Abstract
Stress has been well-documented to have a significant role in the etiopathogenesis of bruxism. Activation of the hypothalamic-pituitary-adrenal axis (HPA) and subsequent release of corticosteroids lead to increased muscle activity. Neurological studies have demonstrated that chronic stress exposure induces neurodegeneration of important neuronal structures and destabilization of the mesocortical dopaminergic pathway. These disruptions impair the abilities to counteract the overactivity of the HPA axis and disinhibit involuntary muscle activity, while at the same time, there is activation of the amygdala. Recent evidence shows that overactivation of the amygdala under stressful stimuli causes rhythmic jaw muscle activity by over activating the mesencephalic and motor trigeminal nuclei. The present review aimed to discuss the negative effects of certain vitamin and mineral deficiencies, such as vitamin D, magnesium, and omega-3 fatty acids, on the central nervous system. It provides evidence on how such insufficiencies may increase stress sensitivity and neuromuscular excitability and thereby reduce the ability to effectively respond to the overactivation of the sympathetic nervous system, and also how stress can in turn lead to these insufficiencies. Finally, the positive effects of individualized supplementation are discussed in the context of diminishing anxiety and oxidative stress, neuroprotection and in the reversal of neurodegeneration, and also in alleviating/reducing neuromuscular symptoms.
Keywords: bruxism, neurodegeneration, vitamin D, magnesium, iron, omega-3 fatty acids
1. Introduction
Bruxism is a parafunctional condition affecting more than two-thirds of the population at some point in their life; however, only 25% of affected individuals are aware of this condition and seek medical attention (1). According to an international consensus, Bruxism is defined as repetitive jaw muscle activity characterized by clenching or grinding of the teeth and/or bracing or thrusting of the mandible; however, it is not regarded a movement disorder or a sleep disorder in otherwise healthy individuals (2). Bruxism can be distinguished into awake and sleep bruxism based on the time of occurrence (circadian manifestation). In a systematic review by Manfredini et al (3), it was shown that the prevalence of awake bruxism ranges from 22 to 31%, whereas sleep bruxism has a prevalence of 12.8%. Finally, women are more prone to bruxism compared to men with a ratio of 5:1(4).
Although numerous factors have been implied to contribute to the etiopathogenesis of bruxism, stress and emotional disturbances are the most commonly accepted (5,6). Emotional stress is associated with an increase in head and muscle tonicity, as well as an increase in non-functional muscle activity (7,8). In addition, the activation of the sympathetic system in response to stressful stimuli leads to increased muscle tone and reduced pain thresholds (8,9). In a systematic review and meta-analysis published in 2021, Fritzen et al (10) verified increased levels of salivary cortisol in bruxists. These increased levels correlate with strong circadian rhythms with peak levels during the activation periods (11). Under experimental stress, there is increased masseter activity, which returns to baseline levels upon relaxation (12). In animal studies, stress-induced muscle hyperactivity was associated with muscle dysfunction and pain (13), whereas humans who experience panic attacks more frequently exhibit tooth clenching, bruxism and nail-biting (14). Overactivation of the sympathetic system and hypothalamic-pituitary-adrenal axis (HPA) axis can make an individual more vulnerable to new stress, as evidenced by animal and human studies (15,16). Patients with temporomandibular disorders (TMD) exhibit higher levels of anxiety (17), while TMD symptoms worsen whenever a person is under stress (18,19).
Based on findings in neurological diseases (20-23) and pharmacological interactions (24-27), it appears that a malfunction of 5-hydroxytryptamine (HT)2 receptors may have a major role in the pathogenesis of bruxism. The role of 5-HT2 receptors in the mediation of orofacial musculature is well documented (28,29). Stress can affect 5-HT receptors, but this effect varies from area to area. For instance, it may induce a decrease in 5-HT1 receptors in the hippocampus and an increase in cortical 5-HT1 (30,31), while leaving the 5-HT2 receptors in the trigeminal nuclei unaffected (30,32). This is also supported by the work of Inan et al (33), who concluded that bruxism occurs as a result of abnormally reduced inhibition of trigeminal motor neurons. In another study by Lauria et al (34), it was shown that a neuropathy in the nigrostriatal system leads to loss of inhibition of the trigeminal nerve. The neuroanatomy of masticatory modulation is a 2-neuron chain where serotonergic neurons from the Raphe nucleus synapse with dopaminergic neurons in the ventral tegmental area (VTA) (35).
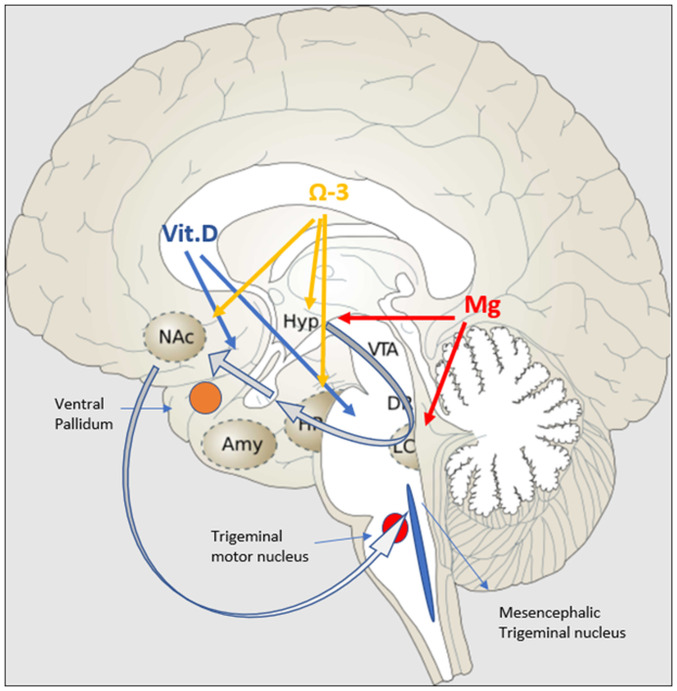
Two important neuronal areas are implicated in the genesis of bruxism and are highly affected by stress: The mesencephalic trigeminal nucleus (Me5) and the mesocortical dopaminergic pathway. Me5 controls the masseter inhibitory reflex, which, when activated, suppresses the trigeminal motor nucleus and inhibits masseter and temporalis contraction. The mesocortical dopaminergic tract connects the VTA with the nucleus accumbens (N.Acc) via either the ventral subiculum (vSub) or amygdala (36). N.Acc receives glutamatergic projections from the hippocampus and basolateral amygdala (BLA) and sends projections to mesencephalic motor effector sites, and is therefore considered to be a motor-limbic interface (37). The mesocortical tract is equally important because it inhibits undesired muscular movements (38). In acute mild stressors, the tract follows the vSub-ventral pallidum (VP)-N.Acc route (39,40). The hippocampus is essential in counteracting overactivation of the HPA axis (41). However, in chronic stressors, the hippocampus presents with neurodegenerative alterations that attenuate this pathway, while at the same time, they activate the BLA-VP-N.Acc pathway (42). Studies in mice have revealed that a direct projection from CeA to trigeminal motor nucleus is highly activated during hunting of prey and that it promotes strong biting attacks (43). Of note, the same pathway has recently been identified in humans, and in higher distribution than BLA-Mo5 (Trigeminal Motor Nucleus) (44), further highlighting that the same association may also apply to humans and warranting further research in this field. Fig. 1 diagrammatically represents the effect of certain nutrients on the mesocortical dopaminergic pathway.
Figure 1.
Effects of vitamin D, omega-3 fatty acids and magnesium on the mesocortical dopaminergic pathway (grey arrows). Vitamin D acts on the mesocortical dopaminergic pathway by protecting dopaminergic neurons (blue arrows). Omega-3 fatty acids suppress overactivity of the HPA axis and increase neurogenesis and synaptic activity in the hippocampus and N.Acc (orange arrows). Magnesium suppresses HPA and SAM axis overactivation by acting on the hypothalamus and LC, respectively (red arrows). Amy, amygdala; HPA, hypothalamic-pituitary-adrenal; HP, hippocampus; Hyp, hypothalamus; LC, locus coereleus; Mg, magnesium; N.Acc, nucleus accubens; SAM, sympathetic adrenal medullary; Vit.D. vitamin D; VTA, ventral tegmental area.
Chronic stress exposure and increased circulating levels of corticosterone impair progenitor cell proliferation, inhibit neuronal differentiation and suppress cell survival in the hippocampal dentate gyrus (45-49), events that affect hippocampal neurogenesis (50-52). This is happening as a result of oxidative stress due to auto-oxidation of catecholamines (53). Oxidative stress due to increased cellular activity induces nitrogen oxide (NO) production, which activates microglia. The latter further releases NO and reactive oxygen species (ROS) that induce neuroinflammation and neurodegeneration (54,55). This in turn negatively modulates the ability of the hippocampus to control hyperactivity of the HPA axis. Neurodegeneration of the hippocampus and attenuation of the vSub-VP-N.Acc pathway invoke alterations in the mesocortical dopaminergic neurons that are implicated in neuropathic and chronic pain (56-58). The dysregulation of dopamine D2 receptor expressing indirect pathway output neurons promotes hypersensitivity to pain and increased impulsivity due to reduced levels of dopamine in N.Acc (59,60). The attenuation of the vSub-VP-N.Acc pathway and the activation of the BLA-VP-N.Acc cause further reduction of dopamine neurons in the VTA (42). All these aforementioned events have a strong effect on Me5. Animal electrophysiological studies have shown that chronic restrained stress induces an increase in the excitability of Me5 and glutamatergic neurotransmission from Me5 to Mo5(61). This was evidenced by increased levels of acetylcholinesterase (Ache) and creatine kinase (CK-MM) in the masseter muscles (61). In another study by Ueno et al (62), it was demonstrated that activation of the ventral part of amygdala in guinea pigs may cause rhythmic jaw movement.
