be linked to a definition of ILD progression specific for SSc, as surrogate outcome, to help guiding decisions for management and monitoring disease course for patients diagnosed with SSc-ILD.
Materials and methods
A systematic search of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to identify papers that include definitions of SSc-ILD severity, progression and outcome (PROSPERO registration number CRD42022379254). We considered outcomes as events that were recorded during a study and had impact on the patients’ health and could be related to ILD. These were different from measurements and definitions of progression, which was only related to ILD and therefore recorded separately.
In summary, Medline, Embase, Web of Science databases and Cochrane Library were searched up to 1 August 2023. A single author (CB) with established experience35–37 performed the literature search using the search, strings detailed in online supplemental file 1.
rmdopen-2023-003426supp001.pdf (319.1KB, pdf)
The citations retrieved from these databases were entered in the software EndNote V.20 and duplicates were removed. The citations were evaluated in a two-step process by teams of two independent evaluators (LP, FB, CC, EF, SP, ST, PB, CC, RDL, AE, JL), including title/abstract evaluation (step 1) and full-text evaluation (step 2). Discrepancies were resolved by direct discussion between the two assessors, otherwise through the opinion of a third evaluator (CB). Randomised clinical trials (RCT), cross sectional or longitudinal studies (both retrospective or prospective) were included in the SLR if they were focused on SSc or on cohorts in which data on patients with SSc could be extracted, considered ILD as primary target (representing at least one of population, exposure, outcome) and included at least 10 adult patients. Exclusion criteria were papers written in languages other than English, non-clinical studies, lung involvement due to other causes (ie, other diseases, smoking or professional exposure, etc), ILD onset as an outcome, literature reviews and no full-text availability.
A reproducibility exercise was conducted on the first 20 abstracts for step 1 and the first 5 full texts for step 2 derived from the selection and performed by all extractors, to ensure homogeneity in evaluation and data extraction (90% of agreement achieved).
Results
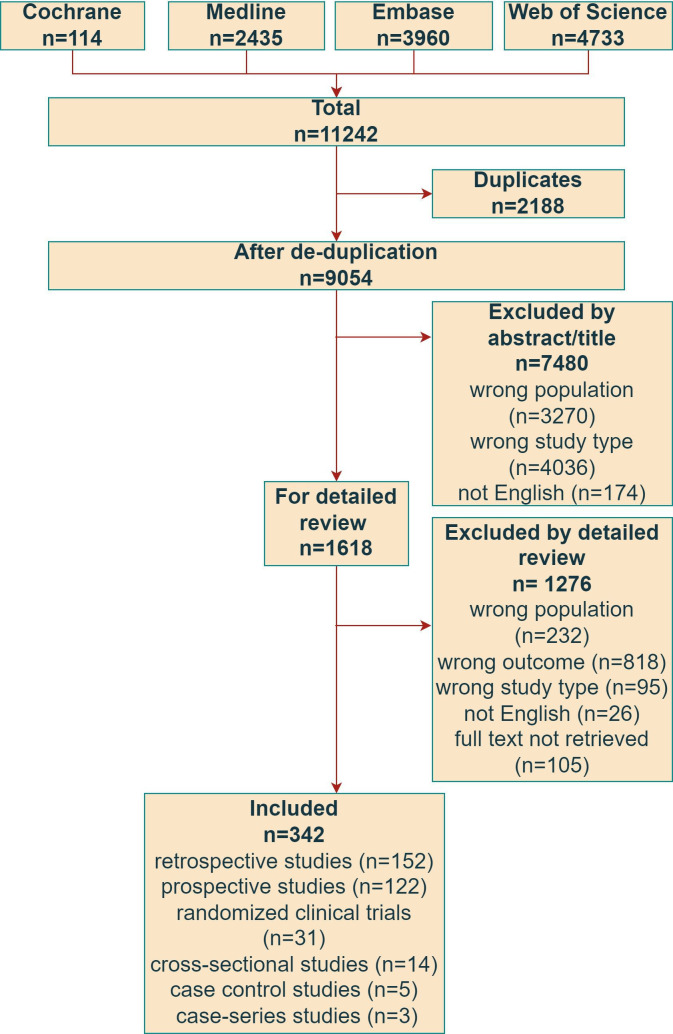
Out of 9054 papers identified by the primary literature search, 1618 articles were selected for full-text analysis (figure 1). The reasons for exclusion from the first selection step were: wrong population (n=3270), wrong study type (n=4036) and not English article (n=174). Among the remaining 1618 papers evaluated as full text in step 2, 1276 manuscripts were excluded due to no definition of severity/progression/outcome presented (n=818), wrong population (n=232), wrong study type (n=95), full texts not retrieved (n=103) and not English paper (n=26). Finally, 342 manuscripts were extracted in step 3, including 43 939 patients with SSc-ILD, range 6–3778 patients per paper (data available for 305/342 papers). Mean age was 50 years and ranged from 32 to 66 years (data available for 141/342 papers), the median age ranged from 18 to 75 years (data available for 29 papers), 68.6% were women (data available for 161/342 papers). The mean disease duration ranged from 1 to 11 years (data available for 165/342 papers). The design of the study was retrospective in 167 cases (49%), prospective in 122 (37%), RCT in 31 (9%), cross-sectional in 14 (4%), case control in 5 (1%) and case-series in 3 (0.9%). Regarding the SSc classification criteria, 147 studies used the 2013 American College of Rheumatology (ACR)/EULAR criteria, 125 the 1980 ARA criteria, in 20 studies patients meet 2013 ACR/EULAR criteria and 1980 ARA criteria simultaneously, 7 papers used other criteria (including Leroy and others) and 43 did not indicate any criteria.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of studies selection. ILD, interstitial lung disease; SSc, systemic sclerosis.
The SLR results are summarised below and divided in three sections: definitions of severity, progression and outcomes of SSc-ILD.
Definitions of SSc-ILD severity
A definition of SSc-ILD severity was included in 155 (45%) papers. It was based on HRCT data in 62 (40%) studies, on combined HRCT and FVC changes in 25 (16%), on FVC changes in 27 (17%), on combined FVC and DLCO changes in 15 (10%) and on DLCO changes in 12 (8%). The most used definitions of SSc-ILD severity are given in table 1, and less frequent are mentioned in online supplemental table 1.
Table 1.
Definitions of SSc-ILD severity
| SSc cohort, n articles | SSc cohort, n (from n of articles) | Among SSc cohort, n ILD (from n of articles) |
Severe ILD in SSc cohort, n (%) (from n of articles) |
References | SSc-ILD cohort, n articles | SSc-ILD cohort, n patients (from n of articles) |
Severe ILD in SSc-ILD cohort, n patients, (%) (from n of articles) |
References | |
| PFTs | |||||||||
| FVC<50% | 11 | 4148 (11) | 2318 (10) | 195 (22%) (9) | 63–72 | 3 | 209 (3) | 11 (7%) (2) | 73–75 |
| FVC<70% | 9 | 6164 (9) | 2151 (7) | 1716 (30%) | 76–84 | 2 | 65 (2) | 37 (60%) (2) | 85 86 |
| DLCO<40% | 3 | 231 (3) | 98 (3) | 39 (43%) (2) | 87–89 | 1 | 19 | – | 90 |
| DLCO<50% | 4 | 1021 (4) | 315 (4) | 175 (16) (4) | 67 84 91 92 | ||||
| DLCO<70% | 4 | 2701 | 156 (1) | 84 (36%) (1) | 85 91 93 | ||||
| DLCO<50% and/or FVC<50% | 12 | 4159 (12) | 1071 (9) | 164 (30) (5) | 94–105 | ||||
| HRCT | |||||||||
| ILD extent>10% | 4 | 1099 (4) | 478 (3) | 104 (24%) (3) | 8 106–108 | 1 | 14 (1) | 8 (57) (1) | 109 |
| ILD extent>20% | 12 | 2255 (12) | 960 (10) | 380 (36%) (10) | 9 30 110–119 | 12 | 1071 (12) | 271 (44%) (10) | 29 61 120–129 |
| ILD extent>30% | 3 | 2706 (3) | 779 (3) | 90 (14%) (2) | 107 130 | ||||
| ILD extent>50% | 2 | 111 (2) | 82 (2) | 8 (14%) (1) | 131 132 | 2 | 144 (4) | – | 133 134 |
| Warrick ILD score>15 points | 3 | 234 (3) | 171 (3) | 29 (28%) (2) | 98 135 136 | ||||
| Warrick score | 2 | 101 (2) | 61 (2) | 4 (44%) (1) | 137 138 | 3 | 173 (2) | 8 (57.1%) (1) | 139–141 |
| According to HRCT score, grade 3 as 50–74% and grade 4 as>75% | 5 | 359 (5) | 16 (6.5%) (2) | 142–146 | |||||
| Combined measurement | |||||||||
| ILD>20% and FVC<70% | 2 | 770 (2) | 273 (2) | 51 (33%) (2) | 147 148 | 7 | 709 (7) | 230 (36%) (6) | 122 149–154 |
| ILD extent>30% or ILD extent 10–30% with FVC<70% | 6 | 876 (6) | 374 (5) | 177 (37%) (5) | 155–160 | 6 | 1103 (5) | 139 (42%) (4) | 161–166 |
DLCO, diffusion capacity of the lung for carbon monoxide; FVC, forced vital capacity; HRCT, high-resolution CT; ILD, interstitial lung disease; PFTs, pulmonary function tests.
ILD extent presented as percentage of affected parenchyma on HRCT was the most frequently used imaging parameter (89 studies, 8327 patients), both alone (62 studies, 4974 patients) or in combination with PFT parameters (27 papers, 3353 patients). Different cut-off points were proposed, ranging from >10% to >50%: the most frequent cutoff was >20% as proposed by the visual Goh et al staging system on five levels,38 which was presented in 24 studies including 2031 patients. According to the modified Goh et al scoring system, extensive disease was defined as lung involvement>20% on HRCT or 10%–30% ILD involvement on HRCT with FVC<70% predicted. Limited disease was determined as ≤20% ILD involvement on HRCT or 10%–30% lung involvement on HRCT and an FVC≥70% predicted.38
The second most frequently used HRCT assessment tool of ILD severity was the semiquantitative scoring proposed by Warrick et al,39 which combines severity and extent of disease. In this score, different abnormalities (ground-glass opacity alone, mixed ground glass and reticular disease, reticular fibrosis alone and honeycombing) correspond to increasingly severe disease and consequently receive increasingly higher scores. The extent is determined based on the total number of bronchopulmonary segments involved for each abnormality, with a maximum score of 31 points. The Warrick score was used in 10 studies, including altogether 519 patients with considered cut-off >15 points (in 3 studies), >10 points (in 1 study) and >7 (in 2 studies).
The other HRCT assessments to present ILD severity were the CT score grade 3 or grade 4 as corresponding to 50%–74% or >75% or lung parenchyma involved (five studies), the predominance of reticular or honeycombing appearance (four studies), extensive-ILD Z score≥3 (two studies), presence of ground glass opacities (one study), presence of linear opacities of >3 mm thickness and presence of honeycombing (one study).
PFTs parameters were used as assessment tools for SSc-ILD severity in 67/155 papers, including altogether 8766 patients (as part of combined indexes in 40 studies) with % predicted FVC as the most frequently used parameter. FVC was used to define severity as a single parameter in 27 papers (5078 patients), in combination with % predicted DLCO in 15 papers (1519 patients) or with other parameters in 25 papers (3287 patients). Among the included studies, a cutoff for FVC<45% was used in 1 paper (246 patients), FVC<50% was used in 27 papers (4026 patients), <60% in 1 paper (20 patients), <70% in 29 papers (5298 patients) and<80% in 2 paper (3867 patients). Per cent predicted DLCO as isolated severity marker of SSc-ILD was presented in 12 studies including 588 patients, with a cutoff <40% (4 papers, 117 patients), <50% (4 papers, 315 patients) or <70% (4 papers, 156 patients).
There were some composite measures for ILD severity in the literature: the ILD—Gender, Age, Physiology index (taking into account the ILD subtype, gender, age, FVC and DLCO), a composed index (including the body mass index, DLCO results, Medical Research Council dyspnoea score (mMRC) and the 6-min walking distance (6MWD)), and the du Bois index. The Medsger severity scale was used in three studies including altogether 63 patients.
The severity of ILD was estimated also according to the clinical symptoms: exercise tolerance/dyspnoea score>1 (1 paper, 57 patients), NYHA functional class III-IV (1 paper, 21 patients), exercise oxygen desaturation (1 paper, 63 patients) and a grade IV on the mMRC (1 paper, 28 patients).
Definitions of SSc-ILD progression
Out of 207 studies including definitions of SSc-ILD progression, 62 (30%) provided a definition referred to combined DLCO and FVC changes, 65 (31%) to isolated FVC changes, 27 (13%) to DLCO changes, 20 (10%) to HRCT extension, 13 (6%) to combination of PFT and HRCT data, 11 (5%) to combination of PFTs, clinical signs and HRCT data, 5 (2%) to vital capacity decline and 4 (2%) to other aspects (tables 2 and 3). The time frame of progression assessment were 6 months (5.3% papers), 12 months (35.3 %), 24 months (18.4% papers), 36 months (7.7%), 60 months (3.4%), more than 60 months (7.7%), other (9.2 %) or unknown (13.0%).
Table 2.
Definitions of SSc-ILD progression based on a single parameter
| SSc mixed cohorts | SSc-ILD cohorts | ||||||||
| Time, months (n papers) |
n patients (n papers) | n ILD patients (n papers) | n progressors (n papers) |
References | Time frame, months (n papers) |
n patients (n papers) | n progressors (n papers) | References | |
| FVC | |||||||||
| Decline FVC>15% | 6 (1) 65 (1) |
200 (2) | 84 (2) | 19 (2) | 167 168 | 12 (2) 60 (1) |
253 (3) | 26 (3) | 29 169 170 |
| Decline FVC>10% | 12 (9) 24 (4) 36 (4) 48 (1) >60 (3) Unk (3) |
11 604 (22) | 2746 (20) | 976 (19) | 20–27 30 71 116 171–179 | 6 (3) 12 (5) 24 (6) >60 (4) |
3007 (18) | 424 (16) | 28 29 74 75 125 150 161 180–190 |
| Decline FVC>5% | 12 (1) Unk (1) |
6527 (2) | 100 (1) | 401 (2) | 191 192 | 12 (4) 24 (3) Unk (1) |
1777 (8) | 589 (6) | 28 124 188 189 193–196 |
| Decline FVC>3.3% | – | – | – | – | – | 12 (1) 48 (1) 52 (1) Unk (1) |
1418 (4) | 571 (3) | 197–200 |
| DLCO | |||||||||
| Decline DLCO>15% | 12 (2) 24 (1) 60 (2) |
568 (5) | 445 (5) | 135 (5) | 24 118 172 173 191 | 6 (2) 12 (2) 36 (1) 60 (2) |
505 (7) | 168 (7) | 29 85 161 170 183 201 202 |
| Decline DLCO>10% | 12 (2) 55 (1) >60 (1) Unk (1) |
340 (5) | 112 (2) | 86 (5) | 167 171 176 203 204 | 24 (2) | 30 (2) | 9 (1) | 140 195 |
| HRCT | |||||||||
| Increase ILD extent>10% | 12 (2) | 347 (2) | 213 (2) | 6 (2) | 30 205 | 24 (2) | 145 (2) | 5 (2) | 31 32 |
| Increased ILD extent>20% | 24 (1) Unk (1) |
22 (2) | 8 (2) | 206 207 | |||||
| Increase ILD extent | 12 (1) 24 (1) 37 (1) 62 (1) |
1103 (4) | 480 (4) | 105 (4) | 148 208–210 | 12 (1) 24 (1) |
217 (2) | 34 (1) | 211 212 |
DLCO, carbon monoxide diffusing capacity; FVC, forced vital capacity; HRCT, high-resolution CT; ILD, interstitial lung disease.
