Abstract
Pulmonary hypertension (PH) is a heterogeneous and life-threatening cardiopulmonary disorder in which mitochondrial dysfunction is believed to drive pathogenesis, although the underlying mechanisms remain unclear. To determine if abnormal SIRT3 (sirtuin 3) activity is related to mitochondrial dysfunction in adventitial fibroblasts from patients with idiopathic pulmonary arterial hypertension (IPAH) and hypoxic PH calves (PH-Fibs) and whether SIRT3 could be a potential therapeutic target to improve mitochondrial function, SIRT3 concentrations in control fibroblasts, PH-Fibs, and lung tissues were determined using quantitative real-time PCR and western blot. SIRT3 deacetylase activity in cells and lung tissues was determined using western blot, immunohistochemistry staining, and immunoprecipitation. Glycolysis and mitochondrial function in fibroblasts were measured using respiratory analysis and fluorescence-lifetime imaging microscopy. The effects of restoring SIRT3 activity (by overexpression of SIRT3 with plasmid, activation SIRT3 with honokiol, and supplementation with the SIRT3 cofactor nicotinamide adenine dinucleotide [NAD+]) on mitochondrial protein acetylation, mitochondrial function, cell proliferation, and gene expression in PH-Fibs were also investigated. We found that SIRT3 concentrations were decreased in PH-Fibs and PH lung tissues, and its cofactor, NAD+, was also decreased in PH-Fibs. Increased acetylation in overall mitochondrial proteins and SIRT3-specific targets (MPC1 [mitochondrial pyruvate carrier 1] and MnSOD2 [mitochondrial superoxide dismutase]), as well as decreased MnSOD2 activity, was identified in PH-Fibs and PH lung tissues. Normalization of SIRT3 activity, by increasing its expression with plasmid or with honokiol and supplementation with its cofactor NAD+, reduced mitochondrial protein acetylation, improved mitochondrial function, inhibited proliferation, and induced apoptosis in PH-Fibs. Thus, our study demonstrated that restoration of SIRT3 activity in PH-Fibs can reduce mitochondrial protein acetylation and restore mitochondrial function and PH-Fib phenotype in PH.
Keywords: mitochondria, SIRT3, honokiol, nicotinamide adenine dinucleotide, pulmonary hypertension
Clinical Relevance
The present study provides novel information and adds to a better understanding of the mechanisms underlying the cell abnormalities that characterize vascular remodeling in pulmonary hypertension and identify potential new therapeutic strategies.
Pulmonary hypertension (PH) is a prevalent comorbid condition that significantly worsens morbidity and mortality in patients with various disorders. Although five classes of drugs have been approved for treating patients with PH, they can alleviate symptoms but do not prevent or reverse the causative pathology (1). It is therefore necessary to explore new strategies for treating PH. Metabolic and mitochondrial reprogramming are increasingly recognized as central drivers of the pathogenesis of the dysregulated vascular cell phenotype and right ventricular dysfunction in PH (2–9). Many PH-initiating factors, including transcription factors (e.g., HIF [hypoxia-induced factor] [10]), cytokines (e.g., complement [11], ILs [12]), growth factors (e.g., TGF-β [transforming growth factor-β] [13]), and epigenetic regulators (14, 15), have all been reported to affect mitochondrial function and metabolism of pulmonary vascular cells. In return, abnormal metabolites play an important role in sustaining functional abnormalities of PH cells, including aberrant proliferation, apoptosis, inflammation, and chromatin (7, 14, 16). Normalizing the metabolic status and mitochondrial function is a proposed effective PH treatment strategy. However, the underlying mechanisms that cause mitochondrial dysfunction in PH and specific treatments targeting mitochondria are still unknown.
We previously showed that persistently activated PH pulmonary artery adventitial fibroblasts (PH-Fibs), both from patients with PH and from hypoxia-induced PH calves, exhibit altered mitochondrial metabolism with increased pyruvate dehydrogenase (PDH) phosphorylation, decreased mitochondrial energy production, increased mitochondrial fragmentation, and complex I deficiency (NDUFS4 [NADH:ubiquinone oxidoreductase subunit S4]) (3, 5, 17). Notably, these significant mitochondrial alterations cannot be recapitulated in vitro when pulmonary artery adventitial fibroblasts from control lungs (CO-Fibs) are exposed to hypoxia, suggesting that the establishment of these distinct, mitochondrial metabolism-driven alterations in PH-Fibs requires multiple signals and that hypoxia/HIF activation is not sufficient (5). Dichloroacetate (DCA), a PDH kinase inhibitor that increases the entry of pyruvate to mitochondria, has been shown to reverse hypoxia-induced mitochondrial abnormalities and induce pulmonary artery smooth muscle cell (PASMC) apoptosis in a monocrotaline-induced pulmonary arterial hypertension (PAH) rat model (5, 18). However, although DCA treatment decreased the inhibition of PDH and suppressed cell proliferation in PH-Fibs, it did not increase mitochondrial respiration or oxygen consumption, indicating continued complex I dysfunction and persistent mitochondrial and cytosolic reactive oxygen species (ROS) production (5). Furthermore, a clinical trial also showed a lack of response to DCA in the subgroup of patients with idiopathic PAH (IPAH) with SIRT3 (sirtuin 3) polymorphisms (19). These studies indicate the involvement of SIRT3 in mitochondrial dysfunction in PH.
SIRT3 is a member of the sirtuin family, including SIRT1–7, and requires nicotinamide adenine dinucleotide (NAD+) as a cofactor for its enzymatic activity. SIRT3 acts as the primary mitochondrial deacetylase that decreases the acetylation and consequently increases the activities of most, if not all, mitochondrial proteins involved in the tricarboxylic acid cycle (GDH [glutamate dehydrogenase], SDH [succinate dehydrogenase], IDH2 [isocitrate dehydrogenase (NADP+) 2]), oxidative phosphorylation (OXPHOS), fatty acid oxidation (long-chain acyl–coenzyme A dehydrogenase), and ROS management (mitochondrial superoxide dismutase [MnSOD2]) (20, 21). Decreased activity of SIRT3 has been reported in various disease conditions, including PH (22–24), affecting the phenotypes of multiple cell types (23, 25–29). SIRT3 was downregulated in the lung tissues of rats with acute pulmonary embolism, and activation of SIRT3 attenuated the loss in lung function, ameliorated inflammatory response and oxidative damage, and inhibited apoptosis in lung tissues (30). Loss of Sirt3 function in rodent and human PASMCs ameliorates mitochondrial function and promotes the development of PAH in rodents and humans (23). Interestingly, loss-of-function SIRT3 polymorphisms have also been shown to be a reason why DCA is not effective in treating some patients with PH (19). These data indicate the importance of SIRT3 in regulating mitochondrial-involved metabolism and pulmonary vascular cell functions. Pulmonary artery adventitial fibroblasts are highly proliferative, apoptosis resistant, and proinflammatory (2–5, 17). However, the role of SIRT3 in pulmonary artery adventitial fibroblasts is unclear. Therefore, in this study, we tested the hypothesis that SIRT3 activity is decreased in PH-Fibs and that restoring its activity can reduce mitochondrial protein acetylation, improve mitochondrial function, and inhibit the proliferation of adventitial fibroblasts in PH.
Methods
Detailed methods are provided in the data supplement.