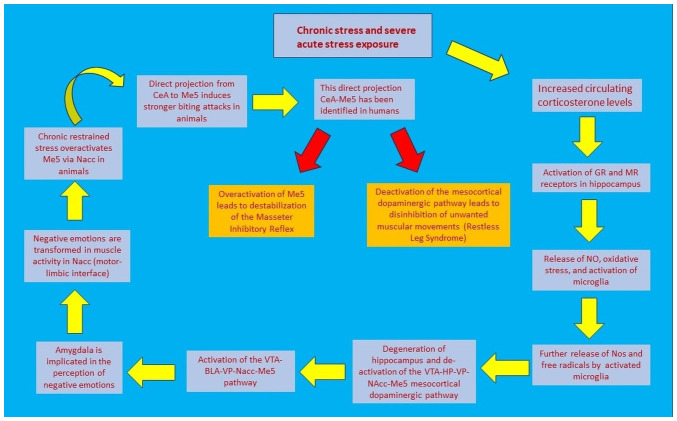
Recent evidence has revealed similar degenerative findings in bruxists. Keskinruzgar et al (63), by studying the retinas of bruxists, found that retinal nerve fiber layer (axon) thickness, inferior parietal lobe (dendrites) and granule cell layer (soma) volume are significantly reduced. The retina is considered to be a continuation of the brain; therefore, any neurodegenerative changes in the retina are representative of those induced in the mesocortical dopaminergic tract. In the same study, an increase in choroidal thickness was also demonstrated. The choroid is an important site of vascularization and is responsible for transport of nutrients and oxygen (63). This study, however, does not specify the cause of neurodegeneration or an association between bruxism and neurodegeneration. The fact that bruxism is a common comorbid condition or symptom in other neurodegenerative disorders such as Parkinson's disease or Parkisonism (64), as well as in rapid eye movement sleep behavior disorder (65), constitutes enough evidence so as to not exclude neurodegeneration from this equation. Ozcan-Kucuk et al (66) concluded that there is a significant increase in oxidative stress and prolidase activity in bruxists. Increased levels of prolidase, an enzyme found in the plasma and several organs, including the brain, are associated with higher levels of proline, a brain neurotransmitter, which can induce oxidative stress and subsequently neurodegeneration (67). These effects have been examined in anxiety (68), several musculoskeletal disorders (e.g. ankylosis, osteoarthritis) (69,70) and neuropsychiatric diseases (Alzheimer's, bipolar disorder) (67,71). Fig. 2 illustrates the sequence of events that connect stress with jaw muscle activity.
Figure 2.
Schematic representation of events that lead from stress to jaw muscle activity. BLA, basolateral amygdala; CeA, central amygdala; GR, glucocorticoids; HP, hippocampus; Me5, mesencephalic trigeminal nucleus; MR, mineralocorticoid; N.Acc, nucleus accubens; NO, nitric oxide; VP, ventral pallidum; VTA, ventral tegmental area.
Based on these findings, particularly on the neurodegeneration of the hippocampus, the attenuation of the vSub-VP-N.Acc pathway and the fact that a malfunction may occur at any given point in the mesocortical dopaminergic tract, it is difficult to identify a therapeutic target. According to a systematic review from 2022 by Minakuchi et al (72), none of the already used treatment modalities is able to achieve universal positive results. As far as pharmacologic agents are concerned, the evidence of efficacy has low to moderate confidence, with numerous adverse effects; at the same time, the efficacy of cognitive behavioral therapy is still low and does not appear to improve the symptoms in <6 months (72). Overwhelming evidence points towards insufficiencies and deficiencies of necessary elements for the homeostasis and normal function of the central nervous system (CNS), with vitamin D (vit.D), magnesium and omega-3 fatty acids being the most studied ones.
2. Vit.D and the pathogenesis of bruxism
Effect on physiological function
Vit.D) is a very important nutrient, not only for maintaining a healthy musculoskeletal system but also for the positive effect on the nervous system. It has long been known that vit.D has a vital role in calcium and phosphorous metabolism, in three main ways: i) By enhancing intestinal absorption, ii) by controlling the synthesis of parathyroid hormone and iii) by promoting the maturation of pre-osteoclasts into osteoclasts (73-75). However, current evidence suggests that vit.D also has a major role in neuroimmune modulation, neuroplasticity and neurological oxidative stress reduction (76). This property is supported by the abundance of vit.D receptors throughout the CNS (77), as well as by its ability to cross the blood brain barrier (78). Vit.D is also known to control ~3% of the human genome via the vit.D receptor (VDR), which forms a heterodimer with the retinoid X receptor (79,80). This heterodimer complex binds to specific DNA sequences and alters the expression of genes that are involved in glutamatergic and GABAergic neurotransmission, calcium regulation and also the expression of neurotrophic factors and genes involved in neuroprotection (81,82).
The strong antioxidant capacity of vit.D helps to improve brain enzyme activity and to reduce lipid peroxidation. Oxidative stress has a major role in neurodegenerative diseases, mainly through auto-oxidation of catecholamines (53,54). Mitochondrial dysfunction leads to a rise in ROS and activated microglia, resulting in nitric oxide (NO) and ROS production during neuroinflammation (55). Vit.D also protects the brain by suppressing inflammatory cytokines such as IL-6(83). However, the most important function of vit.D is the protection of dopaminergic neurons. Although the mechanism has remained to be fully elucidated, there is sufficient evidence to suggest that it can affect calcium metabolism, apoptosis, inflammation, immunomodulation, detoxification and neurotrophin upregulation (84-86). Cholecalciferol (VD3) may directly modulate tyrosine hydroxylase, a rate-limiting enzyme in dopamine synthesis, as evidenced by immunohistochemical staining. The glial cell-derived neurotrophic factor (GDNF) protein also appears to be increased by these effects, thereby supporting the neuroprotective effects of VD3 on dopaminergic neurons (87). Orme et al (87) demonstrated that adding VD3 to mesencephalic cultures of dopaminergic neurons increased their number and upregulated GDNF. Furthermore, VD3 has also been shown to regulate factors involved in dopaminergic system ontogeny (88), as evidenced through studies in animals with altered dopaminergic metabolism due to VD3 deficiency, where VD3 supplementation resulted in a reduction of the degree of dopaminergic denervation (87,89).
Association of vit.D deficiency with anxiety. Vit.D deficiency and insufficiency have been strongly implicated in anxiety and depression. Huang et al (90) showed that a decrease of 1 ng/ml in 25(OH)D (Calcidiol, a hydroxylated form of vit.D) was associated with greater anxiety and depression. Similar results were obtained by Altinbas (91) on intensive care unit personnel. In another study by Al-Atram et al (92), vit.D deficiency was positively correlated with anxiety but not with depression. However, none of these studies clarified the insufficiency and deficiency cut-off points, with the majority accepting <30 ng/ml as insufficiency and <20 ng/ml as deficiency (93). When compared to subjects with normal (adequate) vit.D levels, vit.D deficiency seemed to be associated with a reduction in brain volume, particularly in the hippocampus (94). According to certain researchers, vit.D deficiency has a direct effect on sleep because of the association between sleep-wake regulation brain regions and vit.D target neurons in the diencephalon (95). The main causes of vit.D deficiency or insufficiency are inadequate UVB exposure or decreased bioavailability, as well as through certain types of medication, such as glucocorticoids (GR), antiretrovirals and anticonvulsants (96).
Association of vit.D with bruxism. Recent evidence has unveiled a strong association between bruxism, particularly sleep bruxism (central), and vit.D deficiency. Alkhatatbeh et al (97) showed that there is a significant link between vit.D deficiency and sleep bruxism, with 60% of bruxism patients exhibiting low levels of vit.D compared to 34% of the controls. In the same study, the authors also assessed the daily dietary intake of calcium and reported that only 26% of bruxists had a daily dietary calcium intake of >600 mg/day, compared to 42% of the controls (97). This association can be explained by the neuroprotective role of vit.D. Low vit.D levels may disrupt calcium homeostasis, affecting neuron excitability (98). Furthermore, because vit.D is responsible for calcium homeostasis, low vit.D levels will result in low serum calcium levels, i.e. hypocalcemia, which has an immediate effect on neuromuscular function, and the potential to cause muscle spasms and cramps (95). In another study by Allaf and Abdul-Hak (99), insufficiency or deficiency of vit.D were shown to increase with the severity of bruxism, while in non-bruxists, insufficiency and deficiency was present in 41 and 16% of individuals, respectively. In subjects with mild bruxism, these numbers appeared to rise to 50 and 30%, respectively, and in moderate and severe bruxism to 58%, whereas in extremely severe bruxism, insufficiency and deficiency can reach 72% in total.
Association of vit.D with TMD. An association has been highlighted between low levels of vit.D and TMD. In individuals suffering from osteoarthritis, vit.D is linked to cartilage regeneration (100): vit.D deficiency is observed in individuals suffering from headaches attributed to TMD, as well as from tension-type headaches (101,102), while low levels of vit.D in combination with the Bsml variant of the VDR gene are highly associated with the etiology and pathogenesis of disc displacement with reduction (103). Similarly, Wu et al (104) found an association between low serum levels of vit.D and arthritis, muscle pain and chronic widespread pain. Finally, Demir and Ersoz (105) concluded that the levels of parathyroid hormone in response to vit.D deficiency were significantly higher in TMD subjects as compared to controls. On the other hand, the observation that supplementation of vit.D reduces or alleviates neurological and muscular pain (106-109), is rather encouraging. This effect is thought to occur due to a reduction in oxidative protein damage and in the neuronal levels of Ca.
Vit.D insufficiency and stress. Another well-documented observation during exposure to stress are the low levels of 1,25(OH)2D, the active form of vit.D. This can be the result of increased acute hemodilution and interstitial extravasation, or due to a decrease in the synthesis of binding proteins that augment renal wasting of 25(OH)D (110), or a result of hypocalcemia, a common finding during stress. Specifically, under high stress, particularly conditions that present hyperventilation, the body may respond with respiratory alkalosis, which can lead to hypocalcemia. This may, in turn, cause a compensatory increase in parathyroid hormone (PTH), which could further increase the conversion of 25(OH)D to 1,25(OH)2D in order to maintain calcium homeostasis. In the presence of secondary hyperparathyroidism, 25(OH)D consumption would exacerbate vit.D deficiency (111). Therefore, in acute stress situations, vit.D deficiency may represent a discrepancy between tissue requirement and substrate supply, where the local tissue is unable to generate adequate 1,25(OH)2D, despite maximal stimulation of 1-a-hydroxylase by PTH (112). PTH resistance, which occurs in hypomagnesaemia, renal failure and hypoparathyroidism, can further impair the formation of 1,25(OH)2D (112).
