Abstract
Background
Surgery of primary tumour is the backbone of colorectal cancer treatment (CRC). But in stage III cancer, metastatic or local relapse is often observed (50%). So, adjuvant treatment is always considered in this setting. The best treatment duration of hypothetic disease is not easy to define. Adjuvant chemotherapy for CRC actually lasts 6 months. The choice of optimal duration is based upon old studies using 5‐fluorouracil (5FU). During the last ten years, results of major randomized controlled studies (RCTs) comparing different durations of treatments and different schedules in adjuvant setting were published. Several studies compared a 6‐month chemotherapy with a longer treatment. Conversely, a single study by Chau et al compared a 6 month chemotherapy with continuous treatment lasting 3 months. But the optimal duration of these chemotherapies could be challenged. Even though the optimal duration of chemotherapy in CRC is a major issue, it has never been answered adequately.
Objectives
To evaluate the optimal duration of adjuvant treatment, we performed a meta‐analysis of all RCTs comparing two durations of adjuvant treatment, 6 months versus 9 to 12 months.
Search methods
Publications were identified from PubMed (February 28th, 2009), Embase, and the Cochrane Database of Clinical Controlled Trials (CENTRAL) in the Cochrane Library 2009 issue 1. Reviews and books were also scrutinized. Abstracts were reviewed from ASCO annual meetings proceedings from 1998 to 2009.
Selection criteria
Patients with surgically resected colorectal cancer with high risk of recurrence.
Data collection and analysis
Several RCTs compared shorter versus longer durations of chemotherapy, 6 studies for overall survival (OS) and 7 studies for relapse free survival (RFS), for a total of 10326 patients, mean age 63.1 years, including 9826 colon and 500 rectum cancers.
Main results
Treatments were always based on 5‐FU. Two studies were excluded, an epidemiological study and a study comparing continuous treatment during 3 months with conventional chemotherapy during 6 months. The later because it compared 2 durations less than or equal to 6 months. Shorter duration of chemotherapy (3‐6 months) compared with longer duration (9‐12 months) was not associated to poorer RFS (RR =0.96, 95% CI : 0.90‐1.02) and OS (RR = 0.96 ; 95% CI : 0.91‐1.02).
Authors' conclusions
The present meta‐analysis confirmed that adjuvant chemotherapy of CRC should not last for more than 6 months. Prolonged duration would result in lower benefit to risk ratio. However, the results do not make it possible to favour either 3 or 6 month durations. They should help design a future RCT comparing different durations of continuous treatment.
Plain language summary
Duration of 5FU based chemotherapy in adjuvant setting for colorectal cancer should not exceed 6 months.
The aim of this systematic review was to determine the optimal duration of chemotherapy. Currently, the standard of duration is based upon studies performed in the 90's, and focus on the determination of the best schedule of 5FU treatment alone or in combination with folinic acid or levamisole and the optimal duration of the adjuvant treatment. Different durations of 5FU based treatment were compared: 6 months versus 9‐12 months. Shorter duration of chemotherapy (3‐6 months) compared with longer duration (9‐12 months) did not result in poorer relapse free survival or overall survival. Consequently, the duration of 5FU based chemotherapy in adjuvant setting for colorectal cancer can be reduced to 6 months.
A recently published study compared 3 months continuous infusion of 5FU to 6 months bolus 5FU, and showed the benefit of this 3 months schedule. Therefore, future studies will evaluate the efficacy of shorter chemotherapy with the new gold‐standard: FOLFOX (combining oxaliplatine and 2 days continuous 5FU, bimonthly) in order to minimize the neurotoxicity of oxaliplatine.