Table 3.
Definitions of SSc-ILD progression based on the combination of multiple parameters
| SSc mixed cohorts | SSc-ILD cohorts | ||||||||
| Time, months (n papers) |
n patients (n papers) | n ILD patients (n papers) | n progressors (n papers) |
References | Time frame, months (n papers) |
n patients (n papers) | n progressors (n papers) | References | |
| PFTs | |||||||||
| Decline FVC>10% or DLCO>15% | 6 (1) 12 (6) 24 (3) >36 (5) >60 (1) Unk (6) |
10 476 (18) | 4258 (14) | 1144 (14) | 8 70 116 117 148 208 213–225 | 6 (1) 12 (9) 24 (4) 36 (1) >60 (3) Unk (8) |
1571 (22) | 461 (18) | 32 38 123 152 153 226–241 |
| Decline FVC>10% or DLCO>10% | 6 (1) 12 (2) >36 (4) 60 (1) |
531 (8) | 336 (8) | 181 (8) | 174 205 242–245 | 12 (1) 24 (1) 36 (2) >36 (2) |
489 (6) | 47 (4) | 121 246–250 |
| Decline FVC>10% and/or decline FVC 5%–9% with decline DLCO>15% | 6 (1) 12 (2) 24 (1) |
878 (4) | 495 (4) | 110 (4) | 251–254 | 12 (2) Unk (1) |
772 (3) | 96 (1) | 154 192 255 |
| PFTs+HRCT | |||||||||
| Decline FVC≥10% or DLCO≥15% or ILD extent increase>20% | 12 (1) 21 (1) 24 (1) |
462 (3) | 204 (3) | 55 (3) | 113 256 257 | 12 (1) 56 (1) |
72 (2) | 15 (2) | 258 |
| PFTs or HRCT+clinical signs | |||||||||
| Decline FVC>10% or decline FVC 5%–10% and worsening f respiratory symptoms or/and increase ILD extent |
24 (1) | 36 (1) | 22 (1) | 9 (1) | 259 | 12 (1) 36 (1) Unk (1) |
109 (3) | 48 (1) | 260–262 |
DLCO, carbon monoxide diffusing capacity; FVC, forced vital capacity; HRCT, high-resolution CT; ILD, interstitial lung disease.
Among PFTs parameters, FVC was the most frequently used in 150/207 manuscripts. Among the included manuscripts, various cutoffs for decline were presented, representing FVC decline over a variable follow-up duration (tables 2 and 3 and Supplement table 2,3): >20% in 3 papers (96 patients), >15% in 6 papers (390 patients), >10% in 108 papers (14 237 patients), 5%–10% 13 papers (1656 patients), >5% in 14 papers (2075 patients), >3.3% in 4 papers (1418 patients), > 0.08 L per year in 1 paper (29 patients), any decline in 1 paper (148 patients). In addition, progression to FVC<70% at 36 months was recorded in 2 papers (212 patients) and <55% at follow-up in another paper (44 patients). Percent decline of DLCO as marker of SSc-ILD progression was presented in 103 studies including 10 152 patients. Also for DLCO decline, different cutoffs have been proposed over different time points (tables 2 and 3 and Supplement table 2,3): >30% (1 paper, 34 patients), >20% (2 papers, 53 patients), >15% (69 papers, 8766 patients), >10% (26 papers, 1152 patients), >7% (1 paper, 33 patients), >5% (1 paper, 25 patients), DLCO<70% at longest follow-up (1 paper, 44 patients) and any decline in 1 paper (44 patients).
The most frequently used definition of SSc-ILD progression based on PFT changes were decline FVC>10% or 5%–10% plus DLCO>15% (53 studies, 7553 patients), otherwise as decline FVC>10% or DLCO>10% (14 studies, 825 patients).
Definitions based on HRCT changes included the increase of ILD extent by >5% in 2 paper (102 patients), >10% in 4 papers (358 patients), >20% in 6 papers (308 patients), ≥2% increase in quantitative ILD score40 in 2 papers (269 patients), ≥4% increase in quantitative ILD score40 in 1 paper (97 patients), increase in the ILD score proposed by Kazerooni et al41 ≥ 1 point in 1 paper (73 patients) or ≥2 points in 2 papers (116 patients), otherwise any increase in ILD extent in 6 papers (697 patients). The Kazerooni assessment strategy includes the extent of ground-glass opacity (alveolar score) and honeycombing (interstitial score) on a scale of 0–5 in the three lobes of both lungs as follows: 0—no alveolar disease, 1—ground glass involving <5% of the lobe, 2—ground glass involving up to 25% of the lobe, 3—ground glass involving 25%–49% of the lobe, 4—ground glass involving 50%–75% of the lobe, 5—ground glass involving >75% of the lobe for alveolar score; 0—no interstitial disease, 1—septal thickening without honeycombing, 2—honeycombing involving up to 25% of the lobe, 3—honeycombing involving 25%–49% of the lobe, 4—honeycombing involving 50%–75% of the lobe, 5—honeycombing involving >75% of the lobe for interstitial score.41 The increasing extent ILD was also part of a composite definition in 9 papers (1196 patients), combined with decline FVC and/or DLCO with deterioration of dyspnoea symptoms.
Finally, progression of ILD was also estimated according to clinical symptoms in 11 papers (944 patients), all using worsening of dyspnoea in combination with PFT or HRCT changes.
Complementary to ILD progression, 16/342 papers presented definitions of SSc-ILD improvement. Generally, the most frequently used definitions were based on PFTs changes: isolate increase of FVC (7 studies, 262 patients) with cutoff of 3.6%, 5%, 10% or 15% (Supplementary Table 4), isolate increase DLCO (2 studies, 45 patients) with cutoff of 10% and 15%, increase FVC or DLCO (5 studies, 123 patients), improvement in VC>10% (1 study, 7 patients) and increase 6MWD>15% (1 study, 7 patients).
Definitions of SSc-ILD outcome
The last part of our study was focused on outcomes in SSc-ILD. Information about outcomes of SSc-ILD was present in 175 articles. The most frequent endpoint was mortality (table 4). According to the SRL results, mortality was categorised to mortality due to ILD (12 studies, 2628 patients), SSc-related mortality (8 studies, 2091 patients), 15-year mortality (2 studies, 628 patients), 10-year mortality (16 studies, 2732 patients), 5-year mortality (22 studies, 4771 patients), 3-year mortality (10 studies, 952 patients), 1-year mortality (13 studies, 947 patients), in-hospital mortality (5 studies, 1899 patients), mortality after lung transplantation (1 study, 72 patients) and overall mortality with follow-up duration from 12 months to 360 months (68 studies, 8076 patients). Other popular recorded outcomes of patients with SSc-ILD were hospitalisation (6 papers, 1835 patients), end-stage ILD (6 paper, 501 patients), lung transplantation (3 papers, 375 patients) and infections (2 papers, 82 patients).
Table 4.
Definitions of outcome in SSc-ILD
| SSc mixed cohorts | SSc-ILD cohorts | ||||||||
| Time, months (n papers) |
n patients (n papers) | n ILD patients (n papers) | n of patients with outcome (n papers) | References | Time, months (n papers) |
n patients (n papers) | n of patients with outcome (n papers) | References | |
| Mortality due to ILD | 180 (12) | 6165 (12) | 2628 (10) | 277 (12) | 23 24 65 118 138 167 263–267 | – | – | – | – |
| SSc-related mortality | 25- 200 (7) UNK (1) |
3789 (7) | 1785 (7) | 324 (7) | 23 65 264 266 268 269 | 97 (1) | 306 (1) | 39 (1) | 17 |
| 15-year mortality | – | 1284 (1) | 522 (1) | 260 (1) | 270 | – | 106 (1) | 21 (1) | 154 |
| 10-year mortality | – | 7484 (13) | 2509 (10) | 1991 (13) | 23 78 106 111 130 179 214 267 268 271–273 | – | 223 (3) | 46 (3) | 144 150 274 |
| 5-year mortality | – | 10 604 (16) | 4335 (15) | 1104 (13) | 23 84 106 111 173 225 242 267 268 271–273 275–277 | – | 436 (6) | 117 (6) | 274 278–282 |
| 3-year mortality | – | 1046 (5) | 688 (5) | 134 (5) | 158 173 242 268 275 | – | 264 (5) | 25 (5) | 127 152 279 281 283 |
| 1-year mortality | – | 1153 (5) | 633 (5) | 33 (2) | 254 268 272 273 275 | – | 314 (8) | 12 (6) | 280–287 |
| Mortality | 12–36036 | 23 366 (35) | 5048 (26) | 2338 (31) | 5 8 23 25 64–66 92 114 117–119 131 147 160 174 177 219 244 266–268 288–300 | 12–348 (32) | 3028 (31) | 582 (27) | 17 28 50 71 85 128 141 142 149 151 153 163 170 185 226 238 242 258 269 274 278 301–311 |
| In-hospital mortality | 120 (1) 12 (1) Unk (3) |
14 240 (4) | 1899 (3) | 164 (3) | 312–316 | – | – | – | – |
| Mortality after lung transplantation | 45–721 | 72 (1) | 27 (1) | 317 | |||||
| Hospitalisation | 12 (1) 120 (2) 84 (1) Unk (3) |
6211 (5) | 1261 (4) | 591 (4) | 312 314 316 318 319 | 12 (1) | 574 (1) | 78 (1) | 166 |
| End-stage ILD | 12 (1) 60 (1) 120 (1) 200 (2) |
1646 (5) | 451 (4) | 111 (5) | 65 245 320–322 | 173 (1) | 50 (1) | 16 (1) | 323 |
| Lung transplantation | 120 (1) 200 (1) | 552 (2) | 203 (2) | 17 (2) | 65 179 | 60 (1) | 172 (1) | 3 (1) | 301 |
| Infections | 120 (1) | 95 (1) | 36 (1) | 26 (1) | 312 | 120 (1) | 46 (1) | 9 (1) | 144 |
ILD, interstitial lung disease; SSc, systemic sclerosis; Unk, unknown/not specified.
Parameters of other SSc-related organ progressions or improvement were also considered as outcomes in 21/175 studies (Supplementary Table 5), such as malignancy, changes in the mRSS, left ventricular ejection fraction, new onset of pulmonary hypertension, new onset of digital ulcers, overall disease progression (defined according to the occurrence of at least one organ progression), compression fractures, femoral head necrosis, newly diagnosed arterial hypertension, newly diagnosed diabetes, hyperlipidaemia requiring treatment, abnormal liver function tests and decrease Health Assessment Questionnaire Disability Index. The duration of patients’ follow-up for outcome assessment ranged from 1 to 30 years.
Discussion
In more than a half of the patients with SSc, ILD may determine a meaningful impact on quality of life, morbidity and survival.5 7 Unlike the progressive fibrosing disease course of IPF, the course of SSc-ILD is definitely variable, with phases of rapid or slow disease progression, disease stability or improvement.19 In fact, ILD progression can be observed not only during the first 3 years of disease duration, but also in later stages. In patients with SSc, a recent analysis from the EUSTAR cohort showed that the frequency of ILD progression is over 10% every year, regardless of disease duration, which ranged from less than 3 to more than 15 years after onset of Raynaud’s phenomenon.42 The high heterogeneity of the course of SSc-ILD requires appropriate monitoring and approach to treatment strategy.
Although there is no consensus on which definition of SSc-ILD progression best fits the case of a surrogate outcomes, most ILD progression definitions have been related to increased morbidity and mortality. This stresses the need to identify patients at higher risk of progression, to start an early treatment.43 Overall, there is consensus among SSc-ILD experts that the definition of SSc-ILD progression should cover multiple domain that are usually affected by this complication.44 The recent 2022 ATS/ERS/JRS/ALAT Clinical Practice Guideline on progressive fibrosing ILD based the definition of ILD progression on at least two of worsening of respiratory symptoms, physiological evidence of disease progression and radiological progression, presenting within a 1–2 years’ time frame.33 Despite being currently proposed to define a progressive phenotype also in SSc, the current definition has not been tested so far in this disease, and therefore lacks a data-driven support. Similarly, the "Efficacy and safety of Nintedanib in patients with progressive fibrosing interstitial lung disease" (INBUILD) study defined a progressive ILD phenotype as a relative decline in the FVC predicted≥10% or a relative decline in FVC predicted of 5%–10% associated to worsening of respiratory symptoms or an increased extent of fibrosis on HRCT, or worsening of respiratory symptoms. These events could be recorded over a time observation up to 24 months and manifest despite management with standard of care treatments, excluding nintedanib or pirfenidone.45 However, only a small proportion of SSc-ILD cases were included in this study, which limits the possibility to extrapolate the applicability of this definition to this specific population.
Applying a combined and multidomain definition of progression in the large prospective Canadian Registry for Pulmonary Fibrosis, progression events were recorded in in 49% patients with SSc-ILD within 24 months. Progressive fibrosing-ILD (PF-ILD) was defined as a relative FVC decline ⩾10%, death, lung transplantation or any two of: relative FVC decline ⩾5% and<10%, worsening respiratory symptoms or worsening fibrosis on CT of the chest, all within 24 months from baseline. Based on which definition was used to determine progression, the frequency of events ranged from 8% (relative FVC decline 5%–9% with fibrosis on CT) to 49% (relative FVC decline ⩾10%).46 However, it should be highlighted that pulmonology registry are likely enriched for progressive, severe patients and reflects daily practice in pulmonology, which is not necessarily representative of the daily clinical practice in rheumatology. Although based only on functional decline, the number of progressive events in EUSTAR cohort are, in fact, much lower.19 A study from a large European centre by Nasser et al reported that 168 out of 617 patients (27%) met the PF-ILD criteria during a 7-year period.47 Another retrospective study including nine specialist centres in the UK by Simpson et al identified the new incident cases of PF-ILD rate according to the INBUILD criteria in 14.5% assessed over a 2-year period.48 Finally, another recent analysis of 826 patients with SSc-ILD from the EUSTAR database showed that 27% patients experienced ILD progression defined as a decline in predicted FVC>5% during any 12-month period, and 67% of all patients with SSc-ILD experienced progression at any time over the mean 5-year follow-up.19 However, most patients with SSc-ILD (58%) were stable (decline or increase in FVC<5%) or improved (increase in FVC≥5% predicted) during any 12-month period and only 8% showed rapid, continuously declining FVC.19
Our SLR focuses on definitions of severity, progression and outcome for patients with SSc-ILD, considering the existing literature data until 1 August 2023. As a result of our research, we presented data on 175 articles regarding the SSc-ILD outcomes. The most frequent outcomes were mortality (157 studies), end-stage lung disease (6 studies) and hospitalisation (6 studies). Concerning severity of SSc-ILD, ILD extent on HRCT over 20% of the parenchyma is most frequently used, alone or in combination with PFT parameters. Among PFTs parameters for severity assessment, predicted FVC values <50% or <70% and predicted DLCO values <40%, <50% or <70% were used in most papers. Our review also confirmed the importance of both PFTs (mostly using FVC% predicted and DLCO% predicted), and HRCT as a tool to identify progressive SSc-ILD. Finally, several studies have proposed criteria for progression of SSc-ILD based on a combination of clinical signs (worsening of dyspnoea) in combination with pulmonary function measures and radiographic extent of fibrosis on HRCT.