3. Magnesium
Role of magnesium in physiological function
Magnesium (Mg) is the body's fourth most abundant cation and the second most abundant intracellularly; aerobic and anaerobic metabolism, bioenergetic reactions, metabolic pathway regulation, signal transduction, ion channel activity, cell proliferation, differentiation, apoptosis, angiogenesis and membrane stabilization are all biological processes that involve magnesium (113-115). More than 325 enzymes, many of which are specific to the nervous system, are Mg-dependent, highlighting the importance of this particular mineral in CNS physiological function (116).
Stress is known to promote oxidative stress through catecholamine auto-oxidation: it aggravates lipid peroxidation, increases DNA oxidative damage marker production and decreases plasma anti-oxidative activity (117,118). During a stressful event, there is activation of the HPA axis and release of corticotropin-releasing factor (CRF) from parvocellular neurons. Mg antagonizes the glutamate-stimulated CRF release, stabilizes CRF receptor binding and stimulates the Na/K ATPase, which decreases CRF-receptor activity (119-121). Mg also reduces adrenocorticotropic hormone release by suppressing HPA-axis activity via angiotensin II antagonism (122).
Mg has been shown to have a direct suppressive effect on locus coeruleus (LC) activity and low Mg levels seem to increase sensitivity to stress (123). The release of substance P, a neuropeptide that preferentially activates tachykinin NK1 receptors, can explain many of the effects of Mg deficiency in stress situations: Low Mg concentrations reduce Mg-gated blockade of N-methyl-D-aspartate (NMDA) receptor channels, resulting in the release of substance P and calcitonin gene-related peptide (CGRP) from sensory C fibers, i.e. neuropeptides that are involved in the production of reactive oxygen and nitrogen species and in the induction of neurogenic inflammation that is characterized by an increase in inflammatory cells and cytokines (124,125).
Magnesium is also essential for the synthesis of kynureninase. Therefore, in cases of hypomagnesemia, increased levels of kynurenines are observed in the circulation, which in turn promotes anxiety, an increase in the release of noradrenaline, reduced levels of serotonin, GABA receptor blockade and locomotor hyperactivity (126). The increased neural excitability observed in hypomagnesemia is believed to occur from the increased activity of NMDA receptors, which in turn induces anxiety, increased dopamine release and modulation of serotonin receptors (127).
Magnesium deficiency reduces the activity of B6 by inhibiting alkaline phosphatase, an enzyme necessary for the transformation of the active form of B6, pyridoxal phosphate. This causes increased levels of kynurenines, increased sympathetic activation and increased sensitivity to glucorticoids (128). As B6 is known to participate in decarboxylation of glutamic acid to GABA, of DOPA to dopamine and of 5-hydroxytryptophan to serotonin, it is regarded as a neuroprotective and antitoxic agent (129).
Implication of magnesium in bruxism. The possible involvement of magnesium in the pathogenesis of bruxism can be further supported by the fact that hypomagnesemia is observed in burning mouth syndrome (BMS), particularly in cases where the tongue is involved (130). BMS is caused by hyperactivity of somatosensory fibers of the trigeminal nerve and loss of central inhibition. This hyperactivity may be caused by parafunctional habits. Another important function of Mg is that it exhibits a direct enhancing effect on 5-HT1A serotonin receptor transmission by acting as a cofactor of tryptophan hydroxylase, which has a major role in the dopaminergic system (131). Mg levels are inversely correlated with estrogen levels, showing a sex-related difference (132).
Mg deficiency symptoms include neuromuscular irritability and weakness, headaches, hyperemotionality, generalized anxiety, insomnia, and asthenia (116,133,134). In Mg-deficient animals, there are increased plasma corticosterone levels, increased irritability and aggressive behavior (123,135). Experimentally-induced Mg deficiency in animal studies has been associated with disrupted sleep patterns, while an increase in the amplitude of daily variation of sleep and slow-wave sleep delta power has also been observed (135). Chronic sleep deprivation in humans is associated with progressively decreasing intracellular Mg levels, reduced duration of cardiopulmonary exercise and increased hypersensitivity to sympathetic nervous stimulation (136). In a study by Sarchielli et al (137), patients who suffered from migraine and tension-type headaches, very common symptoms in patients with bruxism and TMD, presented with significantly lower levels of serum and salivary Mg. This is because hypomagnesaemia makes cerebral arteries more sensitive to CO2, which promotes cerebral vasospasm and headache (138,139). Hypomagnesaemia is also linked to exacerbated neural excitability, migraine, orofacial tardive dyskinesia and increased anxiety, symptoms that may be improved by combined supplementation of Mg and B6(140).
Potential implication of magnesium deficiency in stress. Cernak et al (141) showed that stress, chronic and subchronic, causes significant reductions in magnesium concentrations in two ways: First, by stimulating neuroendocrine factors that increase urinary magnesium and second, by inducing complex hormonal alterations that subsequently impair magnesium homeostasis. At the same time, a 9- to 11-fold increase in plasma superoxide anion generation was observed, as well as a reduction in antioxidant enzyme concentrations, such as those of superoxide dismutase (141).
Overall, Mg has a strong neuroprotective role. As mentioned earlier, stress has a deleterious effect on neurons because of the increased levels of IL-6 that initiate microglial activation. Increased inducible NO synthase levels that are expressed during stressful periods impair hippocampal neurogenesis (142,143). Mg deficiency increases neuronal calcium influx and, as a result, NO production, which has been linked to cytotoxic effects (116). Mg counteracts the effects of calcium and reduces ROS production via the phospholipase lipoxygenase and cyclooxygenase pathways (144).
4. Iron
Iron, although not having been linked to bruxism, has recently been associated with restless leg syndrome (RLS), a common neurologic disorder characterized by involuntary muscle movement, which is associated with malfunction of the mesocortical dopaminergic pathway (145). This pathway, as discussed earlier, may be implicated in the pathogenesis of sleep (central) bruxism. Some consider bruxism as a manifestation of RLS, based on the fact that ~40% of patients with RLS report a history of bruxism (146), while others assume that bruxism and RLS are comorbid conditions (147). Both entities share some common features as they are both observed in non-REM sleep and are linked to basal ganglia dysfunction (148).
Brain iron deficiency has a critical role in the pathogenesis of RLS. Anemia and pregnancy are two conditions characterized by systemic iron deficiency, which are highly associated with RLS symptoms (149-151). Similar to bruxists, females are more susceptible to RLS. In a study by Umbreit (152), it was found that RLS without anemia exhibits a sex-dependent preference. Patients with RLS with and without anemia present with more profound tiredness, poorer sleep quality and less energy during the day (151). Depleted iron stores are associated with decreased activity of iron-dependent enzymes, reduced cellular oxidative capacity, as well as decreased energy efficiency (153,154). Previous clinical studies have shown that peripheral iron deficiency increases the risk of RLS prevalence (151). Reduced serum iron concentrations reduce brain iron levels, and iron therapy benefits patients with RLS with low peripheral iron levels (155). In a systematic review and meta-analysis, it was shown that iron therapy, in a way similar to that of a dopamine agonist, reduces restlessness and RLS severity (156).
Both human and animal studies have demonstrated that stress exposure causes a reduction in serum iron ranging from 27 to 44%, a reduction in ferritin by 24% and and a reduction in hemoglobin by 12.5% (157,158).
5. Omega-3 fatty acids
Omega-3 fatty acids-physiological function
Although omega-3 fatty acids have not been directly linked with bruxism, they have been highly implicated in anxiety and emotional mood disorders (159), as well as in neuroinflammation and neurodegeneration (159-162). They are esterified into phospholipids in the sn-2 position of the cell membrane (160), playing a key role in the structure and function of the brain cell membrane. Overwhelming evidence signifies their involvement in hippocampal development and synaptic function (162).
Omega-3 fatty acids may be found in numerous brain regions, but more abundantly in the prefrontal cortex, hippocampus and hypothalamus (163). They have been classified into n-6 polyunsaturated fatty acids (PUFA) and n-3 PUFA, with their main metabolites being arachidonic acid and docosahexaenoic acid (DHA), respectively. The free forms of PUFA are transformed into specific derivatives, such as eicosanoids, specialized proresolving mediators (SPM) and endocannabinoids (eCBs), which strongly regulate inflammation (160). The eCBs are lipids produced by the membrane's fatty acids after neuronal stimulation and bind to cytochrome P450 carbonyl reductase 1(164). Activation of this receptor causes an inhibition in glutamate and GABA release from presynaptic neurons (165). Regulation of eCBs in the brain by PUFA has a strong impact on hippocampal synaptic plasticity and in eCB-dependent plasticity (166), while CB1-associated signaling pathways in the prefrontal cortex (PFC) and N.Acc are also affected. Another signaling pathway, GPR120, which functions as an omega-3 fatty acid receptor, signals DHA activity that is highly prominent in the hypothalamus and N.Acc (167), areas that are closely linked to the pathogenesis of anxiety and bruxism, as discussed earlier.
Consequences of omega-3 fatty acid insufficiency. As these substances cannot be synthesized, they can only be obtained through diet (168). However, in Western civilizations, there is a shift in the consumption of fatty acids towards n-6 and less towards n-3(169), resulting in an imbalance between the two types that is associated with serious consequences, as described below. A decrease in plasma DHA for 49 consecutive days has been shown to cause a 5% DHA reduction in the brain, with the prefrontal cortex and hippocampus being more sensitive (160). DHA, in particular, has been shown to influence neurite growth, synaptogenesis, synapsin and glutamate receptor subunit levels, as well as synaptic activity in hippocampal neurons. This has been supported by in vitro studies demonstrating impaired long-term potentiation, shorter neurites, fewer branches, and fewer synapsin-positive puncta in DHA-deficient cultures, while DHA supplementation appears to restore neurite growth and synaptogenesis (162).