Background
Colorectal cancer (CRC) is one of the leading causes of cancer deaths in the Western world. Surgery remains the only curative therapy for colon cancer, but numerous clinical trials have suggested that systemic chemotherapy (CT) in the adjuvant setting could improve the curative rate for colorectal cancer patients. Chemotherapy in colorectal cancer can be initiated either as adjuvant therapy for high relapse risk patients (stage II and mainly stage III), or in metastatic patients. There remains uncertainties about the best duration of CT in both of these settings. Usually, CT lasts 6 months but the rationale for the choice of this duration in adjuvant treatment relies upon old studies using 5FU O'Connell 1998. But the optimal duration of these chemotherapies could be challenged (Gibson 2006). Even though the optimal duration of CT in CRC is a major issue, it has never been answered adequately. In the adjuvant setting, randomised controlled trials aim to compare various durations of 5FU based CT (between 3 months and 12 months). Nowadays, there is a consensus about an optimal duration of adjuvant CT of 6 months, relying upon studies published at the end of the 1990's, but this consensus could be challenged considering recent publications (Saini 2003). However, adjuvant studies comparing duration of CT are mainly based on 5FU regimens, with no use of oxaliplatin.
Objectives
To evaluate the optimal duration for adjuvant chemotherapy (CT) in patients with localised colorectal cancer, after receiving curative resection, by comparing two duration settings of CT.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria:
We considered Randomised Controlled Trials (RCT) for inclusion, without language restriction in the electronic searches. Included trials reported comparison of different durations of adjuvant CT for colorectal cancer.
Exclusion criteria:
·Non‐randomised studies ·Phase II studies ·Retrospective studies ·Association of CRC to other cancers (non colorectal cancers) ‐ History of other cancers.
Types of participants
Patients with surgically resected, non metastatic colorectal cancer (stage II and III) with high risk of relapse (meaning stage III disease or stage II with some risks factors (p :pathological) pT3 with perforation, occlusion, lymphatic or venous invasion and pT4). These patients were treated with adjuvant CT regimens of varying duration.
Types of interventions
Open labelled randomised controlled trials comparing different durations of 5‐FU in non‐metastatic colorectal cancer patients
Types of outcome measures
Primary outcomes: 1. Relapse free survival 2. Overall survival Secondary outcomes: 1. Chemotherapy‐induced toxicities, considering the limitations related to the various modes of evaluation of adverse drug reactions in the different trials.
Search methods for identification of studies
Publications have been identified by an electronic search using online PubMed, using simultaneously two key words "colorectal cancer, duration of CT". A second query used the key‐words: "colon cancer, duration of chemotherapy : A third query, in adjuvant setting were "colorectal cancer, adjuvant CT duration" A final query were "oral chemotherapy, duration of chemotherapy".
#16Search #7 and (#5 OR #6) Field: Title/Abstract, Limits: Randomized Controlled Trial #8Search #7 and (#5 OR #6) Field: Title/Abstract #15Search #14 NOT #8 Field: Title/Abstract #14Search #11 AND #13 AND # 6 Field: Title/Abstract #6Search duration Field: Title/Abstract #13Search chemotherapy OR fluorouracil* Field: Title/Abstract #12Search chemotherapy OR fluorouracil* OR Field: Title/Abstract #11Search colorectal Field: Title/Abstract #10Search #8 NOT #9 Field: Title #9Search duration Field: Title #7Search #1 AND (#2 OR #3 OR #4) Field: Title/Abstract #5Search time factors Field: MeSH Terms #4Search Neoadjuvant Therapy Field: MeSH Terms #3Search Chemotherapy, Adjuvant Field: MeSH Terms #2Search Antineoplastic Combined Chemotherapy Protocols Field: MeSH Terms #1Search Colorectal Neoplasms Field: MeSH Terms
In addition, we will perform searches in Embase and the Cochrane Database of Clinical Controlled Trials (CENTRAL). Abstracts will be reviewed from ASCO annual meetings proceedings from 1998 to 2009.
From a preliminary search in PubMed we identified one review by Chau 2006, citing 4 primary studies (O'Connell 1998; Andre 2007; Chau 2005; Haller 2005).
References of relevant articles to support of the background for this systematic review were found in a book by P. Rougier et H Bleiberg, in the chapter about duration of CT (Bleiberg 1998). From the last chapter on "Adjuvant systemic treatment of colorectal cancer, duration of chemotherapy", we also identified an ongoing study by Dencausse (Dencausse 2002).
Data collection and analysis
Each eligible publication were assessed by means of a predefined data sheet. G. Des Guetz and B. Uzzan selected the articles.
