To the Editor:
Histological evidence of tissue injury is considered the most relevant defining feature of experimental acute lung injury (ALI). In humans, ALI/acute respiratory distress syndrome (ARDS) criteria follow the 2012 Berlin definition (1): 1) a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ⩽300 mm Hg while receiving invasive or noninvasive ventilation with a tight-fitting mask and positive end-expiratory pressure or continuous positive airway pressure of at least 5 cm H2O; 2) three severity levels of initial arterial hypoxemia (categories of PaO2/FiO2 ratio of ⩽100 mm Hg [severe], 101–200 mm Hg [moderate], and 201–300 mm Hg [mild]), which correlate with mortality (45%, 35%, and 27%, respectively); 3) timing (respiratory failure should have developed within 1 week of a known clinical insult); 4) inability of cardiac failure to fully explain respiratory failure; and 5) radiological evidence of diffuse bilateral pulmonary infiltrates. However, these criteria cannot be directly translated to laboratory animals, especially to small animals. Furthermore, although arterial blood gas analysis, chest radiography, and other ancillary tests can be performed on some animal models, not all laboratories are equipped for these techniques, or their application on an experimental group with many animals is impractical. Thus, the 2011 American Thoracic Society (ATS) workshop report on features and measurements of experimental acute lung injury in animals (2) and the 2022 update (3) emphasized that histological features remain the most relevant feature of ALI, and these should be assessed in a rigorous manner.
In 2011, ATS committee proposed a lung injury scoring (LIS) system to histologically quantify five parameters (alveolar space neutrophils, interstitial neutrophils, hyaline membranes, proteinaceous debris, and septal thickening) in at least 20 random high-power fields (total magnification 400×) with at least 1 high-power field distance between regions of interest (ROIs) and at least 50% of each field occupied by lung alveoli (2). The committee also suggested conditions for preparing lung tissues under physiologic pressure parameters of perfusion and inflation (2, 4). Since the 2011 report (2), LIS has been applied in several studies involving different species, including mouse (5–7), rat (8, 9), pig (10, 11), monkey (12), and human (13).
In the 2022 update (3), participants were surveyed after the meeting regarding how histological injury should be quantified, and 57% respondents reaffirmed the 2011 parameters of blinded assessment in several nonoverlapping fields. Although we agree with that assessment, we also recognize the challenges of selecting random, nonoverlapping microscopic fields. To address this challenge and these concerns and limitations, we developed a custom script to provide standardization for LIS on digitized slides.
Over the past decade, whole-slide imaging (WSI) has become more widely available, and advances in computer processing power have permitted quantitative analysis of these large image files (14). Despite these advancements, manual annotation and assessment by an experienced pathologist remain critical, especially for biologically relevant yet complex histological features. Unbiased sampling is a potential strategy to address the bottleneck caused by manual annotation, and despite the committee’s recommendation, a practical and standardized approach for LIS evaluation is lacking. The ROIs sampling strategy from WSI should 1) represent all the information contained in a single slide, 2) reduce potential sampling bias, and 3) be freely accessible and applicable in any research or pathology laboratory.
The custom script described here selects ROIs from sections using a stratified uniform sampling representative of the whole lung. The lung sections are prepared according to ATS guidelines (2, 4) and as described in the detailed protocol in the data supplement. After scanning and saving an image, the file is opened using QuPath (https://qupath.github.io) (15) and ImageJ (https://imagej.net/ij/index.html)/Fiji (https://fiji.sc) (16), both free open-source applications. The contour of the tissue is outlined by the user, and the script is applied (https://codebeautify.org/alleditor/y234a8635; see the data supplement and Figures E1 and E2 in the data supplement for protocol details). The script automatically generates and saves nonoverlapping ROIs, with at least one high-power field distance between them, with a scale bar at high resolution (see Figures 1 and E3). For easier adoption, we also provide a representative work flow of mouse lung sampling from WSI to ROIs generation (Video E1).
Figure 1.