Results
SIRT3 Expression and Activity were Downregulated in PH Lungs
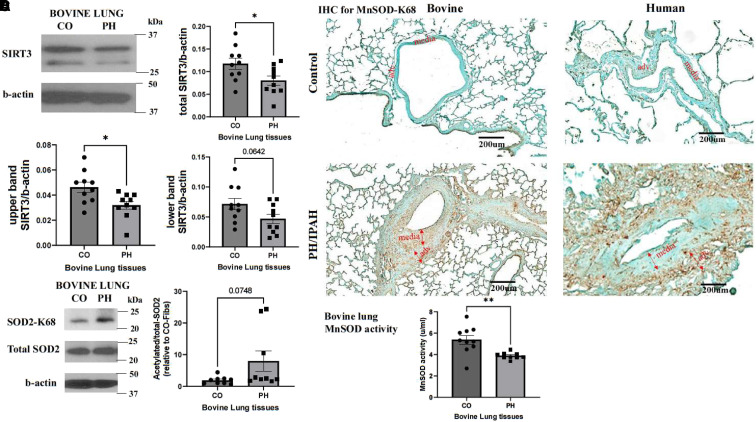
To determine whether there are differences in SIRT3 protein concentrations in the lung tissues between control and hypoxia-induced PH calves, we collected lung protein lysates from the distal part of the left caudal lobes and performed western blot analysis. It has been reported that SIRT3 has two isoforms: a full-length form that is cleaved within mitochondria by matrix metalloprotease to a 28-kD short form. The long isoform is localized to mitochondria, cytoplasm, and nucleus, and the short isoform is localized to mitochondria (31). We observed two bands of SIRT3 protein on western blot analysis. The full-length (upper band/b-actin) SIRT3 protein concentrations were significantly decreased in bovine PH lungs compared with control lungs, while the mitochondrial forms (lower band/b-actin) of SIRT3 showed a trend toward decrease that did not reach statistical significance (P = 0.06). The overall SIRT3 concentrations were significantly decreased in PH lungs (Figure 1A).
Figure 1.
Lung vasculature in pulmonary hypertension (PH) exhibits decreased SIRT3 (sirtuin 3) protein levels, increased acetylation of mitochondrial SOD2 (superoxide dismutase 2), and decreased MnSOD (mitochondrial SOD) activity. (A) Lung tissues were collected from 2-week hypoxia-induced PH young calves and age-matched normoxic control (CO) animals. Protein lysates were isolated, and western blot was performed with an antibody against SIRT3 (n = 10). Images show a representative blot for SIRT3 and b-actin. The full-length isoform (upper band), short-length isoform (lower band), and total SIRT3 protein levels were analyzed as the ratio to b-actin. Data are presented as mean ± SEM; *P < 0.05. (B) IHC staining was performed on lung specimens of hypoxia-induced PH calves, patients with IPAH, and corresponding COs with an antibody that is specific for K68 acetylation of SOD2 (brown color; n = 3 per group; scale bars, 200 μm). (C) Representative western blot images of acetylated MnSOD, total SOD2, and b-actin of bovine PH lungs and CO lungs (n = 9). Data were analyzed as the ratio of acetylated to total SOD2 and are presented as mean ± SEM. (D) MnSOD activity of bovine PH and CO lung tissues was determined using an Invitrogen superoxide dismutase colorimetric assay kit (n = 10). Data are presented as mean ± SEM; **P < 0.01. adv. = pulmonary artery adventitial layer; CO-Fibs = pulmonary artery adventitial fibroblasts from control lungs; IHC = immunohistochemistry; IPAH = idiopathic pulmonary arterial hypertension; K68 = lysine 68; media = pulmonary artery media layer.
The decreased expression of SIRT3, a mitochondrial deacetylase, can result in higher acetylation of its target protein. To investigate this effect, we conducted immunohistochemistry (Figure 1B) and western blot (Figure 1C) analyses on lung specimens from patients with IPAH, young calves with hypoxia-induced PH, and their corresponding controls. Specifically, we examined the acetylation of MnSOD2, a target of SIRT3, using an antibody that is specific for lysine 68 acetylation (acK68) of SOD2 (superoxide dismutase 2). We found that the staining of SOD2-acK68 was increased in the pulmonary arteries of patients with IPAH and bovine PH lung sections compared with controls, though there were variations in expression across different vessels (Figure 1B). Although the western blot analysis showed a trend toward increased acetylated SOD2 expression in bovine PH lung tissues, it did not reach statistical significance (P = 0.07) (Figure 1C). Finally, we examined MnSOD activity in bovine lung tissues and observed a significant decrease in PH lung tissues (Figure 1D).
Decreased SIRT3 levels and NAD+ Availability Are Observed in PH-Fibs
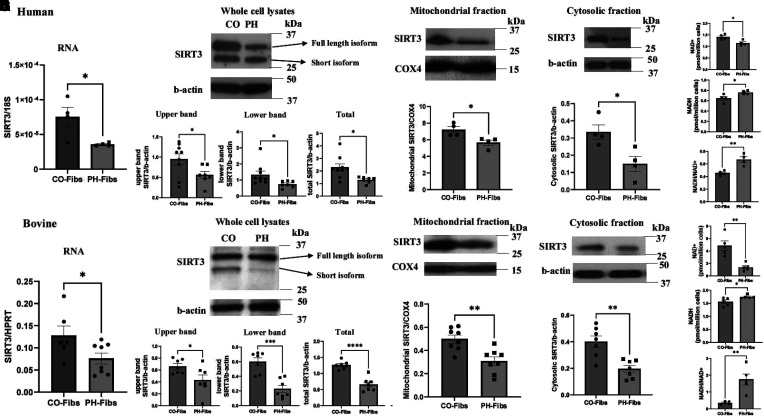
We used an explantation technique to isolate pulmonary artery adventitial fibroblast cells from the lungs of patients with IPAH and cows with PH (PH-Fibs) and from their corresponding control lungs (CO-Fibs). We then quantified the mRNA and protein levels of SIRT3, as well as its cofactor, NAD+. Our results showed that both human and bovine PH-Fibs had significantly lower levels of SIRT3 mRNA compared with CO-Fibs (Figures 2A and 2F). Similarly, we observed significantly decreased protein levels of the full-length form (upper band/b-actin) and mitochondrial short form (lower band/b-actin) of SIRT3 in PH-Fibs, as well as overall SIRT3 levels, compared with CO-Fibs (Figures 2B and 2G). We also separated the mitochondrial and cytosolic compartments of fibroblasts and performed western blot for SIRT3 using b-actin as a cytosolic loading control and COX4 (cytochrome c oxidase subunit 4) as mitochondrial loading control. A single band of SIRT3 was observed in cytosolic and mitochondrial fractions. SIRT3 protein levels were significantly reduced in both the cytosolic and mitochondrial fractions of human and bovine PH-Fibs compared with CO-Fibs (Figures 2C and 2H). We also examined mRNA levels of other sirtuin family members and found that only SIRT3 and SIRT4 mRNA expression was significantly decreased in human and bovine PH-Fibs (see Figure E1 in the data supplement).
Figure 2.
Pulmonary artery adventitial fibroblast cells isolated from lung pulmonary arteries dissected from patients with IPAH and bovine PH (PH-Fibs) exhibit decreased SIRT3 levels as well as decreased SIRT3 cofactor NAD+. PH-Fibs and CO-Fibs were isolated using an explantation technique. (A–D and F–I) mRNA (A and F), whole-cell protein lysate (B and G), mitochondrial (C and H), and cytosolic compartment (D and I) were isolated (human, upper panel; bovine, lower panel), and real-time quantitative PCR (A and F) and western blots (B–D, and G–I) were performed to determine SIRT3 expression. B-actin was used as loading control for whole-cell and cytosolic lysates, and COX4 was used as loading control for mitochondrial lysates. Representative western blot images are shown, and data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. (E and J) NAD+ and NADH concentrations and NADH:NAD+ ratio were measured and calculated with freshly collected human and bovine PH-Fibs and CO-Fibs using an NAD/NADH colorimetric assay kit. Data are presented as mean ± SEM; *P < 0.05 and **P < 0.01. COX4 = cytochrome c oxidase subunit 4; HPRT = hypoxanthine phosphoribosyltransferase; NAD+ = nicotinamide adenine dinucleotide; NADH = nicotinamide adenine dinucleotide reduced.
NAD+ is a cofactor that facilitates the activity of SIRT3. We measured concentrations of oxidized form (NAD+) and reduced form (NADH) in freshly collected fibroblast samples using an NAD/NADH colorimetric assay kit and found that human and bovine PH-Fibs exhibited significantly decreased NAD+, increased NADH, and an increased NADH:NAD+ ratio compared with CO-Fibs (Figures 2E and 2J).