The methodological quality of identified trials was assessed independently by the two reviewers (GDG and BU) taking into account the quality of random allocation concealment and the description of dropouts and withdrawals, as well as blinding of the patients and healthcare providers to the intervention. Any disagreements were resolved by discussion. Studies were excluded if they were not randomised controlled trials in adults. The excluded studies and the reasons for their exclusion have been summarised in the Table of Excluded Studies. Data were analysed using the RevMan Analyses statistical programme in Review Manager 5.
Odds ratios and 95% confidence intervals were calculated for dichotomous outcomes using the Mantel‐Haenszel method and a fixed effect model DerSimonian 1986. Continuous variables were analysed using fixed effect meta‐analyses of (weighted) mean differences (WMD). Continuous variables were processed using mean and standard deviation values. When only means and ranges were available an estimate to the standard deviation was made which is discussed in more detail in the results section.
Subgroup analyses were considered for outcomes, whenever feasible.
Sensitivity analyses were performed upon the detection of statistical heterogeneity.
Publication bias: Upon identification of sufficient number of trials this were assessed using Funnel plots.
Results
Description of studies
Results of the search
Electronic data search using online PubMed retrieved a total of 102 references without RCT limitation and 13 RCT references.
A review provided by PubMed concerning duration of adjuvant CT Chau 2006 cited 2 new articles Andre 2003; Haller 2005 and one duplicate Saini 2003 from the PubMed query.
From analysis of proceedings of ASCO meetings (1998 to 2009), no additional data were found, except an update of a previously published article Andre 2007, originally presented in 2005.
Seven independent studies representing 10326 patients with a mean age of 63.1 years, with 4884 males, 5300 females, from 6 studies (missing data for Ito et al ), included 9826 colon and 500 rectum cancers. In adjuvant setting, classification of patients according to prognostic factors is usually done to better define the benefit of treatment for patients in view of their risk of recurrence. The different studies used Dukes' and Dukes' derived classifications (Astler‐Coller) or TNM staging to describe patients treated in adjuvant setting. These 7 studies included 1721 stage II and 8455 stage III (missing data for Ito et al. Ito 2000). Several studies included other prognostic factors such as grade Haller 2005; Chau 2005;Nakamura 2001, O'Connell 1998 or surgical complications Haller 2005; Andre 2007; O'Connell 1998.
Included studies
To avoid overlapping of different groups of patients with shorter or longer duration of CT between studies, we choose to compare durations of CT shorter than or equal to 6 months with durations longer than 6 months (9‐12 months). Thus, the two main differences in study groups compared were the duration and also the type of CT. Concerning the regimens of CT, all treatments were based on 5FU but the modalities were different from one study to another. Three types of modulation of 5FU could be considered : continuous infusion, bolus infusion with folinic acid or bolus infusion with levamisole Andre 2007; Haller 2005O'Connell 1998 . In the two Japanese studies, different durations of oral 5FU (with Ftoraftur) were compared Ito 2000 , Nakamura 2001
The main characteristics of patients included in the 5 eligible studies Andre 2007; Haller 2005 , Ito 2000 , Nakamura 2001 , O'Connell 1998 plus the RCT by Chau 2005 with an atypical mode of administration of CT and the large retrospective study from Neugut 2006 are summarized in Table 1.
1. characteristics of studies.