Implication of omega-3 fatty acid deficiency in stress. Animal studies have also shown that n-3 PUFA deficiencies induce emotional and neuronal disturbances through adrenal activation that resemble those observed after social defeat stress (161,170,171). Similar to chronic stress, HPA hyperactivity, due to a reduction in CB1 receptor function, invokes a marked increase in corticosterone levels. This action, in combination with a disturbance in the GR-mediated signaling pathway, leads to neuronal atrophy of the PFC by means of pyramidal neuron arborization atrophy. The GR signaling pathway and its downstream target bone-derived neurotrophic factor have a crucial role in neuroplasticity (161,172). As discussed earlier, chronic stress exposure, as well as stress induced by DHA deficiency, eventually cause hippocampal degeneration and attenuation of the mesocortical dopaminergic tract (namely the vSub-VP-N.Acc pathway), whilst at the same time activating the BLA-VP-N.Acc pathway that induces rhythmic jaw activity and possibly bruxism.
Oxidative stress causes NO production, which activates microglia. The latter are glial cells of myeloid origin, important in maintaining brain homeostasis and in protecting nerve cells (173). Activation of microglia causes further production of NO and ROS, leading to neuroinflammation (174). DHA deficiency induces abnormal microglial activation through changes in their membrane fluidity and the modulation of several proinflammatory transcription factors (175). These changes are gender- and area-dependent. Adult females have more microglia than males in the paraventricular nucleus of the hypothalamus, amygdala and hippocampus, particularly in the dentate gyrus (175), which has a crucial role in controlling the HPA axis. This is probably the reason why females appear to be more vulnerable to stress and more prone to bruxism.
Effects of omega-3 supplementation. In vitro evidence has highlighted that application of eicosapentaenoic acid (EPA) on microglial cultures inhibits the production and expression of inflammatory enzymes, such as NO synthase and COX-2, as well as the production of proinflammatory cytokines (IL-1β, IL-6 and TNF-α) (174). Levine et al (176) demonstrated that application of EPA plus DHA increases microglial autophagy, an important process for the homeostasis of immune cells that are able to diminish inflammatory processes. Another SPM form of DHA, Neuroprotectin D1, seems to protect the brain from leukocyte infiltration, reducing cyclooxygenase-2 expression, cytokine production and microglia activation (177). An in vivo study by Lynch et al (178) reported that omega-3 supplementation, and particularly EPA, significantly reduced marker expression of microglial activation and production of IL-1β by microglia, while at the same time, there was an increase in anti-inflammatory IL-4 expression. In neuropathic pain and retina degeneration models, N-3 PUFA supplementation was shown to cause a reduction in microglial density/activation, which can be linked to reduced neuroinflammation (179,180).
In a study by Ferraz et al (159), it was concluded that supplementation of PUFAs to animals under restrained stress was able to counteract the anxiolytic effects by suppressing the HPA axis and reducing the plasma corticosterone levels. It was also shown that there is an increase in serotonin and dopamine levels particularly in the hippocampus, both of which constitute actions that further promote an increase in the brain-derived neurotrophic factor and synaptophysin expression, as well as hippocampal neurogenesis. Similar results were obtained in humans, as shown by Kiecolt-Glaser et al (181), where supplementation of EPA and DHA in medical students for 12 weeks appeared to successfully reduce the release of IL-6 and anxiety symptoms compared to controls.
Finally, noteworthy findings have also been obtained in individuals with morning headaches, a common symptom in bruxists. Morning headaches usually exhibit the characteristics of migraine (182). Numerous hypotheses have been made regarding the mechanism of their genesis, with oxidative stress and activation of the trigeminovascular pathway being the two most prevalent (183). In this context, the role of CGRP has been highlighted both as a mediator and a potential therapeutic target (184). First of all, the release of CGRP has also been shown to be regulated by oxylipin receptors in the trigeminal nerve endings and central pain processing pathways (185,186). Furthermore, a recent study by Marchetti et al (184) showed that supplementation of PUFAs at a ratio of 1.5 omega-6/1 omega-3 induced a marked reduction in migraine symptoms, frequency and pain intensity; the explanation given by the authors was that EPA, DHA and their SPM activate innate and adaptive immune systems, remove ROS, and inhibit COX-2 and NF-κB intracellular signaling pathways. Table I summarizes the main characteristics of the nutrients discussed in this section and evidence that highlights their potential implication in the etiopathogenesis of bruxism.
Table I.
Normal functions of vitamin D, magnesium, iron and omega-3 fatty acids and their implications in the pathogenesis bruxism.
| Nutrient | Normal function | Implication in the pathogenesis of bruxism |
|---|---|---|
| Vitamin D | Neuroimmune modulation, neuroplasticity (76) Oxidative stress reduction (76) IL-6 suppression (83) Regulation of dopamine synthesis and dopaminergic system ontogeny (88,89) Protection of dopaminergic neurons (87) | Deficiency: Increases anxiety and neuromuscular excitability (90,98), worsens with the severity of bruxism (99); associated with osteoarthritis and headaches (100-102,104) Supplementation: Reduces or alleviates neurological and muscular pain symptoms (106-109) |
| Magnesium | Suppresses HPA axis and reduces ACTH release (119-122) Suppresses LC activity (123) Controls the release of Substance P and CGRP (124,125) Essential for the synthesis of kynureninase and B6(126) Enhances 5-HT1A activity (131) | Deficiency: Neuromuscular irritability, headaches, hyperemotionality, general anxiety, hypersensitivity to sympathetic stimulation, disrupted sleep patterns (116,133-135) Supplementation: Counteracts the effect of neuronal calcium influx and reduces ROS production (144) |
| Iron | Increases cellular oxidative capacity through iron-dependent enzymes (153,154) | Deficiency: Tiredness, poor sleep quality, headaches (151), involuntary muscular movements (restless leg syndrome) (145). Linked to stress exposure and basal ganglia dysfunction (148) Supplementation: Acts similarly to dopamine agonist, reduces restlessness (156) |
| Omega-3 fatty acids | Improvement of neurite growth, synaptogenesis and synaptic activity in hippocampal neurons (162,166) Suppression of HPA axis (159) Increase of serotonin and dopamine levels in the hippocampus (159) Inhibition of NO, COX-2, IL-6, IL-1b, TNF-a production by activated microglia (176,178) Neuroprotectin D1 protects brain from neurogenic inflammation (177) | Deficiency: Induces stress and increased corticosterone levels. Causes morphological and functional disturbances in PFC and hippocampus (161,170,171) Supplementation: Reduces anxiety and corticosterone levels (159,181). Reduces neuropathic pain (179). Reduces morning headaches frequency and pain severity (184) |
ACTH, adrenocorticotropic hormone; CGRP, calcitonin gene-related peptide; COX-2, cyclooxygenase-2; HPA, hypothalamic-pituitary-adrenal; LC, locus coeruleus; NO, nitric oxide; PFC, prefrontal cortex; ROS, reactive oxygen species.
6. Concluding remarks
This review has discussed the effects of chronic stress exposure in the pathogenesis of bruxism. In particular, oxidative stress, through the release of NO and the activation of microglia, has been shown to lead to neurodegeneration of the hippocampus and destabilization of the mesocortical dopaminergic pathway. It has also been highlighted that the former impairs the ability to counteract HPA axis overactivity, while the latter results in loss of inhibition of unwanted (involuntary) muscle movements. Based on recent evidence, it appears that attenuation of the vSub-VP-N.Acc route leads to activation of the BLA-VP-N.Acc pathway. The prevailing route has a strong yet opposing effect on the Me5, a nucleus that inhibits the involuntary contraction of masseter muscles by controlling the masseter inhibitory reflex. Indeed, activation of the amygdala appears to induce rhythmic jaw activity, while overactivation of Me5 under chronic stress exposure causes overactivation of Mo5, as evidenced by increased levels of CK-MM and pain in masseter muscles (61).
This report, instead of focusing on pharmacological agents, has shifted the attention towards homeostatic disturbances and deficiencies in important elements, such as vit.D, magnesium, omega-3 fatty acids, and, to a lesser degree, iron. Their neuroprotective properties, their capacity to reduce oxidative stress to suppress the HPA and LC axes, and their positive action on the neuroplasticity and neuronal growth of the hippocampus, particularly in the case of omega-3, further support this notion. Conversely, a decrease in the levels of these elements in the brain is associated with neurodegeneration, increased anxiety, increased neuromuscular excitability, bruxism, muscle spasms and pain. Of note, appropriate and individualized supplementation of these nutrients appears to reduce or alleviate the neurological and musculoskeletal symptoms (108,109). Future studies should focus on whether these concentration alterations have a causal effect or whether they constitute the aftermath of chronic stress exposure, and if re-establishment of the nutrient balance has a significant impact on the pathogenesis and chronicity of bruxism.
The present review has certain limitations. Regarding the pathogenesis of bruxism, the articles included constitute a small number of cases, with self-reporting subjective symptoms, which makes it difficult to provide a definite diagnosis. On the other hand, the actual levels of Mg and omega-3 fatty acids in the patients' circulation (plasma) and brain may not be easily assessed. In addition, patient variability is probably the reason behind the inconsistency in achieving the desired outcome in affected individuals, while there is also a lack of data regarding the optimum duration of nutrient supplementation to achieve the desired outcome. However, despite the small number of studies addressing these issues and their associated limitations, the existing evidence supports the rationale that subsequent research should be conducted in this field, with the aim to further enhance the current understanding of this complex, multifactorial condition and hopefully to provide the affected individuals with alternative and more effective therapeutic modalities.
Acknowledgements
This article is a part of and constitutes a (partial) requirement for the fellowship program on temporomandibular joint (TMJ) disorders of the TMJ Foundation, TMJ Consultancy Services (Bhopal, India) and DARSN Academy for Maxillofacial Education and Research (Damer, India).
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization: IAP. Writing and original draft preparation: IAP and MA. Review and editing: MA, VZ and DAS;.Visualization: IAP and MA. Supervision: VZ and MA. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate.
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
IAP, MA and VZ declare that they have no competing interests. DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision for this article.
