One of the best-documented aspects of the biology of luminous bacteria has been their abundance and distribution in the marine environment (reviewed in reference 51). This has resulted in part from the relative ease of studying these microorganisms; the bioluminescence produced by most of the colonies formed by this group of bacteria makes them readily recognizable upon primary isolation, and a robust and simple phenotype-based taxonomy is available for species-level identification (66). Past studies have demonstrated that marine luminous bacteria, and Vibrio fischeri in particular, are remarkably successful at adapting to a variety of ecological niches (50). They are commonly present as planktonic cells in seawater or sediments (59, 63, 70, 74), and as saprophytes they populate fecal material and the surfaces of dead or dying marine animals (1, 8, 37). V. fischeri and other luminous bacteria also form a variety of pathogenic and cooperative associations with marine animals: they are increasingly recognized as causes of invertebrate diseases (2, 22, 38, 77); they are a common constituent of enteric tract microbial consortia (60, 64, 72); and they encompass the four described species (V. fischeri, Vibrio logei, Photobacterium phosphoreum, and Photobacterium leiognathi) that form stable, cooperative associations in specialized symbiotic organs of marine squids and fishes (18, 51).
Although many studies have reported specific niches and predictable patterns in geographical distribution for luminous bacterial species (17, 50, 59), the factors underlying their ecological dynamics (e.g., their movement between niches and their formation of stable subspecies or biovars) have, until recently, been poorly understood. We discuss here what is currently known about the extent to which the population of planktonic V. fischeri can influence, or be influenced by, its existence in symbiotic niches (24, 50).
GENERAL ECOLOGICAL STUDIES OF V. FISCHERI
Early studies of the ecology of luminous bacteria concluded that different species were more likely to be found in environments with particular patterns of temperature, salinity, nutrient concentration, or solar irradiation (55, 74, 75, 87). These observations led to the idea that the abundance and distribution of each luminous species were driven principally by abiotic environmental factors. While this correlation may apply to bacteria in the planktonic or enteric niches (72), the specificity of light organ symbionts for their host species can dominate these abiotic factors. For example, the occurrence of the typically warm-water species P. leiognathi in the organs of both temperate-water and tropical leiognathid fishes and the occurrence of the typically temperate-water species V. fischeri in the light organs of both tropical and temperate-water monocentrid fishes present convincing evidence of the dominance of the needs of the symbiotic interaction over the effects of the abiotic environment (23, 42). Thus, the role and importance of both abiotic and biotic factors must be considered when we predict what species of luminous bacteria will be found in a given habitat.
The light organ association between V. fischeri and the bobtail sepiolid squid Euprymna scolopes has recently emerged as a model system for investigating the process of bacterial colonization of host tissues and its effects on host development (46, 67, 71). This system has also begun to contribute to our understanding of the role(s) of symbiotic associations in the dynamics of V. fischeri ecology. In the course of examining how the symbiosis is established and maintained, it has become apparent that the exchange of these bacteria between the host and the ambient seawater environment is a fundamental feature (30). This ecological relationship between V. fischeri and the light organs of bobtail squids will be the focus of the following paragraphs.
ROLE OF E. SCOLOPES LIGHT ORGAN SYMBIOSIS IN V. FISCHERI ECOLOGY
Symbiotic associations that employ horizontal transmission of their microbial symbionts between host generations can have a direct impact on the ecology of these symbionts by modulating both their relative abundance and their geographical distribution. For example, the occurrence of a legume host species has a major effect on the presence of its specific Rhizobium species in the ambient soil (29, 86). Unfortunately, little has been reported about the role that aquatic animal hosts play in the ecology of their symbiotic bacterial partners, and there is even less documentation of the effect symbionts may have on the distribution of their hosts (13). In 1980 Ruby et al. proposed that the relatively high concentration of the luminous bacterium P. phosphoreum in oceanic seawater collected at depths of several hundred meters was a result of the expulsion of these bacteria from the symbiotic light organs of fishes that are typically present at these midwater depths (70). Subsequently, Haygood et al. (25) and Nealson et al. (52) demonstrated that there was a continuous release of luminous bacteria from pores in the light organs of both monocentrid and anomalopid fishes held in the laboratory and suggested that, in nature, this release may have a major impact on the abundance of these bacteria in the host’s habitat.
Support for these hypotheses awaited the identification of a more tractable host organism than these fish species and one that inhabits an accessible natural environment about which specific issues could be experimentally addressed. The association between V. fischeri and E. scolopes has provided an understanding of three aspects of symbiotic ecology: (i) the mechanism by which the host serves as a source of bacteria entering the ambient environment, (ii) the magnitude of this activity and its effects on the abundance and distribution of V. fischeri cells, and (iii) the importance of this activity to the continuation of the association from generation to generation.