SIRT3 Deacetylase Activity Is Decreased in PH-Fibs
To determine if decreased SIRT3 expression and/or NAD+ concentrations contribute to the decreased SIRT3 activity, we isolated mitochondrial proteins from human and bovine PH-Fibs and CO-Fibs and performed western blot to determine the acetylation of mitochondrial proteins using an antibody against panacetylated lysine. The results showed that the overall mitochondrial proteins from both human and bovine PH-Fibs (Figure 3A) exhibited significantly higher acetylation compared with their controls, indicating decreased SIRT3 deacetylase activity in PH-Fibs.
Figure 3.
Decreased SIRT3 activity in PH-Fibs is detected by increased mitochondrial protein panacetylation, increased MPC1 (mitochondrial pyruvate carrier 1) and MnSOD acetylation, and decreased MnSOD activity. (A) Mitochondrial proteins were extracted from PH-Fibs and CO-Fibs. Western blot analysis was performed using an antibody against acetylated lysine (panacetylation). COX4 was used as the mitochondrial loading control. Representative images are shown. Densitometry data were calculated as all acetylated proteins/COX4 and are shown as mean ± SEM (n = 4 or 5); *P < 0.05. (B) IP was performed to determine the degree of acetylation of SIRT3-specific target MPC1 on bovine CO-Fibs and PH-Fibs. Protein lysates were first pulled down with MPC1 antibody, followed by western blot to determine the degree of acetylation of MPC1. Data are shown as mean ± SEM (n = 3); *P < 0.05. (C) Western blot was performed on human (n = 4) and bovine (n = 6–8) PH-Fibs and CO-Fibs with antibodies specific for K68 acetylation of SOD2 or total SOD2. Densitometry data were calculated as acetylated/total SOD2 and are shown as mean ± SEM; *P < 0.05. (D) MnSOD activity of human (n = 6 or 7) and bovine (n = 5) PH-Fibs and CO-Fibs was determined using an Invitrogen superoxide dismutase colorimetric assay kit. Data are presented as mean ± SEM; *P < 0.05 and **P < 0.01.
We examined the acetylation of two important mitochondrial proteins: MPC1 (mitochondrial pyruvate carrier 1), which controls the import/uptake of pyruvate into mitochondria (32), and SOD2, which regulates ROS production. The acetylation and function of MPC are reported to be regulated by the deacetylase activity of SIRT3 (33). As there is no antibody to directly determine the acetylation of MPC, we first pulled down MPC1 protein in whole-cell lysates using an MPC1 antibody and IgG beads and then determined the acetylation of MPC1 protein using an antibody against panacetylation in western blot analysis of precipitated MPC1 protein. The results demonstrated that the levels of acetylated MPC1 were increased in human PH-Fibs (P = 0.08) and significantly increased in bovine PH-Fibs compared with CO-Fibs (Figure 3B). The acetylation of MnSOD (MnSOD-acK68) were significantly increased in western blot analysis (Figure 3C), and colorimetric activity analysis showed that MnSOD activity was decreased in both human and bovine PH-Fibs compared with CO-Fibs (Figure 3D).
We also examined the mRNA and protein concentrations of MPC1/2 in CO-Fibs and PH-Fibs and found that both human and bovine PH-Fibs had significantly decreased MPC1/2 mRNA levels compared with CO-Fibs. Human PH-Fibs also showed significantly decreased MPC2 protein expression and a nonsignificant (P = 0.08) decrease in MPC1 protein expression, while bovine PH-Fibs showed significantly decreased MPC1 protein expression. No MPC2 antibody for bovine species is available (see Figure E2).
In summary, these studies demonstrate that PH-Fibs exhibit reduced levels of SIRT3 and its cofactor NAD+ as well as attenuated SIRT3 activity.
Restoration of SIRT3 and NAD+ Decreases Mitochondrial Protein Acetylation and Increases MnSOD Activity in PH-Fibs
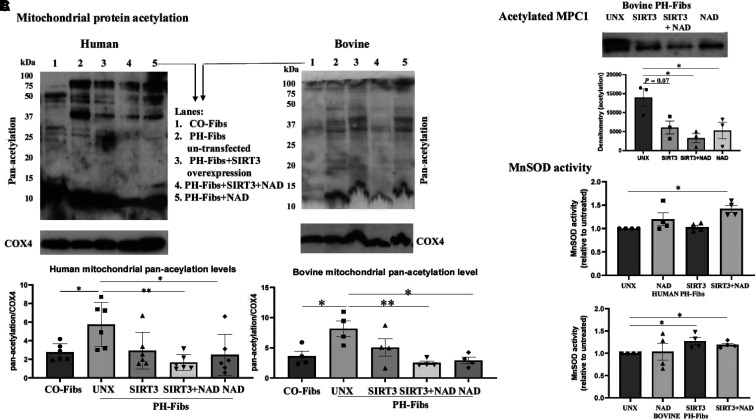
We aimed to investigate whether restoring SIRT3 expression could affect mitochondrial protein acetylation. To achieve this, we overexpressed SIRT3 in PH-Fibs with plasmid transfection, supplemented cells with SIRT3 cofactor NAD+, or both. The effects of plasmid transfection on SIRT3 protein expression are shown in Figure E3. Then, using an antibody against panacetylation, we performed western blots on isolated mitochondrial proteins from CO-Fibs, PH-Fibs, PH-Fibs with SIRT3 transfection, PH-Fibs with SIRT3 transfection plus NAD+ supplementation, and PH-Fibs with NAD+ supplementation alone. We found that in human and bovine PH-Fibs, SIRT3 overexpression alone caused a decrease in mitochondrial protein acetylation, although this was not statistically significant. However, adding NAD+ further reduced mitochondrial protein acetylation (Figure 4A, lane 4), leading to a significant decrease in mitochondrial protein acetylation compared with untreated PH-Fibs (even though it is not statistically different from SIRT3 transfection alone), to a degree similar to that in CO-Fibs.
Figure 4.
Overexpression of SIRT3 and supplementation with NAD+ decreased mitochondrial protein acetylation and increased MnSOD activity in PH-Fibs. (A) Human (n = 6) and bovine (n = 4) PH-Fibs were transfected with SIRT3 plasmid to overexpress SIRT3 in the presence or absence of NAD+ supplement or left untransfected (UNX). Mitochondrial protein lysates were extracted, and a western blot was performed with an antibody against panacetylation. COX4 was used as the mitochondrial loading control. Representative western blots are shown, and data are presented as mean ± SEM. *P < 0.05, **P < 0.01. Lane 1, CO-Fibs; lane 2, PH-Fibs UNX; lane 3, PH-Fibs transfected with SIRT3; lane 4, PH-Fibs plus SIRT3 plus NAD+; lane 5, PH-Fibs plus NAD+. (B) IP was performed to determine the effect of SIRT3 overexpression and NAD+ supplementation on MPC1 acetylation in bovine PH-Fibs (n = 3). Protein lysates were first pulled down with MPC1 antibody, followed by western blot with an antibody against acetylation. Data shown as mean ± SEM. *P < 0.05. (C) The effects of SIRT3 plasmid transfection and NAD+ supplementation on MnSOD activity in human and bovine (n = 4) PH-Fibs were determined using an Invitrogen superoxide dismutase colorimetric assay kit. Data are presented as mean ± SEM; *P < 0.05.
We then examined the effect of SIRT3 overexpression and NAD+ supplementation on the acetylation of MPC1 and the activity of MnSOD. We found that SIRT3 overexpression decreased MPC1 acetylation (P = 0.07) and that NAD+ supplementation significantly (P = 0.049) decreased MPC1 acetylation, and the combination of SIRT3 plasmid and NAD+ supplement further decreased the acetylation of MPC1 (P = 0.017) in bovine PH-Fibs (Figure 4B) and significantly increased the activity of MnSOD (Figure 4C).
There are three members of the sirtuin family in mitochondria: SIRT3, SIRT4, and SIRT5 (34). Even though SIRT3 is the major mitochondrial deacetylase, we explored the possibility that the decreased mitochondrial protein acetylation observed after overexpressing SIRT3 could involve the deacetylase activity of SIRT4 and/or SIRT5. Therefore, we determined the mRNA levels of SIRT4 and SIRT5 in PH-Fibs transfected with SIRT3 plasmid and found that manipulating SIRT3 did not affect mRNA concentrations of SIRT4 and SIRT5 in PH-Fibs (see Figure E4). This result suggests that the decrease in mitochondrial protein acetylation is due mainly to increased SIRT3 levels rather than SIRT4 or SIRT5.