Independent of the definition used, it is important to detect ILD progression, as it can represent a surrogate outcome for future severe events.49 Using data from the SLS I and II, the post-hoc analysis by Volkmann et al demonstrated that radiographic progression of SSc-ILD (defined by a≥2% increase in total quantitative ILD score—QILD score) over the course of 1–2 years is associated with an increased risk of long-term mortality.50 Even after adjusting for other factors known to affect survival in patients with SSc, worsening of ILD on HRCT remained associated with mortality, in particular in the SLS II subgroup.50 The ability of PFT changes at 1 and 2 years to predict long-term mortality over more than 15 years was estimated in study by Goh et al.29 The changes in the FVC% predicted in 1 year provided the most accurate prognostic information, with the optimal expression of FVC change consisting of either a ≥10% decline in the FVC from baseline or a 5%–9% decline in the FVC with a ≥15% decline in the DLCO. The prognostic value of PFT trends in the whole cohort was entirely explained by the disease progression in patients with extensive lung fibrosis, defined as ILD affecting more than 20% of the lung parenchyma. The categorical changes in the carbon monoxide transfer coefficient (KCO) and the FVC:DLCO ratio in those with limited lung fibrosis and change in the composite categorical decline (a≥10% decline in the FVC from baseline or a 5%–9% decline in the FVC with a ≥15% decline in the DLCO) in those with extensive lung fibrosis at 2 years were independent prediction factors of mortality. The prognostic value of the PFT changes was further confirmed in a mortality prediction model using data from the SLS I and II trials.17 The final SLS II survival models demonstrated that older age, higher baseline values of modified Rodnan’s skin score, as well as a more meaningful decline of FVC%-predicted and DLCO%-predicted over 24 months were associated with worse survival. Despite the established value of PFT as a surrogate marker of mortality in SSc-ILD, the EUSTAR study by Hoffmann-Vold AM et al could not identify significant differences in mortality between patients with a≥10% decline in the FVC (12%), decline in FVC 5%–10% predicted (15%) or decline or increase in FVC<5% (9%) over the initial 12-month period.19
Severe disease and long-term outcomes are the cornerstone of SSc-ILD management, as they represent the target that should be avoided or delayed through medications. The currently available RCTs on SSc-ILD used FVC decline as an outcome due to its good surrogacy for survival [SLS I—percentage of the predicted FVC value at 12 months, SLS II—percentage of the predicted FVC value at 24 months, the "Safety and Efficacy of Nintedanib in Systemic Sclerosis" (SENSCIS) study —the annual rate of absolute decline in FVC assessed over 52 weeks, FaSScinate and FocuSSced trial—percentage of the predicted FVC value at 48 weeks).26 28 51–53 In comparison to SSc-ILD, the field of RCTs in PAH has moved forward from using pure functional outcomes as primary endpoints, aiming at preventing morbidity and mortality events. For example, the primary outcome in the "study with an ERA in Pulmonary arterial Hypertension to Improve cliNical outcome" (SERAPHIN) trial was the time from the initiation of treatment to the first occurrence of a composite end point of death, or worsening of pulmonary arterial hypertension (defined as events such as atrial septostomy, lung transplantation, initiation of treatment with intravenous or subcutaneous prostanoids).54 Similarly, the "study of first-line Ambrisentan and Tadalafil combination therapy in subjects with pulmonary arterial hypertension" (AMBITION) trial used the first event of clinical failure as primary endpoint, which was defined as the first occurrence among death, hospitalisation for worsening pulmonary arterial hypertension, disease progression or unsatisfactory long-term clinical response.55 Therefore, the combination of long-term outcomes and severe disease worsening, including both functional, radiological and clinical events, could help to create a combined morbidity–mortality outcome also for SSc-ILD. This might be used as a primary endpoint in SSc-ILD trials, further supporting the beneficial long-term effects of currently used medications. In addition, a long-term severe outcome endpoint may also support the creation of a data-driven, SSc-specific definition of ILD progression, which could then represent a solid short-term surrogate outcome and allow the identification of patients at higher risk of progression.
Risk stratification is another successful achievement in PAH, allowing the identification of patients who should then be treated more aggressively and followed up more tightly.56 In line with lack of agreed long-term outcomes and progression definitions, preliminary data are also available for risk risk strafication of SSc-ILD. Data from a post hoc analysis of the patients with SSc-ILD receiving placebo in the SENSCIS trial identified a modest association between larger extent of fibrotic ILD at baseline and a greater decline in FVC % predicted at week 52.57 Conversely, a post hoc analysis of the FocuSSced trial did not show the significant differences in FVC% declining in placebo group based on QILD severity, possibly related to the different outcome (decline in absolute vs % predicted FVC) and the different inclusion criteria.58 The prognostic value of higher FVC % predicted at baseline as a factor determining a higher risk of a decline in FVC>10% predicted over the next 12 months was also confirmed in recent analysis of patients with SSc-ILD in the EUSTAR database.19
In addition to pulmonary and other ILD features, other SSc-related extrapulmonary characteristics have shown association with ILD progression. In the same EUSTAR analysis, Hoffmann-Vold et al identified male sex, presence of reflux/dysphagia symptoms and high baseline mRSS as the strongest independent predictors of 5-year FVC decline, with significant interaction effects between time and these variables.19 The association between oesophageal symptoms severity, even in the presence of PPI therapy, and future radiographic ILD progression was also confirmed in post analysis of SLS II study.59 In addition to oesophageal features, early disease duration (<18 months since first non-Raynaud symptom), elevated inflammatory markers or extensive skin fibrosis (with mRSS≥18) at baseline have been also confirmed as risk factors for significant FVC decline in both the FocuSSced and the Senscis RCTs26 60 Finally, lower SpO2 after 6MWT and arthritis ever were identified as independent predictors of SSc-ILD progression defined as a relative decrease in FVC % ≥15%, or FVC%≥10% combined with DLCO % ≥15% at 1-year follow-up.61
There are some limitations of this overview. First, this SLR included only articles with available full text and in English language; therefore, additional relevant studies might have been missed. Second, if an article was not identified by title or abstract as highlighting severity, progression or outcomes in SSc-ILD, it may not be detected during the screening process. Additionally, SLR was conducted in the four major electronic databases, Medline, Embase, Web of Science databases Cochrane Library, while other sources were not included.
In summary, the large heterogeneity of definitions of SSc-ILD ‘progression’, ‘severity’ and ‘outcome’ emphasise the need for further studies to develop standardised, consensus definitions, in which also patients’ perspective must be included.62 The results of the present SLR may represent the basis for the development process to create a disease-specific model to define progression of SSc-ILD, anchored to a predetermined specific definition of severe SSc-ILD. Overall, this initiative will aim at a better understanding of the variability of the course of SSc-ILD, thus allowing an accurate stratification of patients with SSc-ILD, timely treatment initiation and development of treatments goals.43
Acknowledgments
on behalf of the SILPRO project investigators.
Footnotes
Twitter: @cosimobruni
Contributors: Criterion 1: (a) substantial contributions to study conception and design: CB, OD. (b) Substantial contributions to acquisition of data: LP, FB, CC, EF, SP, ST, PB, CC, RDL, AE, JL, CB. (c) Substantial contributions to analysis and interpretation of data: all authors. Criterion 2: drafting the article or revising it critically for important intellectual content: all authors. Criterion 3: final approval of the version of the article to be published: all authors. Guarantor: CB.
Funding: The study was supported by the 2021 Betty Z Benedict research grant by the Scleroderma Clinical Trial Consortium (SCTC) and the Scleroderma Research Foundation (SRF).
Competing interests: Liubov Petelytska Grant/research support from: received research grant from Swiss National Research Foundation/Scholars at risk. Francesco Bonomi, Carlo Cannistrà, Elisa Fiorentini, Silvia Peretti, Sara Torracchi, Pamela Bernardini, Carmela Coccia, Riccardo De Luca, Alessio Economou, Juela Levani: None declared. Marco Matucci-Cerinic: Speakers bureau: Actelion, Janssen, Inventiva, Bayer, Biogen, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Lilly, Pfizer, Roche., Consultant of: Actelion, Janssen, Inventiva, Bayer, Biogen, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Lilly, Pfizer, Roche. Distler O: Consultancy relationship with and/or has received research funding from and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Prometheus, Redxpharma, Roivant and Topadur. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). Co-founder of CITUS AG. Editorial Board member for Annals of the Rheumatic Diseases, RMD Open. Cosimo Bruni Speakers bureau: Eli- Lilly, Consultant of: Boehringer Ingelheim, Grant/research support from: Gruppo Italiano Lotta alla Sclerodermia (GILS), European Scleroderma Trials and Research Group (EUSTAR), Foundation for research in Rheumatology (FOREUM), Scleroderma Clinical Trials Consortium (SCTC), Scleroderma Research Foundation (SRF). Educational grants from AbbVie and Wellcome Trust. Congress Support from: Boehringer Ingelheim.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data will be made available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. Journal of Scleroderma and Related Disorders 2017;2:137–52. 10.5301/jsrd.5000249 [DOI] [Google Scholar]
- 2.Nihtyanova SI, Denton CP. Pathogenesis of systemic sclerosis associated interstitial lung disease. J Scleroderma Relat Disord 2020;5:6–16. 10.1177/2397198320903867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elhai M, Meune C, Boubaya M, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. 10.1136/annrheumdis-2017-211448 [DOI] [PubMed] [Google Scholar]
- 4.Volkmann ER, Fischer A. Update on morbidity and mortality in systemic sclerosis-related interstitial lung disease. J Scleroderma Relat Disord 2021;6:11–20. 10.1177/2397198320915042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. 10.1136/ard.2009.114264 [DOI] [PubMed] [Google Scholar]
- 6.Fretheim H, Halse A-K, Seip M, et al. Multidimensional tracking of phenotypes and organ involvement in a complete nationwide systemic sclerosis cohort. Rheumatology (Oxford) 2020;59:2920–9. 10.1093/rheumatology/keaa026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottin V, Brown KK. Interstitial lung disease associated with systemic sclerosis (SSC-ILD). Respir Res 2019;20:13. 10.1186/s12931-019-0980-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann-Vold A-M, Fretheim H, Halse A-K, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med 2019;200:1258–66. 10.1164/rccm.201903-0486OC [DOI] [PubMed] [Google Scholar]
- 9.Suliman YA, Dobrota R, Huscher D, et al. Brief report: pulmonary function tests: high rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis Rheumatol 2015;67:3256–61. 10.1002/art.39405 [DOI] [PubMed] [Google Scholar]
- 10.Rahaghi FF, Hsu VM, Kaner RJ, et al. Expert consensus on the management of systemic sclerosis-associated interstitial lung disease. Respir Res 2023;24:6. 10.1186/s12931-022-02292-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann-Vold A-M, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. The Lancet Rheumatology 2020;2:e71–83. 10.1016/S2665-9913(19)30144-4 [DOI] [PubMed] [Google Scholar]
- 12.Bruni C, Chung L, Hoffmann-Vold AM, et al. High-resolution computed tomography of the chest for the screening, re-screening and follow-up of systemic sclerosis-associated interstitial lung disease: a EUSTAR-SCTC survey. Clin Exp Rheumatol 2022;40:1951–5. 10.55563/clinexprheumatol/7ry6zz [DOI] [PubMed] [Google Scholar]
- 13.Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR scleroderma trials and research group database. Ann Rheum Dis 2007;66:754–63. 10.1136/ard.2006.062901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peoples C, Medsger TA, Lucas M, et al. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. J Scleroderma Relat Disord 2016;1:177–240. 10.5301/jsrd.5000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein H, Lee P, Chau C, et al. The effect of male sex on survival in systemic sclerosis. J Rheumatol 2014;41:2193–200. 10.3899/jrheum.140006 [DOI] [PubMed] [Google Scholar]
- 16.Al-Sheikh H, Ahmad Z, Johnson SR. Ethnic variations in systemic sclerosis disease manifestations, internal organ involvement, and mortality. J Rheumatol 2019;46:1103–8. 10.3899/jrheum.180042 [DOI] [PubMed] [Google Scholar]
- 17.Volkmann ER, Tashkin DP, Sim M, et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis 2019;78:122–30. 10.1136/annrheumdis-2018-213708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vonk MC, Walker UA, Volkmann ER, et al. Natural variability in the disease course of SSC-ILD: implications for treatment. Eur Respir Rev 2021;30:200340. 10.1183/16000617.0340-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann-Vold A-M, Allanore Y, Alves M, et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219–27. 10.1136/annrheumdis-2020-217455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avouac J, Lepri G, Smith V, et al. Sequential Nailfold Videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum 2017;47:86–94. 10.1016/j.semarthrit.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 21.Becker M, Graf N, Sauter R, et al. Predictors of disease worsening defined by progression of organ damage in diffuse systemic sclerosis: a European scleroderma trials and research (EUSTAR) analysis. Ann Rheum Dis 2019;78:1242–8. 10.1136/annrheumdis-2019-215145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrota R, Jordan S, Juhl P, et al. Circulating collagen Neo-epitopes and their role in the prediction of fibrosis in patients with systemic sclerosis: a Multicentre cohort study. The Lancet Rheumatology 2021;3:e175–84. 10.1016/S2665-9913(20)30385-4 [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira Martins LV, Oliveira SM, Silvatti J, et al. Mortality in systemic sclerosis-associated interstitial lung disease in Brazil: A real-life, long-term follow-up observational study. J Clin Rheumatol 2022;28:e532–8. 10.1097/RHU.0000000000001792 [DOI] [PubMed] [Google Scholar]