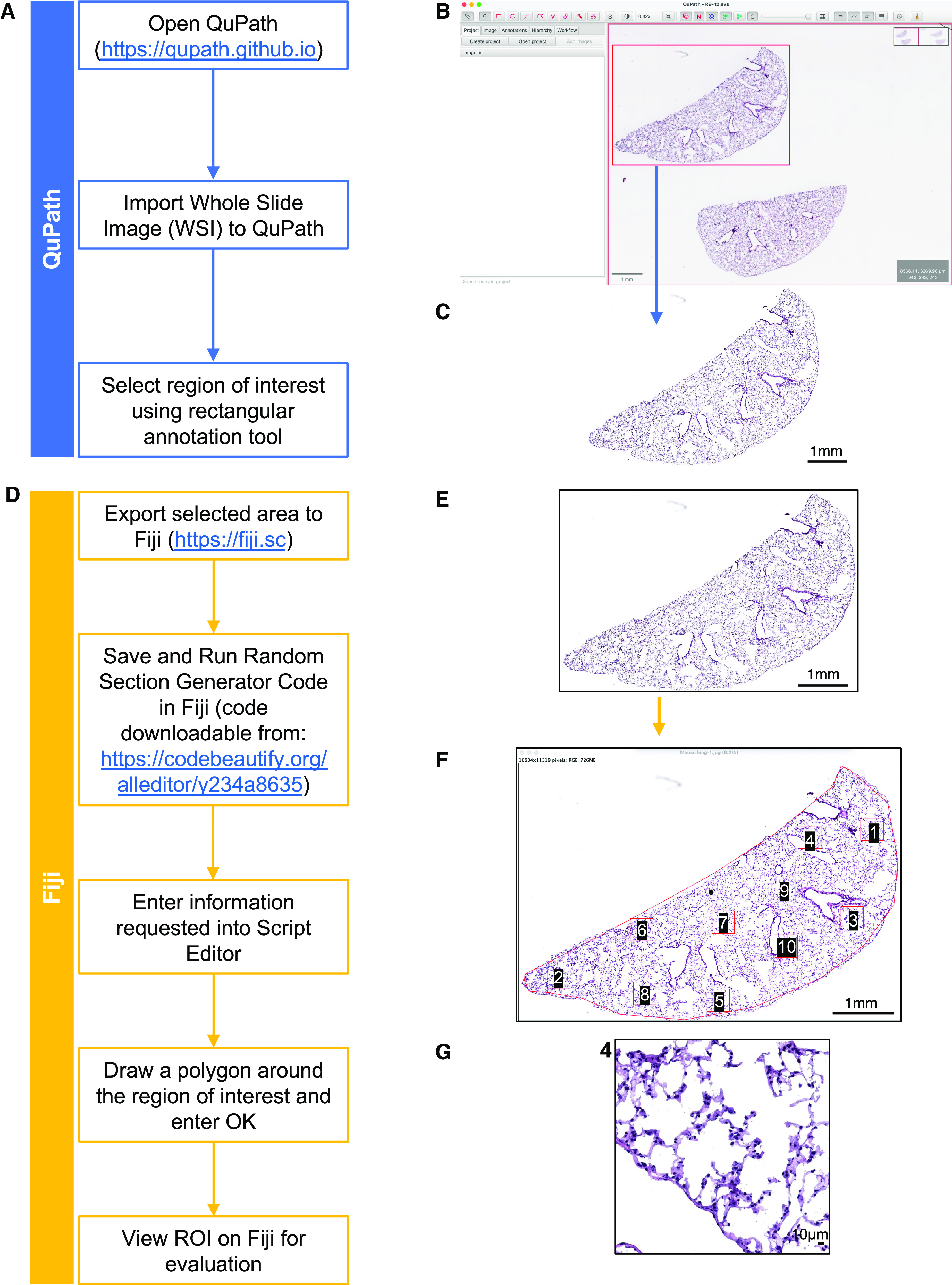
Highlights of the code applied to a mouse lung tile scan. (A–G) Left: flowchart for each step of the protocol. Right: examples of mouse lung images corresponding to each step. (A) Flowchart of QuPath steps. (B) Tile scan of whole mouse lung section opened in QuPath and selection of lung area to be exported in the red square. (C) Selected area opened in ImageJ and saved in .jpeg file format. (D) Flowchart of Fiji steps. (E) Selected area from C opened in Fiji. (F) Random regions of interest (ROIs) are selected by running the code. (G) Example of a high-magnification ROI, number 4 in this case. Scale bars: (B), (C), (E), (F), 1mm; (G), 10 μm.
We applied the custom script to both mouse and rat lungs after different models of lung injury—bleomycin in mice and lipopolysaccharide (LPS)/hydrochloric acid (HCl) and detergent ([3-(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate [CHAPS]) in rats—with reproducible sampling and analysis (see Figures E4 and E5). All images retain high-quality histological details to allow the grading of LIS parameters by a pathologist using a standard computer monitor, without other special equipment.
The number of ROIs for each species will vary with its size, and randomly selecting more ROIs than are required for the analysis permits the exclusion of those with predominantly large airway lumens or vessels and those composed of <50% of lung alveoli. Because of the patchy nature of the lung injury, the ATS guidelines (2) suggest independent, blinded scoring of at least 20 random high-power ROIs for murine total lung area for each tested condition. In our case, to standardize lung sampling, we designed a lung map of three representative sections of the upper, middle, and lower regions of the lung corresponding to routinely examined sections (see Figure E2). To strengthen the statistical power of our data, we selected 20 ROIs from each section, for a total of 60 ROIs/mouse. In the case of rat lungs, considering the lung size, we selected a total of 300 ROIs for total lung area to obtain a representative sample of lung injury (see Figure E2 for the lung map).
We also applied this code to samples from other species, such as human and pig, obtaining the same yield in terms of resolution and random selection of ROIs (see Figure E6).