Restoration of SIRT3 and NAD+ Improves Mitochondrial Function in PH-Fibs
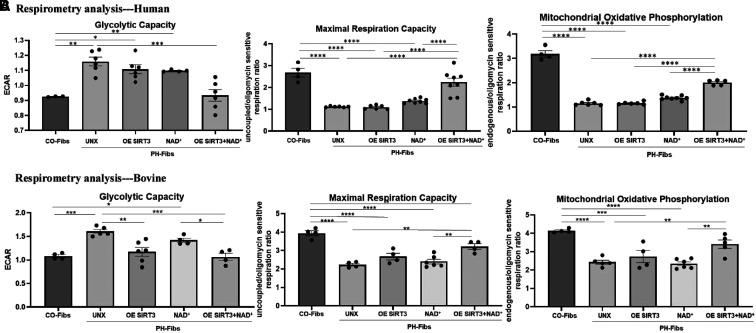
Our data thus far indicate that the activity of mitochondrial deacetylase SIRT3 is downregulated in PH-Fibs and PH lungs; in particular, the acetylation of MPC1 and MnSOD was increased. This led to an examination of the metabolic state and mitochondrial respiration in control and PH-Fibs, as well as the role of SIRT3 in regulating metabolism. Respirometry analysis revealed that both human (Figure 5A) and bovine (Figure 5B) PH-Fibs exhibited significantly increased glycolytic capacity (extracellular acidification rate [ECAR]) and inhibited mitochondrial respiratory activity with significantly decreased maximal respiration capacity and suppressed mitochondrial OXPHOS (Figures 5A and 5B). Overexpression of SIRT3 with plasmid or increasing NAD+ availability alone did not show significant effects on human and bovine fibroblast metabolism, with the exception that SIRT3 plasmid significantly reduced bovine PH-Fib glycolysis (Figure 5B), and the combination of SIRT3 overexpression and NAD+ supplementation synergistically and significantly decreased glycolytic capacity and increased mitochondrial maximal respiration and mitochondrial OXPHOS to degrees similar to CO-Fibs (Figures 5A and 5B). In general, the combination of SIRT3 plasmid with NAD+ supplementation was the most effective in regulating metabolism in both human and bovine PH-Fibs.
Figure 5.
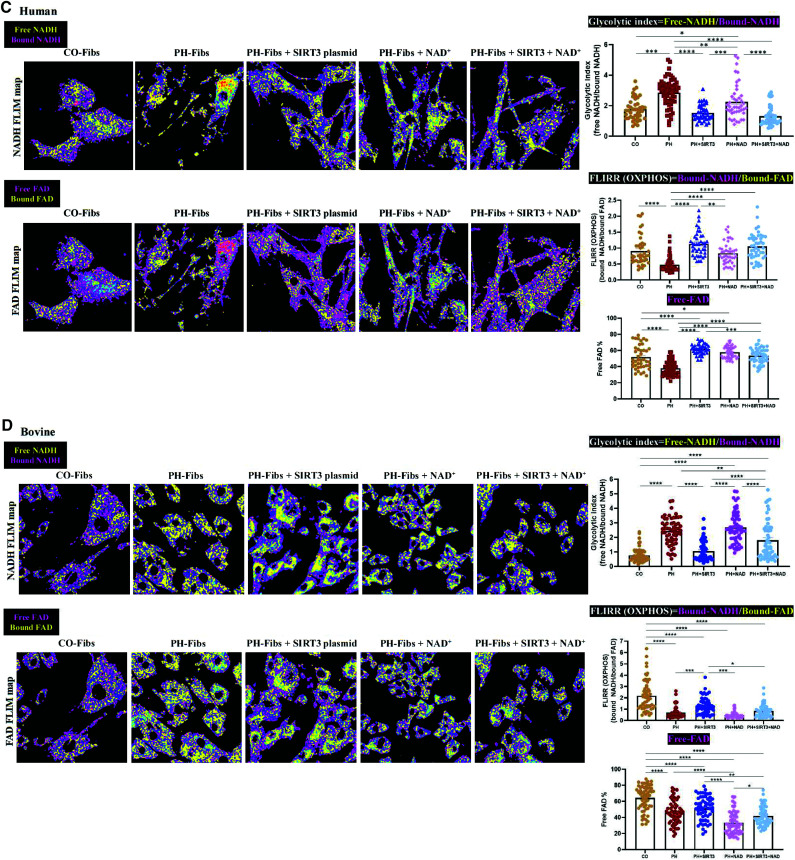
Respiratory analysis and FLIM demonstrated that overexpressing (OE) SIRT3 and supplementation with NAD+ decreased glycolysis and increased mitochondrial maximal respiration and oxidative phosphorylation (OXPHOS) in PH-Fibs. (A and B) Glycolytic capacity (ECAR), mitochondrial maximal respiration, and mitochondrial OXPHOS (state 3/4) were measured using respirometry analysis on the following cells: human (A) and bovine (B) CO-Fibs, PH-Fibs, and PH-Fibs transfected with SIRT3 plasmid with or without NAD+ supplementation or with NAD+ supplementation alone. The results showed that glycolytic capacity was increased and mitochondrial function was impaired in human and bovine PH-Fibs. OE SIRT3 and supplementation with NAD+ improved mitochondrial functions and decreased glycolysis in PH-Fibs. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. (C and D) Intracellular metabolic changes in human (C) and bovine (D) CO-Fibs, PH-Fibs, and PH-Fibs transfected with SIRT3 plasmid with or without NAD+ supplementation or with NAD+ supplementation alone were examined using FLIM. Top: NADH (yellow, free NADH; purple, enzyme-bound NADH) maps are shown. Bottom: flavin adenine dinucleotide (FAD) (purple, free FAD; yellow, enzyme-bound FAD) maps are shown. Glycolytic index (free NADH fraction/enzyme-bound NADH fraction), FLIRR (enzyme-bound NADH fraction/enzyme-bound FAD fraction), and free FAD fraction were quantified. Each dot represents a single cell. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. ECAR = extracellular acidification rate; FLIM = fluorescence-lifetime imaging microscopy; FLIRR = fluorescence-lifetime imaging redox ratio.
NAD and flavin adenine dinucleotide (FAD) are two metabolic coenzymes that play important roles in cellular oxidation and reduction. We used fluorescence-lifetime image microscopy (FLIM), as a complementary method, to examine the dynamics of these fluorescent molecules in CO-Fibs and PH-Fibs and in response to SIRT3 overexpression and NAD+ supplementation. The fractions of free and enzyme-bound NADH and FAD were measured on the basis of their different lifetimes when these molecules were reduced or oxidized. The glycolytic index (the ratio of free to enzyme-bound NADH) and the fluorescence-lifetime redox ratio (FLIRR; the ratio of enzyme-bound NADH to enzyme-bound FAD) were calculated to estimate their respective glycolytic and OXPHOS contributions to the metabolic balance in cells.
Quantifying the metabolic ratios revealed that in human PH-Fibs (Figure 5C), SIRT3 overexpression significantly decreased the glycolytic index, making it comparable with that of CO-Fibs. In addition, FLIRR (OXPHOS) activity was significantly increased, indicating improved mitochondrial activity. SIRT3 also significantly increased free FAD percentage, indicating increased electron transport chain activity. The combination of SIRT3 plasmid and NAD+ supplementation further slightly, but not significantly, decreased the glycolytic index. In bovine PH-Fibs (Figure 5D), SIRT3 overexpression also significantly decreased the glycolytic index, bringing it to a value similar to that of CO-Fibs. Although FLIRR (OXPHOS) in bovine PH-Fibs also demonstrated a significant improvement, it did not reach the same degree as in CO-Fibs, and the free FAD percentage was not improved with SIRT3 transfection. Unlike in adult human cells, NAD+ supplementation alone did not affect the metabolic activity, and no synergetic effect was observed with SIRT3 transfection in PH-Fibs from young calves. This discrepancy may be due to species differences or a greater shortage of NAD+ in bovine PH-Fibs (Figure 1J) compared with human PH-Fibs (Figure 1E).