- 24.De Santis M, Bosello SL, Peluso G, et al. Bronchoalveolar Lavage fluid and progression of scleroderma interstitial lung disease. Clin Respir J 2012;6:9–17. 10.1111/j.1752-699X.2010.00228.x [DOI] [PubMed] [Google Scholar]
- 25.Jaafar S, Lescoat A, Huang S, et al. Clinical characteristics, visceral involvement, and mortality in at-risk or early diffuse systemic sclerosis: a longitudinal analysis of an observational prospective multicenter US cohort. Arthritis Res Ther 2021;23:170. 10.1186/s13075-021-02548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna D, Lin CJF, Furst DE, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2020;8:963–74. 10.1016/S2213-2600(20)30318-0 [DOI] [PubMed] [Google Scholar]
- 27.Tiwari V, Rigby WFC. Journal club: efficacy of Tocilizumab in early systemic sclerosis-related interstitial lung disease. ACR Open Rheumatol 2022;4:119–22. 10.1002/acr2.11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Distler O, Gahlemann M, Maher TM. Nintedanib for systemic sclerosis-associated interstitial lung disease. reply. N Engl J Med 2019;381:1596–7. 10.1056/NEJMc1910735 [DOI] [PubMed] [Google Scholar]
- 29.Goh NS, Hoyles RK, Denton CP, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017;69:1670–8. 10.1002/art.40130 [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann-Vold A-M, Aaløkken TM, Lund MB, et al. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol 2015;67:2205–12. 10.1002/art.39166 [DOI] [PubMed] [Google Scholar]
- 31.Boonstra M, Meijs J, Dorjée AL, et al. Rituximab in early systemic sclerosis. RMD Open 2017;3:e000384. 10.1136/rmdopen-2016-000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carnevale A, Silva M, Maietti E, et al. Longitudinal change during follow-up of systemic sclerosis: correlation between high-resolution computed tomography and pulmonary function tests. Clin Rheumatol 2021;40:213–9. 10.1007/s10067-020-05375-y [DOI] [PubMed] [Google Scholar]
- 33.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kafaja S, Clements PJ, Wilhalme H, et al. Reliability and minimal clinically important differences of forced vital capacity: results from the scleroderma lung studies (SLS-I and SLS-II). Am J Respir Crit Care Med 2018;197:644–52. 10.1164/rccm.201709-1845OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruni C, Buch MH, Furst DE, et al. Primary systemic sclerosis heart involvement: A systematic literature review and preliminary data-driven, consensus-based WSF/HFA definition. J Scleroderma Relat Disord 2022;7:24–32. 10.1177/23971983211053246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruni C, De Luca G, Lazzaroni M-G, et al. Screening for pulmonary arterial hypertension in systemic sclerosis: A systematic literature review. Eur J Intern Med 2020;78:17–25. 10.1016/j.ejim.2020.05.042 [DOI] [PubMed] [Google Scholar]
- 37.Bruni C, Buch MH, Djokovic A, et al. Consensus on the assessment of systemic sclerosis–associated primary heart involvement: world scleroderma foundation/heart failure Association guidance on screening, diagnosis, and follow-up assessment. J Scleroderma Relat Disord 2023;8:169–82. 10.1177/23971983231163413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goh NSL, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248–54. 10.1164/rccm.200706-877OC [DOI] [PubMed] [Google Scholar]
- 39.Warrick JH, Bhalla M, Schabel SI, et al. High resolution computed tomography in early scleroderma lung disease. J Rheumatol 1991;18:1520–8. [PubMed] [Google Scholar]
- 40.Kim HJ, Brown MS, Elashoff R, et al. Quantitative texture-based assessment of one-year changes in Fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. Eur Radiol 2011;21:2455–65. 10.1007/s00330-011-2223-2 [DOI] [PubMed] [Google Scholar]
- 41.Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997;169:977–83. 10.2214/ajr.169.4.9308447 [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann-Vold AM, Brunborg C, Airò P, et al. Pos0063 progressive interstitial lung disease is frequent also in late disease stages in systemic sclerosis patients from Eustar. Ann Rheum Dis 2022;81:248. 10.1136/annrheumdis-2022-eular.4051 [DOI] [Google Scholar]
- 43.Bruni C, Campochiaro C, Vries-Bouwstra JK. Interstitial lung disease: how should Therapeutics be implemented? Rheum Dis Clin North Am 2023;49:279–93. [DOI] [PubMed] [Google Scholar]
- 44.Roofeh D, Brown KK, Kazerooni EA, et al. Systemic sclerosis associated interstitial lung disease: a conceptual framework for Subclinical, clinical and progressive disease. Rheumatology (Oxford) 2023;62:1877–86. 10.1093/rheumatology/keac557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing interstitial lung diseases. N Engl J Med 2019;381:1718–27. 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 46.Hambly N, Farooqi MM, Dvorkin-Gheva A, et al. Prevalence and characteristics of progressive Fibrosing interstitial lung disease in a prospective Registry. Eur Respir J 2022;60:2102571. 10.1183/13993003.02571-2021 [DOI] [PubMed] [Google Scholar]
- 47.Nasser M, Larrieu S, Si-Mohamed S, et al. Progressive Fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study). Eur Respir J 2021;57:2002718. 10.1183/13993003.02718-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson T, Barratt SL, Beirne P, et al. The burden of progressive Fibrotic interstitial lung disease across the UK. Eur Respir J 2021;58:2100221. 10.1183/13993003.00221-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landini N, Orlandi M, Bruni C, et al. n.d. Computed tomography predictors of mortality or disease progression in systemic sclerosis-interstitial lung disease: A systematic review. Front Med;8. 10.3389/fmed.2021.807982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkmann ER, Tashkin DP, Roth MD, et al. Early radiographic progression of scleroderma: lung disease predicts long-term mortality. Chest 2022;161:1310–9. 10.1016/j.chest.2021.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khanna D, Denton CP, Jahreis A, et al. Safety and efficacy of subcutaneous Tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. The Lancet 2016;387:2630–40. 10.1016/S0140-6736(16)00232-4 [DOI] [PubMed] [Google Scholar]
- 52.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016;4:708–19. 10.1016/S2213-2600(16)30152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655–66. 10.1056/NEJMoa055120 [DOI] [PubMed] [Google Scholar]
- 54.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809–18. 10.1056/NEJMoa1213917 [DOI] [PubMed] [Google Scholar]
- 55.Galiè N, Barberà JA, Frost AE, et al. Initial use of Ambrisentan plus Tadalafil in pulmonary arterial hypertension. N Engl J Med 2015;373:834–44. 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 56.Humbert M, Kovacs G, Hoeper MM, et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023;61:2200879. 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 57.Denton CP, Goh NS, Humphries SM, et al. Extent of fibrosis and lung function decline in patients with systemic sclerosis and interstitial lung disease: data from the SENSCIS trial. Rheumatology (Oxford) 2023;62:1870–6. 10.1093/rheumatology/keac535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roofeh D, Lin CJF, Goldin J, et al. Tocilizumab prevents progression of early systemic sclerosis–associated interstitial lung disease. Arthritis Rheumatol 2021;73:1301–10. 10.1002/art.41668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volkmann ER, Tashkin DP, Leng M, et al. Association of symptoms of gastroesophageal reflux, Esophageal dilation, and progression of systemic sclerosis-related interstitial lung disease. Arthritis Care Res (Hoboken) 2023;75:1690–7. 10.1002/acr.25070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khanna D, Maher TM, Volkmann ER, et al. Effect of Nintedanib in patients with systemic sclerosis-associated interstitial lung disease and risk factors for rapid progression. RMD Open 2023;9:e002859. 10.1136/rmdopen-2022-002859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu W, Jordan S, Becker MO, et al. Prediction of progression of interstitial lung disease in patients with systemic sclerosis: the SPAR model. Ann Rheum Dis 2018;77:1326–32. 10.1136/annrheumdis-2018-213201 [DOI] [PubMed] [Google Scholar]
- 62.Bruni C, Heidenreich S, Duenas A, et al. Patient preferences for the treatment of systemic sclerosis-associated interstitial lung disease: a discrete choice experiment. Rheumatology (Oxford) 2022;61:4035–46. 10.1093/rheumatology/keac126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aguila LA, da Silva HC, Medeiros-Ribeiro AC, et al. Is exposure to environmental factors associated with a characteristic clinical and laboratory profile in systemic sclerosis? A retrospective analysis. Rheumatol Int 2021;41:1143–50. 10.1007/s00296-020-04693-3 [DOI] [PubMed] [Google Scholar]
- 64.Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010;12:R166. 10.1186/ar3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Callejas-Moraga EL, Guillén-Del-Castillo A, Perurena-Prieto J, et al. Anti-RNPC-3 antibody predicts poor prognosis in patients with interstitial lung disease associated to systemic sclerosis. Rheumatology (Oxford) 2021;61:154–62. 10.1093/rheumatology/keab279 [DOI] [PubMed] [Google Scholar]
- 66.Cottrell TR, Wise RA, Wigley FM, et al. The degree of skin involvement identifies distinct lung disease outcomes and survival in systemic sclerosis. Ann Rheum Dis 2014;73:1060–6. 10.1136/annrheumdis-2012-202849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Santis M, Bosello S, La Torre G, et al. Functional, radiological and biological markers of Alveolitis and infections of the lower respiratory tract in patients with systemic sclerosis. Respir Res 2005;6:96. 10.1186/1465-9921-6-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deepa AS, Rachel RP, Ramchandran P, et al. Pulmonary involvement in systemic sclerosis: A clinical profile. Lung India 2016;33:144–7. 10.4103/0970-2113.177439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Sergany M, Shahba A, Ghazy M, et al. Increased expression of soluble Fractalkine (Cx3Cl1) in systemic sclerosis - possible role in vascular inflammation. The Egyptian Rheumatologist 2011;33:93–8. 10.1016/j.ejr.2011.03.004 [DOI] [Google Scholar]
- 70.Gilson M, Zerkak D, Wipff J, et al. Prognostic factors for lung function in systemic sclerosis: prospective study of 105 cases. Eur Respir J 2010;35:112–7. 10.1183/09031936.00060209 [DOI] [PubMed] [Google Scholar]
- 71.Guillen-Del Castillo A, Pilar Simeón-Aznar C, Fonollosa-Pla V, et al. Good outcome of interstitial lung disease in patients with scleroderma associated to anti-PM/Scl antibody. Semin Arthritis Rheum 2014;44:331–7. 10.1016/j.semarthrit.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 72.Janardana R, Nair AM, Surin AK, et al. Unique clinical and autoantibody profile of a large Asian Indian cohort of scleroderma-do South Asians have a more aggressive disease Clin Rheumatol 2019;38:3179–87. 10.1007/s10067-019-04659-2 [DOI] [PubMed] [Google Scholar]
- 73.Khanna D, Clements PJ, Furst DE, et al. Correlation of the degree of Dyspnea with health-related quality of life, functional abilities, and diffusing capacity for carbon Monoxide in patients with systemic sclerosis and active Alveolitis: results from the scleroderma lung study. Arthritis Rheum 2005;52:592–600. 10.1002/art.20787 [DOI] [PubMed] [Google Scholar]
- 74.Sari A, Guven D, Armagan B, et al. Rituximab experience in patients with long-standing systemic sclerosis-associated interstitial lung disease: A series of 14 patients. J Clin Rheumatol 2017;23:411–5. 10.1097/RHU.0000000000000584 [DOI] [PubMed] [Google Scholar]
- 75.Shenoy PD, Bavaliya M, Sashidharan S, et al. Cyclophosphamide versus mycophenolate mofetil in scleroderma interstitial lung disease (SSC-ILD) as induction therapy: A single-centre, retrospective analysis. Arthritis Res Ther 2016;18:123. 10.1186/s13075-016-1015-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arandia NI, Simeón-Aznar CP, Castillo AG, et al. Influence of antibody profile in clinical features and prognosis in a cohort of Spanish patients with systemic sclerosis. Clin Exp Rheumatol 2017;35:S98–105. [PubMed] [Google Scholar]
- 77.Dapena MF, Rivera A, Sopeña B, et al. Clinical and Epidemiological differences between men and women with systemic sclerosis: A study in a Spanish systemic sclerosis cohort and literature review. Clin Exp Rheumatol 2017;35:S89–97. [PubMed] [Google Scholar]
- 78.Iniesta Arandia N, Espinosa G, Guillén Del Castillo A, et al. Anti-polymyositis/Scl antibodies in systemic sclerosis: clinical associations in a Multicentric Spanish cohort and review of the literature. J Clin Rheumatol 2022;28:e180–8. 10.1097/RHU.0000000000001676 [DOI] [PubMed] [Google Scholar]
- 79.Iniesta Arandia N, Simeón-Aznar CP, Guillén Del Castillo A, et al. Influence of antibody profile in clinical features and prognosis in a cohort of Spanish patients with systemic sclerosis. Clin Exp Rheumatol 2017;35 Suppl 106:98–105. [PubMed] [Google Scholar]
- 80.Iniesta Arandia N, Espinosa G, Tolosa Vilella C, et al. Serodiscordant patients with systemic sclerosis: when antibody does not correspond to skin involvement. Clin Exp Rheumatol 2020;38 Suppl 125:106–14. [PubMed] [Google Scholar]
- 81.Hoffmann-Vold A-M, Tennøe AH, Garen T, et al. High level of Chemokine Ccl18 is associated with pulmonary function deterioration, lung fibrosis progression, and reduced survival in systemic sclerosis. Chest 2016;150:299–306. 10.1016/j.chest.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 82.Perosa F, Favoino E, Favia IE, et al. Subspecificities of Anticentromeric protein A antibodies identify systemic sclerosis patients at higher risk of pulmonary vascular disease. Medicine 2016;95:e3931. 10.1097/MD.0000000000003931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steelandt A, Benmostefa N, Avouac J, et al. Ethnic influence on the phenotype of French patients with systemic sclerosis. Joint Bone Spine 2021;88:105081. 10.1016/j.jbspin.2020.09.013 [DOI] [PubMed] [Google Scholar]
- 84.van den Hombergh WM, Knaapen-Hans HK, van den Hoogen FH, et al. Prediction of organ involvement and survival in systemic sclerosis patients in the first 5Years from diagnosis. Journal of Scleroderma and Related Disorders 2020;5:57–65. 10.1177/2397198319869564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Binks M. Phase I/II trial of Autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis 2001;60:577–84. 10.1136/ard.60.6.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Espinosa G, Simeón CP, Plasín MÁ, et al. Efficacy of Cyclophospamide in the treatment of interstitial lung disease associated with systemic sclerosis. Arch Bronconeumol 2011;47:239–45. 10.1016/j.arbres.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 87.Hoang-Duc H, Pham-Huy Q, Vu-Minh T, et al. Study of the correlation between HRCT semi-quantitative scoring, concentration of alveolar nitric oxide, and clinical-functional parameters of systemic sclerosis-induced interstitial lung disease. Yale J Biol Med 2020;93:657–67. [PMC free article] [PubMed] [Google Scholar]