EXPULSION OF V. FISCHERI CELLS INTO SEAWATER BY E. SCOLOPES
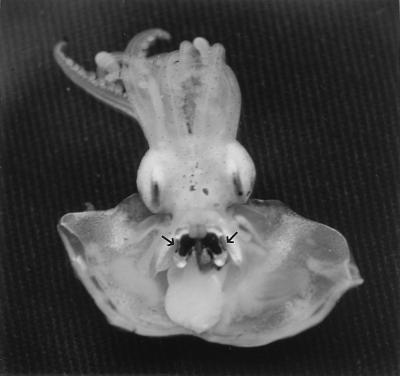
During its nighttime foraging activity in shallow-water reefs of Hawaii (4), E. scolopes is believed to use the ventrally directed light of its bacterial symbionts in a camouflaging behavior called counterillumination (41). Throughout the bobtail squid’s life cycle, the light organ retains pores that connect its internal symbiont-containing crypts with the ambient seawater (Fig. 1) (44, 47). These observations provided morphological evidence that the host has the potential to expel symbiotic bacteria into the surrounding environment. The appearance of V. fischeri cells in seawater containing adult E. scolopes confirmed that such a release occurred from squid light organs (34). However, unlike the apparently continuous expulsion activity reported for fishes (25, 52), symbiont release by E. scolopes occurred as a single pulse that exhibited a 24-h periodicity (34); that is, each morning approximately 90 to 95% of the symbiont population was expelled within a discrete 1-h period following sunrise or other environmental illumination. Studies of newly colonized squid hatchlings revealed that a similar light-initiated pattern of symbiont expulsion occurs in juvenile squids and that the pattern actually begins within the first 24 h of colonization (7). This release pattern is consistent with what is known of the normal behavior of the host in the field. In the natural environment, the onset of daylight signals the end of the normal nocturnal activity of the squid (41, 76). By this time the squid has buried itself in the sandy bottom of its shallow-water habitat, and not only is there no longer a need to produce luminescence, but even if the animal is disturbed and must leave the sand, its bioluminescence capacity is far too weak to be of any use in counterillumination during daylight. Thus, the diel expulsion of a large portion of its symbiotic population probably has no detrimental effect on the host’s survival. It has been suggested that by expelling the symbionts, the metabolic cost of maintaining them and their luminescence may be significantly reduced (25); however, neither the percentage of the host’s metabolic energy that is typically consumed by its symbiotic bacterial population nor the relative cost of repopulating the light organ each day is known. In addition, there is no evidence that V. fischeri cells can continue to luminesce in a nongrowing state indefinitely; thus, unlike some rhizobium species that continue to fix nitrogen in a terminally differentiated bacteroid state (80), luminous symbionts, in order to produce light in the host, may have to be part of a growing culture. As a result, the host would have to expel a portion of its bacteria, either continuously or periodically, to maintain a roughly uniform symbiont population size within the organ.
FIG. 1.
Light-emitting organ of E. scolopes. Shown is a ventral dissection of an adult specimen revealing the mantle cavity, within which lies the large bilobed silver and black bioluminescent organ. Every morning the contents of the organ, composed of symbiotic V. fischeri cells and the fluid surrounding them, are expelled from a pair of pores (arrows) on the lateral surfaces of the organ.
The net growth rate of the squid symbionts can be estimated from the number of cells expelled and the number remaining to repopulate the organ to its typical maximum density. This calculation is based on the assumption that there is no degradation or lysis of V. fischeri cells in the crypts (47). Because a preponderance of the bacteria are expelled as a pulse each morning, those bacteria remaining within the light organ must subsequently proliferate to a functionally useful level within the next 12 h, after which the host begins to forage again in the water column (76). As yet, the growth kinetics of the remaining bacteria are not known: do they begin to grow immediately at a low, continuous rate; do they grow rapidly and cease growth once they fully repopulate; or do they remain dormant until just before dark, at which time they rapidly repopulate the organ? Because of the individual variation in symbiont population size among hosts and the need to sacrifice the animals for each determination of the number of bacteria present, there has not yet been sufficient precision in these measurements to answer this question. However, if one assumes a constant rate of growth between expulsions, an average doubling time of 4.8 h can be calculated (34), a value that is only about 10% of the bacteria’s maximum growth rate (5). Similar calculations of the average growth rate of symbionts in the light organs of monocentrid and anomalopid fishes (25, 52) and leiognathid fishes (15), as well as reports of the paucity of dividing bacterial cells in monocentrid light organs (78), indicate that a low average symbiont growth rate during the maintenance of the association may be a general feature of luminous bacterial symbioses.
While the usual source of cells to repopulate the light organ is certainly the bacteria that remain in the crypts after the expulsion event, there is evidence that secondary colonization events can occur subsequent to the initial event. When juvenile animals colonized by one strain of V. fischeri were placed in seawater containing another, competitively dominant strain, the latter was able to initiate a secondary infection and displace the already established symbiont (33). That this reinfection process can occur in a 2-day-old juvenile can be explained by the fact that the juvenile retains features of the newly hatched state for several days before developing into the mature adult (43). It was, perhaps, more surprising to find that adult animals could continue to be reinfected by V. fischeri cells in the ambient seawater through the two pores remaining on the light organ’s surface (34). Thus, the light organ crypts remain a dynamic and open environment with their own microecology, perhaps continually selecting for more competitive symbiont strains. This continuous interaction may be responsible for producing the apparently mixed cultures of distinct V. fischeri strains that have been observed in the light organs of adult E. scolopes (6) and other bobtail squid species (18).
ABUNDANCE AND TYPES OF V. FISCHERI IN THE E. SCOLOPES HABITAT
V. fischeri cells are transmitted horizontally between generations of E. scolopes hosts (45, 71); that is, newly hatched juvenile bobtail squids are free of symbionts but, within hours, obtain them from the surrounding seawater (82). Thus, to understand the dynamics of the association’s development, it is essential to determine the abundance of symbiotically competent V. fischeri cells in the environment. The large populations of bacteria carried in adult light organs (between 108 and 109 cells in mature animals) and the viable nature of the newly expelled cells (34, 69) suggest that symbiont expulsion will have a very significant effect on the abundance of V. fischeri in those habitats where the host is abundant. For instance, it can be calculated that a single expulsion by one adult bobtail squid would produce enough symbiont cells to fill 10,000 m3 of seawater with a concentration of V. fischeri that is equivalent to what is typically found in coastal waters (34, 73). Thus, it was predicted that the concentration of CFU of V. fischeri in natural seawater inhabited by a population of E. scolopes is relatively elevated.