One of the advantages of FLIM is that it indicates signal distribution within cells. The FLIM map images (Figure 5C and 5D) showed that at basal condition, glycolysis (free [yellow] and bound [purple] NADH) and OXPHOS (bound NADH [purple] and bound FAD [yellow]) activities were distributed evenly throughout both human and bovine CO-Fibs. PH-Fibs, however, showed more free NADH (yellow) and more bound FAD (yellow) compared with CO-Fibs. After treatment, both human and bovine cells exhibited compartment-separated metabolic activities, with some glycolysis remaining around the nucleus (free NADH, yellow) and increased OXPHOS (bound NADH, purple) observed in the periphery of the cells, revealing a proportional shift from glycolysis toward OXPHOS. Supplementation with NAD+ and SIRT3 transfection further increased the proportion of OXPHOS to glycolysis.
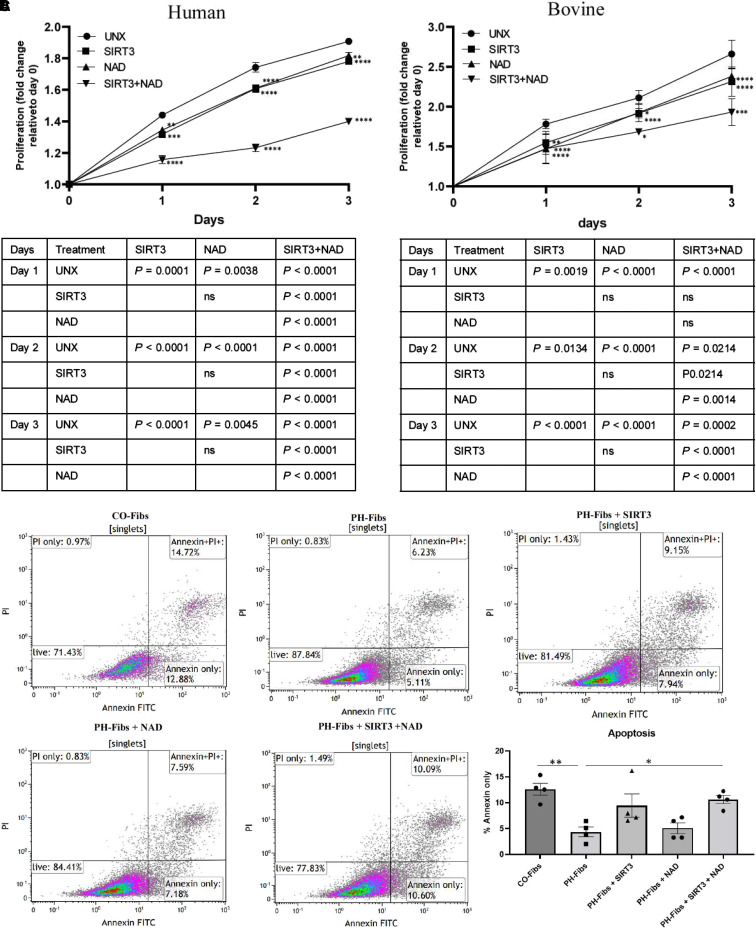
SIRT3 and NAD+ Regulate Proliferation and Apoptosis in PH-Fibs
We previously demonstrated that PH-Fibs exhibit highly proliferative and apoptosis-resistant phenotypes (3, 35). This is consistent with the well-known phenomenon that mitochondrial abnormalities can enhance proliferation while hindering apoptosis in most vascular cells, including endothelial cells, smooth muscle cells, and fibroblasts (36). Thus, we investigated the impact of overexpressing SIRT3 and increasing NAD+ concentrations on the proliferation properties of PH-Fibs. Our findings using CyQUANT analysis (invitrogen) and annexin V–FITC apoptosis staining revealed that SIRT3 and NAD+ acted in synergy to inhibit the proliferation of both human and bovine PH-Fibs (Figures 6A and 6B) and induce PH-Fib apoptosis (Figure 6C). Moreover, we explored the effects of SIRT3 overexpression and NAD+ supplementation on the expression of genes associated with cell proliferation and apoptosis. We found that in human PH-Fibs, SIRT3 overexpression decreased the expression of proproliferative genes (CCND3 [cyclin D3], Mki67 [marker of proliferation Ki-67]), decreased the expression of antiapoptotic genes (Bcl2, Birc5 [baculoviral IAP repeat containing 5]), and increased the expression of proapoptosis genes (NOXA [NADPH oxidase activator], PERP [P53 apoptosis effector related to PMP22]). NAD+ showed synergetic effects with the SIRT3 plasmid (see Figure E5A). Similar outcomes were observed for bovine PH-Fibs regarding the impact of SIRT3 overexpression and NAD+ supplementation (see Figure E5B).
Figure 6.
Overexpression of SIRT3 and supplementation with NAD+ decreased proliferation and induced apoptosis in PH-Fibs. (A and B) PH-Fibs from (A) patients with IPAH and (B) 2-week hypoxia-induced neonatal PH calves were seeded in 24-well plates in equal numbers and were transfected with plasmid to overexpress SIRT3 in the presence or absence of NAD+ for 72 hours or supplemented with NAD+ alone. Plates were collected daily, stored at −80 °C, and analyzed for cell proliferation using CyQUANT methods. Data were analyzed using two-way ANOVA and multiple comparisons. Data are presented as fold change relative to Day 0 and presented as mean ± SEM. The P values for each pair of comparisons are shown in the table below the corresponding graphs. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with untreated within the same day. (C) The apoptosis assay was performed with bovine fibroblast cells and measured using annexin V–FITC/PI labeling in flow cytometry. PH-Fibs have a significantly lower percentage of apoptotic cells compared with CO-Fibs, and overexpressing SIRT3 plus NAD+ supplementation synergistically induced cell apoptosis in PH-Fibs. *P < 0.05 and **P < 0.01. PI = propidium iodide.
The Transcriptional Corepressor C-Terminal Binding Protein-1 Regulates SIRT3 Expressions in PH-Fibs
To explore the possible reasons for the downregulated expression of SIRT3 in PH-Fibs, we aimed to investigate the regulation of SIRT3 expression in PH, which has not been extensively studied. Previous research has indicated that a transcriptional corepressor, CTBP1 (C-terminal binding protein 1), binds to the promoter region of SIRT3 and represses SIRT3 gene expression in breast cancer (37). In our previous study, we observed an upregulation of CTBP1 in PH-Fibs (3). To examine the role of CTBP1 in regulating SIRT3 expression in fibroblasts, we conducted experiments and found that knockdown of CTBP1 in both human and bovine PH-Fibs significantly increased SIRT3 mRNA and protein expression. Conversely, overexpression of CTBP1 with plasmid in human and bovine CO-Fibs significantly decreased SIRT3 mRNA expression (see Figure E6).