|
Author Reference Year of publication |
N (M/F) |
Age (median) |
Primary Tumor Colon/ rectum |
Stage II/III |
Durations compared (Nb patients) |
Chemotherapy Protocols |
Total number of Relapses Short/Long |
Total number of deaths Short/Long |
Toxicity |
| HALLER | 3561 (1944/1617) |
63.7 | 2732/773 | 694/2867 | 6 months (1577) vs 12 months (835) 8 months excluded |
low dose LV + 5FU +/‐levamisole vs high dose LV + 5FU bolus weekly for 8 months vs levamisole + 5FU bolus weekly | 829/468 | 780/435 | ND |
| ANDRE | 905 (489/416) |
60 | ND | 389/516 | 6 months (454) vs 9 months (451) | LV/5FU2 vs FULV | 128/123 | 57/75 | Less toxicity for LVFU2 |
| NAKAMURA | 293 (152/141) |
< 75 | 275/5 | 156/136 | 6 months (150) vs 12 months (143) | oral carmofur | 47/49 | 39/49 | ND |
| ITO | 144 | < 75 | ND | 3 months (69) vs 12 months (75) | HCFU oral (8 mg/kg/j) . | 22/24 | 18/16 | Side effects : n=23 : 10 (3 mo) vs 13 (12 mo). | |
| O'CONNELL | 891 (454/437) |
65 | 647/268 | 157/733 | 6 months (445) vs 12 months (446) | 5FU weekly (450 mg/m2) vs 5FU (370 mg/m2) bolus for 5 days every 5 weeks | 170/170 | 160/167 | more grade 3/4 diarrhea and stomatitis. for longer duration No difference for grade 3/4 leucopenia |
| CHAU | 801 (431/370) |
62.5 | 685/84 | 325/470 | 3 months vs (397) 6 months (404) | 5FU/LV (Mayo Clinic) versus 5FU (300 mg/m2/d continuous | 104/127 | 99/121 | Less toxicity for short treatment (diarrhea, nausea, vomiting, alopecia, neutropenia, anemia, thrombopenia but more hand/foot syndromes) |
| NEUGUT | 3733 (1414/2319) | > 65 | ND | 0/3733 | 1‐4 months (488) vs 5‐7 months (1091) | 5FU | ND/ND | 154/188 | ND |
Finally, five studies were selected for analyses.
Excluded studies
References which were not RCT's and did not meet our inclusion criteria were excluded.
We excluded the atypical study by Chau 2005 comparing 3‐month protracted infusion of CT with 6‐month conventional CT, and the retrospective epidemiologic study by Neugut 2006, mainly devoted to the optimum duration of CT among patients older than 65 years. Using the fixed effect model, we did not find statistically significant heterogeneity. Inclusion in a sensitivity analysis of the large retrospective study introduced significant heterogeneity.
For one article found in the book from Rougier et al. (Bleiberg 1998), additional data were needed for statistical analysis (Dencausse 2002), and were requested from the principal author, but he did not reply. These authors randomised patients into 6 or 12 months 5FU/leucovorin (5FU/LV) with or without levamisole, but compared the pooled 6 and 12 months regimens of 5FU/LV with 12 months of 5FU/LV plus levamisole. Separate data according to duration of treatment were not provided. Thus, we could not include this trial in this review.
Risk of bias in included studies
Our analyses did not rely upon enough studies to perform a funnel plot.
Allocation
All studies were randomised (although not explicitly mentioned by Ito (Ito 2000)), except for Andre (Andre 2007), who used a minimisation to stratify patients which can be accepted, using a centralised registration system
Blinding
All studies were open‐labeled.
Incomplete outcome data
In both Japanese studies many patients did not receive the scheduled treatment ; Andre 2007 and O'Connell 1998 used explicitly intention to treat analyses.
Effects of interventions
In both Japanese studies many patients did not receive the scheduled treatment ; Andre 2007 and O'Connell 1998 used explicitly intention to treat analyses.
Discussion
Summary of main results
In the MA assessing 5 RCTs (after exclusion of the retrospective study by Neugut 2006 and the atypical study by Chau 2005), shorter duration of CT (3‐6 months) did not predict better Relapse Free Survival, RFS, (RR =0.96, 95% CI : 0.90‐1.02 ; p = 0.17) (Figure 1) and Overall Survival, OS, (RR = 0.96 ; 95% CI : 0.91‐1.02 ; p = 0.22) (Figure 2) than longer duration of CT (9‐12 months). Although the inclusion of the RCT by Chau comparing 3 months continuous infusion and 6 months bolus treatment in a sensitivity analysis improved the results of shorter versus longer duration of CT, they did not reach statistical significance. Considering toxicities, more grade 3/4 diarrhoeas and stomatitis were observed for longer duration and conversely less grade 3/4 diarrhoeas and stomatitis for shorter duration of CT for the two opposite studies with the shortest (3 months, continuous infusion) and longest 5FU treatment (12 months, bolus injection). The toxicities were poorly evaluated in the majority of studies.