To test this prediction it was necessary to identify and quantify V. fischeri CFU from environmental samples. Luminous bacteria are routinely isolated from seawater by plating samples on a nutrient agar, marine salts medium, and subsequently observing the plates for luminescent colonies (51). Unfortunately, colonies of V. fischeri that arise from cells released by E. scolopes light organs generally do not produce visible luminescence (5) and thus escape detection by a visual screen of the isolation plates. This low level of luminescence is probably the reason that few strains of V. fischeri were reported in early analyses of Hawaiian seawater by Reichelt and Baumann (66). Those authors were careful to point out that their use of an initial screen for luminescence eliminated from identification any non-visibly luminous (NVL) strains of typically luminous bacterial species. The difficulty in recognizing colonies of such NVL strains was subsequently overcome with the development of species-specific lux gene DNA probes (84) that could be used to identify V. fischeri colonies by their ability to hybridize with these probes in colony-lift experiments. By this approach, seawater and sediment samples were collected from several coastal locations in Hawaii, as well as in southern California and Massachusetts, and the numbers both of visibly luminous and of NVL (but probe-hybridizing) V. fischeri organisms were determined (32). This work has allowed several conclusions to be drawn: (i) seawater in the habitat of E. scolopes contained approximately 200 CFU of V. fischeri organisms per 100 ml, almost all of which were of the NVL type characteristic of E. scolopes symbionts (32); (ii) sediment samples taken in the same locations contained about a 70-fold-higher concentration of these cells (34), similar to a result reported in a study of other luminous bacteria in a different environment (63); and (iii) the concentration of these V. fischeri CFU decreased as a direct function of the distance a seawater sample was taken from the nearshore habitat of E. scolopes (34). There was also no evidence of NVL V. fischeri CFU in environments with no known host species, e.g., southern California or Massachusetts coastal seawater (35).
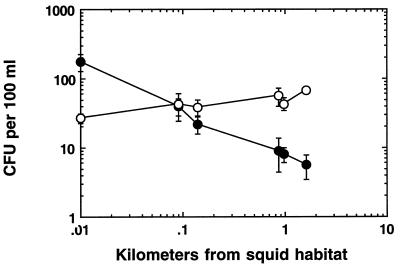
It appears, then, that colonized E. scolopes squids ensure the maintenance of a high concentration of potentially infective V. fischeri cells in the seawater that their offspring will subsequently use as the inoculum for their own nascent light organs. Once expelled into the ambient seawater, however, the released V. fischeri cells, like other aquatic bacteria, are subject to competition for the use of available nutrients and predation by protists and bacteriophages (26). Even with the daily input of released squid symbionts, these depleting factors may make it difficult for V. fischeri cells to remain at high concentrations in seawater. This suggestion is made because, although the closely related but nonsymbiotic species Vibrio harveyi maintains a relatively constant, but low, population density across a several kilometer transect of ocean leading from the nearshore squid habitat into offshore waters, the abundance of V. fischeri CFU becomes markedly reduced with increasing distance from the host’s habitat (Fig. 2) (34).
FIG. 2.
Concentrations of luminous bacteria in seawater at various distances away from a population of E. scolopes. Seawater samples were taken at points along a transect in Kaneohe Bay, Hawaii, beginning near a population of squids on the reefs at Coconut Island (34) and extending 2 km seaward. CFU of both V. fischeri (•) and the closely related (but nonsymbiotic) species V. harveyi (○) in the samples were determined as described previously (34). Error bars, when larger than the symbol, indicate one standard deviation in the data.
Finally, it should be noted that the abundance and distribution of sediment, enteric, and other populations of V. fischeri have not been adequately determined for Hawaiian habitats. Thus, the role of these populations in the normal process of infection of juveniles remains unknown. For example, in their natural environment, are juvenile animals typically infected by cells encountered in the ambient seawater or, instead, while they are buried in the V. fischeri-enriched sandy sediment during the daylight hours (34)?
While the evidence presented in these studies was correlative in nature, the results suggest that relatively high concentrations of V. fischeri can be expected in the vicinity of a population of E. scolopes. These were among the first studies of an animal host apparently exerting a significant influence on the abundance of its symbiotic bacterial partner, and this light organ association may serve as a model for the study of other aquatic symbioses in which bacterial symbionts are transmitted horizontally (9, 11, 21).
PHYSIOLOGICAL STATE OF SYMBIOTICALLY COMPETENT V. FISCHERI IN HAWAIIAN SEAWATER
Ecological studies of bacteria in natural samples are often based upon the appearance of CFU and thus reveal only the presence of cells that are capable of producing colonies on a given isolation medium. Until recently, it was believed that all viable V. fischeri cells have a 100% plating efficiency (48) and thus that they could be enumerated by counting CFU. However, reports that other Vibrio species can enter an apparently dormant state in which they retain viability but are no longer able to form colonies on standard isolation media (the so-called viable but nonculturable [VNC] state) suggested that undiscovered symbiotically competent V. fischeri cells might exist in such a state as well. VNC is an unfortunate term that emphasizes our present inability to isolate a particular bacterium by typical culturing methods; it does not explain what the characteristics of the state are or how the state is entered and exited. Temperature change (81, 85), nutrient availability (58, 83), and/or solar irradiation (61) has been proposed to play a role in transforming other vibrios and enteric bacteria from a culturable to a VNC condition. More recently, studies have begun to examine both the entry into this state in the natural environment (56, 57) and the genes responsible for controlling the entry process itself (65).
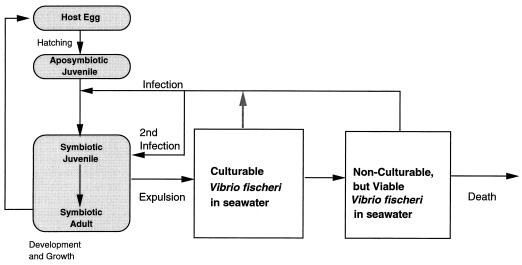
Seawater samples collected from the bobtail squid habitats in Hawaii contain only a few V. fischeri CFU per ml. However, when these samples were subjected to molecular analyses, the presence of several hundred to a thousand copies of the V. fischeri luxA gene was revealed (35). This observation suggested that greater than 99% of the V. fischeri cells present in those samples might be in an unculturable state. Further evidence for the presence of these VNC-like cells came when diluted seawater samples containing fewer than 1 CFU of V. fischeri organisms were shown to be capable of consistently initiating an infection of juvenile E. scolopes (35), a phenomenon reminiscent of that demonstrated for VNC Vibrio vulnificus cells in the pathogenic infection of mice (56). Because of their numerical dominance in seawater, VNC-like V. fischeri cells probably are the usual form that initiates the colonization of E. scolopes juveniles in nature, and thus, it is these cells that play an essential role in the life cycle of the host (Fig. 3). For this reason it will be of considerable interest to determine what characteristics of the juvenile squid’s nascent light organ are responsible for efficiently initiating a conversion of these dormant bacteria into growing forms. It will also be important to understand whether these VNC V. fischeri cells are indeed metabolically inactive, as has been suggested for other VNC bacteria (10, 16, 39). If they are truly dormant, then certain questions arise: (i) how are they able to colonize the squid when active motility is apparently required (20) and (ii) how do they initiate growth in the light organ without a discernible lag period (69)?