- 88.Legány N, Toldi G, Distler JHW, et al. Increased plasma soluble Urokinase plasminogen activator receptor levels in systemic sclerosis: possible association with Microvascular abnormalities and extent of fibrosis. Clin Chem Lab Med 2015;53:1799–805. 10.1515/cclm-2015-0079 [DOI] [PubMed] [Google Scholar]
- 89.Zompatori M, Leone MB, Giannotta M, et al. Pulmonary hypertension and systemic sclerosis: the role of high-resolution computed tomography. Radiol Med 2013;118:1360–72. 10.1007/s11547-013-0934-1 [DOI] [PubMed] [Google Scholar]
- 90.Silver RM, Metcalf JF, Stanley JH, et al. Interstitial lung disease in scleroderma. analysis by Bronchoalveolar Lavage. Arthritis Rheum 1984;27:1254–62. 10.1002/art.1780271107 [DOI] [PubMed] [Google Scholar]
- 91.Grosicka A, Manasar A, Kucharz EJ, et al. Serum concentration of surfactant protein D in patients with systemic sclerosis: the potential marker of the interstitial lung disease severity. Best Pract Res Clin Rheumatol 2018;32:541–9. 10.1016/j.berh.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 92.Trad S, Huong DLT, Frances C, et al. Impaired carbon Monoxide diffusing capacity as a marker of limited systemic sclerosis. Eur J Intern Med 2011;22:e80–6. 10.1016/j.ejim.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 93.Kumánovics G, Minier T, Radics J, et al. Comprehensive investigation of novel serum markers of pulmonary fibrosis associated with systemic sclerosis and Dermato/polymyositis. Clin Exp Rheumatol 2008;26:414–20. [PubMed] [Google Scholar]
- 94.Abignano G, Mennillo GA, Lettieri G, et al. Arthritis Mutilans in systemic sclerosis. Arthritis Rheumatol 2019;71:120. 10.1002/art.40723 [DOI] [PubMed] [Google Scholar]
- 95.Atabati E, Shariati Sarabi Z, Jokar MH, et al. The correlation between Helicobacter Pylori infection and disease severity in patients with systemic sclerosis. Middle East J Dig Dis 2021;13:253–8. 10.34172/mejdd.2021.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benyamine A, Heim X, Resseguier N, et al. Elevated serum Krebs von den Lungen-6 in systemic sclerosis: a marker of lung fibrosis and severity of the disease. Rheumatol Int 2018;38:813–9. 10.1007/s00296-018-3987-3 [DOI] [PubMed] [Google Scholar]
- 97.Caimmi C, Caramaschi P, Venturini A, et al. Malnutrition and Sarcopenia in a large cohort of patients with systemic sclerosis. Clin Rheumatol 2018;37:987–97. 10.1007/s10067-017-3932-y [DOI] [PubMed] [Google Scholar]
- 98.Çakir Edis E, Hatipoğlu ON, Pamuk ÖN, et al. Effectiveness of Thoracic Ultrasonography in the evaluation of the severity of pulmonary involvement in patients with systemic sclerosis. Arch Rheumatol 2016;31:364–70. 10.5606/ArchRheumatol.2016.5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Derk CT, Grace E, Shenin M, et al. A prospective open-label study of mycophenolate mofetil for the treatment of diffuse systemic sclerosis. Rheumatology (Oxford) 2009;48:1595–9. 10.1093/rheumatology/kep295 [DOI] [PubMed] [Google Scholar]
- 100.Derk CT, Huaman G, Jimenez SA. A retrospective randomly selected cohort study of D-penicillamine treatment in rapidly progressive diffuse cutaneous systemic sclerosis of recent onset. Br J Dermatol 2008;158:1063–8. 10.1111/j.1365-2133.2008.08452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diab S, Dostrovsky N, Hudson M, et al. Systemic sclerosis sine scleroderma: a multicenter study of 1417 subjects. J Rheumatol 2014;41:2179–85. 10.3899/jrheum.140236 [DOI] [PubMed] [Google Scholar]
- 102.Hudson M, Pope J, Mahler M, et al. Clinical significance of antibodies to Ro52/Trim21 in systemic sclerosis. Arthritis Res Ther 2012;14:R50. 10.1186/ar3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaeger VK, Wirz EG, Allanore Y, et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: A longitudinal EUSTAR study. PLoS One 2016;11:e0163894. 10.1371/journal.pone.0163894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lanteri A, Sobanski V, Langlois C, et al. Serum free light chains of Immunoglobulins as biomarkers for systemic sclerosis characteristics, activity and severity. Autoimmun Rev 2014;13:974–80. 10.1016/j.autrev.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 105.Liu X, Mayes MD, Pedroza C, et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis Arthritis Care Res (Hoboken) 2013;65:1375–80. 10.1002/acr.21968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hax V, Bredemeier M, Didonet Moro AL, et al. Clinical Algorithms for the diagnosis and prognosis of interstitial lung disease in systemic sclerosis. Semin Arthritis Rheum 2017;47:228–34. 10.1016/j.semarthrit.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 107.Wechsler RJ, Steiner RM, Spirn PW, et al. The relationship of Thoracic Lymphadenopathy to pulmonary interstitial disease in diffuse and limited systemic sclerosis: CT findings. AJR Am J Roentgenol 1996;167:101–4. 10.2214/ajr.167.1.8659350 [DOI] [PubMed] [Google Scholar]
- 108.Wuttge DM, Bozovic G, Hesselstrand R, et al. Increased alveolar nitric oxide in early systemic sclerosis. Clin Exp Rheumatol 2010;28(5 Suppl 62):S5–9. [PubMed] [Google Scholar]
- 109.Caccavo D, Afeltra A, Rigon A, et al. Antibodies to carbonic Anhydrase in patients with connective tissue diseases: relationship with lung involvement. Int J Immunopathol Pharmacol 2008;21:659–67. 10.1177/039463200802100320 [DOI] [PubMed] [Google Scholar]
- 110.Ariani A, Silva M, Bravi E, et al. Operator-independent quantitative chest computed tomography versus standard assessment of interstitial lung disease related to systemic sclerosis: A multi-centric study. Mod Rheumatol 2015;25:724–30. 10.3109/14397595.2015.1016200 [DOI] [PubMed] [Google Scholar]
- 111.Ewert R, Ittermann T, Habedank D, et al. Prognostic value of cardiopulmonary exercise testing in patients with systemic sclerosis. BMC Pulm Med 2019;19:230. 10.1186/s12890-019-1003-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frauenfelder T, Winklehner A, Nguyen TDL, et al. Screening for interstitial lung disease in systemic sclerosis: performance of high-resolution CT with limited number of slices: a prospective study. Ann Rheum Dis 2014;73:2069–73. 10.1136/annrheumdis-2014-205637 [DOI] [PubMed] [Google Scholar]
- 113.Meier C, Freiburghaus K, Bovet C, et al. Serum metabolites as biomarkers in systemic sclerosis-associated interstitial lung disease. Sci Rep 2020;10:21912. 10.1038/s41598-020-78951-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moore DF, Kramer E, Eltaraboulsi R, et al. Increased morbidity and mortality of scleroderma in African Americans compared to non-African Americans. Arthritis Care Res (Hoboken) 2019;71:1154–63. 10.1002/acr.23861 [DOI] [PubMed] [Google Scholar]
- 115.Olewicz-Gawlik A, Danczak-Pazdrowska A, Kuznar-Kaminska B, et al. Interleukin-17 and Interleukin-23: importance in the pathogenesis of lung impairment in patients with systemic sclerosis. Int J Rheum Dis 2014;17:664–70. 10.1111/1756-185X.12290 [DOI] [PubMed] [Google Scholar]
- 116.Shirai Y, Fukue R, Kaneko Y, et al. Clinical relevance of the serial measurement of Krebs von den Lungen-6 levels in patients with systemic sclerosis-associated interstitial lung disease. Diagnostics (Basel) 2021;11:2007. 10.3390/diagnostics11112007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vanaken L, Landini N, Lenaerts J, et al. Progressive lung fibrosis and mortality can occur in early systemic sclerosis patients without pulmonary abnormalities at baseline assessment. Clin Rheumatol 2020;39:3393–400. 10.1007/s10067-020-05105-4 [DOI] [PubMed] [Google Scholar]
- 118.Vilela VS, Vanhaecke A, da Silva BRA, et al. Is there a link between Nailfold Videocapillaroscopy and pulmonary function tests in systemic sclerosis patients? A 24-month follow-up Monocentric study. J Clin Rheumatol 2022;28:26–32. 10.1097/RHU.0000000000001798 [DOI] [PubMed] [Google Scholar]
- 119.Ramahi A, Lescoat A, Roofeh D, et al. Risk factors for lung function decline in systemic sclerosis-associated interstitial lung disease in a large single-centre cohort. Rheumatology (Oxford) 2023;62:2501–9. 10.1093/rheumatology/keac639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bergmann C, Distler JHW, Treutlein C, et al. 68Ga-FAPI-04 PET-CT for molecular assessment of fibroblast activation and risk evaluation in systemic sclerosis-associated interstitial lung disease: a single-centre, pilot study. The Lancet Rheumatology 2021;3:e185–94. 10.1016/S2665-9913(20)30421-5 [DOI] [PubMed] [Google Scholar]
- 121.Iudici M, Cuomo G, Vettori S, et al. Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSC-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum 2015;44:437–44. 10.1016/j.semarthrit.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 122.Khanna D, Nagaraja V, Tseng C, et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Res Ther 2015;17. 10.1186/s13075-015-0872-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moore OA, Proudman SM, Goh N, et al. Quantifying change in pulmonary function as a Prognostic marker in systemic sclerosis-related interstitial lung disease. Clin Exp Rheumatol 2015;33:S111–6. [PubMed] [Google Scholar]
- 124.Nawata T, Shirai Y, Suzuki M, et al. Chest wall muscle atrophy as a contributory factor for forced vital capacity decline in systemic sclerosis-associated interstitial lung disease. Rheumatology 2021;60:250–5. 10.1093/rheumatology/keaa322 [DOI] [PubMed] [Google Scholar]
- 125.Sircar G, Goswami RP, Sircar D, et al. Intravenous cyclophosphamide vs Rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford) 2018;57:2106–13. 10.1093/rheumatology/key213 [DOI] [PubMed] [Google Scholar]
- 126.Yamakawa H, Takemura T, Iwasawa T, et al. Emphysematous change with scleroderma-associated interstitial lung disease: the potential contribution of Vasculopathy? BMC Pulm Med 2018;18:25. 10.1186/s12890-018-0591-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Young A, Vummidi D, Visovatti S, et al. Prevalence, treatment, and outcomes of coexistent pulmonary hypertension and interstitial lung disease in systemic sclerosis. Arthritis Rheumatol 2019;71:1339–49. 10.1002/art.40862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pugnet G, Petermann A, Collot S, et al. Changes on chest HRCT in systemic sclerosis-related interstitial lung disease after Autologous haematopoietic stem cell transplantation. Rheumatology (Oxford) 2023;62:SI32–42. 10.1093/rheumatology/keac319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sarı A, Önder Ö, Armağan B, et al. Pleuroparenchymal Fibroelastosis in systemic sclerosis-associated interstitial lung disease. Turk J Med Sci 2022;52:83–8. 10.3906/sag-2107-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fairley JL, Hansen D, Proudman S, et al. Clinical features of systemic sclerosis-mixed connective tissue disease and systemic sclerosis overlap syndromes. Arthritis Care Res (Hoboken) 2021;73:732–41. 10.1002/acr.24167 [DOI] [PubMed] [Google Scholar]
- 131.Fraticelli P, Gabrielli B, Pomponio G, et al. Low-dose oral Imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot study. Arthritis Res Ther 2014;16:R144. 10.1186/ar4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dupont A, Koether V, Labreuche J, et al. Dual-energy CT lung perfusion in systemic sclerosis: preliminary experience in 101 patients. Eur Radiol 2023;33:401–13. 10.1007/s00330-022-09016-7 [DOI] [PubMed] [Google Scholar]
- 133.Maeda M, Ichiki Y, Aoyama Y, et al. Surfactant protein D (SP-D) and systemic scleroderma (SSC). J Dermatol 2001;28:467–74. 10.1111/j.1346-8138.2001.tb00013.x [DOI] [PubMed] [Google Scholar]
- 134.Rizzi M, Sarzi-Puttini P, Airoldi A, et al. Performance capacity evaluated using the 6-minute walk test: 5-year results in patients with diffuse systemic sclerosis and initial interstitial lung disease. Clin Exp Rheumatol 2015;33:S142–7. [PubMed] [Google Scholar]
- 135.Tumsatan P, Wongwiwatchai J, Apinives C, et al. Mediastinal Lymphadenopathy in patients with systemic sclerosis. J Med Assoc Thai 2016;99:348–53. [PubMed] [Google Scholar]
- 136.Salaffi F, Di Carlo M, Carotti M, et al. Relationship between interstitial lung disease and Oesophageal dilatation on chest high-resolution computed tomography in patients with systemic sclerosis: a cross-sectional study. Radiol Med 2018;123:655–63. 10.1007/s11547-018-0894-3 [DOI] [PubMed] [Google Scholar]
- 137.Englert H, Richards BL, Angelides S, et al. 99M)Tc-labelled Glucosamine in the assessment of systemic sclerosis inflammatory lung disease: a novel inexpensive investigative tool with predictive value. Ann Nucl Med 2021;35:1157–66. 10.1007/s12149-021-01653-0 [DOI] [PubMed] [Google Scholar]
- 138.Trad S, Amoura Z, Beigelman C, et al. Pulmonary arterial hypertension is a major mortality factor in diffuse systemic sclerosis, independent of interstitial lung disease. Arthritis Rheum 2006;54:184–91. 10.1002/art.21538 [DOI] [PubMed] [Google Scholar]
- 139.Daoussis D, Liossis S-NC, Tsamandas AC, et al. Experience with Rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 2010;49:271–80. 10.1093/rheumatology/kep093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Griffiths B, Miles S, Moss H, et al. Systemic sclerosis and interstitial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol 2002;29:2371–8. [PubMed] [Google Scholar]
- 141.Winstone TA, Hague CJ, Soon J, et al. Oesophageal diameter is associated with severity but not progression of systemic sclerosis-associated interstitial lung disease. Respirology 2018;23:921–6. 10.1111/resp.13309 [DOI] [PubMed] [Google Scholar]
- 142.Kwon HM, Kang EH, Park JK, et al. A decision model for the watch-and-wait strategy in systemic sclerosis-associated interstitial lung disease. Rheumatology (Oxford) 2015;54:1792–6. 10.1093/rheumatology/kev121 [DOI] [PubMed] [Google Scholar]
- 143.Ricci A, Mariotta S, Bronzetti E, et al. Serum CA 15-3 is increased in pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2009;26:54–63. [PubMed] [Google Scholar]
- 144.Xie M. Long-term efficacy and low adverse events of methylprednisolone pulses combined to low-dose glucocorticoids for systemic sclerosis: A retrospective clinical study of 10 years’ follow-up [DOI] [PMC free article] [PubMed]
- 145.Hochhegger B, Lonzetti L, Rubin A, et al. Chest MRI with CT in the assessment of interstitial lung disease progression in patients with systemic sclerosis. Rheumatology 2022;61:4420–6. 10.1093/rheumatology/keac148 [DOI] [PubMed] [Google Scholar]
- 146.Pan H-F, Wang J, Leng R-X, et al. Interleukin-18: friend or foe for systemic sclerosis Journal of Investigative Dermatology 2011;131:2495. 10.1038/jid.2011.224 [DOI] [PubMed] [Google Scholar]
- 147.Renaud A, Pautre R, Morla O, et al. Thoracic Lymphadenopathies in diffuse systemic sclerosis: an observational study on 48 patients using computed tomography. BMC Pulm Med 2022;22:44. 10.1186/s12890-022-01837-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vandecasteele E, Melsens K, Vanhaecke A, et al. Incidence, prevalence and long-term progression of Goh algorithm rated interstitial lung disease in systemic sclerosis in two independent cohorts in Flanders: A retrospective cohort study. Semin Arthritis Rheum 2021;51:969–76. 10.1016/j.semarthrit.2021.07.018 [DOI] [PubMed] [Google Scholar]