1.
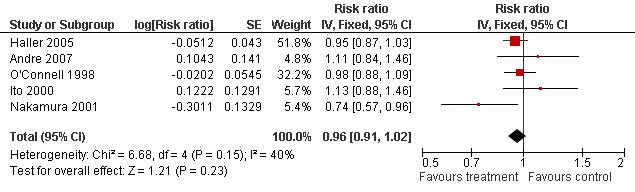
Forest plot of comparison: 2 Overall survival (OS), outcome: 2.1 Overall survival.
2.

Forest plot of comparison: 1 Relapse free survival (RFS), outcome: 1.1 Relapse free survival.
Overall completeness and applicability of evidence
The PubMed query retrieved a total of 8 studies comparing various durations of CT. Of the 8 studies, one should have been completely re‐analysed for the purpose of this MA Dencausse 2002 and one Neugut 2006 was a retrospective study that we did not include in the MA.
Inclusion of the epidemiologic study by Neugut 2006 in our MA introduced statistical heterogeneity, validating our decision not to include this retrospective non‐randomised study. This study was carried out by extracting files of patients of SEER database. Many biases could explain the differences between this study and the others. It only provided OS data.
One RCT compared durations of CT of 3 and 6 months Chau 2005, administered by different methods (protracted infusion for 3‐month duration and conventional IV infusion for 6‐month duration). We chose to exclude this latter study from our MA, to avoid overlapping of shorter and longer durations of CT between studies.
The 5 remaining RCTs did not show statistical heterogeneity allowing us to used a fixed effect model.
Although the evidence is rather scarse (both Japanese studies are small and do not contribute importantly to the results) it has been accepted by the vast majority of oncologists, who consider than 6‐month is the optimal duration of adjuvant CT in CRC;
Paradoxically, we observed the best recurrence free survival (RFS) for patients treated by 3 month continuous regimen in the study by Chau 2005. This study was characterized by a continuous infused CT during 3 months. This single study could not definitely convince us of the final benefit of short course CT, but it appeared interesting in several respects. CT was given at low doses but in continuous infusion, without any stop during 3 months. This mode of administration raises practical issues both for nurses and patients and also economic issues. This completely continuous administration was rendered possible by the low dose of 5FU administered daily and hence the daily metabolism of this low dose.
Quality of the evidence
All 5 included studies were randomised controlled studies (Andre 2007 used minimisation, (an accepted method to allocate patients without formal randomisation according to several prognostic factors). Two studies, Andre 2007 and O'Connell 1998, performed intention to treat analyses. Both Japanese studies were of poor quality, with many protocol violations and cross‐over concerning the duration of CT. Two Japanese studies compared different durations of oral CT based on UFT. However, these studies were not very contributive due to the small sample sizes of both studies (155 patients randomised in Ito's study) and their large confidence intervals. It is nevertheless noticeable that the capecitabine in the X‐Act study showed a superiority in terms of toxicity and less clearly in terms of efficacy compared to bolus 5FU.
Although each study in this MA was not weighted by a quality score, the two Japanese studies of poorest quality did not contribute largely to the results and the 3 remaining RCTs were of good and similar quality.
Potential biases in the review process
Publication bias is improbable.
In the 80's and the beginning of 90's, the main debated issue about CT concerned the mode of administration of 5FU. Thus, CT was based upon 5FU potentiated by folinic acid or levamisole alone or in combination. Two modalities of intravenous (IV) infusion of 5FU were also assessed, bolus or continuous infusion. The different groups compared in the RCTs differed not only by duration of CT but also by mode of administration of 5FU. Therefore, some trials used a 2x2 factorial design.
Authors' conclusions
Implications for practice.
Our MA of all published randomised controlled trials comparing two durations of adjuvant CT in CRC shows no benefit and even a slight harm to prolong such treatment for more than 6 months, when administered by IV bolus 5FU. However, we were not able to compare the effects of 3 or 6 month durations of CT, because the study mostly favouring a 3 month duration used protracted venous infusion and thus the relative influence of both of these factors (duration of CT, mode of administration of CT) was impossible to assess independently.