FIG. 3.
Proposed model for the dynamic interaction between the life cycle of the bioluminescent bobtail squid E. scolopes and the ecological cycle of its symbiont, V. fischeri.
Entry into the VNC state is believed to be a mechanism evolved by numerous gram-negative bacterial species for enhancing survival over the potentially long periods between favorable growth environments (e.g., a specific host tissue). It does not appear that symbiotic V. fischeri cells released as a result of the natural daily expulsion process are either more or less suited to survival than cells cultured in laboratory medium: both appear equally infective, and both enter the VNC-like state at the same apparent rate (30).
POPULATION BIOLOGY OF V. FISCHERI: EVIDENCE FOR SYMBIOTIC BIOVARS?
Although the possibility of coevolution between V. fischeri and its several host species has been suggested in the past (49), until recently (31, 42) the prevailing assumption has been that there is no pattern of genetic differences among strains of V. fischeri isolated from different niches. Instead, it was believed that there is only a random exchange of cells from one niche to another, without any differentiation of specialized subgroups within a species. No substantive differences in phenotypic traits have been observed among isolates of V. fischeri obtained from different geographic areas or biological niches. Indeed, it is this uniformity that has made the traditional taxonomic methods so valuable for categorizing this and other species of luminous bacteria (51, 66). However, the recent discovery of distinct levels of symbiotic competence among geographically cooccurring V. fischeri strains has suggested that strains with ecological specializations might share affinities at the genetic level (33).
Genotypic analyses of a number of isolates of V. fischeri from fish and squid light organs, and from water samples collected at different locations, have suggested that there are genetically distinguishable subspecies, or biovars, of V. fischeri and that some of these biovars are more suited to be, and thus more likely to be found as, the light organ symbionts of specific animal hosts (31). Among light organ symbionts from different species in the genus Euprymna, the phylogenetic position of the host from which a strain of V. fischeri was isolated is a better predictor of the bacterium’s genetic subgrouping than is the geographical location of the host (36). These observations provided additional evidence against the hypothesis that the V. fischeri strains present in a region make up a common pool from which any species of host will be inoculated, a condition that would be expected to lead to a genetically and symbiotically uniform set of strains within a given location, without regard to the source of the strains.
Two colonization characteristics that distinguish these biovars of V. fischeri are (i) the minimum infective dose (i.e., the concentration of cells necessary to promote a symbiotic infection) and (ii) the maximum symbiont population size achieved. Using several strains of V. fischeri obtained from the light organs of either E. scolopes, its Japanese congener Euprymna morsei (28), or two species of monocentrid fishes, Cleidopus gloriamaris and Monocentris japonica, experiments were performed to compare the symbiosis competencies of the strains. The results of these experiments showed that monocentrid light organ symbionts were less effective in colonizing E. scolopes juveniles than were bobtail squid symbionts (Table 1). For example, there were both a 10- to 100-fold-higher minimum infective dose and a 10- to 100-fold-lower level of cells present in the symbiotic population. Surprisingly, when presented at low concentrations, E. morsei strains were even more effective than E. scolopes strains in colonizing E. scolopes juveniles and the two types of strains colonized to about the same extent (Table 1). Nevertheless, when presented together, E. scolopes strains did eventually outcompete E. morsei strains for dominance of the E. scolopes light organ (54), suggesting that different determinants play a role in initiating colonization and in persisting in one.
TABLE 1.
Comparative colonization characteristics of V. fischeri strains
| V. fischeri strain | Host speciesa | Inoculum concn (CFU/ml) resulting in a 50% infection rateb | Total CFU per light organ (104) after 48 hc |
|---|---|---|---|
| ES114 | E. scolopes | 750 | 42 |
| ES12 | E. scolopes | 400 | 25 |
| ES213 | E. scolopes | ND | 46 |
| ES401 | E. scolopes | ND | 90 |
| EM17 | E. morsei | 200 | 42 |
| EM18 | E. morsei | 100 | 31 |
| EM24 | E. morsei | 70 | 17 |
| MJ11 | M. japonica | 10,000 | 4.5 |
| CG101 | C. gloriamaris | 2,000 | 0.62 |
Strains were isolated from light organ homogenates of the indicated host species (5). Each isolate was obtained from a different animal.
For each strain, groups of 10 newly hatched E. scolopes squids were exposed for 3 h to seawater containing between 102 and 105 V. fischeri cells per ml. The approximate concentrations of cells that were required to infect 50% of the juvenile animals are given. ND, not determined.
Symbiont cell numbers (CFU) were estimated by homogenizing the light organ and plating serial dilutions on a nutrient medium (69).
While juvenile animals of only a few species of bobtail squid (E. scolopes and some Sepiola species [40]) are currently available, experiments are under way to compare the abilities of a more extensive list of symbionts from 6 species of squid to colonize juveniles of several species of Euprymna and Sepiola (18, 46, 54). The results of such reciprocal crosses between pairs of hosts and symbionts will reveal not only whether the host and symbiont species are coevolving but also what mechanisms may be responsible for their specificities.