References
- 1.Simoes WA. Occlusal plane: A clinical evaluation. J Clin Pediatr Dent. 1995;19:75–81. [PubMed] [Google Scholar]
- 2.Lobbezoo F, Ahlberg J, Raphael KG, Wetselaar P, Glaros AG, Kato T, Santiago V, Winocur E, De Laat A, De Leeuw R, et al. International consensus on the assessment of bruxism: Report of a work in progress. J Oral Rehabil. 2018;45:837–844. doi: 10.1111/joor.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: A systematic review of the literature. J Orofac Pain. 2013;27:99–110. doi: 10.11607/jop.921. [DOI] [PubMed] [Google Scholar]
- 4.Manfredini D, Piccotti F, Ferronato G, Guarda-Nardini L. Age peaks of different RDC/TMD diagnoses in a patient population. J Dent. 2010;38:392–399. doi: 10.1016/j.jdent.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: Dichotomous effects of COMT in neuropathic vs. nociceptive pain modalities. CNS Neurol Disord Drug Targets. 2012;11:222–235. doi: 10.2174/187152712800672490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Fierro N, Martinez-Fierro M, Cerda-Flores RM, Gómez-Govea MA, Delgado-Enciso I, Martínez-De-Villarreal LE, González-Ramírez MT, Rodríguez-Sánchez IP. The phenotype, psychotype and genotype of bruxism. Biomed Rep. 2018;8:264–268. doi: 10.3892/br.2018.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson CR, Okeson JP, Falace DA, Nitz AJ, Curran SL, Anderson D. Comparison of psychologic and physiologic functioning between patients with masticatory muscle pain and matched controls. J Orofac Pain. 1993;7:15–22. [PubMed] [Google Scholar]
- 8.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain. 2013;14 (12 Suppl):T75–T90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertoli E, de Leeuw R, Schmidt JE, Okeson JP, Carlson CR. Prevalence and impact of post-traumatic stress disorder symptoms in patients with masticatory muscle or temporomandibular joint pain: Differences and similarities. J Orofac Pain. 2007;21:107–119. [PubMed] [Google Scholar]
- 10.Fritzen VM, Colonetti T, Cruz MVB, Ferraz SD, Ceretta L, Tuon L, DA Rosa MI, Ceretta RA. Levels of salivary cortisol in adults and children with bruxism diagnosis: A systematic review and meta-analysis. J Evid Based Dent Pract. 2022;22(101634) doi: 10.1016/j.jebdp.2021.101634. [DOI] [PubMed] [Google Scholar]
- 11.Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: Its regulation and clinical implications. Biochim Biophys Acta. 2011;1812:581–591. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CM, Chou SL, Gale EN, McCall WD Jr. Human masticatory muscle activity and jaw position under experimental stress. J Oral Rehabil. 2002;29:44–51. doi: 10.1046/j.1365-2842.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- 13.de Leeuw JR, Steenks MH, Ros WJ, Lobbezoo-Scholte AM, Bosman F, Winnubst JA. Multidimensional evaluation of craniomandibular dysfunction. I: Symptoms and correlates. J Oral Rehabil. 1994;21:501–514. doi: 10.1111/j.1365-2842.1994.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 14.Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain. 2009;23:153–166. [PubMed] [Google Scholar]
- 15.Miyake H, Mori D, Katayama T, Fujiwara S, Sato Y, Azuma K, Kubo KY. Novel stress increases hypothalamic-pituitary-adrenal activity in mice with a raised bite. Arch Oral Biol. 2016;68:55–60. doi: 10.1016/j.archoralbio.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Aizawa H, Cui W, Tanaka K, Okamoto H. Hyperactivation of the habenula as a link between depression and sleep disturbance. Front Hum Neurosci. 2013;7(826) doi: 10.3389/fnhum.2013.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gameiro GH, da Silva Andrade A, Nouer DF, Ferraz de Arruda Veiga MC. How may stressful experiences contribute to the development of temporomandibular disorders? Clin Oral Investig. 2006;10:261–268. doi: 10.1007/s00784-006-0064-1. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Zhou KX, Yin NN, Zhang CK, Shi MH, Zhang HY, Wang DM, Xu ZJ, Zhang JD, Li JL, Wang MQ. Malocclusion generates anxiety-like behavior through a putative lateral habenula-mesencephalic trigeminal nucleus pathway. Front Mol Neurosci. 2019;12(174) doi: 10.3389/fnmol.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suvinen TI, Hanes KR, Gerschman JA, Reade PC. Psychophysical subtypes of temporomandibular disorders. J Orofac Pain. 1997;11:200–205. [PubMed] [Google Scholar]
- 20.Lavigne GJ, Kato T, Kolta A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14:30–46. doi: 10.1177/154411130301400104. [DOI] [PubMed] [Google Scholar]
- 21.Blanchet PJ, Rompre PH, Lavigne GJ, Lamarche C. Oral dyskinesia: A clinical overview. Int J Prosthodont. 2005;18:10–19. [PubMed] [Google Scholar]
- 22.Clark GT, Ram S. Four oral motor disorders: Bruxism, dystonia, dyskinesia and drug-induced dystonic extrapyramidal reactions. Dent Clin North Am. 2007;51:225–243. doi: 10.1016/j.cden.2006.09.002. viii-ix. [DOI] [PubMed] [Google Scholar]
- 23.Kwak YT, Han IW, Lee PH, Yoon JK, Suk SH. Associated conditions and clinical significance of awake bruxism. Geriatr Gerontol Int. 2009;9:382–390. doi: 10.1111/j.1447-0594.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 24.Winocur E, Gavish A, Voikovitch M, Emodi-Perlman A, Eli I. Drugs and bruxism: A critical review. J Orofac Pain. 2003;17:99–111. [PubMed] [Google Scholar]
- 25.Garrett AR, Hawley JS. SSRI-associated bruxism: A systematic review of published case reports. Neurol Clin Pract. 2018;8:135–141. doi: 10.1212/CPJ.0000000000000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendtsen L, Jensen R, Olesen J. Amitriptyline, a combined serotonin and noradrenaline re-uptake inhibitor, reduces exteroceptive suppression of temporal muscle activity in patients with chronic tension-type headache. Electroencephalogr Clin Neurophysiol. 1996;101:418–422. [PubMed] [Google Scholar]
- 27.Falisi G, Rastelli C, Panti F, Maglione H, Quezada Arcega R. Psychotropic drugs and bruxism. Expert Opin Drug Saf. 2014;13:1319–1326. doi: 10.1517/14740338.2014.947262. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto K, Imbe H, Tashiro A, Kimura A, Donishi T, Tamai Y, Senba E. The role of peripheral 5HT2A and 5HT1A receptors on the orofacial formalin test in rats with persistent temporomandibular joint inflammation. Neuroscience. 2005;130:465–474. doi: 10.1016/j.neuroscience.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi O, Ishikawa T. Involvement of peripheral 5-HT2A receptor activation in inflammatory pain. Nihon Rinsho. 2001;59:1675–1680. (In Japanese) [PubMed] [Google Scholar]
- 30.Chaouloff F. Regulation of 5-HT receptors by corticosteroids: Where do we stand? Fundam Clin Pharmacol. 1995;9:219–233. doi: 10.1111/j.1472-8206.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 31.Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Ann N Y Acad Sci. 1997;836:106–134. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- 32.Yeung LY, Kung HF, Yew DT. Localization of 5-HT1A and 5-HT2A positive cells in the brainstems of control age-matched and Alzheimer individuals. Age (Dordr) 2010;32:483–495. doi: 10.1007/s11357-010-9152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inan R, Senel GB, Yavlal F, Karadeniz D, Gunduz A, Kiziltan ME. Sleep bruxism is related to decreased inhibitory control of trigeminal motoneurons, but not with reticulobulbar system. Neurol Sci. 2017;38:75–81. doi: 10.1007/s10072-016-2711-x. [DOI] [PubMed] [Google Scholar]
- 34.Lauria G, Majorana A, Borgna M, Lombardi R, Penza P, Padovani A, Sapelli P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain. 2005;115:332–337. doi: 10.1016/j.pain.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Bostwick JM, Jaffee MS. Buspirone as an antidote to SSRI-induced bruxism in 4 cases. J Clin Psychiatry. 1999;60:857–860. doi: 10.4088/jcp.v60n1209. [DOI] [PubMed] [Google Scholar]
- 36.Belujon P, Grace AA. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci. 2015;282(20142516) doi: 10.1098/rspb.2014.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floresco SB. Dopaminergic regulation of limbic-striatal interplay. J Psychiatry Neurosci. 2007;32:400–411. [PMC free article] [PubMed] [Google Scholar]
- 38.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 39.Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: Cellular mechanisms of input selection. J Neurosci. 2001;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 42.Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: Response alteration by stress pre-exposure. Eur J Neurosci. 2012;35:1312–1321. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han W, Tellez LA, Rangel MJ Jr, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell. 2017;168:311–324 e318. doi: 10.1016/j.cell.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaya B, Geha P, de Araujo I, Cioffi I, Moayedi M. Identification of central amygdala and trigeminal motor nucleus connectivity in humans: An ultra-high field diffusion MRI study. Hum Brain Mapp. 2023;44:1309–1319. doi: 10.1002/hbm.26104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubo KY, Iinuma M, Chen H. Mastication as a stress-coping behavior. Biomed Res Int. 2015;2015(876409) doi: 10.1155/2015/876409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Iinuma M, Onozuka M, Kubo KY. Chewing maintains hippocampus-dependent cognitive function. Int J Med Sci. 2015;12:502–509. doi: 10.7150/ijms.11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muramoto T, Takano Y, Soma K. Time-related changes in periodontal mechanoreceptors in rat molars after the loss of occlusal stimuli. Arch Histol Cytol. 2000;63:369–380. doi: 10.1679/aohc.63.369. [DOI] [PubMed] [Google Scholar]