Implications for research.
Our results should help design future RCTs comparing different durations of continuous CT, like capecitabine or continuous infusion of 5FU. An international study (IDEA study), now open for inclusion will include 10500 patients (equivalence study) and compare 3 and 6 months of Oxaliplatin based CT. In future clinical trials assessment of precise duration of CT should take into account prognostic factors.
Acknowledgements
We thank Dr Mathilde Tardieu (resident) her help in translating this manuscript.
Data and analyses
Comparison 1. Overall survival (OS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 5 | Risk ratio (Fixed, 95% CI) | 0.96 [0.91, 1.02] |
1.1. Analysis.

Comparison 1 Overall survival (OS), Outcome 1 Overall survival.
Comparison 2. Relapse free survival (RFS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse free survival | 5 | Risk ratio (Fixed, 95% CI) | 0.96 [0.90, 1.02] |
2.1. Analysis.

Comparison 2 Relapse free survival (RFS), Outcome 1 Relapse free survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andre 2007.
| Methods | RCT(minimization). 2X2 factorial design | |
| Participants | surgically resected colorectal cancer | |
| Interventions | 6 months treated versus 9 months LV5FU 2 (5FU bolus 400 mg/m2 then 600 mg/m2 for 22h d1, d2) every 2 weeks versus FULV each month d1‐d5, 400mg/m2 |
|
| Outcomes | RFS and OS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? All outcomes | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | Intention to treat (ITT) analysis. |
| Free of other bias? | Low risk | |
Haller 2005.
| Methods | RCT | |
| Participants | surgically resected colorectal cancer | |
| Interventions | low dose LV + 5FU 425 mg/m2 bolus d1‐d5 each month (6 cycles) +/‐levamisole vs high dose LV + 5FU 500mg/m2 bolus weekly for 8 months vs levamisole + 5FU 450 mg/m2 bolus d1‐d5 then once weekly for a year |
|
| Outcomes | RFS and OS | |
| Notes | ||
Ito 2000.
| Methods | RCT | |
| Participants | surgically resected colorectal cancer | |
| Interventions | HCFU per os 8 mg/kg/d for 3 months versus 12 months | |
| Outcomes | RFS and OS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Centralized registration |
| Allocation concealment? | Low risk | randomisation in one centre |
| Blinding? All outcomes | High risk | |
| Free of other bias? | High risk | |
Nakamura 2001.
| Methods | RCT | |
| Participants | surgically resected colorectal cancer | |
| Interventions | Carmofur per os 6 months versus 12 months | |
| Outcomes | RFS and OS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Centralized registration |
| Allocation concealment? | Low risk | computer randomisation |
| Blinding? All outcomes | High risk | |
| Free of other bias? | High risk | |
O'Connell 1998.
| Methods | RCT. 2X2 factorial design | |
| Participants | surgically resected colorectal cancer | |
| Interventions | 5FU weekly (450 mg/m2) vs 5FU (370 mg/m2) bolus for 5 days every 5 week 6 months vs 12 months |
|
| Outcomes | RFS and OS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | |
| Blinding? All outcomes | High risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chau 2005 | comparison between 3 months and 6 months treatment. Protracted infusion |
| Neugut 2006 | Retrospective epidemiological study |
Contributions of authors
G. Des Guetz, cancerologist, and B. Uzzan, pharmacologist and methodologist, wrote the protocol. G. Des Guetz, who is the promotor of these meta‐analyses, wrote the protocol, read the selected articles (putting the emphasis on clinical and oncologic aspects) in association with BU (putting the emphasis on clinical and methodological aspects). GDG and BU made decisions of inclusion or rejection of each eligible article in each meta‐analysis (metastatic setting and adjuvant setting), and were the main contributors to this systematic review. Dr P. NICOLAS, pharmacologist and statistician, downloaded RevMan 5 (the software of managing reviews and protocols), and gathered the numerical data needed for the statistical calculations, participated in the decisions of inclusion or rejection of the articles, choose the best statistical method to answer to each issue, provided results as tables and figures, and elaborated slides for oral presentation. Dr J.F. MORERE, oncologist and Dr G. PERRET, pharmacologist provided supervision and guidance to this work, and participated in the writing the review manuscript.