The existence of host-species-specific biovars has been well-documented among Rhizobium species, most notably within the species Rhizobium leguminosarum (27). In this bacterium, the mechanism responsible for the genetic isolation of biovars has been linked to the presence of surface specificity determinants (called Nod factors) that determine the range of host plant species that R. leguminosarum strains can nodulate (14). These lipochitin oligosaccharide signal compounds are structurally related but distinct and have host-specific effects on plant gene expression. The genes encoding these surface factors appear to be primarily plasmid borne in Rhizobium species, though not in the related genus Bradyrhizobium (80). In this regard it is relevant to point out that V. fischeri symbionts of different Euprymna species may be more like Bradyrhizobium: there is no evidence that any V. fischeri symbiosis-competency genes are located extrachromosomally (6, 67).
EFFECT OF V. FISCHERI DISTRIBUTION ON HOST HABITAT DISTRIBUTION
It has been demonstrated that without colonization by V. fischeri cells, the light organ of E. scolopes remains functionally and morphologically undeveloped (12, 45), but how important is symbiosis to the host? Because each one of the hundreds of animals collected to date from the natural environment has been found to be colonized and luminescent (68), symbiosis must be the normal state of the host and one that starts within a few days of hatching. Nevertheless, uninfected juveniles can be maintained in the laboratory without any apparent ill effects. Thus, it remains to be experimentally tested whether the absence of a functioning light organ seriously compromises the survival of an E. scolopes individual in nature, and, if so, we must determine at what stage in its posthatch development this absence becomes critical. If a capacity to luminesce is, in fact, important for survival in nature, then the continuance of a population of E. scolopes must require the presence in its habitat of symbiotically competent V. fischeri cells in a sufficient concentration to ensure that most juvenile animals are infected (19). Because there is evidence neither that adult squids remain near the egg clutches they lay (76) nor that there is a sufficient inoculum of V. fischeri present as a coating on the clutch (68), the host population apparently depends upon the presence of a critical density of symbiotically competent V. fischeri cells in the seawater or the sediments of its habitat (Fig. 2). Such a condition exists within the confines of at least portions of Kaneohe Bay, Hawaii, where the best-studied E. scolopes populations are found (34, 35). However, the conditions remain undescribed for host populations that exist on exposed shorelines of the Hawaiian Islands, where intrusion of vast volumes of open ocean water by regular tidal activity can be expected to continuously dilute the concentration of expelled V. fischeri cells in the ambient seawater to a very low value (34). How do juvenile animals obtain an inoculum of bacteria under such conditions? Perhaps studies of the stability of sediment populations of V. fischeri will reveal an answer.
On a broader ecological time scale, it is not known how new populations of bobtail squids are established in previously unpopulated regions: is a critical number of adults required to establish such a population (i.e., to have an impact on the local environmental density of V. fischeri), or is immigration confined to locations that already have a sufficient abundance of established, and symbiotically competent, V. fischeri? In either case, the biogeography of the host may well be regulated by the presence of bacteria in sufficient numbers to sustain the infection of the next generation of juveniles. Experiments designed to address such issues for the host squids remain to be conducted, but their results may help identify factors that affect the distribution of other symbiotic species with horizontally transmitted symbionts (62, 79).
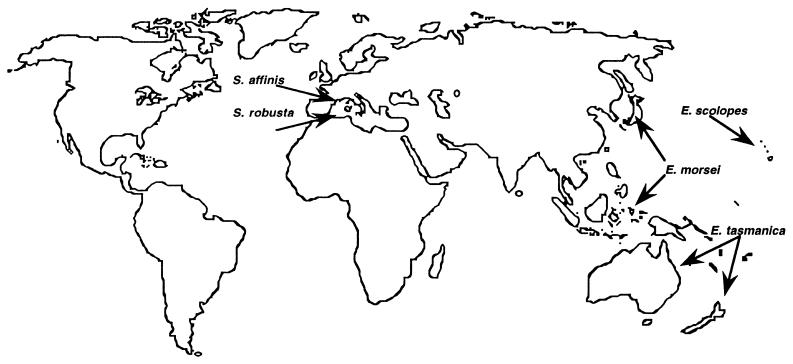
It is interesting to speculate upon the geographical distribution of those bobtail squid species that have light organ associations (Fig. 4). In the Pacific and Indian Oceans at least six recognized species of Euprymna have symbiotic light organs (53), and all three of the species that have been examined to date (E. scolopes, E. morsei, and Euprymna tasmanica) maintain V. fischeri as their partner (6, 54, 69). It must be remembered that the ecology, behavior, and even developmental biology of these other animal host species may differ from those of E. scolopes, so it cannot be assumed that the relationship of these hosts with their bacterial symbiont is identical to that determined for the Hawaiian species. In addition, there are nine species of Sepiola in the Atlantic Ocean and Mediterranean Sea (40) and the two species (Sepiola affinis and Sepiola robusta) that have recently been examined contain as their predominant light organ symbiont V. logei (18), the closest congener of V. fischeri (3). Perhaps by a comparison of these different species, and the discovery of distinctions in their strategies for ensuring the colonization of subsequent generations, we will be able to infer the ancestral pattern of symbiotic ecology.
FIG. 4.
Approximate geographic distributions of some bobtail squid species of the genera Euprymna and Sepiola. The Mediterranean species of Sepiola have roughly overlapping ranges (40).
CONCLUDING STATEMENT
Understanding the processes driving the ecology of any marine bacterial species is a difficult and perplexing problem, in part because of the many biotic and abiotic influences that must be considered. The luminous bacterium V. fischeri is a widespread and ecologically versatile organism, but in at least some habitats its symbiotic relationship with the bobtail squid E. scolopes appears to be a dominant factor in controlling its abundance and distribution. It is likely that the ecological patterns of many other marine bacteria are similarly driven by an association with a specific animal or plant host. An examination of these dynamic processes, and the biological mechanisms that underlie them, will play an important part in future discoveries in marine microbiology.
ACKNOWLEDGMENTS
We thank M. McFall-Ngai for valuable discussions.