- 48.Mori D, Katayama T, Miyake H, Fujiwara S, Kubo KY. Occlusal disharmony leads to learning deficits associated with decreased cellular proliferation in the hippocampal dentate gyrus of SAMP8 mice. Neurosci Lett. 2013;534:228–232. doi: 10.1016/j.neulet.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Mori D, Miyake H, Mizutani K, Shimpo K, Sonoda S, Yamamoto T, Fujiwara S, Kubo KY. Effects of occlusal disharmony on the hippocampal dentate gyrus in aged senescence-accelerated mouse prone 8 (SAMP8) Arch Oral Biol. 2016;65:95–101. doi: 10.1016/j.archoralbio.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Azuma K, Ogura M, Kondo H, Suzuki A, Hayashi S, Iinuma M, Onozuka M, Kubo KY. Maternal active mastication during prenatal stress ameliorates prenatal stress-induced lower bone mass in adult mouse offspring. Int J Med Sci. 2017;14:348–355. doi: 10.7150/ijms.18298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki A, Iinuma M, Hayashi S, Sato Y, Azuma K, Kubo KY. Maternal chewing during prenatal stress ameliorates stress-induced hypomyelination, synaptic alterations, and learning impairment in mouse offspring. Brain Res. 2016;1651:36–43. doi: 10.1016/j.brainres.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Onishi M, Iinuma M, Tamura Y, Kubo KY. Learning deficits and suppression of the cell proliferation in the hippocampal dentate gyrus of offspring are attenuated by maternal chewing during prenatal stress. Neurosci Lett. 2014;560:77–80. doi: 10.1016/j.neulet.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson's disease. Front Neuroanat. 2015;9(91) doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berridge MJ. Vitamin D and Depression: Cellular and regulatory mechanisms. Pharmacol Rev. 2017;69:80–92. doi: 10.1124/pr.116.013227. [DOI] [PubMed] [Google Scholar]
- 55.Tohari AM, Zhou X, Shu X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem Funct. 2016;34:82–94. doi: 10.1002/cbf.3167. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe M, Narita M, Hamada Y, Yamashita A, Tamura H, Ikegami D, Kondo T, Shinzato T, Shimizu T, Fukuchi Y, et al. Activation of ventral tegmental area dopaminergic neurons reverses pathological allodynia resulting from nerve injury or bone cancer. Mol Pain. 2018;14(1744806918756406) doi: 10.1177/1744806918756406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martikainen IK, Nuechterlein EB, Pecina M, Love TM, Cummiford CM, Green CR, Stohler CS, Zubieta JK. Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. J Neurosci. 2015;35:9957–9965. doi: 10.1523/JNEUROSCI.4605-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282–297. doi: 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 60.Ramdani C, Carbonnell L, Vidal F, Beranger C, Dagher A, Hasbroucq T. Dopamine precursors depletion impairs impulse control in healthy volunteers. Psychopharmacology (Berl) 2015;232:477–487. doi: 10.1007/s00213-014-3686-z. [DOI] [PubMed] [Google Scholar]
- 61.Zhao YJ, Liu Y, Wang J, Li Q, Zhang ZM, Tu T, Lei R, Zhang M, Chen YJ. Activation of the mesencephalic trigeminal nucleus contributes to masseter hyperactivity induced by chronic restraint stress. Front Cell Neurosci. 2022;16(841133) doi: 10.3389/fncel.2022.841133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueno Y, Higashiyama M, Haque T, Masuda Y, Katagiri A, Toyoda H, Uzawa N, Yoshida A, Kato T. Motor representation of rhythmic jaw movements in the amygdala of guinea pigs. Arch Oral Biol. 2022;135(105362) doi: 10.1016/j.archoralbio.2022.105362. [DOI] [PubMed] [Google Scholar]
- 63.Keskinruzgar A DDS, PhD Kalenderoglu A MD, Yapici Yavuz G DDS, PhD Koparal M DDS, PhD Simsek A MD, Karadag AS MD, Utkun M DDS. Investigation of neurodegenerative and inflammatory processes in sleep bruxism. Cranio. 2020;38:358–364. doi: 10.1080/08869634.2018.1543829. [DOI] [PubMed] [Google Scholar]
- 64.Verhoeff MC, Koutris M, Berendse HW, van Dijk KD, Lobbezoo F. Parkinson's disease, temporomandibular disorder pain and bruxism and its clinical consequences: A protocol of a single-centre observational outpatient study. BMJ Open. 2022;12(e052329) doi: 10.1136/bmjopen-2021-052329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe S, Gagnon JF, Montplaisir JY, Postuma RB, Rompré PH, Huynh NT, Kato T, Kawano F, Lavigne GJ. Sleep bruxism and oromandibular myoclonus in rapid eye movement sleep behavior disorder: A preliminary report. Sleep Med. 2013;14:1024–1030. doi: 10.1016/j.sleep.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Ozcan-Kucuk A, Ege B, Koparal M, Gonel A, Koyuncu I. Evaluation of the oxidative stress level and serum prolidase activity in patients with sleep bruxism. Comb Chem High Throughput Screen. 2021;24:286–293. doi: 10.2174/1386207323999200729114410. [DOI] [PubMed] [Google Scholar]
- 67.Selek S, Altindag A, Saracoglu G, Celik H, Aksoy N. Prolidase activity and its diagnostic performance in bipolar disorder. J Affect Disord. 2011;129:84–86. doi: 10.1016/j.jad.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Ercan AC, Bahceci B, Polat S, Cenker OC, Bahceci I, Koroglu A, Sahin K, Hocaoglu C. Oxidative status and prolidase activities in generalized anxiety disorder. Asian J Psychiatr. 2017;25:118–122. doi: 10.1016/j.ajp.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Altay MA, Erturk C, Bilge A, Yapti M, Levent A, Aksoy N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: Relationships with radiographic severity and clinical parameters. Rheumatol Int. 2015;35:1725–1731. doi: 10.1007/s00296-015-3290-5. [DOI] [PubMed] [Google Scholar]
- 70.Baspinar S, Kirnap M, Baspinar O, Dizdar OS, Kocer D. Serum prolidase level in ankylosing spondylitis: Low serum levels as a new potential gold standard biomarker for disease activity. Rheumatol Int. 2016;36:1609–1616. doi: 10.1007/s00296-016-3536-x. [DOI] [PubMed] [Google Scholar]
- 71.Arikanoglu A, Akil E, Varol S, Yucel Y, Yuksel H, Cevik MU, Palanci Y, Unan F. Relationship of cognitive performance with prolidase and oxidative stress in Alzheimer disease. Neurol Sci. 2013;34:2117–2121. doi: 10.1007/s10072-013-1346-4. [DOI] [PubMed] [Google Scholar]
- 72.Minakuchi H, Fujisawa M, Abe Y, Iida T, Oki K, Okura K, Tanabe N, Nishiyama A. Managements of sleep bruxism in adult: A systematic review. Jpn Dent Sci Rev. 2022;58:124–136. doi: 10.1016/j.jdsr.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 74.Brown AJ, Dusso AS, Slatopolsky E. Vitamin D analogues for secondary hyperparathyroidism. Nephrol Dial Transplant. 2002;17 (Suppl 10):S10–S19. doi: 10.1093/ndt/17.suppl_10.10. [DOI] [PubMed] [Google Scholar]
- 75.Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007:1–235. [PMC free article] [PubMed] [Google Scholar]
- 76.Berridge MJ. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2016;371(20150434) doi: 10.1098/rstb.2015.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koundourakis NE, Avgoustinaki PD, Malliaraki N, Margioris AN. Muscular effects of vitamin D in young athletes and non-athletes and in the elderly. Hormones (Athens) 2016;15:471–488. doi: 10.14310/horm.2002.1705. [DOI] [PubMed] [Google Scholar]
- 78.Gascon-Barre M, Huet PM. Apparent [3H]1,25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am J Physiol. 1983;244:E266–E271. doi: 10.1152/ajpendo.1983.244.3.E266. [DOI] [PubMed] [Google Scholar]
- 79.Chau YY, Kumar J. Vitamin D in chronic kidney disease. Indian J Pediatr. 2012;79:1062–1068. doi: 10.1007/s12098-012-0765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groves NJ, McGrath JJ, Burne TH. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr. 2014;34:117–141. doi: 10.1146/annurev-nutr-071813-105557. [DOI] [PubMed] [Google Scholar]
- 82.Dimitrakis E, Katsarou MS, Lagiou M, Papastefanopoulou V, Spandidos DA, Tsatsakis A, Papageorgiou S, Moutsatsou P, Antoniou K, Kroupis C, Drakoulis N. Association of vitamin D receptor gene haplotypes with late-onset Alzheimer's disease in a Southeastern European Caucasian population. Exp Ther Med. 2022;24(584) doi: 10.3892/etm.2022.11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ikonen H, Palaniswamy S, Nordstrom T, Järvelin MR, Herzig KH, Jääskeläinen E, Seppälä J, Miettunen J, Sebert S. Vitamin D status and correlates of low vitamin D in schizophrenia, other psychoses and non-psychotic depression-The Northern Finland Birth Cohort 1966 study. Psychiatry Res. 2019;279:186–194. doi: 10.1016/j.psychres.2019.02.060. [DOI] [PubMed] [Google Scholar]
- 84.Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 85.Klotz B, Mentrup B, Regensburger M, Zeck S, Schneidereit J, Schupp N, Linden C, Merz C, Ebert R, Jakob F. 1,25-dihydroxyvitamin D3 treatment delays cellular aging in human mesenchymal stem cells while maintaining their multipotent capacity. PLoS One. 2012;7(e29959) doi: 10.1371/journal.pone.0029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cass WA, Peters LE, Fletcher AM, Yurek DM. Calcitriol promotes augmented dopamine release in the lesioned striatum of 6-hydroxydopamine treated rats. Neurochem Res. 2014;39:1467–1476. doi: 10.1007/s11064-014-1331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orme RP, Middleditch C, Waite L, Fricker RA. The role of vitamin D(3) in the development and neuroprotection of midbrain dopamine neurons. Vitam Horm. 2016;100:273–297. doi: 10.1016/bs.vh.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193–203. doi: 10.1016/j.neuroscience.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 89.Kesby JP, Cui X, O'Loan J, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology (Berl) 2010;208:159–168. doi: 10.1007/s00213-009-1717-y. [DOI] [PubMed] [Google Scholar]
- 90.Huang JY, Arnold D, Qiu CF, Miller RS, Williams MA, Enquobahrie DA. Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J Womens Health (Larchmt) 2014;23:588–595. doi: 10.1089/jwh.2013.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Altınbas A. The effect of vitamin D levels on the mood disorders of the operating room and intensive care unit staff. J Clin Anal Med. 2019;10:156–161. [Google Scholar]