- 149.Forestier A, Le Gouellec N, Béhal H, et al. Evolution of high-resolution CT-scan in systemic sclerosis-associated interstitial lung disease: description and prognosis factors. Semin Arthritis Rheum 2020;50:1406–13. 10.1016/j.semarthrit.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 150.Le Gouellec N, Duhamel A, Perez T, et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS One 2017;12:e0181692. 10.1371/journal.pone.0181692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morisset J, Vittinghoff E, Elicker BM, et al. Mortality risk prediction in scleroderma-related interstitial lung disease: the SADL model. Chest 2017;152:999–1007.:S0012-3692(17)31078-4. 10.1016/j.chest.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van den Hombergh WMT, Simons SO, Teesselink E, et al. Intravenous cyclophosphamide pulse therapy in interstitial lung disease associated with systemic sclerosis in a retrospective open-label study: influence of the extent of inflammation on pulmonary function. Clin Rheumatol 2018;37:2715–22. 10.1007/s10067-018-4171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Watanabe S, Kase K, Saeki K, et al. Kinetic changes in serum KL-6 levels predict disease progression in patients with systemic sclerosis-associated interstitial lung disease. Respiratory Medicine 2022;191:106689. 10.1016/j.rmed.2021.106689 [DOI] [PubMed] [Google Scholar]
- 154.Jang HJ, Woo A, Kim SY, et al. Characteristics and risk factors of mortality in patients with systemic sclerosis-associated interstitial lung disease. Ann Med 2023;55:663–71. 10.1080/07853890.2023.2179659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ayano M, Tsukamoto H, Kohno K, et al. Increased Cd226 expression on Cd8+ T cells is associated with upregulated cytokine production and endothelial cell injury in patients with systemic sclerosis. J Immunol 2015;195:892–900. 10.4049/jimmunol.1403046 [DOI] [PubMed] [Google Scholar]
- 156.Elhai M, Hoffmann-Vold AM, Avouac J, et al. Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol 2019;71:972–82. 10.1002/art.40815 [DOI] [PubMed] [Google Scholar]
- 157.Kozij NK, Granton JT, Silkoff PE, et al. Exhaled nitric oxide in systemic sclerosis lung disease. Can Respir J 2017;2017:6736239. 10.1155/2017/6736239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Volkmann ER, Saggar R, Khanna D, et al. Improved transplant-free survival in patients with systemic sclerosis-associated pulmonary hypertension and interstitial lung disease. Arthritis Rheumatol 2014;66:1900–8. 10.1002/art.38623 [DOI] [PubMed] [Google Scholar]
- 159.Yuan Y-P, Yuan P, Su Y-L, et al. Clinical phenotypes, hemodynamic characteristics and prognosis of Chinese patients with systemic sclerosis-associated Precapillary pulmonary hypertension: a retrospective study. Clin Rheumatol 2022;41:1675–86. 10.1007/s10067-021-06016-8 [DOI] [PubMed] [Google Scholar]
- 160.Guillén-Del-Castillo A, Meseguer ML, Fonollosa-Pla V, et al. Impact of interstitial lung disease on the survival of systemic sclerosis with pulmonary arterial hypertension. Sci Rep 2022;12:12947. 10.1038/s41598-022-17525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goh NSL, Desai SR, Anagnostopoulos C, et al. Increased epithelial permeability in pulmonary fibrosis in relation to disease progression. European Respiratory Journal 2011;38:184–90. 10.1183/09031936.00010910 [DOI] [PubMed] [Google Scholar]
- 162.Ufuk F, Demirci M, Altinisik G. Quantitative computed tomography assessment for systemic sclerosis-related interstitial lung disease: comparison of different methods. Eur Radiol 2020;30:4369–80. 10.1007/s00330-020-06772-2 [DOI] [PubMed] [Google Scholar]
- 163.Yamakawa H, Hagiwara E, Kitamura H, et al. Clinical features of idiopathic interstitial pneumonia with systemic sclerosis-related autoantibody in comparison with interstitial pneumonia with systemic sclerosis. PLoS One 2016;11:e0161908. 10.1371/journal.pone.0161908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ciaffi J, van Leeuwen NM, Boonstra M, et al. Evolution of systemic sclerosis-associated interstitial lung disease one year after hematopoietic stem cell transplantation or cyclophosphamide. Arthritis Care & Research 2022;74:433–41. 10.1002/acr.24451 Available: https://acrjournals.onlinelibrary.wiley.com/toc/21514658/74/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roeser A, Sese L, Chassagnon G, et al. The association between air pollution and the severity at diagnosis and progression of systemic sclerosis-associated interstitial lung disease: results from the retrospective Scleropol study. Respir Res 2023;24:151. 10.1186/s12931-023-02463-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kreuter M, Del Galdo F, Miede C, et al. Impact of lung function decline on time to Hospitalisation events in systemic sclerosis-associated interstitial lung disease (SSC-ILD): a joint model analysis. Arthritis Res Ther 2022;24:19. 10.1186/s13075-021-02710-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Becker MO, Radic M, Schmidt K, et al. Serum Cytokines and their predictive value in pulmonary involvement of systemic sclerosis. Sarcoidosis Vasc Diffuse Lung Dis 2019;36:274–84. 10.36141/svdld.v36i4.7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu Q, Zhang J, Fan L, et al. Clinical features and risk factors of rapidly progressive systemic sclerosis in a single center in China: anti-RNA polymerase III antibodies as a Predictor. Discov Med 2023;35:193–200. 10.24976/Discov.Med.202335175.20 [DOI] [PubMed] [Google Scholar]
- 169.Plastiras SC, Karadimitrakis SP, Ziakas PD, et al. Scleroderma lung: initial forced vital capacity as Predictor of pulmonary function decline. Arthritis Rheum 2006;55:598–602. 10.1002/art.22099 [DOI] [PubMed] [Google Scholar]
- 170.Yiannopoulos G, Pastromas V, Antonopoulos I, et al. Combination of intravenous pulses of cyclophosphamide and Methylprednizolone in patients with systemic sclerosis and interstitial lung disease. Rheumatol Int 2007;27:357–61. 10.1007/s00296-006-0217-1 [DOI] [PubMed] [Google Scholar]
- 171.Becker MO, Schohe A, Weinert K, et al. Responders to cyclophosphamide: results of a single-centre analysis among systemic sclerosis patients. Ann Rheum Dis 2012;71:2061–2. 10.1136/annrheumdis-2011-200389 [DOI] [PubMed] [Google Scholar]
- 172.Bosello SL, De Luca G, Rucco M, et al. Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthritis Rheum 2015;44:428–36. 10.1016/j.semarthrit.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 173.De Lauretis A, Sestini P, Pantelidis P, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol 2013;40:435–46. 10.3899/jrheum.120725 [DOI] [PubMed] [Google Scholar]
- 174.Tiev KP, Hua-Huy T, Kettaneh A, et al. Alveolar concentration of nitric oxide predicts pulmonary function deterioration in scleroderma. Thorax 2012;67:157–63. 10.1136/thoraxjnl-2011-200499 [DOI] [PubMed] [Google Scholar]
- 175.Kumánovics G, Görbe E, Minier T, et al. Follow-up of serum KL-6 lung fibrosis biomarker levels in 173 patients with systemic sclerosis. Clin Exp Rheumatol 2014;32:S–138. [PubMed] [Google Scholar]
- 176.White B, Moore WC, Wigley FM, et al. Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and Alveolitis. Ann Intern Med 2000;132:947–54. 10.7326/0003-4819-132-12-200006200-00004 [DOI] [PubMed] [Google Scholar]
- 177.Campochiaro C, Hoffmann-Vold A-M, Avouac J, et al. Sex influence on outcomes of patients with systemic sclerosis-associated interstitial lung disease: a EUSTAR database analysis. Rheumatology 2023;62:2483–91. 10.1093/rheumatology/keac660 [DOI] [PubMed] [Google Scholar]
- 178.Ghuman A, Khanna D, Lin CJF, et al. Prognostic and predictive markers of systemic sclerosis-interstitial lung disease in a clinical trial and long-term observational cohort. Rheumatology (Oxford) 2023:kead234. 10.1093/rheumatology/kead234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meridor K, Sagy I, Molad Y. Anti-Ro/SS-A antibody is associated with worse pulmonary outcome and reduced overall survival in systemic sclerosis. Mod Rheumatol 2022;32:1086–93. 10.1093/mr/roab118 [DOI] [PubMed] [Google Scholar]
- 180.Boin F, De Fanis U, Bartlett SJ, et al. T cell polarization identifies distinct clinical phenotypes in scleroderma lung disease. Arthritis Rheum 2008;58:1165–74. 10.1002/art.23406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.De Luca G, Bosello SL, Berardi G, et al. Tumour-associated antigens in systemic sclerosis patients with interstitial lung disease: association with lung involvement and cancer risk. Rheumatology (Oxford) 2015;54:1991–9. 10.1093/rheumatology/kev204 [DOI] [PubMed] [Google Scholar]
- 182.Dheda K, Lalloo UG, Cassim B, et al. Experience with azathioprine in systemic sclerosis associated with interstitial lung disease. Clin Rheumatol 2004;23:306–9. 10.1007/s10067-004-0906-7 [DOI] [PubMed] [Google Scholar]
- 183.Lopes AJ, Capone D, Mogami R, et al. Pneumonia Intersticial Associada À Esclerose Sistêmica: Avaliação DA Função Pulmonar no Período de Cinco Anos. J Bras Pneumol 2011;37:144–51. 10.1590/S1806-37132011000200003 [DOI] [PubMed] [Google Scholar]
- 184.Owen C, Ngian G-S, Elford K, et al. Mycophenolate mofetil is an effective and safe option for the management of systemic sclerosis-associated interstitial lung disease: results from the Australian scleroderma cohort study. Clin Exp Rheumatol 2016;34:170–6. [PubMed] [Google Scholar]
- 185.Schupp J, Becker M, Günther J, et al. Serum Ccl18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur Respir J 2014;43:1530–2. 10.1183/09031936.00131713 [DOI] [PubMed] [Google Scholar]
- 186.Tzelepis GE, Plastiras SC, Karadimitrakis SP, et al. Determinants of pulmonary function improvement in patients with scleroderma and interstitial lung disease. Clin Exp Rheumatol 2007;25:734–9. [PubMed] [Google Scholar]
- 187.Acharya N, Sharma SK, Mishra D, et al. Efficacy and safety of Pirfenidone in systemic sclerosis-related interstitial lung disease—a randomised controlled trial. Rheumatol Int 2020;40:703–10. 10.1007/s00296-020-04565-w [DOI] [PubMed] [Google Scholar]
- 188.Allanore Y, Khanna D, Smith V, et al. Effects of Nintedanib in patients with limited cutaneous systemic sclerosis and interstitial lung disease. Rheumatology 2023. 10.1093/rheumatology/kead280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Assassi S, Distler O, Allanore Y, et al. Effect of Nintedanib on progression of systemic sclerosis-associated interstitial lung disease over 100 weeks: data from a randomized controlled trial. ACR Open Rheumatol 2022;4:837–44. 10.1002/acr2.11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kreuter M, Bonella F, Blank N, et al. Anti-acid therapy in SSC-associated interstitial lung disease: long-term outcomes from the German network for systemic sclerosis. Rheumatology 2023;62:3067–74. 10.1093/rheumatology/kead023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Showalter K, Hoffmann A, Richardson C, et al. Esophageal dilation and other clinical factors associated with pulmonary function decline in patients with systemic sclerosis. J Rheumatol 2021;48:1830–8. 10.3899/jrheum.210533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Campochiaro C, De Luca G, Lazzaroni M-G, et al. Real-life efficacy and safety of Nintedanib in systemic sclerosis-interstitial lung disease: data from an Italian Multicentre study. RMD Open 2023;9:e002850. 10.1136/rmdopen-2022-002850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jandali B, Salazar GA, Hudson M, et al. The effect of anti-Scl-70 antibody determination method on its predictive significance for interstitial lung disease progression in systemic sclerosis. ACR Open Rheumatol 2022;4:345–51. 10.1002/acr2.11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Volkmann ER, Tashkin DP, Kuwana M, et al. Progression of interstitial lung disease in systemic sclerosis: the importance of Pneumoproteins Krebs von den Lungen 6 And Ccl18. Arthritis Rheumatol 2019;71:2059–67. 10.1002/art.41020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Barešić M, Novak S, Perković D, et al. Real world experience with Nintedanib in connective tissue disease-related interstitial lung disease: a retrospective cohort study. Clin Rheumatol 2023;42:2897–903. 10.1007/s10067-023-06689-3 [DOI] [PubMed] [Google Scholar]
- 196.Cerro-Chiang G, Ayres M, Rivas A, et al. Protein biomarkers of disease progression in patients with systemic sclerosis associated interstitial lung disease. Sci Rep 2023;13:8645. 10.1038/s41598-023-35840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Highland KB, Distler O, Kuwana M, et al. Efficacy and safety of Nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021;9:96–106. 10.1016/S2213-2600(20)30330-1 [DOI] [PubMed] [Google Scholar]
- 198.Khanna D, Tseng C-H, Furst DE, et al. Minimally important differences in the Mahler’s transition dyspnoea index in a large randomized controlled trial--results from the scleroderma lung study. Rheumatology (Oxford) 2009;48:1537–40. 10.1093/rheumatology/kep284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kuwana M, Allanore Y, Denton CP, et al. Nintedanib in patients with systemic sclerosis-associated interstitial lung disease: subgroup analyses by autoantibody status and skin score. Arthritis Rheumatol 2022;74:518–26. 10.1002/art.41965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang MM, Balmert LC, Marangoni RG, et al. Circulating Ctrp9 is associated with severity of systemic sclerosis–associated interstitial lung disease. Arthritis Care Res (Hoboken) 2023;75:152–7. 10.1002/acr.24749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic Subsets of Fibrosing Alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002;165:1581–6. 10.1164/rccm.2106012 [DOI] [PubMed] [Google Scholar]
- 202.Jinnin M, Ihn H, Asano Y, et al. Effect of D-penicillamine on pulmonary fibrosis in patients with systemic sclerosis. Ann Rheum Dis 2003;62:1019–20. 10.1136/ard.62.10.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.de Clerck LS, Dequeker J, Francx L, et al. D-penicillamine therapy and interstitial lung disease in scleroderma. A long-term followup study. Arthritis Rheum 1987;30:643–50. 10.1002/art.1780300607 [DOI] [PubMed] [Google Scholar]
- 204.Scorza R, Caronni M, Mascagni B, et al. Effects of long-term cyclic Iloprost therapy in systemic sclerosis with Raynaud’s phenomenon. A randomized, controlled study. Clin Exp Rheumatol 2001;19:503–8. [PubMed] [Google Scholar]
- 205.Davas EM, Peppas C, Maragou M, et al. Intravenous cyclophosphamide pulse therapy for the treatment of lung disease associated with scleroderma. Clinical Rheumatology 1999;18:455–61. 10.1007/s100670050138 [DOI] [PubMed] [Google Scholar]
- 206.Felício CHC, Parra ER, Capelozzi VL. Idiopathic and collagen vascular disease nonspecific interstitial pneumonia: clinical significance of remodeling process. Lung 2007;185:39–46. 10.1007/s00408-006-0104-2 [DOI] [PubMed] [Google Scholar]