Work reviewed here from E.G.R.’s laboratory was supported by grants from the Office of Naval Research (N00014-93-I0846), the National Science Foundation (IBN96-01155), and the University of Southern California Sea Grant office.
REFERENCES
- 1.Andrews C C, Karl D M, Small L F, Fowler S W. Metabolic activity and bioluminescence of oceanic faecal pellets and sediment trap particles. Nature. 1984;307:539–541. [Google Scholar]
- 2.Angulo L, Lopez J E, Vicente J A, Saborido A M. Haemorrhagic areas in the mouth of farmed turbot, Scophthalmus maximus (L.) J Fish Dis. 1994;17:163–169. [Google Scholar]
- 3.Bang S S, Baumann P, Nealson K H. Phenotypic characterization of Photobacterium logei (sp. nov.), a species related to P. fischeri. Curr Microbiol. 1978;1:285–288. [Google Scholar]
- 4.Berry S S. The cephalopoda of the Hawaiian Islands. Bulletin of the Bureau of Fisheries. 1912;32:255–362. [Google Scholar]
- 5.Boettcher K J, Ruby E G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boettcher K J, Ruby E G. Occurrence of plasmid DNA in the sepiolid squid symbiont Vibrio fischeri. Curr Microbiol. 1994;29:279–286. [Google Scholar]
- 7.Boettcher K J, Ruby E G, McFall-Ngai M J. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J Comp Physiol. 1996;179:65–73. [Google Scholar]
- 8.Buchner P. Endosymbiosis of animals with plant microorganisms. New York, N.Y: Interscience Publishers; 1965. pp. 543–571. . (Revised English version of Endosymbiose der Tiere mit pflanzlichen Mikroorganismen, 1953.) [Google Scholar]
- 9.Cary S C, Warren W, Anderson E, Giovannoni S J. Identification and localization of bacterial endosymbionts in hydrothermal vent taxa with symbiont-specific polymerase chain reaction amplification and in situ hybridization techniques. Mol Mar Biol Biotechnol. 1993;2:51–62. [PubMed] [Google Scholar]
- 10.Colwell R R, Huq A. Vibrios in the environment: viable but nonculturable Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 117–133. [Google Scholar]
- 11.Distel D L, DeLong E F, Waterbury J B. Phylogenetic characterization and in situ localization of the bacterial symbiont of shipworms (Teredinidae: Bivalvia) by using 16S rRNA sequence analysis and oligodeoxynucleotide probe hybridization. Appl Environ Microbiol. 1991;57:2376–2382. doi: 10.1128/aem.57.8.2376-2382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doino J, McFall-Ngai M J. A transient exposure to symbiosis-competent bacteria induces light organ morphogenesis in the host squid. Biol Bull. 1995;189:347–355. doi: 10.2307/1542152. [DOI] [PubMed] [Google Scholar]
- 13.Douglas A E. Symbiotic interactions. Oxford, United Kingdom: Oxford University Press; 1994. [Google Scholar]
- 14.Downie J A. Signalling strategies for nodulation of legumes by rhizobia. Trends Microbiol. 1994;2:318–324. doi: 10.1016/0966-842x(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 15.Dunlap P D. Physiological and morphological state of the symbiotic bacteria from light organs of ponyfish. Biol Bull. 1984;167:410–425. doi: 10.2307/1541286. [DOI] [PubMed] [Google Scholar]
- 16.Effendi I, Austin B. Dormant/unculturable cells of the fish pathogen Aeromonas salmonicida. Microb Ecol. 1995;30:183–192. doi: 10.1007/BF00172573. [DOI] [PubMed] [Google Scholar]
- 17.Feldman K A, Buck J D. Distribution and characterization of luminescent bacteria in a temperate estuary. Estuaries. 1984;7:93–97. [Google Scholar]
- 18.Fidopiastis P M, von Boletzky S, Ruby E G. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J Bacteriol. 1998;180:59–64. doi: 10.1128/jb.180.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giere O, Conway N M, Gastrock G, Schmidt C. ‘Regulation’ of gutless annelid ecology by endosymbiotic bacteria. Mar Ecol Prog Ser. 1991;68:287–299. [Google Scholar]
- 20.Graf J, Dunlap P V, Ruby E G. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J Bacteriol. 1994;176:6986–6991. doi: 10.1128/jb.176.22.6986-6991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gros O, Darrasse A, Durand P, Frenkiel L, Mouëza M. Environmental transmission of a sulfur-oxidizing bacterial gill endosymbiont in the tropical lucinid bivalve Codakia orbicularis. Appl Environ Microbiol. 1996;62:2324–2330. doi: 10.1128/aem.62.7.2324-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris L, Owens L, Smith S. A selective medium for Vibrio harveyi. Appl Environ Microbiol. 1996;62:3548–3550. doi: 10.1128/aem.62.9.3548-3550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastings J W, Makemson J, Dunlap P V. How are growth and luminescence regulated independently in light organ symbionts? Symbiosis. 1987;4:3–24. [Google Scholar]
- 24.Haygood M G. Light organ symbioses in fishes. Crit Rev Microbiol. 1994;19:191–216. doi: 10.3109/10408419309113529. [DOI] [PubMed] [Google Scholar]
- 25.Haygood M G, Tebo B M, Nealson K H. Luminous bacteria of a monocentrid fish (Monocentris japonicus) and two anomalopid fishes (Photoblepharon palpebratus and Kryptophanaron alfredi): population sizes and growth within the light organs, and rates of release into the seawater. Mar Biol (Berlin) 1984;78:249–254. [Google Scholar]
- 26.Hidaka T, Kobayashi M. Characterization of the bacteriophages infecting marine luminous bacterium Vibrio fischeri. Mem Fac Fish Kagoshima Univ. 1988;37:161–172. [Google Scholar]
- 27.Jordan D C. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int J Syst Bacteriol. 1982;32:136–139. [Google Scholar]
- 28.Kishitani T. Ueber das Leuchtorgan von Euprymna morsei Verrill und die symbiotischen Leuchtbakterien. Proceedings of the Imperial Academy. 1928;4:306–309. [Google Scholar]