The custom script provides advantages over current manual methods of selecting ROIs for LIS. First, compared with commercial options that focus on downstream analysis but not automated sampling, this script is freely accessible and does not require a paid subscription. Second, it is standardized, which means that it can be used to generate ROIs in a consistent and reproducible manner. Third, it is random and eliminates inadvertent sampling bias in the selection of ROIs. Fourth, it is easy to use, which means that it can be used by researchers and pathologists in any laboratory, requiring minimal training. Last, ROIs can be presented in a blinded manner to a single scorer or shared electronically with multiple investigators and pathologists worldwide uninvolved in the original imaging processing for interobserver studies. We also envision that this code can be applied to other organs and across different species.
In summary, the open-source custom script described here is an easily adaptable tool for researchers and pathologists evaluating ALI in laboratory animals and provides a well-established method for the random selection of lung fields emphasized in the 2022 update of the lung injury workshop (3). Future directions include the application of this strategy to artificial intelligence and deep neural networks for semiautomated LIS evaluation. Although training for artificial intelligence may require a large, well-annotated dataset, the method presented here provides a practical tool to assist in the annotation process and LIS evaluation.
Acknowledgments
Acknowledgment
The authors thank the following collaborators and supporters: the Institute of Comparative Medicine veterinary staff, including Rivka L. Shoulson and Dominik Hajosi for supporting animal studies, and the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, funded in part through National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30CA013696.
Footnotes
Supported by Driscoll Children’s Fund Scholar (N.V.D.), the Louis V. Gerstner Jr. Scholarship Fund (N.V.D.), the Stony Wold-Herbert Fund (N.V.D.), and National Heart, Lung, and Blood Institute grant U01HL134760-07 (N.V.D., co-investigator).
Author Contributions: C.P., D.I., and N.V.D. designed the study. C.P. and D.I. optimized the code with T.S. and validated on mouse (D.I.), rat, human, and pig (C.P.). C.P., D.I., A.S., T.S., and N.V.D. interpreted and analyzed the data. N.V.D. wrote the manuscript with support from C.P., M.L.M., T.S., and A.S. All the authors read and approved the final manuscript.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2. Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. Acute Lung Injury in Animals Study Group An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol . 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kulkarni HS, Lee JS, Bastarache JA, Kuebler WM, Downey GP, Albaiceta GM, et al. Update on the features and measurements of experimental acute lung injury in animals: an official American Thoracic Society workshop report. Am J Respir Cell Mol Biol . 2022;66:e1–e14. doi: 10.1165/rcmb.2021-0531ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsia CCW, Hyde DM, Ochs M, Weibel ER, ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med . 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi Y-Y, Liu T-J, Fu J-H, Xu W, Wu LL, Hou AN, et al. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol Med Rep . 2016;13:1186–1194. doi: 10.3892/mmr.2015.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blondonnet R, Audard J, Belville C, Clairefond G, Lutz J, Bouvier D, et al. RAGE inhibition reduces acute lung injury in mice. Sci Rep . 2017;7:7208. doi: 10.1038/s41598-017-07638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel BV, Wilson MR, Takata M. Resolution of acute lung injury and inflammation: a translational mouse model. Eur Respir J . 2012;39:1162–1170. doi: 10.1183/09031936.00093911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin F, Fan Z, Xu M, Wang Z, Dong Y, Qu C, et al. Amelioration of ambient particulate matter (PM2.5)-induced lung injury in rats by aerobic exercise training. Front Physiol . 2021;12:731594. doi: 10.3389/fphys.2021.731594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schranc Á, Fodor GH, Südy R, Tolnai J, Babik B, Peták F. Exaggerated ventilator-induced lung injury in an animal model of type 2 diabetes mellitus: a randomized experimental study. Front Physiol . 2022;13:889032. doi: 10.3389/fphys.2022.889032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Neill JD, Guenthart BA, Kim J, Chicotka S, Queen D, Fung K, et al. Cross-circulation for extracorporeal support and recovery of the lung. Nat Biomed Eng . 2017;1:0037. [Google Scholar]
- 11.LaPar DJ, Laubach VE, Emaminia A, Crosby IK, Hajzus VA, Sharma AK, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–894. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toth A, Steinmeyer S, Kannan P, Gray J, Jackson CM, Mukherjee S, et al. Inflammatory blockade prevents injury to the developing pulmonary gas exchange surface in preterm primates. Sci Transl Med . 2022;14:eabl8574. doi: 10.1126/scitranslmed.abl8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hozain AE, O’Neill JD, Pinezich MR, Tipograf Y, Donocoff R, Cunningham KM, et al. Xenogeneic cross-circulation for extracorporeal recovery of injured human lungs. Nat Med . 2020;26:1102–1113. doi: 10.1038/s41591-020-0971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horai Y, Akatsuka A, Mizukawa M, Niishina H, Nishikawa S, Ono Y, et al. Current status and prospects for quantitative analysis of digital image of pathological specimen using image processing software including artificial intelligence. Transl Regul Sci . 2020;2:72–79. [Google Scholar]
- 15. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep . 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods . 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