- 207.Yilmaz N, Can M, Kocakaya D, et al. Two-year experience with mycophenolate mofetil in patients with scleroderma lung disease: a case series. Int J Rheum Dis 2014;17:923–8. 10.1111/1756-185X.12399 [DOI] [PubMed] [Google Scholar]
- 208.Chassagnon G, Vakalopoulou M, Régent A, et al. Elastic registration-driven deep learning for Longitudinal assessment of systemic sclerosis interstitial lung disease at CT. Radiology 2021;298:189–98. 10.1148/radiol.2020200319 [DOI] [PubMed] [Google Scholar]
- 209.Launay D, Remy-Jardin M, Michon-Pasturel U, et al. High resolution computed tomography in Fibrosing Alveolitis associated with systemic sclerosis. J Rheumatol 2006;33:1789–801. [PubMed] [Google Scholar]
- 210.Bruni C, Occhipinti M, Pienn M, et al. Lung vascular changes as biomarkers of severity in systemic sclerosis-associated interstitial lung disease. Rheumatology (Oxford 2023;62:696–706. 10.1093/rheumatology/keac311 [DOI] [PubMed] [Google Scholar]
- 211.Wangkaew S, Tungteerabunditkul S, Prasertwittayakij N, et al. Comparison of clinical presentation and incidence of cardiopulmonary complications between male and female Thai patients with early systemic sclerosis: inception cohort study. Clin Rheumatol 2020;39:103–12. 10.1007/s10067-019-04551-z [DOI] [PubMed] [Google Scholar]
- 212.Volkmann ER, McMahan ZH, Smith V, et al. Risk of malnutrition in patients with systemic sclerosis-associated interstitial lung disease treated with Nintedanib. Arthritis Care Res (Hoboken) June 25, 2023. 10.1002/acr.25176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boonstra M, Bakker JA, Grummels A, et al. Association of anti-Topoisomerase I antibodies of the Igm Isotype with disease progression in anti-Topoisomerase I-positive systemic sclerosis. Arthritis Rheumatol 2020;72:1897–904. 10.1002/art.41403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bournia V-K, Kallianos A, Panopoulos S, et al. Cardiopulmonary exercise testing and prognosis in patients with systemic sclerosis without baseline pulmonary hypertension: a prospective cohort study. Rheumatol Int 2022;42:303–9. 10.1007/s00296-021-04937-w [DOI] [PubMed] [Google Scholar]
- 215.Bruni C, Tashkin DP, Steen V, et al. Intravenous versus oral cyclophosphamide for lung and/or skin fibrosis in systemic sclerosis: an indirect comparison from EUSTAR and randomised controlled trials. Clin Exp Rheumatol 2020;38 Suppl 125:161–8. [PubMed] [Google Scholar]
- 216.Cao X-Y, Hu S-S, Xu D, et al. Serum levels of Krebs von den Lungen-6 as a promising marker for predicting occurrence and deterioration of systemic sclerosis-associated interstitial lung disease from a Chinese cohort. Int J Rheum Dis 2019;22:108–15. 10.1111/1756-185X.13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Celeste S, Santaniello A, Caronni M, et al. Carbohydrate antigen 15.3 as a serum biomarker of interstitial lung disease in systemic sclerosis patients. Eur J Intern Med 2013;24:671–6. 10.1016/j.ejim.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 218.Lambrecht S, Smith V, De Wilde K, et al. Growth differentiation factor 15, a marker of lung involvement in systemic sclerosis, is involved in fibrosis development but is not indispensable for fibrosis development. Arthritis Rheumatol 2014;66:418–27. 10.1002/art.38241 [DOI] [PubMed] [Google Scholar]
- 219.Legendre P, Blanchet B, Porcher R, et al. Mycophenolic acid drug monitoring in patients with systemic sclerosis associated with diffuse skin and/or pulmonary involvement: A Monocentric and retrospective French study. J Scleroderma Relat Disord 2021;6:87–95. 10.1177/2397198320944342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu S, Chung MP, Ley B, et al. Peripheral blood Leucocyte Telomere length is associated with progression of interstitial lung disease in systemic sclerosis. Thorax 2021;76:1186–92. 10.1136/thoraxjnl-2020-215918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maher TM, Mayes MD, Kreuter M, et al. Effect of Nintedanib on lung function in patients with systemic sclerosis-associated interstitial lung disease: further analyses of a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2021;73:671–6. 10.1002/art.41576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ross L, Stevens W, Wilson M, et al. Can patient-reported symptoms be used to measure disease activity in systemic sclerosis. Arthritis Care Res (Hoboken) 2020;72:1459–65. 10.1002/acr.24053 [DOI] [PubMed] [Google Scholar]
- 223.Wu W, Jordan S, Graf N, et al. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European scleroderma trials and research (Eustar) cohort. Ann Rheum Dis 2019;78:648–56. 10.1136/annrheumdis-2018-213455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Elhai M, Boubaya M, Distler O, et al. Outcomes of patients with systemic sclerosis treated with Rituximab in contemporary practice: a prospective cohort study. Ann Rheum Dis 2019;78:979–87. 10.1136/annrheumdis-2018-214816 [DOI] [PubMed] [Google Scholar]
- 225.Kreuter M, Hoffmann-Vold A-M, Matucci-Cerinic M, et al. Impact of lung function and baseline clinical characteristics on patient-reported outcome measures in systemic sclerosis-associated interstitial lung disease. Rheumatology (Oxford) 2023;62:SI43–53. 10.1093/rheumatology/keac325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bérezné A, Ranque B, Valeyre D, et al. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: A retrospective multicenter open-label study. J Rheumatol 2008;35:1064–72. [PubMed] [Google Scholar]
- 227.De Santis M, Inzitari R, Bosello SL, et al. Beta-Thymosins and interstitial lung disease: study of a scleroderma cohort with a one-year follow-up. Respir Res 2011;12:22. 10.1186/1465-9921-12-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ebata S, Yoshizaki A, Fukasawa T, et al. n.d. Increased red blood cell distribution width in the first year after diagnosis predicts worsening of systemic sclerosis-associated interstitial lung disease at 5 years: A pilot study. Diagnostics;11:2274. 10.3390/diagnostics11122274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Janardana R, Irodi A, Chebbi PP, et al. Mycophenolate in scleroderma-associated interstitial lung disease: real-world data from rheumatology and Pulmonology clinics in South Asia. Journal of Scleroderma and Related Disorders 2021;6:271–6. 10.1177/23971983211024410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kaenmuang P, Navasakulpong A. Short-term lung function changes and predictors of progressive systemic sclerosis-related interstitial lung disease. Tuberc Respir Dis (Seoul) 2020;83:312–20. 10.4046/trd.2020.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kase K, Watanabe S, Saeki K, et al. Fractional analysis of Bronchoalveolar Lavage in systemic sclerosis-associated interstitial lung disease. J Thorac Dis 2021;13:4146–55. 10.21037/jtd-20-2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Launay D, Buchdahl A-L, Berezné A, et al. Mycophenolate mofetil following cyclophosphamide in worsening systemic sclerosis-associated interstitial lung disease. Journal of Scleroderma and Related Disorders 2016;1:234–40. 10.5301/jsrd.5000205 [DOI] [Google Scholar]
- 233.Lepri G, Avouac J, Airò P, et al. Effects of Rituximab in connective tissue disorders related interstitial lung disease. Clin Exp Rheumatol 2016;34 Suppl 100:181–5. [PubMed] [Google Scholar]
- 234.Occhipinti M, Bosello S, Sisti LG, et al. Quantitative and semi-quantitative computed tomography analysis of interstitial lung disease associated with systemic sclerosis: A longitudinal evaluation of pulmonary parenchyma and vessels. PLoS One 2019;14:e0213444. 10.1371/journal.pone.0213444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Panopoulos ST, Bournia VK, Trakada G, et al. Mycophenolate versus cyclophosphamide for progressive interstitial lung disease associated with systemic sclerosis: a 2-year case control study. Lung 2013;191:483–9. 10.1007/s00408-013-9499-8 [DOI] [PubMed] [Google Scholar]
- 236.Poormoghim H, Rezaei N, Sheidaie Z, et al. Systemic sclerosis: comparison of efficacy of oral cyclophosphamide and azathioprine on skin score and pulmonary involvement-a retrospective study. Rheumatol Int 2014;34:1691–9. 10.1007/s00296-014-3026-y [DOI] [PubMed] [Google Scholar]
- 237.Seibold JR, Denton CP, Furst DE, et al. Randomized, prospective, placebo-controlled trial of Bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis Rheum 2010;62:2101–8. 10.1002/art.27466 [DOI] [PubMed] [Google Scholar]
- 238.Volpinari S, La Corte R, Bighi S, et al. Bronchoalveolar Lavage in systemic sclerosis with lung involvement: role and correlations with functional, radiological and Scintigraphic parameters. Rheumatol Int 2011;31:1183–8. 10.1007/s00296-010-1390-9 [DOI] [PubMed] [Google Scholar]
- 239.Yoshida Y, Omoto T, Kohno H, et al. Lower Ch50 as a Predictor for intractable or recurrent lupus Enteritis: A retrospective observational study. Modern Rheumatology 2021;31:643–8. 10.1080/14397595.2020.1812871 [DOI] [PubMed] [Google Scholar]
- 240.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 2006;54:3962–70. 10.1002/art.22204 [DOI] [PubMed] [Google Scholar]
- 241.Simeon-Aznar C. Intravenous cyclophosphamide pulse therapy in the treatment of systemic sclerosis-related interstitial lung disease: a long term study. TORMJ 2008;2:39–45. 10.2174/1874306400802010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Henrique-Neto Á, Vasconcelos MYK, Dias JBE, et al. Hematopoietic stem cell transplantation for systemic sclerosis: Brazilian experience. Adv Rheumatol 2021;61:9. 10.1186/s42358-021-00166-8 [DOI] [PubMed] [Google Scholar]
- 243.Kloth C, Henes J, Xenitidis T, et al. Chest CT texture analysis for response assessment in systemic sclerosis. Eur J Radiol 2018;101:50–8. 10.1016/j.ejrad.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 244.Rivière S, Hua-Huy T, Tiev KP, et al. High baseline serum Clara cell 16 kDa predicts subsequent lung disease worsening in systemic sclerosis. J Rheumatol 2018;45:242–7. 10.3899/jrheum.170440 [DOI] [PubMed] [Google Scholar]
- 245.Schmidt K, Martinez-Gamboa L, Meier S, et al. Bronchoalveoloar Lavage fluid Cytokines and Chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther 2009;11:R111. 10.1186/ar2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kloth C, Maximilian Thaiss W, Preibsch H, et al. Quantitative chest CT analysis in patients with systemic sclerosis before and after Autologous stem cell transplantation: comparison of results with those of pulmonary function tests and clinical tests. Rheumatology (Oxford) 2016;55:1763–70. 10.1093/rheumatology/kew259 [DOI] [PubMed] [Google Scholar]
- 247.Mittoo S, Wigley FM, Wise RA, et al. Long term effects of cyclophosphamide treatment on lung function and survival in scleroderma patients with interstitial lung disease. Open Rheumatol J 2011;5:1–6. 10.2174/1874312901105010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wanchu A, Suryanaryana BS, Sharma S, et al. High-dose prednisolone and bolus cyclophosphamide in interstitial lung disease associated with systemic sclerosis: a prospective open study. Int J Rheum Dis 2009;12:239–42. 10.1111/j.1756-185X.2009.01417.x [DOI] [PubMed] [Google Scholar]
- 249.Weigold F, Günther J, Pfeiffenberger M, et al. Antibodies against Chemokine receptors Cxcr3 and Cxcr4 predict progressive deterioration of lung function in patients with systemic sclerosis. Arthritis Res Ther 2018;20:52. 10.1186/s13075-018-1545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zamora AC, Wolters PJ, Collard HR, et al. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respiratory Medicine 2008;102:150–5. 10.1016/j.rmed.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 251.Campochiaro C, De Luca G, Lazzaroni MG, et al. Safety and efficacy of Rituximab Biosimilar (CT-P10) in systemic sclerosis: an Italian Multicentre study. Rheumatology 2020;59:3731–6. 10.1093/rheumatology/keaa136 [DOI] [PubMed] [Google Scholar]
- 252.Chikhoune L, Brousseau T, Morell-Dubois S, et al. Association between routine laboratory parameters and the severity and progression of systemic sclerosis. J Clin Med 2022;11:5087. 10.3390/jcm11175087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kuster S, Jordan S, Elhai M, et al. Effectiveness and safety of Tocilizumab in patients with systemic sclerosis: a propensity score matched controlled observational study of the EUSTAR cohort. RMD Open 2022;8:e002477. 10.1136/rmdopen-2022-002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yomono K, Kuwana M. Outcomes in patients with systemic sclerosis undergoing early vs delayed intervention with potential disease-modifying therapies. Rheumatology (Oxford) 2022;61:3677–85. 10.1093/rheumatology/keab931 [DOI] [PubMed] [Google Scholar]
- 255.Denton CP, Goh NS, Humphries SM, et al. Extent of fibrosis and lung function decline in patients with systemic sclerosis and interstitial lung disease: data from the SENSCIS trial. Rheumatology 2023;62:1870–6. 10.1093/rheumatology/keac535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gargani L, Bruni C, De Marchi D, et al. Lung magnetic resonance imaging in systemic sclerosis: a new promising approach to evaluate pulmonary involvement and progression. Clin Rheumatol 2021;40:1903–12. 10.1007/s10067-020-05491-9 [DOI] [PubMed] [Google Scholar]
- 257.Gargani L, Bruni C, Romei C, et al. Prognostic value of lung ultrasound B-lines in systemic sclerosis. Chest 2020;158:1515–25. 10.1016/j.chest.2020.03.075 [DOI] [PubMed] [Google Scholar]
- 258.Mena-Vázquez N, Rojas-Gimenez M, Romero-Barco CM, et al. n.d. Characteristics and predictors of progression interstitial lung disease in rheumatoid arthritis compared with other autoimmune disease: A retrospective cohort study. Diagnostics;11:1794. 10.3390/diagnostics11101794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ledoult E, Morelle M, Soussan M, et al. 18)F-FDG positron emission tomography scanning in systemic sclerosis-associated interstitial lung disease: a pilot study. Arthritis Res Ther 2021;23:76. 10.1186/s13075-021-02460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Broens B, Zwezerijnen B, Laken CJ, et al. Quantification of 68Ga-FA. The Lancet Rheumatology 2021;3:e475. 10.1016/S2665-9913(21)00143-0 [DOI] [PubMed] [Google Scholar]
- 261.Hoffmann-Vold A-M, Weigt SS, Saggar R, et al. Endotype-Phenotyping may predict a treatment response in Progressive Fibrosing interstitial lung disease. EBioMedicine 2019;50:379–86. 10.1016/j.ebiom.2019.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kiboshi T, Kotani T, Konma J, et al. Comparison of therapeutic effects of combination therapy with prednisolone and tacrolimus or azathioprine on progressive interstitial pneumonia with systemic sclerosis. Mod Rheumatol 2022;32:358–64. 10.1080/14397595.2021.1918864 [DOI] [PubMed] [Google Scholar]
- 263.Aggarwal R, Lucas M, Fertig N, et al. Anti-U3 RNP Autoantibodies in systemic sclerosis. Arthritis Rheum 2009;60:1112–8. 10.1002/art.24409 [DOI] [PubMed] [Google Scholar]