- 29.Kucey R M N, Hynes M F. Populations of Rhizobium leguminosarum biovars phaseoli and viceae in fields after bean or pea in rotation with nonlegumes. Can J Microbiol. 1989;35:661–667. [Google Scholar]
- 30.Lee K-H. Ecology of Vibrio fischeri, the light organ symbiont of the Hawaiian sepiolid squid Euprymna scolopes. Ph.D. thesis. Los Angeles: University of Southern California; 1995. [Google Scholar]
- 31.Lee K-H, Gray K M, Ruby E G. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. Genetic diversity among symbiotic and planktonic isolates of Vibrio fischeri, abstr. H-222; p. 239. [Google Scholar]
- 32.Lee K-H, Ruby E G. Detection of the light organ symbiont, Vibrio fischeri, in Hawaiian seawater by using lux gene probes. Appl Environ Microbiol. 1992;58:942–947. doi: 10.1128/aem.58.3.942-947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K-H, Ruby E G. Competition between Vibrio fischeri strains during initiation and maintenance of a light organ symbiosis. J Bacteriol. 1994;176:1985–1991. doi: 10.1128/jb.176.7.1985-1991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K-H, Ruby E G. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl Environ Microbiol. 1994;60:1565–1571. doi: 10.1128/aem.60.5.1565-1571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K-H, Ruby E G. Symbiotic role of the viable but nonculturable state of Vibrio fischeri in Hawaiian coastal seawater. Appl Environ Microbiol. 1995;61:278–283. doi: 10.1128/aem.61.1.278-283.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, K.-H., and E. G. Ruby. Unpublished data.
- 37.Lingle W L, Gibson B G, O’Kane D J. Characterization of amphipods infected by bioluminescent vibrios. In: Campbell K, Kricka L J, Stanley P E, editors. Bioluminescence and chemiluminescence: fundamental and applied aspects. Chichester, England: John Wiley & Sons Ltd.; 1994. pp. 89–92. [Google Scholar]
- 38.Liu P-C, Lee K-K, Yii K-C, Kou G H, Chen S-N. Isolation of Vibrio harveyi from diseased Kuruma prawns Penaeus japonicus. Curr Microbiol. 1996;33:129–132. doi: 10.1007/s002849900087. [DOI] [PubMed] [Google Scholar]
- 39.Magariños B, Romalde J L, Barja J L, Toranzo A E. Evidence of a dormant but infective state of the fish pathogen Pasteurella piscicida in seawater and sediment. Appl Environ Microbiol. 1994;60:180–186. doi: 10.1128/aem.60.1.180-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangold K, von Boletzky S. Mediterranean cephalopod fauna. In: Clarke M R, Trueman E R, editors. The mollusca. 12. Paleontology and neontology of cephalopods. San Diego, Calif: Academic Press; 1988. pp. 315–330. [Google Scholar]
- 41.McFall-Ngai M J. Crypsis in the pelagic environment. Am Zool. 1990;30:175–188. [Google Scholar]
- 42.McFall-Ngai M J. Luminous bacterial symbiosis in fish evolution: adaptive radiation among the leiognathid fishes. In: Margulis L, Fester R, editors. Symbiosis as a source of evolutionary innovation. Cambridge, Mass: The M. I. T. Press; 1991. pp. 381–409. [Google Scholar]
- 43.McFall-Ngai M J. Animal-bacterial interactions in the early life history of marine invertebrates: the Euprymna scolopes/Vibrio fischeri symbiosis. Am Zool. 1995;34:554–561. [Google Scholar]
- 44.McFall-Ngai M J, Montgomery M K. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda:Sepiolidae) Biol Bull. 1990;179:332–339. doi: 10.2307/1542325. [DOI] [PubMed] [Google Scholar]
- 45.McFall-Ngai M, Ruby E G. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- 46.McFall-Ngai, M., and E. G. Ruby. Sepiolids and vibrios: when first they meet. BioScience, in press.
- 47.Montgomery M K, McFall-Ngai M J. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development. 1994;120:1719–1729. doi: 10.1242/dev.120.7.1719. [DOI] [PubMed] [Google Scholar]
- 48.Nealson K H. Isolation, identification, and manipulation of luminous bacteria. Methods Enzymol. 1978;57:153–166. [Google Scholar]
- 49.Nealson K H. Alternative strategies of symbiosis of marine luminous fishes harboring light-emitting bacteria. Trends Biochem Sci. 1979;4:105–110. [Google Scholar]
- 50.Nealson K H, Hastings J W. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev. 1979;43:496–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nealson K H, Hastings J W. The luminous bacteria. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications. 2nd ed. New York, N.Y: Springer-Verlag; 1991. pp. 3892–3906. [Google Scholar]
- 52.Nealson K H, Haygood M G, Tebo B M, Roman M, Miller E, McCosker J E. Contribution by symbiotically luminous fishes to the occurrence and bioluminescence of luminous bacteria in seawater. Microb Ecol. 1984;10:69–77. doi: 10.1007/BF02011596. [DOI] [PubMed] [Google Scholar]
- 53.Nesis K N. Cephalopods of the world. Neptune City, N.J: T. F. H. Publications; 1987. [Google Scholar]
- 54.Nishiguchi, M. K., E. G. Ruby, and M. J. McFall-Ngai. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in the sepiolid-Vibrio symbioses. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 55.O’Brien C H, Sizemore R K. Distribution of the luminous bacterium Beneckea harveyi in a semitropical estuarine environment. Appl Environ Microbiol. 1979;38:928–933. doi: 10.1128/aem.38.5.928-933.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oliver J D, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliver J D, Hite F, McDougald D, Andon N L, Simpson L M. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl Environ Microbiol. 1995;61:2624–2630. doi: 10.1128/aem.61.7.2624-2630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliver J D, Nilsson L, Kjelleberg S. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol. 1991;57:2640–2644. doi: 10.1128/aem.57.9.2640-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orndorff S A, Colwell R R. Distribution and identification of luminous bacteria from the Sargasso Sea. Appl Environ Microbiol. 1980;39:983–987. doi: 10.1128/aem.39.5.983-987.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortigosa M, Garay E, Pujalte M-J. Numerical taxonomy of Vibrionaceae isolated from oysters and seawater along an annual cycle. Syst Appl Microbiol. 1994;17:216–225. [Google Scholar]