The SIRT3 Activator Honokiol Reduced Mitochondrial Protein Acetylation, Improved Mitochondrial Function, and Ameliorated PH-Fib Phenotype
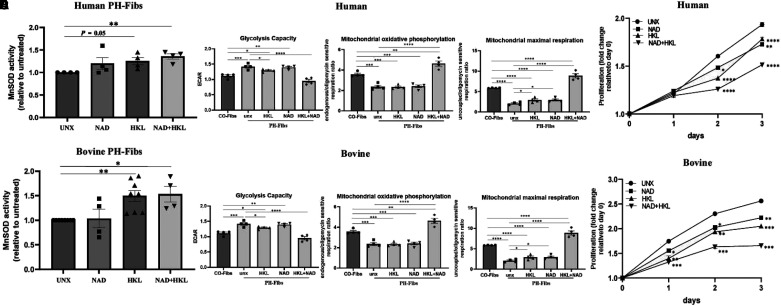
PH-targeted treatments primarily involve vasodilators, but none of them, either alone or in combination, is curative. Thus, exploring molecular targets in other pathways, such as mitochondrial dysfunction, is crucial for developing effective therapies. In this study, we used a small molecule, honokiol, to investigate the clinical relevance of manipulating SIRT3 in regulating PH fibroblast mitochondrial function and phenotype, a role that has never been reported in pulmonary fibroblasts. We first tested different doses of honokiol (5, 10, 20, and 30 μM) and confirmed that honokiol induces SIRT3 protein expression in adventitial fibroblasts, which is consistent with findings in cancer cells reported by other researchers. Representative dose and blots are shown in Figure E7A. On the basis of the western blot results, we treated human and bovine PH-Fibs with honokiol (20 μM) and demonstrated that honokiol decreased PH-Fib mitochondrial protein acetylation (see Figure E7B). Finally, we analyzed the functional phenotypes of human and bovine PH-Fibs with treatment with honokiol (20 μM) alone, NAD+ alone, or the combination of honokiol and NAD+ for 3 days. We analyzed MnSOD activity, metabolic status, and cell proliferation. The results showed that honokiol alone increased MnSOD activity in human (P = 0.055), and bovine (P < 0.01) PH-Fibs, and honokiol in combination with NAD+ significantly increased MnSOD activity in both human and bovine PH-Fibs (Figures 7A and 7D). Respiratory analysis demonstrated that the combination of honokiol and NAD+ was most effective in increasing mitochondrial OXPHOS and mitochondrial maximal respiration in both human and bovine PH-Fibs (Figures 7B and 7E). The proliferation assay showed that honokiol significantly inhibited PH-Fib proliferation, and NAD+ plus honokiol further attenuated PH-Fib proliferation (Figures 7C and 7F).
Figure 7.
Honokiol (HKL) and NAD+ synergistically increased MnSOD activity, increased mitochondrial oxidative phosphorylation and maximal respiration, and inhibited proliferation in PH-Fibs. (A and D) The effects of honokiol (20 μM, 72 h) and NAD+ supplementation on MnSOD activity of human (A) and bovine (D) PH-Fibs were determined using an Invitrogen superoxide dismutase colorimetric assay kit. Data are presented as mean ± SEM; *P < 0.05 and **P < 0.01. (B and E) Glycolytic capacity (ECAR), mitochondrial maximal respiration, and mitochondrial oxidative phosphorylation (state 3/4) were measured using respirometry analysis on human (B) and bovine (E) PH-Fibs and PH-Fibs treated with honokiol, NAD+ supplementation, or the combination of honokiol and NAD+. The results indicated that the combination of honokiol and NAD+ synergistically decreased glycolysis and increased mitochondrial oxidative phosphorylation and maximal respiration in both human and bovine PH-Fibs. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. (C and F) Human (C) and bovine (F) PH-Fibs were seeded in 24-well plated with equal numbers and were treated with honokiol (20 μM), NAD+ (500 mM), or the combination of honokiol and NAD+ for 3 days. Plates were collected daily, stored at −80 °C, and analyzed for cell proliferation using CyQUANT methods. Data were analyzed using two-way ANOVA and multiple comparisons. Data are presented as fold change relative to Day 0, compared with untreated within the same day, and expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Discussion
PH is a common condition that significantly worsens outcomes in patients with a variety of cardiopulmonary disorders. Current treatments, developed primarily for their vasodilating effects, do not reverse the underlying pathology. There is thus intensive investigation aimed at determining underlying contributing factors. Significant evidence supports the idea that metabolic and mitochondrial reprogramming could be significant contributors to the development and perpetuation of PH, but the specific mechanisms causing mitochondrial dysfunction are unknown. Mitochondria are central to cellular activities. They perform crucial cellular reactions, including the production of energy through the mitochondrial respiratory chain, regulation of cell death, calcium metabolism, and the production of ROS (6–9, 16). Many cellular functions rely on intact mitochondrial metabolism, and any dysfunction can have significant effects on cellular and tissue function. Thus, there is intense interest in developing strategies to restore mitochondrial function. Currently, mitochondrial-specific therapies include drugs targeting different metabolic pathways. These include DCA, an inhibitor of PDH kinase (19, 38); trimetazidine and ranolazine, which decrease long-chain fatty acid oxidation; and vitamin E (39) and coenzyme Q (40) to improve redox balance. However, different patients respond differently to these treatments. For example, a subset of patients who have loss-of-function genetic polymorphisms in SIRT3 and UCP2 (uncoupling protein 2) do not respond to DCA treatment (19). A better understanding of the underlying mechanisms of mitochondrial dysfunction in cells contributing to the pathophysiology of PH is thus needed.
In this study, we investigated the role of SIRT3 dysfunction in PH and its contribution to mitochondrial dysfunction. We found the following results. First, in bovine PH lungs, SIRT3 protein expression is decreased; the acetylation of the SIRT3 target, MnSOD, is increased; and MnSOD activity is decreased compared with CO-lungs, indicating the decreased deacetylase activity of SIRT3.
Second, in human and bovine PH-Fibs, the mRNA and protein concentrations of SIRT3, and its cofactor NAD+, are decreased compared with CO-Fibs. Third, the general acetylation of isolated mitochondrial protein is increased in human and bovine PH-Fibs, particularly the acetylation of the SIRT3-specific targets MPC1 and MnSOD. In addition, the MnSOD activity is increased in PH-Fibs.
Fourth, overexpressing SIRT3 with a plasmid and in conjunction with NAD+ supplementation decreased mitochondrial protein acetylation, restored MnSOD activity, decreased glycolysis, improved mitochondrial function, inhibited proliferation, and induced apoptosis of PH-Fibs.
Fifth, treatment with honokiol, a small-molecule SIRT3 activator, resulted in similar effects as the SIRT3 plasmid and reduced mitochondrial protein acetylation. Honokiol together with NAD+ improved mitochondrial function and inhibited the proliferation of PH-Fibs.
Studies from other groups have also provided evidence regarding the role of SIRT3 in regulating metabolism and the development of PH. It was demonstrated that monocrotaline-induced PAH is associated with energetic dysfunction in the right heart, and SIRT3 activation exerted cardiomyocyte protective effects (22). The Michelakis group demonstrated that heterozygous and homozygous SIRT3 depleted mice spontaneously developed pulmonary vascular lesions, PH, and metabolic dysfunction (23). Zhang and colleagues reported that in a prospective cohort of 60 patients with PAH, nearly 70% carried an SNP on at least one SIRT3 or UCP2 allele (41). The traditional Chinese medicine compound shufeiya recipe was reported to improve monocrotaline-induced PH in rats by regulating SIRT3/FOXO3a (forkhead box O3a), downregulating serum concentrations of ROS, and upregulating the activity of MnSOD (42). Paulin and colleagues nicely demonstrated that SIRT3 was downregulated in human and rodent PASMCs and demonstrated an important role of SIRT3 in regulating metabolism, PDH inhibition, and NFAT–HIF1a–STAT3 (signal transducer and activator of transcription 3) activation of PASMCs using wild-type and SIRT3-knockout mice (129/Sv strain). They showed that loss of function of SIRT3 promoted PAH, and adenoviruses carrying SIRT3 treatment protected against PAH development in a rat model (23). On the other hand, Waypa and colleagues studied the role of SIRT3 in PASMCs using wild-type or SIRT3/knockout mice (C57BL/6 background) and found that the absence of SIRT3 in PASMCs had no effect on acute hypoxia–induced changes in ROS or calcium signaling. SIRT3 depletion was not associated with an increase in HIF1a stabilization in hypoxia and did not augment chronic hypoxia–induced PH (43). The contrasting results of these two studies may be due to the strains of mice used (129/Sv vs. C57BL/6) and the methods used to assess mitochondrial function: mass spectrometry for metabolite analysis, mitochondrial staining, and mitochondrial membrane potential measurements in the Paulin and colleagues’ study (23) versus the biosensor redox-sensitive GFP in that of Waypa and colleagues (43). In our study, we used a completely different animal model: hypoxia-induced PH in young calves. The cell type analyzed in this study is also different. We focused on pulmonary artery adventitial fibroblasts from bovine hypoxia-induced PH and patients with IPAH. We previously demonstrated dramatic remodeling in the perivascular/adventitial area of the pulmonary artery in PH, closely similar to what we observed in human patients, as well as the significant role of adventitial fibroblasts in the pathogenesis of PH (2, 35, 44).