Declarations of interest
None known
New
References
References to studies included in this review
Andre 2007 {published data only}
- André T. Phase III Study Comparing a Semimonthly With a Monthly Regimen of Fluorouracil and Leucovorin As Adjuvant Treatment for Stage II and III Colon Cancer Patients: Final Results of GERCOR C96.1. J Clin Oncol 2007;25(24):3732‐8. [DOI] [PubMed] [Google Scholar]
Haller 2005 {published data only}
- Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, Mayer RJ. Phase III study of fluorouracil, leucovorin, and levamisole in high‐risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005;23(34):8671‐8678. [DOI] [PubMed] [Google Scholar]
Ito 2000 {published data only}
- Ito K, Kato T, Koike A, Miura K, Yamaguchi A, Sakou T, Takagi H. Optimum duration of oral adjuvant chemotherapy of HCFU for colorectal cancer; review of 5‐year follow‐up.. Anticancer Res 2000;20(6C):4681‐6. [PubMed] [Google Scholar]
Nakamura 2001 {published data only}
- Nakamura T, Ohno M, Tabuchi Y, Kamigaki T, Fujii H, Yamagishi H, Kuroda Y, Kansai Carmofur Study Group. Optimal duration of oral adjuvant chemotherapy with Carmofur in the colorectal cancer patients: the Kansai Carmofur Study Group trial III.. Int J Oncol 2001;19(2):291‐8. [PubMed] [Google Scholar]
O'Connell 1998 {published data only}
- O'Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ Jr, Erlichman C, Shepherd L, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high‐risk colon cancer. J Clin Oncol 1998;16(1):295‐300. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chau 2005 {published data only}
- Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, Hill M, Hickish T, Lofts F, Jodrell D, Webb A, Oates JR. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer.. Ann Oncol 2005;16(4):549‐557. [DOI] [PubMed] [Google Scholar]
Neugut 2006 {published data only}
- Neugut AI, Matasar M, Wang X, McBride R, Jacobson JS, Tsai WY, Grann VR, Hershman DL. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol 2006;24(15):2368‐2375. [DOI] [PubMed] [Google Scholar]
Additional references
Andre 2003
- Andre T, Colin P, Louvet C, Gamelin E, Bouche O, Achille E, Colbert N, Boaziz C, Piedbois P, Tubiana‐Mathieu N, Boutan‐Laroze A, Flesch M, Buyse M, Gramont A. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial.. J Clin Oncol 2003;21(15):2896‐2903. [DOI] [PubMed] [Google Scholar]
Bleiberg 1998
- Bleiberg H, Rougier P, Wilke HJ. Management of Colorectal Cancer. London: Martin Dunitz, 1998. [Google Scholar]
Chau 2006
- Chau I, Cunningham D. Adjuvant therapy in colon cancer ‐ what, when and how?. Ann Oncol 2006;17(9):1347‐1359. [DOI] [PubMed] [Google Scholar]
Dencausse 2002
- Dencausse Y, Hartung G, Sturm J, Kopp‐Schneider A, Hagmuller E, Wojatschek C, Lindemann H, Fritze D, Queisser W. Adjuvant chemotherapy in stage III colon cancer with 5‐fluorouracil and levamisole versus 5‐fluorouracil and leucovorin.. Onkologie 2002;25(5):426‐30. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
Gibson 2006
- Gibson TB, Grothey A. Do all patients with metastatic colorectal cancer need chemotherapy until disease progression?. Clin Colorectal Cancer 2006;6(3):196‐201. [DOI] [PubMed] [Google Scholar]
Saini 2003
- Saini A, Norman AR, Cunningham D, Chau I, Hill M, Tait D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5‐FU) is as effective as 6 months of bolus 5‐FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer 2003;88(12):1859‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]