- 61.Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell R R, Cormier M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/aem.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Provasoli L, Yamasu T, Manton I. Experiments on the resynthesis of symbiosis in Convoluta roscoffensis with different flagellate cultures. J Mar Biol Assoc U K. 1968;48:465–479. [Google Scholar]
- 63.Ramesh A, Loganathan B G, Venugopalan V K. Seasonal distribution of luminous bacteria in the sediments of a tropical estuary. J Gen Appl Microbiol. 1989;35:363–368. [Google Scholar]
- 64.Ramesh A, Nandakumar R, Venugopalan V K. Enteric luminous microflora of the pond-cultured milk fish Chanos chanos (Forskal) Microb Ecol. 1986;12:231–235. doi: 10.1007/BF02011207. [DOI] [PubMed] [Google Scholar]
- 65.Ravel J, Hill R T, Colwell R R. Isolation of a Vibrio cholerae transposon-mutant with an altered viable but nonculturable response. FEMS Microbiol Lett. 1994;120:57–62. doi: 10.1111/j.1574-6968.1994.tb07007.x. [DOI] [PubMed] [Google Scholar]
- 66.Reichelt J L, Baumann P. Taxonomy of the marine, luminous bacteria. Arch Mikrobiol. 1973;94:283–330. doi: 10.1007/BF00424970. [DOI] [PubMed] [Google Scholar]
- 67.Ruby E G. Lessons from a cooperative bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu Rev Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.591. [DOI] [PubMed] [Google Scholar]
- 68.Ruby, E. G. Unpublished data.
- 69.Ruby E G, Asato L M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch Microbiol. 1993;159:160–167. doi: 10.1007/BF00250277. [DOI] [PubMed] [Google Scholar]
- 70.Ruby E G, Greenberg E P, Hastings J W. Planktonic marine luminous bacteria: species distribution in the water column. Appl Environ Microbiol. 1980;39:302–306. doi: 10.1128/aem.39.2.302-306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruby E G, McFall-Ngai M J. A squid that glows in the night: development of an animal-bacterial mutualism. J Bacteriol. 1992;174:4865–4870. doi: 10.1128/jb.174.15.4865-4870.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruby E G, Morin J G. Specificity of symbiosis between deep-sea fishes and psychrotrophic luminous bacteria. Deep-Sea Res. 1978;25:161–167. [Google Scholar]
- 73.Ruby E G, Nealson K H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica: a model of symbiosis based on bacterial studies. Biol Bull. 1976;151:574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- 74.Ruby E G, Nealson K H. Seasonal changes in the species composition of luminous bacteria in nearshore seawater. Limnol Oceanogr. 1978;23:530–533. [Google Scholar]
- 75.Shilo M, Yetinson T. Physiological characteristics underlying the distribution patterns of luminous bacteria in the Mediterranean Sea and the Gulf of Elat. Appl Environ Microbiol. 1979;38:577–584. doi: 10.1128/aem.38.4.577-584.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singley C T. Euprymna scolopes. In: Boyle P R, editor. Cephalopod life cycles. 1. Species accounts. London, United Kingdom: Academic Press, Inc. Ltd.; 1983. pp. 69–74. [Google Scholar]
- 77.Sutton D C, Garrick R. Bacterial disease of cultured giant clam Tridacna gigas larvae. Dis Aquat Org. 1993;16:47–53. [Google Scholar]
- 78.Tebo B M, Linthicum D S, Nealson K H. Luminous bacteria and light emitting fish: ultrastructure of the symbiosis. BioSystems. 1979;11:269–280. doi: 10.1016/0303-2647(79)90027-3. [DOI] [PubMed] [Google Scholar]
- 79.Trench R K. Microalgal-invertebrate symbioses: a review. Endocytobiosis Cell Res. 1993;9:135–175. [Google Scholar]
- 80.van Rhijn P, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wai S N, Moriya T, Kondo K, Misumi H, Amako K. Resuscitation of Vibrio cholerae O1 strain TSI-4 from a viable but nonculturable state by heat-shock. FEMS Microbiol Lett. 1996;136:187–191. doi: 10.1111/j.1574-6968.1996.tb08047.x. [DOI] [PubMed] [Google Scholar]
- 82.Wei S L, Young R E. Development of symbiotic bacterial bioluminescence in a nearshore cephalopod, Euprymna scolopes. Mar Biol. 1989;103:541–546. [Google Scholar]
- 83.Whitesides M D, Oliver J D. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol. 1997;63:1002–1005. doi: 10.1128/aem.63.3.1002-1005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wimpee C F, Nadeau T-L, Nealson K H. Development of species-specific hybridization probes for marine luminous bacteria by using in vitro DNA amplification. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf P, Oliver J D. Temperature effects on the viable but nonculturable state of Vibrio vulnificus. FEMS Microbiol Ecol. 1992;101:33–39. [Google Scholar]
- 86.Woomer P, Singleton P W, Bohlool B B. Ecological indicators of native rhizobia in tropical soils. Appl Environ Microbiol. 1988;54:1112–1116. doi: 10.1128/aem.54.5.1112-1116.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yetinson T, Shilo M. Seasonal and geographic distribution of luminous bacteria in the eastern Mediterranean Sea and Gulf of Elat. Appl Environ Microbiol. 1979;37:1230–1238. doi: 10.1128/aem.37.6.1230-1238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]