In this study, we used two different methods to determine cell metabolism: respiratory analysis and FLIM. Respiratory analysis is a technique used to measure the metabolic function of cells in real-time. It measures the oxygen consumption rate and ECAR of cells in response to different conditions or treatments and provides information on the energy production and glycolytic activity of the cells. FLIM is used to study the dynamics of fluorescent molecules in cells. It involves the measurement of the fluorescence lifetime, which is the time required for a fluorescent molecule to decay from its excited state to its ground state. In this study, both respiratory analysis and FLIM detected increased glycolysis and decreased mitochondrial respiration of PH-Fibs at basal levels compared with CO-Fibs. FLIM demonstrated that the combination of SIRT3 overexpression and NAD+ supplementation significantly decreased the glycolytic index and restored OXPHOS as well as electron transport chain activity to the degree of CO-Fibs. However, we did not observe significant synergetic effects, as measured using respiratory analysis. This may be due to the subtle differences in the principles of the two methods used to calculate metabolic state. Glycolytic capacity (ECAR) in respiratory analysis does not necessarily represent glycolytic index by FLIM (the ratio of free to bound NADH).
Immunohistochemistry staining of MnSOD2-acK68 (Figure 3) confirmed the upregulation of MnSOD2 acetylation in the pulmonary vascular wall, indicating that fibroblasts may not be the only cell type whose acetylation is regulated by SIRT3. It is possible that mitochondrial protein acetylation and the mitochondrial function of endothelial cells and smooth muscle cells may also be affected by the downregulated SIRT3 activity in PH. Further studies are needed to explore this possibility.
The sirtuin deacetylation reaction involves the removal of an acetyl group from target substrates via the conversion of NAD+ to nicotinamide and O-acetyl-ADP-ribose (45). NAD+ has been detected in the cytoplasm, nucleus, and mitochondria of cells, with its highest concentration in mitochondria (46). The ratio of the oxidized and reduced forms of NAD+ (NAD+:NADH) is essential for maintaining cellular redox homeostasis and modulating energy metabolism. NAD+ is also a cofactor for poly-ADP-ribose polymerases, sirtuins, and CD38/157 ectoenzymes, which function as essential components of cellular processes (47). A consensus is emerging that NAD+ concentrations decline at the cellular, tissue/organ, and organismal levels during the aging process (48). As downstream effectors, NAD-dependent enzymes such as sirtuins are involved in the pathophysiology of multiple diseases. Small-molecule activators of sirtuins and NAD+ boosters have shown promising cardiometabolic effects in animal models and are well tolerated in healthy volunteers (49). Supplementing NAD intermediates, such as nicotinamide mononucleotide and nicotinamide riboside (vitamin B3), provides proof of concept for the development of an effective metabolic intervention. The data from this study strongly support the synergistic effects of SIRT3 and NAD+ in decreasing mitochondrial protein acetylation, leading to improved mitochondrial function in PH-Fibs.
It remains unclear what causes the decreased concentrations of SIRT3 in fibroblasts in PH. Several studies have shown that the degree of deacetylation of SIRT3 is closely related to the cellular metabolic state and can change with the nutritional state of the cell. PGC-1a (peroxisome proliferator–activated receptor-γ coactivator-1α) has been identified as a key regulator of SIRT3 induction in muscle cells, hepatocytes, and brown adipocytes (50, 51). SIRT3 expression can be regulated by the profibrotic cytokine TGF-β1 in fibroblasts in patients with idiopathic pulmonary fibrosis (28). Kwon and colleagues noted that low concentrations of SIRT1 could indirectly lead to high acetylation and decreased activity of SIRT3, affecting the deacetylation of mitochondrial proteins (52). Furthermore, it has also been reported that micro-RNAs and long noncoding RNAs are involved in the regulation of SIRT3 expression (21). In the present study, we demonstrated the novel finding that a metabolic sensor and transcriptional corepressor, CTBP1, which is dimerized and activated when the cellular free NADH is increased, plays a role in regulating SIRT3 mRNA and protein expression in fibroblasts. However, CTBP1 per se was not sufficient to affect mitochondrial protein deacetylation (data not shown).
Conclusions
The data from this study establish a causative link between reduced SIRT3 deacetylase activity and mitochondrial dysfunction in adventitial fibroblasts in PH. The important and novel findings of this study demonstrate decreased SIRT3 deacetylase activity and increased general mitochondrial protein acetylation, including MPC and MnSOD, in patients with IPAH and bovine PH-Fibs. Restoring SIRT3 and supplementing NAD+ can normalize metabolism and cell phenotype. These results provide valuable insights into the mechanisms of mitochondrial dysfunction and may aid in the development of better personalized treatments for selected patients with PH.
Acknowledgments
Acknowledgment
The authors acknowledge Marcia McGowan and Andy Poczobutt for their help with manuscript preparation and submission as well as financial administration.
Footnotes
Supported by U.S. Department of Defense grant DOD W81XWH2010249; National Heart, Lung, and Blood Institute grant P01HL152961 and Ministry of Education, Czech Republic, grant LTAUSA18107.
Author Contributions: M.L. contributed to the experimental design; prepared samples; and performed experiments, data analysis, results explanation, and manuscript preparation. L.P.-H. performed experiments, data analysis, and manuscript preparation related to respiratory analysis and contributed to the overall experimental design. E.D. performed experiments, data analysis, and manuscript preparation related to fluorescence-lifetime image microscopy. A.G. and R.M.T. contributed to lung tissue immunohistochemistry staining and results explanation. S.R. contributed to sample preparation and nicotinamide adenine dinucleotide measurement. S.K. helped with statistical analysis. R.R.P. contributed to the cell apoptosis assay. B.A.M. contributed to sample preparation. A.L. contributed to the plasmid preparation. K.R.S., C.-J.H., and H.Z. contributed to the experimental design, results explanation, and manuscript preparation.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2022-0360OC on June 21, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lau EMT, Giannoulatou E, Celermajer DS, Humbert M. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol . 2017;14:603–614. doi: 10.1038/nrcardio.2017.84. [DOI] [PubMed] [Google Scholar]
- 2. D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, Ježek P, Li M, Zhang H, et al. Hallmarks of pulmonary hypertension: mesenchymal and inflammatory cell metabolic reprogramming. Antioxid Redox Signal . 2018;28:230–250. doi: 10.1089/ars.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li M, Riddle S, Zhang H, D’Alessandro A, Flockton A, Serkova NJ, et al. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation . 2016;134:1105–1121. doi: 10.1161/CIRCULATIONAHA.116.023171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenmark KR, Tuder RM, El Kasmi KC. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) . 2015;119:1164–1172. doi: 10.1152/japplphysiol.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plecitá-Hlavatá L, Tauber J, Li M, Zhang H, Flockton AR, Pullamsetti SS, et al. Constitutive reprogramming of fibroblast mitochondrial metabolism in pulmonary hypertension. Am J Respir Cell Mol Biol . 2016;55:47–57. doi: 10.1165/rcmb.2015-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res . 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 7. Dasgupta A, Wu D, Tian L, Xiong PY, Dunham-Snary KJ, Chen KH, et al. Mitochondria in the pulmonary vasculature in health and disease: oxygen-sensing, metabolism, and dynamics. Compr Physiol . 2020;10:713–765. doi: 10.1002/cphy.c190027. [DOI] [PubMed] [Google Scholar]