- 264.Freire M, Rivera A, Sopeña B, et al. Clinical and Epidemiological differences between men and women with systemic sclerosis: a study in a Spanish systemic sclerosis cohort and literature review. Clin Exp Rheumatol 2017;35 Suppl 106:89–97. [PubMed] [Google Scholar]
- 265.Rosato E, Leodori G, Gigante A, et al. Reduced ventilatory efficiency during exercise predicts major vascular complications and mortality for interstitial lung disease in systemic sclerosis. Clin Exp Rheumatol 2020;38 Suppl 125:85–91. [PubMed] [Google Scholar]
- 266.Santosa A, Tan CS, Teng GG, et al. Lung and gastrointestinal complications are leading causes of death in SCORE, a multi-ethnic Singapore systemic sclerosis cohort. Scand J Rheumatol 2016;45:499–506. 10.3109/03009742.2016.1153141 [DOI] [PubMed] [Google Scholar]
- 267.Simeón CP, Armadans L, Fonollosa V, et al. Mortality and Prognostic factors in Spanish patients with systemic sclerosis. Rheumatology (Oxford) 2003;42:71–5. 10.1093/rheumatology/keg033 [DOI] [PubMed] [Google Scholar]
- 268.Foocharoen C, Peansukwech U, Mahakkanukrauh A, et al. Clinical characteristics and outcomes of 566 Thais with systemic sclerosis: A cohort study. Int J Rheum Dis 2020;23:945–57. 10.1111/1756-185X.13859 [DOI] [PubMed] [Google Scholar]
- 269.Potjewijd J, Tobal R, Silvertand D, et al. Favorable long term effects of intensified immunosuppression combined with therapeutic plasma exchange in patients with early-onset progressive systemic sclerosis-related interstitial lung disease. J Transl Autoimmun 2022;5:100174. 10.1016/j.jtauto.2022.100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rubio-Rivas M, Corbella X, Guillén-Del-Castillo A, et al. Spanish scleroderma risk score (RESCLESCORE) to predict 15-year all-cause mortality in scleroderma patients at the time of diagnosis based on the RESCLE cohort: derivation and internal validation. Autoimmun Rev 2020;19:102507. 10.1016/j.autrev.2020.102507 [DOI] [PubMed] [Google Scholar]
- 271.Hyldgaard C, Bendstrup E, Pedersen AB, et al. Interstitial lung disease in connective tissue diseases: survival patterns in a population-based cohort. J Clin Med 2021;10:4830. 10.3390/jcm10214830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tomiyama F, Watanabe R, Ishii T, et al. High prevalence of acute exacerbation of interstitial lung disease in Japanese patients with systemic sclerosis. Tohoku J Exp Med 2016;239:297–305. 10.1620/tjem.239.297 [DOI] [PubMed] [Google Scholar]
- 273.Le Gall A, Hoang-Thi T-N, Porcher R, et al. Prognostic value of automated assessment of interstitial lung disease on CT in systemic sclerosis. Rheumatology (Oxford) 2023:kead164. 10.1093/rheumatology/kead164 [DOI] [PubMed] [Google Scholar]
- 274.Zuhur F, Zuhur SS, Zuhur C, et al. Survival in Progressive systemic sclerosis with pulmonary involvement: a single-center experience in Istanbul, Turkey. Rheumatol Int 2012;32:1655–61. 10.1007/s00296-011-1842-x [DOI] [PubMed] [Google Scholar]
- 275.Pradère P, Tudorache I, Magnusson J, et al. Lung transplantation for scleroderma lung disease: an international, multicenter, observational cohort study. J Heart Lung Transplant 2018;37:903–11. 10.1016/j.healun.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 276.Abhishek A, Yazdani R, Pearce F, et al. Outcome of systemic sclerosis associated interstitial lung disease treated with intravenous cyclophosphamide. Clin Rheumatol 2011;30:1099–104. 10.1007/s10067-011-1734-1 [DOI] [PubMed] [Google Scholar]
- 277.Alba MA, Velasco C, Simeón CP, et al. Early- versus late-onset systemic sclerosis differences in clinical presentation and outcome in 1037 patients. Medicine (Baltimore) 2014;93:73-81. 10.1097/MD.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chan C, Ryerson CJ, Dunne JV, et al. Demographic and clinical predictors of progression and mortality in connective tissue disease-associated interstitial lung disease: a retrospective cohort study. BMC Pulm Med 2019;19:192. 10.1186/s12890-019-0943-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chan TY, Hansell DM, Rubens MB, et al. Cryptogenic Fibrosing Alveolitis and the Fibrosing Alveolitis of systemic sclerosis: morphological differences on computed Tomographic scans. Thorax 1997;52:265–70. 10.1136/thx.52.3.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and Autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood 2007;110:1388–96. 10.1182/blood-2007-02-072389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Su R, Bennett M, Jacobs S, et al. An analysis of connective tissue disease-associated interstitial lung disease at a US tertiary care center: better survival in patients with systemic sclerosis. J Rheumatol 2011;38:693–701. 10.3899/jrheum.100675 [DOI] [PubMed] [Google Scholar]
- 282.Nakayama Y, Nakashima R, Handa T, et al. Prognosis of patients with systemic sclerosis-related interstitial lung disease on the lung transplant waiting list: a retrospective study. Sci Rep 2023;13:10150. 10.1038/s41598-023-37141-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Domiciano DS, Bonfá E, Borges CTL, et al. A long-term prospective randomized controlled study of non-specific interstitial pneumonia (NSIP) treatment in scleroderma. Clin Rheumatol 2011;30:223–9. 10.1007/s10067-010-1493-4 [DOI] [PubMed] [Google Scholar]
- 284.Pakas I, Ioannidis JPA, Malagari K, et al. Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol 2002;29:298–304. [PubMed] [Google Scholar]
- 285.Sottile PD, Iturbe D, Katsumoto TR, et al. Outcomes in systemic sclerosis-related lung disease after lung transplantation. Transplantation 2013;95:975–80. 10.1097/TP.0b013e3182845f23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ryerson CJ, O’Connor D, Dunne JV, et al. Predicting mortality in systemic sclerosis-associated interstitial lung disease using risk prediction models derived from idiopathic pulmonary fibrosis. Chest 2015;148:1268–75. 10.1378/chest.15-0003 [DOI] [PubMed] [Google Scholar]
- 287.Mena-Vázquez N, Redondo-Rodríguez R, Rojas-Gimenez M, et al. Efficacy and safety of Rituximab in autoimmune disease—associated interstitial lung disease: A prospective cohort study. J Clin Med 2022;11:927. 10.3390/jcm11040927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum 2010;39:269–77. 10.1016/j.semarthrit.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 289.Avouac J, Walker UA, Hachulla E, et al. Joint and tendon involvement predict disease progression in systemic sclerosis: a EUSTAR prospective study. Ann Rheum Dis 2016;75:103–9. 10.1136/annrheumdis-2014-205295 [DOI] [PubMed] [Google Scholar]
- 290.Bauer PR, Schiavo DN, Osborn TG, et al. Influence of interstitial lung disease on outcome in systemic sclerosis: a population-based historical cohort study. Chest 2013;144:571–7. 10.1378/chest.12-2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Guler SA, Winstone TA, Murphy D, et al. Does systemic sclerosis-associated interstitial lung disease burn out? specific phenotypes of disease progression. Ann Am Thorac Soc 2018;15:1427–33. 10.1513/AnnalsATS.201806-362OC [DOI] [PubMed] [Google Scholar]
- 292.Hashimoto A, Tejima S, Tono T, et al. Predictors of survival and causes of death in Japanese patients with systemic sclerosis. J Rheumatol 2011;38:1931–9. 10.3899/jrheum.100298 [DOI] [PubMed] [Google Scholar]
- 293.Jacobsen S, Ullman S, Shen GQ, et al. Influence of clinical features, serum Antinuclear antibodies, and lung function on survival of patients with systemic sclerosis. J Rheumatol 2001;28:2454–9. [PubMed] [Google Scholar]
- 294.Kim J, Park SK, Moon KW, et al. The Prognostic factors of systemic sclerosis for survival among Koreans. Clin Rheumatol 2010;29:297–302. 10.1007/s10067-009-1324-7 [DOI] [PubMed] [Google Scholar]
- 295.Domsic RT, Nihtyanova SI, Wisniewski SR, et al. Derivation and validation of a prediction rule for two-year mortality in early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol 2014;66:1616–24. 10.1002/art.38381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Poormoghim H, Lakeh MM, Mohammadipour M, et al. Pulmonary survival study in 91 patients with systemic sclerosis. Rheumatol Int 2011;31:1577–82. 10.1007/s00296-010-1501-7 [DOI] [PubMed] [Google Scholar]
- 297.Tiev KP, Hua-Huy T, Kettaneh A, et al. Serum CC Chemokine Ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J 2011;38:1355–60. 10.1183/09031936.00004711 [DOI] [PubMed] [Google Scholar]
- 298.Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, et al. Registry of the Spanish network for systemic sclerosis: survival, Prognostic factors, and causes of death. Medicine (Baltimore) 2015;94:e1728. 10.1097/MD.0000000000001728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Goh NSL, Veeraraghavan S, Desai SR, et al. Bronchoalveolar Lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum 2007;56:2005–12. 10.1002/art.22696 [DOI] [PubMed] [Google Scholar]
- 300.Sangani RA, Lui JK, Gillmeyer KR, et al. Clinical characteristics and outcomes in pulmonary manifestations of systemic sclerosis: contribution from pulmonary hypertension and interstitial lung disease severity. Pulm Circ 2022;12:e12117. 10.1002/pul2.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ahmed SS, Johnson SR, Meaney C, et al. Lung function and survival in systemic sclerosis interstitial lung disease. J Rheumatol 2014;41:2326–8. 10.3899/jrheum.140156 [DOI] [PubMed] [Google Scholar]
- 302.Benad M, Koschel D, Herrmann K, et al. Effects of cyclophosphamide and Rituximab in patients with connective tissue diseases with severe interstitial lung disease. Clin Exp Rheumatol 2022;40:483–8. 10.55563/clinexprheumatol/o5t1f7 [DOI] [PubMed] [Google Scholar]
- 303.Chartrand S, Lee JS, Swigris JJ, et al. Clinical characteristics and natural history of autoimmune forms of interstitial lung disease: A single-center experience. Lung 2019;197:709–13. 10.1007/s00408-019-00276-7 [DOI] [PubMed] [Google Scholar]
- 304.Fischer A, Swigris JJ, Groshong SD, et al. Clinically significant interstitial lung disease in limited scleroderma: Histopathology, clinical features, and survival. Chest 2008;134:601–5. 10.1378/chest.08-0053 [DOI] [PubMed] [Google Scholar]
- 305.Furst DE, Tseng C-H, Clements PJ, et al. Adverse events during the scleroderma lung study. Am J Med 2011;124:459–67. 10.1016/j.amjmed.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 306.Hsu VM, Chung L, Hummers LK, et al. Risk factors for mortality and cardiopulmonary hospitalization in systemic sclerosis patients at risk for pulmonary hypertension, in the PHAROS Registry. J Rheumatol 2019;46:176–83. 10.3899/jrheum.180018 [DOI] [PubMed] [Google Scholar]
- 307.Jhajj A, Gill HP, Hague CJ, et al. Pulmonary physiology is poorly associated with radiological extent of disease in systemic sclerosis-associated interstitial lung disease. Eur Respir J 2019;53:1802182. 10.1183/13993003.02182-2018 [DOI] [PubMed] [Google Scholar]
- 308.Okamoto, M. “Corrigendum to “A retrospective cohort study of outcome in systemic sclerosis-associated interstitial lung disease” [Respir. Investig. 54 (2016) 445–453]”. Respiratory Investigation 2017;55:252. 10.1016/j.resinv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 309.Swigris JJ, Zhou X, Wamboldt FS, et al. Exercise peripheral oxygen saturation (Spo2) accurately reflects arterial oxygen saturation (Sao2) and predicts mortality in systemic sclerosis. Thorax 2009;64:626–30. 10.1136/thx.2008.111393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Takei R, Arita M, Kumagai S, et al. Radiographic fibrosis score predicts survival in systemic sclerosis-associated interstitial lung disease. Respirology 2018;23:385–91. 10.1111/resp.13175 [DOI] [PubMed] [Google Scholar]
- 311.Yamakawa H, Ogura T, Sato S, et al. The potential utility of anterior upper lobe honeycomb-like lesion in interstitial lung disease associated with connective tissue disease. Respiratory Medicine 2020;172:106125. 10.1016/j.rmed.2020.106125 [DOI] [PubMed] [Google Scholar]
- 312.Caetano J, Batista F, Amaral MC, et al. Acute hospitalization in a cohort of patients with systemic sclerosis: a 10-year retrospective cohort study. Rheumatol Int 2022;42:1393–402. 10.1007/s00296-021-04983-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ram Poudel D, George M, Dhital R, et al. Mortality, length of stay and cost of hospitalization among patients with systemic sclerosis: results from the National inpatient sample. Rheumatology (Oxford) 2018;57:1611–22. 10.1093/rheumatology/key150 [DOI] [PubMed] [Google Scholar]
- 314.Sankar S, Habib M, Jaafar S, et al. Hospitalisations related to systemic sclerosis and the impact of interstitial lung disease. analysis of patients hospitalised at the University of Michigan, USA. Clin Exp Rheumatol 2021;39 Suppl 131:43–51. 10.55563/clinexprheumatol/9ivp9g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chung L, Krishnan E, Chakravarty EF. Hospitalizations and mortality in systemic sclerosis: results from the nationwide inpatient sample. Rheumatology (Oxford) 2007;46:1808–13. 10.1093/rheumatology/kem273 [DOI] [PubMed] [Google Scholar]
- 316.Trang A, Kambhatla S, Manadan A. Risk factors for respiratory failure in patients hospitalized with systemic sclerosis: an analysis of the National inpatient sample. Cureus 2023;15:e35797. 10.7759/cureus.35797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Crespo MM, Bermudez CA, Dew MA, et al. Lung transplant in patients with scleroderma compared with pulmonary fibrosis Short- and long-term outcomes. Annals ATS 2016;13:784–92. 10.1513/AnnalsATS.201503-177OC [DOI] [PubMed] [Google Scholar]
- 318.Kreuter M, Del Galdo F, Miede C, et al. Impact of lung function decline on time to Hospitalisation events in systemic sclerosis-associated interstitial lung disease (SSC-ILD): a joint model analysis. Arthritis Res Ther 2022;24. 10.1186/s13075-021-02710-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Morrisroe K, Stevens W, Sahhar J, et al. The clinical and economic burden of systemic sclerosis related interstitial lung disease. Rheumatology (Oxford) 2020;59:1878–88. 10.1093/rheumatology/kez532 [DOI] [PubMed] [Google Scholar]
- 320.Hoshino K, Satoh T, Kawaguchi Y, et al. Association of hepatocyte growth factor promoter polymorphism with severity of interstitial lung disease in Japanese patients with systemic sclerosis. Arthritis & Rheumatism 2011;63:2465–72. 10.1002/art.30415 [DOI] [PubMed] [Google Scholar]
- 321.Lescoat A, Huscher D, Schoof N, et al. Systemic sclerosis-associated interstitial lung disease in the EUSTAR database: analysis by region. Rheumatology 2022. [DOI] [PubMed] [Google Scholar]
- 322.Morgan C, Knight C, Lunt M, et al. Predictors of end stage lung disease in a cohort of patients with scleroderma. Ann Rheum Dis 2003;62:146–50. 10.1136/ard.62.2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kuwana M, Shirai Y, Takeuchi T. Elevated serum Krebs von den Lungen-6 in early disease predicts subsequent deterioration of pulmonary function in patients with systemic sclerosis and interstitial lung disease. J Rheumatol 2016;43:1825–31. 10.3899/jrheum.160339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003426supp001.pdf (319.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data will be made available upon reasonable request to the corresponding author.