- 92.Al-Atram A, Raghunath G, Kannan SK. The Relationship between Vitamin D and Mental Health among Dental Students in Saudi Arabia: A Descriptive Cross-Sectional Study. J Clin Diag Res. 2020;14:VC07–VC10. [Google Scholar]
- 93.Silva MRM, Barros WMA, Silva MLD, Silva JMLD, Souza APDS, Silva ABJD, Fernandes MSS, Souza SL, Souza VON. Relationship between vitamin D deficiency and psychophysiological variables: A systematic review of the literature. Clinics (Sao Paulo) 2021;76(e3155) doi: 10.6061/clinics/2021/e3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Croll PH, Boelens M, Vernooij MW, van de Rest O, Zillikens MC, Ikram MA, Voortman T. Associations of vitamin D deficiency with MRI markers of brain health in a community sample. Clin Nutr. 2021;40:72–78. doi: 10.1016/j.clnu.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 95.Johnson MM, Patel S, Williams J. Don't Take It ‘Lytely’: A case of acute tetany. Cureus. 2019;11(e5845) doi: 10.7759/cureus.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Straube S, Derry S, Moore RA, McQuay HJ. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2010;2010(CD007771) doi: 10.1002/14651858.CD007771.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alkhatatbeh MJ, Hmoud ZL, Abdul-Razzak KK, Alem EM. Self-reported sleep bruxism is associated with vitamin D deficiency and low dietary calcium intake: A case-control study. BMC Oral Health. 2021;21(21) doi: 10.1186/s12903-020-01349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anjum I, Jaffery SS, Fayyaz M, Samoo Z, Anjum S. The role of vitamin D in brain health: A mini literature review. Cureus. 2018;10(e2960) doi: 10.7759/cureus.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allaf BAW, Abdul-Hak M. Association between bruxism severity and serum concentrations of 25-hydroxyvitamin D levels. Clin Exp Dent Res. 2022;8:827–835. doi: 10.1002/cre2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garfinkel RJ, Dilisio MF, Agrawal DK. Vitamin D and its effects on articular cartilage and osteoarthritis. Orthop J Sports Med. 2017;5(2325967117711376) doi: 10.1177/2325967117711376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nowaczewska M, Wicinski M, Osinski S, Kazmierczak H. The role of vitamin D in primary headache-from potential mechanism to treatment. Nutrients. 2020;12(243) doi: 10.3390/nu12010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sohn JH, Chu MK, Park KY, Ahn HY, Cho SJ. Vitamin D deficiency in patients with cluster headache: A preliminary study. J Headache Pain. 2018;19(54) doi: 10.1186/s10194-018-0886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yildiz S, Tümer MK, Yigit S, Nursal AF, Rustemoglu A, Balel Y. Relationship of Vitamin D and Bsml variant with temporomandibular diseases in Turkish population. Br J Oral Maxillofac Surg. 2021;59:555–560. doi: 10.1016/j.bjoms.2020.08.101. [DOI] [PubMed] [Google Scholar]
- 104.Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. The association between vitamin D concentration and pain: A systematic review and meta-analysis. Public Health Nutr. 2018;21:2022–2037. doi: 10.1017/S1368980018000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Demir CY, Ersoz ME. Biochemical changes associated with temporomandibular disorders. J Int Med Res. 2019;47:765–771. doi: 10.1177/0300060518811009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lima LAR, Lopes MJP, Costa RO, Lima FAV, Neves KRT, Calou IBF, Andrade GM, Viana GSB. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J Neuroinflammation. 2018;15(249) doi: 10.1186/s12974-018-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin X, Antony B, Wang X, Persson MS, McAlindon T, Arden NK, Srivastava S, Srivastava R, Van Middelkoop M, Bierma-Zeinstra SM, et al. Effect of vitamin D supplementation on pain and physical function in patients with knee osteoarthritis (OA): An OA Trial Bank protocol for a systematic review and individual patient data (IPD) meta-analysis. BMJ Open. 2020;10(e035302) doi: 10.1136/bmjopen-2019-035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manoy P, Yuktanandana P, Tanavalee A, Anomasiri W, Ngarmukos S, Tanpowpong T, Honsawek S. Vitamin D supplementation improves quality of life and physical performance in osteoarthritis patients. Nutrients. 2017;9(799) doi: 10.3390/nu9080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 111.Quraishi SA, Camargo CA Jr. Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15:625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee P. Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:769–781. doi: 10.1016/j.beem.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Szewczyk B, Poleszak E, Sowa-Kucma M, Siwek M, Dudek D, Ryszewska-Pokraśniewicz B, Radziwoń-Zaleska M, Opoka W, Czekaj J, Pilc A, Nowak G. Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol Rep. 2008;60:588–589. [PubMed] [Google Scholar]
- 114.Vink R, Nechifor M (eds) Magnesium in the Central Nervous System. University of Adelaide Press, Adelaide, AU, 2011. [PubMed] [Google Scholar]
- 115.Wolf FI, Maier JA, Nasulewicz A, Feillet-Coudray C, Simonacci M, Mazur A, Cittadini A. Magnesium and neoplasia: From carcinogenesis to tumor growth and progression or treatment. Arch Biochem Biophys. 2007;458:24–32. doi: 10.1016/j.abb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 116.Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses. 2006;67:362–370. doi: 10.1016/j.mehy.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 117.Seelig MS. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications (a review) J Am Coll Nutr. 1994;13:429–446. doi: 10.1080/07315724.1994.10718432. [DOI] [PubMed] [Google Scholar]
- 118.Sivoňová M, Žitňanová I, Hlinčíková L, Škodáček I, Trebatická J, Ďuračková Z. Oxidative stress in university students during examinations. Stress. 2004;7:183–188. doi: 10.1080/10253890400012685. [DOI] [PubMed] [Google Scholar]
- 119.Cratty MS, Birkle DL. N-methyl-D-aspartate (NMDA)-mediated corticotropin-releasing factor (CRF) release in cultured rat amygdala neurons. Peptides. 1999;20:93–100. doi: 10.1016/s0196-9781(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 120.Perrin MH, Haas Y, Rivier JE, Vale WW. Corticotropin-releasing factor binding to the anterior pituitary receptor is modulated by divalent cations and guanyl nucleotides. Endocrinology. 1986;118:1171–1179. doi: 10.1210/endo-118-3-1171. [DOI] [PubMed] [Google Scholar]
- 121.De Souza EB. Corticotropin-releasing factor receptors: Physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 122.Murck H. Magnesium and affective disorders. Nutr Neurosci. 2002;5:375–389. doi: 10.1080/1028415021000039194. [DOI] [PubMed] [Google Scholar]
- 123.Henrotte JG, Franck G, Santarromana M, Frances H, Mouton D, Motta R. Mice selected for low and high blood magnesium levels: A new model for stress studies. Physiol Behav. 1997;61:653–658. doi: 10.1016/s0031-9384(96)00506-9. [DOI] [PubMed] [Google Scholar]
- 124.Kramer JH, Spurney C, Iantorno M, Tziros C, Mak IT, Tejero-Taldo MI, Chmielinska JJ, Komarov AM, Weglicki WB. Neurogenic inflammation and cardiac dysfunction due to hypomagnesemia. Am J Med Sci. 2009;338:22–27. doi: 10.1097/MAJ.0b013e3181aaee4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tejero-Taldo MI, Kramer JH, Mak IuT, Komarov AM, Weglicki WB. The nerve-heart connection in the pro-oxidant response to Mg-deficiency. Heart Fail Rev. 2006;11:35–44. doi: 10.1007/s10741-006-9191-7. [DOI] [PubMed] [Google Scholar]
- 126.Rickards H, Dursun SM, Farrar G, Betts T, Corbett JA, Handley SL. Increased plasma kynurenine and its relationship to neopterin and tryptophan in Tourette's syndrome. Psychol Med. 1996;26:857–862. doi: 10.1017/s0033291700037880. [DOI] [PubMed] [Google Scholar]
- 127.Nakamura M, Abe S, Goto Y, Chishaki A, Akazawa K, Kato M. In vivo assessment of prevention of white-noise-induced seizure in magnesium-deficient rats by N-methyl-D-aspartate receptor blockers. Epilepsy Res. 1994;17:249–256. doi: 10.1016/0920-1211(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 128.Planells E, Lerma A, Sanchez-Morito N, Aranda P, LLopis J. Effect of magnesium deficiency on vitamin B2 and B6 status in the rat. J Am Coll Nutr. 1997;16:352–356. doi: 10.1080/07315724.1997.10718697. [DOI] [PubMed] [Google Scholar]
- 129.Fowler B. Recent advances in the mechanism of pyridoxine-responsive disorders. J Inherit Metab Dis. 1985;8 (Suppl 1):S76–S83. doi: 10.1007/BF01800664. [DOI] [PubMed] [Google Scholar]
- 130.Henkin RI, Gouliouk V, Fordyce A. Distinguishing patients with glossopyrosis from those with oropyrosis based upon clinical differences and differences in saliva and erythrocyte magnesium. Arch Oral Biol. 2012;57:205–210. doi: 10.1016/j.archoralbio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 131.Abaamrane L, Raffin F, Gal M, Avan P, Sendowski I. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2009;247:137–145. doi: 10.1016/j.heares.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 132.Muneyyirci-Delale O, Nacharaju VL, Dalloul M, Altura BM, Altura BT. Serum ionized magnesium and calcium in women after menopause: Inverse relation of estrogen with ionized magnesium. Fertil Steril. 1999;71:869–872. doi: 10.1016/s0015-0282(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 133.Touyz RM. Magnesium in clinical medicine. Front Biosci. 2004;9:1278–1293. doi: 10.2741/1316. [DOI] [PubMed] [Google Scholar]
- 134.Durlach J, Bac P, Durlach V, Bara M, Guiet-Bara A. Neurotic, neuromuscular and autonomic nervous form of magnesium imbalance. Magnes Res. 1997;10:169–195. (In English, French) [PubMed] [Google Scholar]
- 135.Caddell JL. A triple-risk model for the sudden infant death syndrome (SIDS) and the apparent life-threatening episode (ALTE): The stressed magnesium deficient weanling rat. Magnes Res. 2001;14:227–238. [PubMed] [Google Scholar]