- 8. Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation . 2015;131:1691–1702. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu W, Janocha AJ, Erzurum SC. Metabolism in pulmonary hypertension. Annu Rev Physiol . 2021;83:551–576. doi: 10.1146/annurev-physiol-031620-123956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pullamsetti SS, Mamazhakypov A, Weissmann N, Seeger W, Savai R. Hypoxia-inducible factor signaling in pulmonary hypertension. J Clin Invest . 2020;130:5638–5651. doi: 10.1172/JCI137558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahman J, Singh P, Merle NS, Niyonzima N, Kemper C. Complement’s favourite organelle—mitochondria? Br J Pharmacol . 2021;178:2771–2785. doi: 10.1111/bph.15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srinivasan K, Pandey AK, Livingston A, Venkatesh S. Roles of host mitochondria in the development of COVID-19 pathology: could mitochondria be a potential therapeutic target? Mol Biomed . 2021;2:38. doi: 10.1186/s43556-021-00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun X, Lu Q, Yegambaram M, Kumar S, Qu N, Srivastava A, et al. TGF-β1 attenuates mitochondrial bioenergetics in pulmonary arterial endothelial cells via the disruption of carnitine homeostasis. Redox Biol . 2020;36:101593. doi: 10.1016/j.redox.2020.101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation . 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Napoli C, Benincasa G, Loscalzo J. Epigenetic inheritance underlying pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol . 2019;39:653–664. doi: 10.1161/ATVBAHA.118.312262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest . 2018;128:3704–3715. doi: 10.1172/JCI120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, et al. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation . 2017;136:2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res . 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 19. Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med . 2017;9:eaao4583. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 20. Cao M, Zhao Q, Sun X, Qian H, Lyu S, Chen R, et al. Sirtuin 3: emerging therapeutic target for cardiovascular diseases. Free Radic Biol Med . 2022;180:63–74. doi: 10.1016/j.freeradbiomed.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Shen X, Pang M, Sun Z, Qian Y, Xue W, et al. Role of histone deacetylase Sirt3 in the development and regression of atherosclerosis. Life Sci . 2021;272:119178. doi: 10.1016/j.lfs.2021.119178. [DOI] [PubMed] [Google Scholar]
- 22. Bernal-Ramírez J, Silva-Platas C, Jerjes-Sánchez C, Ramos-González MR, Vázquez-Garza E, Chapoy-Villanueva H, et al. Resveratrol prevents right ventricle dysfunction, calcium mishandling, and energetic failure via SIRT3 stimulation in pulmonary arterial hypertension. Oxid Med Cell Longev . 2021;2021:9912434. doi: 10.1155/2021/9912434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab . 2014;20:827–839. doi: 10.1016/j.cmet.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 24. Lajoie AC, Potus F. Sirtuin 3 and uncouplin protein 2, the missing link between genetics, metabolism, and pulmonary arterial hypertension. J Am Heart Assoc . 2021;10:e023065. doi: 10.1161/JAHA.121.023065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colloca A, Balestrieri A, Anastasio C, Balestrieri ML, D’Onofrio N. Mitochondrial sirtuins in chronic degenerative diseases: new metabolic targets in colorectal cancer. Int J Mol Sci . 2022;23:3212. doi: 10.3390/ijms23063212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jing E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes . 2013;62:3404–3417. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peugnet V, Chwastyniak M, Mulder P, Lancel S, Bultot L, Fourny N, et al. Mitochondrial-targeted therapies require mitophagy to prevent oxidative stress induced by SOD2 inactivation in hypertrophied cardiomyocytes. Antioxidants . 2022;11:723. doi: 10.3390/antiox11040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rehan M, Kurundkar D, Kurundkar AR, Logsdon NJ, Smith SR, Chanda D, et al. Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nat Aging . 2021;1:205–217. doi: 10.1038/s43587-021-00027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Gong W, Xu M, Zhang S, Shen J, Zhu M, et al. Necroptosis inhibition by hydrogen sulfide alleviated hypoxia-induced cardiac fibroblasts proliferation via sirtuin 3. Int J Mol Sci . 2021;22:11893. doi: 10.3390/ijms222111893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu C, Han J, Jia D, Cai J, Yuan J, Ge X. SIRT3 confers protection against acute pulmonary embolism by anti-inflammation, anti-oxidative stress, anti-apoptosis: participation of AMPK/mTOR pathway. Exp Anim . 2023 doi: 10.1538/expanim.22-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bao J, Lu Z, Joseph JJ, Carabenciov D, Dimond CC, Pang L, et al. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J Cell Biochem . 2010;110:238–247. doi: 10.1002/jcb.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science . 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang L, Li Q, Huang L, Li D, Li X. Sirt3 binds to and deacetylates mitochondrial pyruvate carrier 1 to enhance its activity. Biochem Biophys Res Commun . 2015;468:807–812. doi: 10.1016/j.bbrc.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 34. Ji Z, Liu GH, Qu J. Mitochondrial sirtuins, metabolism, and aging. J Genet Genomics . 2022;49:287–298. doi: 10.1016/j.jgg.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 35. Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol . 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol . 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 37. Di LJ, Byun JS, Wong MM, Wakano C, Taylor T, Bilke S, et al. Genome-wide profiles of CtBP link metabolism with genome stability and epithelial reprogramming in breast cancer. Nat Commun . 2013;4:1449. doi: 10.1038/ncomms2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao L, Ashek A, Wang L, Fang W, Dabral S, Dubois O, et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation . 2013;128:1214–1224. doi: 10.1161/CIRCULATIONAHA.113.004136. [DOI] [PubMed] [Google Scholar]
- 39. Iqbal M, Cawthon D, Wideman RF, Jr, Bottje WG. Lung mitochondrial dysfunction in pulmonary hypertension syndrome. I. Site-specific defects in the electron transport chain. Poult Sci . 2001;80:485–495. doi: 10.1093/ps/80.4.485. [DOI] [PubMed] [Google Scholar]
- 40. Sharp J, Farha S, Park MM, Comhair SA, Lundgrin EL, Tang WH, et al. Coenzyme Q supplementation in pulmonary arterial hypertension. Redox Biol . 2014;2:884–891. doi: 10.1016/j.redox.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Zervopoulos SD, Boukouris AE, Lorenzana-Carrillo MA, Saleme B, Webster L, et al. SNPs for genes encoding the mitochondrial proteins sirtuin3 and uncoupling protein 2 are associated with disease severity, type 2 diabetes, and outcomes in patients with pulmonary arterial hypertension and this is recapitulated in a new mouse model lacking both genes. J Am Heart Assoc . 2021;10:e020451. doi: 10.1161/JAHA.120.020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jia Z, Yan H, Wang S, Wang L, Cao Y, Lin S, et al. Shufeiya recipe improves monocrotaline-induced pulmonary hypertension in rats by regulating SIRT3/FOXO3a and its downstream signaling pathways. Dis Markers . 2022;2022:3229888. doi: 10.1155/2022/3229888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waypa GB, Osborne SW, Marks JD, Berkelhamer SK, Kondapalli J, Schumacker PT. Sirtuin 3 deficiency does not augment hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol . 2013;49:885–891. doi: 10.1165/rcmb.2013-0191OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stenmark KR, Nozik-Grayck E, Gerasimovskaya E, Anwar A, Li M, Riddle S, et al. The adventitia: essential role in pulmonary vascular remodeling. Compr Physiol . 2011;1:141–161. doi: 10.1002/cphy.c090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature . 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 46. Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, et al. Biosensor reveals multiple sources for mitochondrial NAD+ Science . 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verdin E. The many faces of sirtuins: coupling of NAD metabolism, sirtuins and lifespan. Nat Med . 2014;20:25–27. doi: 10.1038/nm.3447. [DOI] [PubMed] [Google Scholar]
- 48. Yoshino J, Baur JA, Imai SI. Nad(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab . 2018;27:513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grootaert MOJ, Bennett MR. Sirtuins in atherosclerosis: guardians of healthspan and therapeutic targets. Nat Rev Cardiol . 2022;19:668–683. doi: 10.1038/s41569-022-00685-x. [DOI] [PubMed] [Google Scholar]
- 50. Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE . 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giralt A, Hondares E, Villena JA, Ribas F, Díaz-Delfín J, Giralt M, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J Biol Chem . 2011;286:16958–16966. doi: 10.1074/jbc.M110.202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK. Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem . 2017;292:17312–17323. doi: 10.1074/jbc.M117.778720. [DOI] [PMC free article] [PubMed] [Google Scholar]