- 136.Omiya K, Akashi YJ, Yoneyama K, Osada N, Tanabe K, Miyake F. Heart-rate response to sympathetic nervous stimulation, exercise, and magnesium concentration in various sleep conditions. Int J Sport Nutr Exerc Metab. 2009;19:127–135. doi: 10.1123/ijsnem.19.2.127. [DOI] [PubMed] [Google Scholar]
- 137.Sarchielli P, Coata G, Firenze C, Morucci P, Abbritti G, Gallai V. Serum and salivary magnesium levels in migraine and tension-type headache. Results in a group of adult patients. Cephalalgia. 1992;12:21–27. doi: 10.1046/j.1468-2982.1992.1201021.x. [DOI] [PubMed] [Google Scholar]
- 138.Thomas J, Tomb E, Thomas E, Faure G. Migraine treatment by oral magnesium intake and correction of the irritation of buccofacial and cervical muscles as a side effect of mandibular imbalance. Magnes Res. 1994;7:123–127. [PubMed] [Google Scholar]
- 139.Bagheri G, Rezaee R, Tsarouhas K, Docea AO, Shahraki J, Shahriari M, Wilks MF, Jahantigh H, Tabrizian K, Moghadam AA, et al. Magnesium sulfate ameliorates carbon monoxide-induced cerebral injury in male rats. Mol Med Rep. 2019;19:1032–1039. doi: 10.3892/mmr.2018.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia-Lopez R, Perea-Milla E, Garcia CR, Rivas-Ruiz F, Romero-Gonzalez J, Moreno JL, Faus V, Aguas Gdel C, Diaz JC. New therapeutic approach to Tourette Syndrome in children based on a randomized placebo-controlled double-blind phase IV study of the effectiveness and safety of magnesium and vitamin B6. Trials. 2009;10(16) doi: 10.1186/1745-6215-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cernak I, Savic V, Kotur J, Prokic V, Kuljic B, Grbovic D, Veljovic M. Alterations in magnesium and oxidative status during chronic emotional stress. Magnes Res. 2000;13:29–36. [PubMed] [Google Scholar]
- 142.Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23:199–208. [PubMed] [Google Scholar]
- 143.Esch T, Stefano GB, Fricchione GL, Benson H. Stress-related diseases-a potential role for nitric oxide. Med Sci Monit. 2002;8:RA103–RA118. [PubMed] [Google Scholar]
- 144.Truelsen T, Nielsen N, Boysen G, Gronbaek M. Self-reported stress and risk of stroke: The Copenhagen City Heart Study. Stroke. 2003;34:856–862. doi: 10.1161/01.STR.0000062345.80774.40. Copenhagen City Heart Study. [DOI] [PubMed] [Google Scholar]
- 145.Ella B, Ghorayeb I, Burbaud P, Guehl D. Bruxism in movement disorders: A comprehensive review. J Prosthodont. 2017;26:599–605. doi: 10.1111/jopr.12479. [DOI] [PubMed] [Google Scholar]
- 146.Dickoff D. Bruxism Associated with Restless Limbs Syndrome (RLS): A Dopamine Responsive Movement Disorder (P02.063) Neurology. 2013;80 (Suppl 7)(P02.063) [Google Scholar]
- 147.Grimaldi BL. The central role of magnesium deficiency in Tourette's syndrome: Causal relationships between magnesium deficiency, altered biochemical pathways and symptoms relating to Tourette's syndrome and several reported comorbid conditions. Med Hypotheses. 2002;58:47–60. doi: 10.1054/mehy.2001.1447. [DOI] [PubMed] [Google Scholar]
- 148.Rizzo G, Li X, Galantucci S, Filippi M, Cho YW. Brain imaging and networks in restless legs syndrome. Sleep Med. 2017;31:39–48. doi: 10.1016/j.sleep.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Ondo WG, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–309. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 150.Allen RP, Earley CJ. The role of iron in restless legs syndrome. Mov Disord. 2007;22 (Suppl 18):S440–S448. doi: 10.1002/mds.21607. [DOI] [PubMed] [Google Scholar]
- 151.Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88:261–264. doi: 10.1002/ajh.23397. [DOI] [PubMed] [Google Scholar]
- 152.Umbreit J. Iron deficiency: A concise review. Am J Hematol. 2005;78:225–231. doi: 10.1002/ajh.20249. [DOI] [PubMed] [Google Scholar]
- 153.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 154.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 155.Allen RP, Picchietti DL, Auerbach M, Cho YW, Connor JR, Earley CJ, Garcia-Borreguero D, Kotagal S, Manconi M, Ondo W, et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: An IRLSSG task force report. Sleep Med. 2018;41:27–44. doi: 10.1016/j.sleep.2017.11.1126. [DOI] [PubMed] [Google Scholar]
- 156.Trotti LM, Becker LA. Iron for the treatment of restless legs syndrome. Cochrane Database Syst Rev. 2019;1(CD007834) doi: 10.1002/14651858.CD007834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wei C, Zhou J, Huang X, Li M. Effects of psychological stress on serum iron and erythropoiesis. Int J Hematol. 2008;88:52–56. doi: 10.1007/s12185-008-0105-4. [DOI] [PubMed] [Google Scholar]
- 158.Singh A, Smoak BL, Patterson KY, LeMay LG, Veillon C, Deuster PA. Biochemical indices of selected trace minerals in men: Effect of stress. Am J Clin Nutr. 1991;53:126–131. doi: 10.1093/ajcn/53.1.126. [DOI] [PubMed] [Google Scholar]
- 159.Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P, Andersen ML, Tufik S, Lima MM. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res. 2011;219:116–122. doi: 10.1016/j.bbr.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 160.Laye S, Nadjar A, Joffre C, Bazinet RP. Anti-Inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol Rev. 2018;70:12–38. doi: 10.1124/pr.117.014092. [DOI] [PubMed] [Google Scholar]
- 161.Larrieu T, Hilal ML, Fourrier C, De Smedt-Peyrusse V, Sans N, Capuron L, Layé S. Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl Psychiatry. 2014;4(e437) doi: 10.1038/tp.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Joffre C, Gregoire S, De Smedt V, Acar N, Bretillon L, Nadjar A, Layé S. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot Essent Fatty Acids. 2016;114:1–10. doi: 10.1016/j.plefa.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 164.Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol. 2008;286 (1-2 Suppl 1):S60–S65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 166.Larrieu T, Laye S. Food for mood: Relevance of nutritional omega-3 fatty acids for depression and anxiety. Front Physiol. 2018;9(1047) doi: 10.3389/fphys.2018.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Auguste S, Fisette A, Fernandes MF, Hryhorczuk C, Poitout V, Alquier T, Fulton S. Central Agonism of GPR120 acutely inhibits food intake and food reward and chronically suppresses anxiety-like behavior in mice. Int J Neuropsychopharmacol. 2016;19(pyw014) doi: 10.1093/ijnp/pyw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12:207–227. [PubMed] [Google Scholar]
- 169.Simopoulos AP. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 170.Carrie I, Clement M, de Javel D, Frances H, Bourre JM. Phospholipid supplementation reverses behavioral and biochemical alterations induced by n-3 polyunsaturated fatty acid deficiency in mice. J Lipid Res. 2000;41:473–480. [PubMed] [Google Scholar]
- 171.Bondi CO, Taha AY, Tock JL, Totah NK, Cheon Y, Torres GE, Rapoport SI, Moghaddam B. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol Psychiatry. 2014;75:38–46. doi: 10.1016/j.biopsych.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: Relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- 173.Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME. Microglia across the lifespan: From origin to function in brain development, plasticity and cognition. J Physiol. 2017;595:1929–1945. doi: 10.1113/JP272134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moon DO, Kim KC, Jin CY, Han MH, Park C, Lee KJ, Park YM, Choi YH, Kim GY. Inhibitory effects of eicosapentaenoic acid on lipopolysaccharide-induced activation in BV2 microglia. Int Immunopharmacol. 2007;7:222–229. doi: 10.1016/j.intimp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 175.De Smedt-Peyrusse V, Sargueil F, Moranis A, Harizi H, Mongrand S, Laye S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105:296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- 176.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, Ma DW, Serhan CN, Bazinet RP. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127:378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, Roche OJ, O'Connell F, Lynch MA. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging. 2007;28:845–855. doi: 10.1016/j.neurobiolaging.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 179.Huang CT, Tsai YJ. Docosahexaenoic acid confers analgesic effects after median nerve injury via inhibition of c-Jun N-terminal kinase activation in microglia. J Nutr Biochem. 2016;29:97–106. doi: 10.1016/j.jnutbio.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 180.Mirza M, Volz C, Karlstetter M, Langiu M, Somogyi A, Ruonala MO, Tamm ER, Jägle H, Langmann T. Progressive retinal degeneration and glial activation in the CLN6 (nclf) mouse model of neuronal ceroid lipofuscinosis: A beneficial effect of DHA and curcumin supplementation. PLoS One. 2013;8(e75963) doi: 10.1371/journal.pone.0075963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. Headache Classification Committee of the International Headache Society (IHS) [DOI] [PubMed] [Google Scholar]
- 183.Ashina M. Migraine. N Engl J Med. 2020;383:1866–1876. doi: 10.1056/NEJMra1915327. [DOI] [PubMed] [Google Scholar]
- 184.Marchetti M, Gualtieri P, De Lorenzo A, Trombetta D, Smeriglio A, Ingegneri M, Cianci R, Frank G, Schifano G, Bigioni G, Di Renzo L. Dietary ω-3 intake for the treatment of morning headache: A randomized controlled trial. Front Neurol. 2022;13(987958) doi: 10.3389/fneur.2022.987958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ramsden CE, Zamora D, Faurot KR, MacIntosh B, Horowitz M, Keyes GS, Yuan ZX, Miller V, Lynch C, Honvoh G, et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: Randomized controlled trial. BMJ. 2021;374(n1448) doi: 10.1136/bmj.n1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, MacIntosh BA, Gaylord S, Ringel A, Hibbeln JR, Feldstein AE, Mori TA, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain. 2013;154:2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.